Abstract
Background
Ambulatory clinics attend to COVID-19 patients, often in spaces with less than ideal ventilation. Testing and treatments can often include aerosol-generating procedures. Portable high efficiency particulate air (HEPA) filtration units have been used to remove airborne contaminants in these areas.
Methods
A particle counter was used to evaluate the effectiveness of portable HEPA filtration units when a proxy airborne contaminant (powder) was actuated into the air. The Center for Disease Control and Prevention's (CDC) Airborne Contaminant Removal table served as a basis for initial particle readings at 6 minutes.
Results
Percent decrease was calculated post powder actuation at the 6-minute and 12-minute mark. There was a statistically significant decrease in smaller particles at the 6-minute and 12-minute mark when the HEPA filtration units were used.
Conclusion
As an adjunct infection control intervention, portable HEPA filtration units can make outpatient exam rooms safer for patients and staff by decreasing cumulative airborne particles.
Key Words: Portable HEPA filtration, Portable HEPA units, Air scrubber, Airborne transmission, Engineering controls, Exam room
Introduction
During the beginning of 2020, as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic swelled in the United States, many healthcare facilities began exploring engineering controls to mitigate the respiratory spread of illness within their facilities. The CDC recognizes that SARS-CoV-2 spreads more easily indoors where the concentration of viral particles is often higher, and endorses ventilation strategies to reduce viral particle concentration.1
Ideally, patients with suspected or confirmed COVID-19 would be placed in airborne infection isolation rooms (AIIR) where air is exhausted to the outside with at least 12 air changes an hour (ACH).2 While AIIR rooms are available in limited number in acute care hospitals, most ambulatory clinics do not have any rooms that meet these engineering recommendations. The building design of many ambulatory clinics presents further challenges when considering older heating, ventilation, and air conditioning (HVAC) systems, windows that do not open, and repurposed space used as exam rooms. When patient volumes are high, this could mean an influx of sick patients with less than ideal ventilation. When a symptomatic COVID-19 patient is seen and tested in an exam room (of note, nasopharyngeal specimen collection is considered an aerosol generating procedure when this study was performed), there should be some consideration to patients who will occupy that room after the sick patients whose COVID-19 status may be unknown. These patients are not fit tested for a respirator and do not have eye protection available.
It is incumbent on the healthcare facility to recognize and mitigate risks of transmission. The CDC has recognized inhalation as one of the three principal ways SARS-CoV-2 spreads with the other ways being: deposition (of droplets and particles on mucous membranes) and touching.3 The CDC further asserts that larger droplets settle faster than smaller droplets. Droplet size has been categorized in various studies with different possible implications. World Health Organization categorizes larger droplet particles (>5-10 μm in diameter) as respiratory droplets, but anything <5 μm in diameter as droplet nuclei which are associated with airborne transmission.4 Anand and Mayya posit that “the virus-laden droplets of sizes of about 20 μm (equivalent to ∼ 10 μm desiccated residue diameter) or less ejected from human ejecta of infected persons are matter of potential concern from the hazard perspective of viral transmission by airborne route in confined environments.”5 Human ejecta does not limit itself to one size particle and this complexity within the context of a global pandemic often lends itself to creative risk mitigation.
The CDC and The American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE) have endorsed using portable HEPA units to assist with ventilation for suboptimal areas where other ventilation options are not available to assist in clearing potentially infectious particles.6 The CDC provides an air contaminant removal table based on air changes per hour corresponding to how many minutes would need to transpire to reach a desired percent decrease in air contaminants.7 , 8 This table is assuming a perfect air mix which is unlikely in real world scenarios. This study evaluated the efficacy of portable HEPA units (“air scrubbers”) while using the CDC air contaminant removal table as a guide.
