Abstract
Background: Liposarcoma of the larynx is a rare entity. Well-differentiated spindle cell liposarcoma of the larynx has not been yet reported in the literature. Case Report: We report the first case of well-differentiated spindle cell liposarcoma of the larynx on a 59-year-old male who presented with change of voice and phlegmy cough for several months. Laryngoscopy revealed a mucosal covered pedunculated mass on the supraglottis. Computerized tomography (CT) scan showed a low-attenuation mass causing moderate narrowing of the airway. The lesion was excised. Grossly, a 4.2 cm ovoid, solid and soft mass with homogeneously white-gray and rubbery cut surface was identified. Microscopic examination revealed a well-demarcated neoplasm composed of predominantly atypical and pleomorphic spindle cells distributed in collagenous stroma, with admixed adipocytes showing variation in cell size and rare lipoblasts. Immunohistochemical stains showed that the spindle cells were positive for MDM2, CDK4, and CD34. Overall, the histology and immunoprofile are consistent with a well-differentiated liposarcoma, spindle cell type. Due to the positive resection margin, the patient subsequently received endoscopic local re-excision with a carbon dioxide laser. He did well at 4 months after primary excision. Conclusion: This case illustrates that while well-differentiated spindle cell liposarcoma rarely occurs in the larynx, it should be considered in the differential diagnosis of patients with laryngeal lesions. A panel of immunohistochemistry markers including MDM2, CDK4 and CD34 is helpful to render accurate diagnosis. Wide excision with long-term follow-up is necessary for this rare variant of liposarcoma.
Keywords: Well-differentiated liposarcoma, spindle cell, larynx, MDM2, CDK4
Liposarcoma is the most common type of soft tissue sarcoma accounting for 20% of all mesenchymal malignancies (1). Liposarcomas of the head and neck represent only 3% of all liposarcomas and are often subcutaneous, low grade, early stage, and with fewer nodal metastases than liposarcomas in non-head and neck sites (2). Well-differentiated spindle cell liposarcoma (WDSCL) was first described by Des Tos AP et al. in 1994 (3). It represents a rare variant of well-differentiated liposarcoma composed predominantly of spindle cells admixed with atypical lipogenic cells. Histologically, the tumor contains CD34-positive spindle cells with slightly enlarged, fusiform nuclei in short fascicles in a collagenous or fibromyxoid stroma. WDSCL is frequently subcutaneous and occurs in the trunk, lower extremities, and head and neck region. It is a rare and locally aggressive growing mesenchymal tumor and may recur, whereas metastasis and dedifferentiation have been rarely reported. Therefore, wide local excision with long-term follow-up looking for recurrence and metastasis is necessary in this rare variant of liposarcoma (4,5). Herein we report the first case, to our knowledge, of WDSCL of the larynx.