Materials and methods
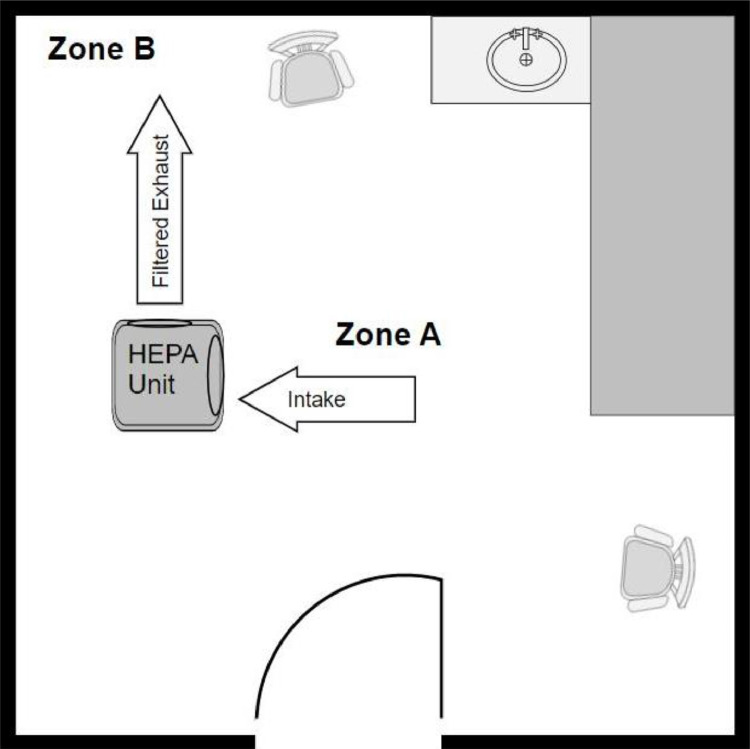
Two BlueDri 550 portable HEPA filtration units (Aerindustries Azusa, CA) were utilized which have an airflow range of 250-500 cubic feet per minute (CFM) of which only the maximum cfm (500) setting was used. Three exam rooms were utilized, and a schedule was designed wherein two exam rooms would serve as the interventional rooms containing a HEPA unit and a third exam room would not have a HEPA unit, serving as the control. The volume of each exam room was 643 ft3. The rooms were labeled (exam room #1, #2, and #3). The schedule randomized all three rooms as interventional or control, HEPA units (labeled #1 and #2), and whether the HEPA unit was on a chair or the floor using a random number generator. Each session involved testing three rooms, two experimental and one control. Each room had two designated areas labeled Zone A (in the center of the room) and Zone B (in a designated corner, see Fig. 1 ). Nine sessions were performed (2:1 experimental to control) for a total of 18 rooms tested with a HEPA unit and 9 rooms tested with no HEPA unit.
Fig 1.
Representation of the layout in an interventional room.
Glo Germ (Glo Germ Moab, UT) powder was used as a proxy air contaminant (1/16 of a tsp) for all sessions. This was aerosolized into the air using a DeVilbiss Powder Blower (DeVilbiss Healthcare Somerset, PA) until all visible powder was out of the glass bottle which was consistently around 40 actuations of the bulb of the powder blower.
To measure particles in the air, an Extech Particle Counter (VPC300 model) (Extech Nashua, NH) was employed. The particle counter was calibrated to National Institute of Standards and Technology (NIST) standards within six months of this study. The flow rate of the particle counter is 2.83L/min. Samples were taken in the cumulative mode with a 21 second sample time which yielded cumulative values for 6 different channels (particle sizes) of 0.3 µm, 0.5 µm, 1 µm, 2.5 µm, 5 µm, 10 µm. The particle counter was zeroed after each actuation of powder and again at the 6 and 12-minute mark readings.
ASHRAE provides the following formula to reach a desired air exchange rate in air changes per hour (ACH):6
The volume size of the small exam room used was 643ft3 and the HEPA unit was used at the max setting of 500 cfm:
This study used Table S3-1 from the CDC and Occupational Safety and Health Administration (OSHA) as a guide to determine when to initially take particle readings.7 , 8 At 46 air changes per hour, after 6 minutes there should theoretically be 99% removal efficiency (in situations of perfect air mixing). It was decided to double this and also take a second reading at 12 minutes given the reality of imperfect mixing. Particle readings were taken in Zone A in the center of the room and immediately following that, readings were taken in Zone B in the corner of the room.
For each session, three rooms were set up according to the randomization schedule; HEPA units were placed in two assigned interventional rooms and one room did not have a HEPA unit. The HEPA units were either placed in a chair with the intake facing the center of the room or on the floor with the intake facing the center of the room. Supply and return vents to each room were sealed using plastic to remove cycling of the HVAC system as a variable. Investigators donned goggles and N-95 respirators. Ambient (pre-interventional) particle count readings (all six particle sizes) were taken for each room and recorded in a lab log book for Zone A and Zone B. Glo Germ was aerosolized in Zone A using a powder blower. Immediately after actuation of the powder, particle readings were taken of Zone A and Zone B and recorded in the lab log book. At this point, in interventional rooms, the HEPA unit was turned on and a stop watch was started for 6 minutes. In the rooms without a HEPA filter, readings were taken after 6 minutes of actuation of the powder. All rooms had particle readings before actuation of powder, immediately post actuation of powder, and then at the 6 and 12-minute mark. Times were recorded for each particle reading in the lab log book for each zone. Particle readings were taken with the door closed and care was taken opening the door not to cause undue turbulence. Investigators consistently left the room between particle readings after powder was aerosolized. The particle counter was zeroed after post-actuation reading, after the 6-minute mark reading, and after the 12-minute mark reading.
Capturing 6 particle sizes for each zone at ambient, post-actuation, and at the 6-minute and 12-minute mark produced a large amount of data points (n = 1,296). This data was collated on a spreadsheet by room type (control vs. interventional), zone, and interval. Percent decrease was calculated using the formula 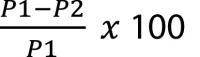 with P1 being the initial particle reading (post actuation) and P2 being the second particle reading (either at the 6 or 12-minute mark). These calculations were zone dependent so that Zone A (post actuation) was compared to Zone A at the 6 and 12-minute mark. The mean percent decrease of each data grouping was also calculated.
with P1 being the initial particle reading (post actuation) and P2 being the second particle reading (either at the 6 or 12-minute mark). These calculations were zone dependent so that Zone A (post actuation) was compared to Zone A at the 6 and 12-minute mark. The mean percent decrease of each data grouping was also calculated.
Results
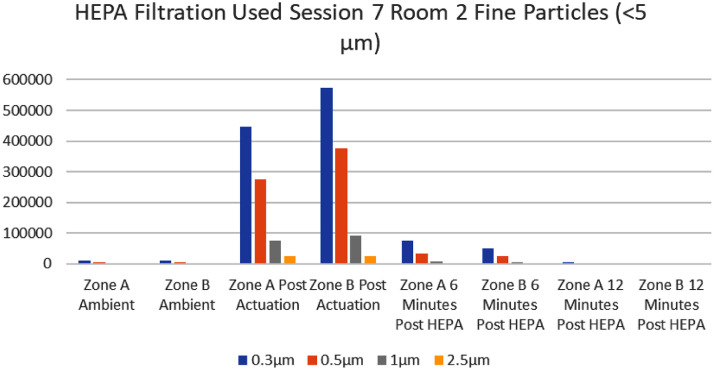
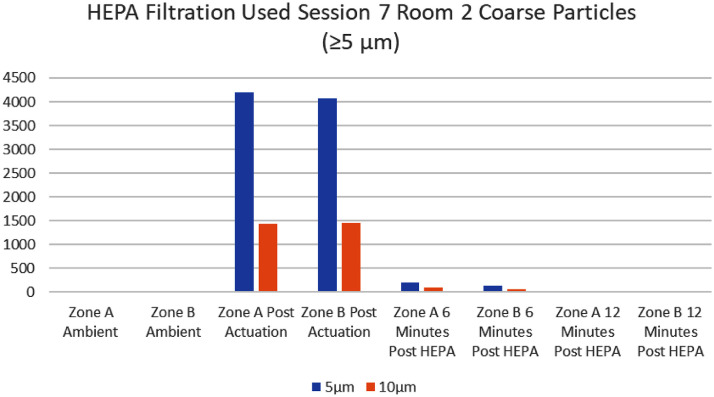
There was a consistent trend of a marked decrease in cumulative particle counts after the HEPA filter unit was turned on when compared to the control room (Fig 2, Fig 3 ). Every room (interventional and control) displayed an expected increase in cumulative particle capture for all sizes after actuation of the Glo Germ powder. All zones (A and B) in interventional rooms, except one, showed a marked decrease in cumulative particle count readings after 6 minutes of HEPA filtration. All zones (A and B) in interventional rooms showed a decrease in cumulative particle count readings from actuation of powder to the 12-minute mark.