Case Report
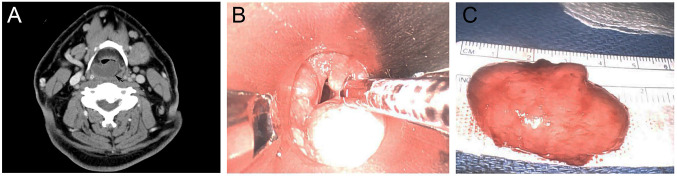
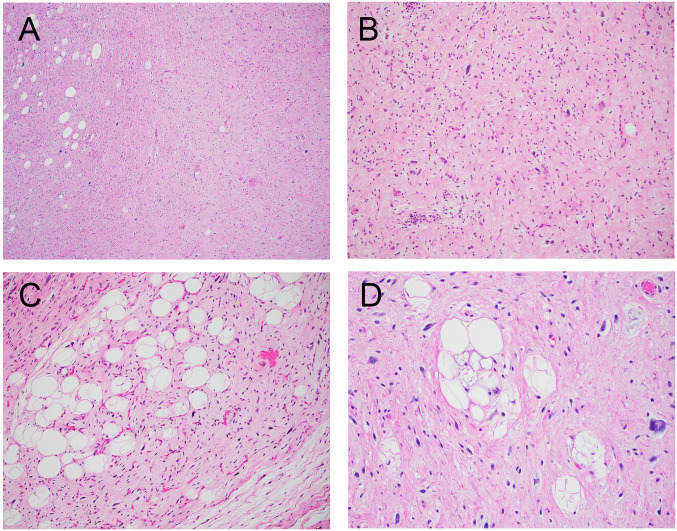
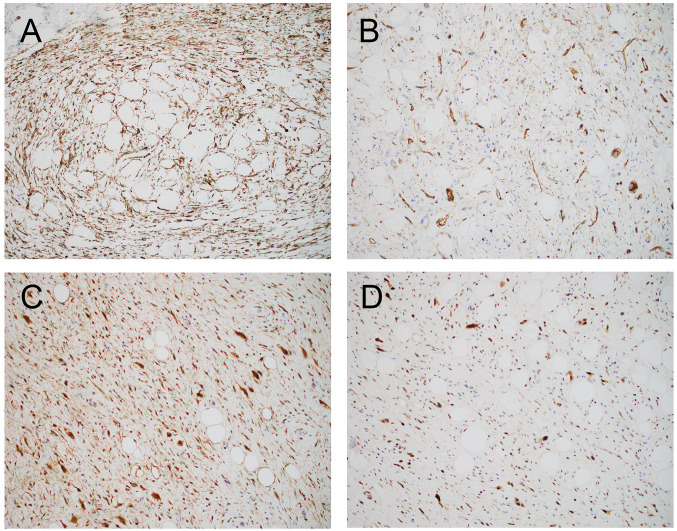
A 59-year-old male with no significant past medical history presented with change of voice and phlegmy cough for several months. Laryngoscopy revealed a mucosal covered pedunculated mass on the supraglottis. A computerized tomography (CT) scan of the neck showed a well-defined low-attenuation mass causing moderate narrowing of the airway (Figure 1A). A laryngoscopy-driven excisional biopsy was performed. Intraoperatively, a smooth solid mass extending from the right supraglottic region underneath the right arytenoid and interarytenoid mucosa was noted (Figure 1B). Grossly, the mass was ovoid, solid and soft with homogeneously white-gray and rubbery cut surface and measured 4.2 cm in greatest dimension (Figure 1C). Microscopically, sections of this mass revealed a well-demarcated neoplasm composed of predominantly atypical and focally pleomorphic spindle cells distributed in a prominent collagenous stroma, with admixed adipocytes showing variation in cell size and rare lipoblasts (Figure 2). There was no tumor necrosis. A rare mitotic figure was noted. Immunohistochemically, the spindle cells were strongly positive for MDM2 and CDK4. There was also multifocal positivity for CD34 and rare cells positive for desmin, while S100 protein was negative (Figure 3). Staining for retinoblastoma (Rb) was positive (normal/retained). The combined histology and immunoprofile are consistent with WDSCL. Due to positive resection margin, the patient subsequently received endoscopic local wide re-excision with carbon dioxide laser. At 4 months after primary excision, the patient was doing well with no evidence of recurrence or metastasis.
Figure 1. Radiological finding and gross image of laryngeal WDSCL. (A): CT scan showing a well-defined low-attenuation mass causing moderate narrowing of the airway. (B): Laryngoscopy revealing a pedunculated solid mass extending from the right supraglottis. (C): Gross picture of the mass showing a 4.2 cm ovoid and soft mass with smooth mucosal surface.
Figure 2. Histological features (Hematoxylin and Eosin staining) of laryngeal WDSCL. (A, B): Low- and high-power view of the tumor showing predominantly atypical and focally pleomorphic spindle cells admixed with adipocytes (C) in a fibromyxoid background. (D): High-power magnification showing nuclear pleomorphism and atypical vacuolated lipoblasts. Magnifications: A, ×20; B and C, ×100; D, ×200.
Figure 3. Immunohistochemical stains of laryngeal WDSCL. (A, B): Spindled tumor cells are diffusely positive for CD34. (C, D): Atypical and pleomorphic spindled tumor cells showing a strong nuclear expression of CDK4 (C) and MDM2 (D). Magnification: ×100.
Discussion
WDSCL occurs most commonly in the subcutaneous tissue of extremities, trunk, and neck and cheek region but rarely seen in the larynx. Morphologically, WDSCL consists of spindled tumor cells in short fascicles set in a fibrous, collagenous or fibromyxoid stroma. Areas of traditional lipoma-like morphology can be found. The differential diagnoses of this case include reactive or benign spindle cell lesions such as nodular fasciitis, fibromatosis, spindle cell lipoma, inflammatory myofibroblastic tumor, and leiomyoma, or malignant mesenchymal tumors such as liposarcoma, myxofibrosarcoma, malignant peripheral nerve sheath tumor, and low-grade fibromyxoid sarcoma. Laryngeal fibromatosis in adults is a rare, locally infiltrative and rapidly progressive disease (6). It is composed of relatively bland and collagenous spindled cells without significant cytological atypia or mitosis. Fibromatosis is characterized by mutations in the beta-catenin gene or the adenomatous polyposis coli (APC) gene, most are sporadic but some are associated with several syndromes such as Gardner’s syndrome. Diffuse nuclear beta-catenin stain is helpful to make the diagnosis. Laryngeal inflammatory myofibroblastic tumors consist of fibroblastic and myofibroblastic spindle cells in a myxoid background with significant mixed inflammatory infiltrate. The diagnosis can be confirmed by the presence of anaplastic lymphoma kinase (ALK) overexpression and by fluorescence in situ hybridization (FISH) rearrangement (7). Spindle cell lipoma is a benign lipomatous tumor composed of admixture of mature adipocytes and fibroblast-like spindle cells in myxoid stroma. Compared to liposarcoma, spindle cell lipoma does not contain lipoblasts and lacks MDM2 and CDK4 amplification (8). WDSCL and other sarcomas such as myxofibrosarcoma or low-grade fibromyxoid sarcoma share many clinical and histological features making the differential diagnosis difficult in some cases. Morphologic features that can help differentiate between those tumors include the presence of lipoblasts and prominent “chicken wire” pattern vasculature in WDSCL (9,10).
Ancillary tests including immunohistochemistry and molecular studies can be very helpful to make the correct diagnosis of WDSCL. Immunohistochemically, the spindle cells in all WDSCL cases are at least focally positive for CD34. Only a portion of the WDSCL cases were positive for MDM2 (5). Although MDM2 amplification has been estimated to occur in the majority of well-differentiated liposarcoma/atypical lipomatous tumors and dedifferentiated liposarcomas (~98%) (11), previous studies showed that soft tissue WDSCLs of the extremities do not contain MDM2 gene amplifications. Instead, it has been reported to harbor RB1 gene deletions and loss of RB expression (5,12). More recently, in-frame TRIO-TERT fusion gene has been described in a case of spindle cell liposarcoma of the thigh through next-generation sequencing (13). This type of in-frame TRIO-TERT fusion has been identified in some non-translocation-related sarcomas such as dedifferentiated liposarcoma, undifferentiated pleomorphic sarcoma, or leiomyosarcoma. The gene arrangement can lead to significantly increased TERT mRNA expression levels, causing increased telomerase activation in these sarcomas. The TRIO-TERT fusions have not been identified in any RB1-deleted spindle cell lipomatous tumors, suggesting that this may represent a biologically distinct pathway (14). Our case is unique in that the spindled tumor cells are strongly and diffusely positive for MDM2 and CDK4, consistent with amplification of these genes on the long arm of chromosome 12. No RB loss of expression is identified. The findings suggest that laryngeal WDSCL exhibits similar molecular alterations compared to conventional well-differentiated liposarcoma. Identifying molecular genetic alterations of this rare entity with additional cases in the future are required to better classify this type of spindle cell lipomatous neoplasm.
WDSCL is a locally aggressive mesenchymal tumor with recurrent potential and low risk of metastasis (5). Wide local excision with negative margins is the preferred treatment (15). In general, anatomic site is the most important prognostic factor for conventional well-differentiated liposarcoma, with higher risk of recurrence in tumors of body cavities compared to those in extremities (16). Our patient recovered uneventfully after the surgery. No evidence of recurrence or metastasis was noted at 4 months after primary excision. Due to the limited number of case reports, the prognosis of WDSCL, particularly those in the larynx, is still unknown.
In conclusion, we report the first case of WDSCL of the larynx which was completely excised. The results suggest that WDSCL should be considered in the differential diagnosis of patients presenting with a spindle cell neoplasm in the larynx. Ancillary tests are helpful to make the diagnosis. Long-term follow-up is required to monitor the progression of this rare tumor.