Fig 2.
Interventional Rooms Where HEPA filtration was used. Particles < 5 µm.
Fig 3.
Interventional Rooms where HEPA filtration was used. Particles ≥ 5 µm.
Examining the one aberrant data set for Zone A for Session 3 Room 3, one can see a slight increase in cumulative particle count readings for sizes 0.5 µm, 1 µm, 2.5 µm, 5 µm and 10 µm at 6 minutes in Zone A despite HEPA filtration. The readings in Zone B a minute later conformed more to the usual observed pattern of decrease. The 12-minute readings for all sizes in Zone A for the same room (Session 3 Room 3) showed a less than expected decrease, while Zone B showed >99% decrease in particles from the initial post-actuation reading in Zone B. Readings from Zone A at the 6-minute and 12-minute mark will be excluded from analysis as an outlier but will be revisited in the discussion.
The mean percent decrease for interventional rooms at 6 minutes post HEPA filtration in Zone A was 92.71%. The mean percent decrease for interventional rooms at 6 minutes post HEPA filtration in Zone B was 95.99%. The mean percent decrease for interventional rooms at the 12-minute mark for Zones A and B were 99.49% and 99.47% respectively.
A chi-square analysis for differences in proportions was performed on a representative sample comparing an interventional room (with HEPA filtration) to a control room and this showed a statistically significant decrease in particles of sizes 0.3 µm, 0.5 µm, 1 µm (P= <.0001) at the 6-minute mark and a statistically significant decrease in particle sizes 0.3 µm, 0.5 µm, 1 µm, and 2.5 µm (P= <.0001) at the 12-minute mark. Larger particles are expected to fall regardless of HEPA filtration, so this is an expected finding.
In the majority of experiments, the resulting particle counts at 12 minutes were lower than the ambient (pre-intervention/actuation) particle count readings when the HEPA unit was used. Of the 216 data points (all particle sizes) at 12 minutes for both zones, only 14 data points showed an increase in cumulative particles from the baseline readings to the 12-minute mark post HEPA intervention, showing that most of the interventional rooms were cleaner after HEPA filtration than they were before powder was actuated.
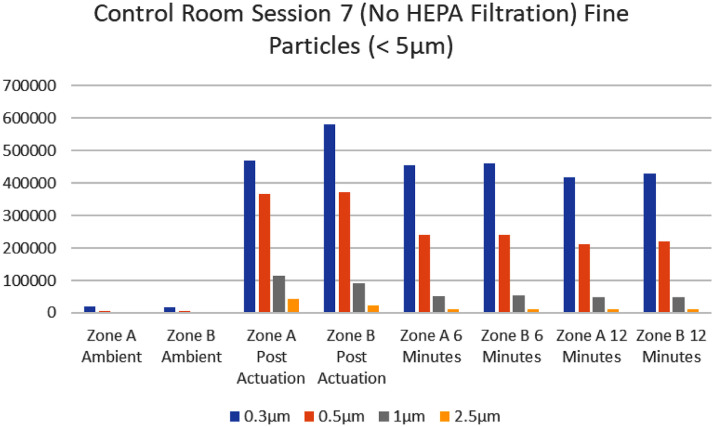
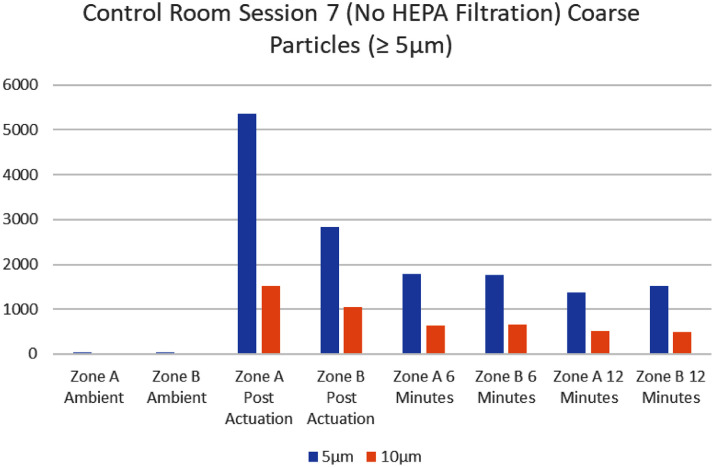
In contrast, the control rooms (where no HEPA filter was used) showed a noticeable carryover of higher cumulative readings in the 6 and 12 minute-mark readings (Fig 4, Fig 5 ). This was especially true for the smallest particles, where the concern for airborne transmission of disease occurs. Once the powder was actuated, particles measuring 0.3 µm remained >150,000 for readings at both the 6-minute and 12-minute mark in Zones A and B (with a range of 192,661 to 492,727). The average pre-actuation ambient capture for particles in control rooms measuring 0.3 µm was 10,080 with a range of 2,137 to 18,618 for Zones A and B. Of the 108 data points collected in Zone A and B (at the 6-minute mark post actuation of the powder), 18 showed an increase in particle readings as particles diffused in the room. At the 12-minute mark, 12 of the 108 data points showed an increase from the post-actuation reading. There was an observed general decrease in most data points from actuation to the 6-minute and 12-minute mark. Excluding the data points that showed increase, the largest percent decrease was seen in at the 12-minute mark for particles >2.5 µm where the average percent decrease was >50%. Generally larger particles are expected to fall.
Fig 4.
Control Room-Where NO HEPA Filtration was used. Particles < 5 µm.
Fig 5.
Control Room-Where NO HEPA filtration was used. Particles ≥ 5 µm.
The majority of ambient readings of particle size 0.3 µm were <20,000 (for control and interventional rooms) with one notable room having an ambient capture of 43,047 for the 3 µm particle size. After powder actuation, the majority readings of 0.3 µm particles captured measured >200,000.
Discussion
It became apparent that readings in Zone A post actuation of powder were often less than Zone B which were taken about a minute after the Zone A reading. With the actuation of the powder being aerosolized from Zone A in such a small exam room, the plume would inevitably travel away from the center. This phenomenon became observable early in the immediate post-actuation readings. Given the limitation of only having one instrument to measure, Zone B was always measured approximately a minute after the reading of Zone A. This could account for the higher percent decrease seen in Zone B.
Regarding the aberrant data set in one interventional room (Session 3 Room 3), some possible explanations might include contamination of the investigator with powder which re-aerosolized from clothing unexpectedly, or perhaps a less than ideal air pattern developed due to a chair or other furniture that was not foreseen and cannot be definitively determined in hindsight. The design schedule called for the HEPA unit to be on the floor during this session, but this phenomenon was not seen in the other seven experiments where the HEPA unit was placed on the ground.
The powder used, which shows bright white under black light, did not show any noticeable pattern when checked during testing and as such no unforeseen patterns that might be of interest were noted on the floor or in the room when checked with the black light. The powder used was dry and would not show the usual desiccation process of wet droplets.
It should be noted that the average percent decrease in 0.3 µm particle size had no real change in the control room (once powder was actuated cumulative counts remained >150,000 at the 6-minute and 12-minute mark). In the interventional rooms, for particle size 0.3 µm, there was between an 80%-93% decrease by 6 minutes and 98.4%-99.6% average decrease by 12 minutes (cumulative counts for 0.3. µm were all <7,000 at the 12-minute mark). One could extrapolate that the HEPA filter effectively decreased fine particles which can reach the lungs.
This study did not look at particles < 0.3 µm due to instrument limitation. The SARS-CoV-2 virus has a diameter less than 0.3 µm but is often found in larger droplets. The HEPA filter itself has a 0.3 µm filter but does filter smaller particles using diffusion/Brownian motion. It would have been helpful to have had instrumentation that reflects ultrafine particle capture.
Additional limitations included an imprecise method of powder aerosolization. In real world situations, not all aerosolizations (sneeze or cough etc.) produce the same cumulative particle counts, so the percent decrease post intervention was the significant method used to assess for change. It would have been interesting to have a precise number of particles (though theoretically difficult) as this would facilitate data display and trend recognition which imperfect delivery of powder complicates. Trends of cumulative capture for each particle size was observed as the experiments progressed.
Table S3-1 (Air Contaminant Removal Table) is a publicly available resource to calculate air contaminant removal based on air changes per hour. The caveat with the table lies in the fact that its own assertions assume a perfect mixing of air which is unlikely in real world application. But nevertheless, it provided a theoretical baseline timeframe wherein the experiment could evaluate air contaminant removal. The majority of the measurements at 6 minutes which corresponds to the S3-1 table did not reach 99% clearance. This clearance percentage came much closer at the 12-minute mark. There was still an impressive reduction in cumulative particle count readings at 6 minutes when compared to the control room.
It would be beneficial for further studies to evaluate HEPA filter units in different sized rooms with different HVAC systems. All three rooms used in this study were of the same size which created an ease of replicating the experiment based on a calculated ACH. However, given the reality of a diversity of spaces in the outpatient healthcare market, further studies incorporating larger rooms and varying ACH would provide a more complete picture of portable HEPA filter unit capabilities.
Conclusion
Portable HEPA filter units can make the exam rooms safer for the next patient, and safer for the staff. Not all patients seen in outpatient clinics have COVID-19, so it is important that healthcare clinics minimize risks for the next patient seen in that space. Table S3-1 can only be used when the potential source for infectious aerosols (the patient) has left the room. This should not limit the use of the portable HEPA filter units while the patient is in the room but rather this provides a basis to start the clock to allow for contaminant clearance before the next patient occupies that space. The expected time shown in Table S3-1 will likely not produce the expected percent clearance of airborne contaminant given imperfect air mixing. In the small exam room used in this study, doubling the time from 6 minutes to 12 minutes after the patient leaves the room, provided a closer estimation of 99% clearance. The overall takeaway is that the longer the portable HEPA filter unit runs, the cleaner the air becomes which can make less than ideal ventilated spaces safer. While this seems self-apparent, this study sought to contextualize portable HEPA units for smaller exam rooms. Portable HEPA units should not replace appropriate PPE and N95 masks when aerosol-generating procedures are performed but, as an adjunct infection control intervention, these units can decrease inhalation risk by decreasing the overall particle concentrations in small exam rooms.
Footnotes
Conflicts of interest: None to report.
References
- 1.Centers for Disease Control and Prevention . 2021. Ventilation in Building.https://www.cdc.gov/coronavirus/2019-ncov/community/ventilation.html [Internet] Atlanta, Georgia. Available at: Accessed June 2, 2021. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Isolation precautions V.D. Airborne Precautions. Available at: https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Accessed June 2, 2021.
- 3.Centers for Disease Control and Prevention. Scientific brief: SARS-CoV-2 transmission [Internet]. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html. Accessed June 2, 2021. [PubMed]
- 4.World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions [Internet]. 2021. Available at: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. Accessed June 2, 2021.
- 5.Anand S, Mayya YS. Size distribution of virus laden droplets from expiratory ejecta of infected subjects. Sci Rep. 2020;10 doi: 10.1038/s41598-020-78110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Society of Heating, Refrigerating and air-conditioning engineers (ASHRAE). Filtration Disinfect [Internet]. 2020. Available at: https://www.ashrae.org/technical-resources/filtration-disinfection. Accessed June 2, 2021.
- 7.Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities [Internet]. 2003. Available at: https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html. Accessed June 2, 2021.
- 8.Occupational Safety and Health Administration (OSHA). Guidelines for preventing the transmission of mycobacterium tuberculosis in health-care facilities [Internet].1994. Available at:https://www.osha.gov/OshDoc/Directive_pdf/CPL_2_106_APP_A.pdf. Accessed June 2, 2021.