Conflicts of Interest
The Authors declare no conflicts of interest with regard to the study.
Authors’ Contributions
X.L. and D.Z. contributed to the design and implementation of the study, to the analysis of the results and to the writing of the article. S.F. contributed to the data collection.
References
- 1.Dei Tos AP. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol. 2000;4(4):252–266. doi: 10.1053/adpa.2000.8133. [DOI] [PubMed] [Google Scholar]
- 2.Gerry D, Fox NF, Spruill LS, Lentsch EJ. Liposarcoma of the head and neck: analysis of 318 cases with comparison to non-head and neck sites. Head Neck. 2014;36(3):393–400. doi: 10.1002/hed.23311. [DOI] [PubMed] [Google Scholar]
- 3.Dei Tos AP, Mentzel T, Newman PL, Fletcher CD. Spindle cell liposarcoma, a hitherto unrecognized variant of liposarcoma. Analysis of six cases. Am J Surg Pathol. 1994;18(9):913–921. doi: 10.1097/00000478-199409000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Laurino L, Furlanetto A, Orvieto E, Dei Tos AP. Well-differentiated liposarcoma (atypical lipomatous tumors) Semin Diagn Pathol. 2001;18(4):258–262. [PubMed] [Google Scholar]
- 5.Mentzel T, Palmedo G, Kuhnen C. Well-differentiated spindle cell liposarcoma (‘atypical spindle cell lipomatous tumor’) does not belong to the spectrum of atypical lipomatous tumor but has a close relationship to spindle cell lipoma: clinicopathologic, immunohistochemical, and molecular analysis of six cases. Mod Pathol. 2010;23(5):729–736. doi: 10.1038/modpathol.2010.66. [DOI] [PubMed] [Google Scholar]
- 6.Mirra M, Calò S, Salviato T, Libera DD, Falconieri G. Aggressive fibromatosis of the larynx: report of a new case in an adult patient and review of the literature. Pathol Res Pract. 2001;197(1):51–55. doi: 10.1078/0344-0338-00008. discussion 56-8. [DOI] [PubMed] [Google Scholar]
- 7.Pierry C, Pérot G, Karanian-Philippe M, Neuville A, Gomez-Brouchet A, Crestani S, Coindre JM. Polypoid laryngeal inflammatory myofibroblastic tumors: misleading lesions: description of six cases showing ALK overexpression. Am J Clin Pathol. 2015;144(3):511–516. doi: 10.1309/AJCPCG8D6JAQBVLG. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Huang H, He S, Wang W, Zhao R, Li L, Cui Z, Zhang R. Spindle cell lipoma: clinicopathologic characterization of 40 cases. Int J Clin Exp Pathol. 2019;12(7):2613–2621. [PMC free article] [PubMed] [Google Scholar]
- 9.Mentzel T, Calonje E, Wadden C, Camplejohn RS, Beham A, Smith MA, Fletcher CD. Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;20(4):391–405. doi: 10.1097/00000478-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: Clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60–67. doi: 10.1016/j.anndiagpath.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Weaver J, Downs-Kelly E, Goldblum JR, Turner S, Kulkarni S, Tubbs RR, Rubin BP, Skacel M. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol. 2008;21(8):943–949. doi: 10.1038/modpathol.2008.84. [DOI] [PubMed] [Google Scholar]
- 12.Mariño-Enriquez A, Nascimento AF, Ligon AH, Liang C, Fletcher CD. Atypical spindle cell lipomatous tumor: Clinicopathologic characterization of 232 cases demonstrating a morphologic spectrum. Am J Surg Pathol. 2017;41(2):234–244. doi: 10.1097/PAS.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 13.Suster DI, Deshpande V, Chebib I, Taylor MS, Mullen J, Bredella MA, Nielsen GP. Spindle cell liposarcoma with a TRIO-TERT fusion transcript. Virchows Arch. 2019;475(3):391–394. doi: 10.1007/s00428-019-02545-5. [DOI] [PubMed] [Google Scholar]
- 14.Creytens D, Mentzel T, Ferdinande L, Lecoutere E, van Gorp J, Atanesyan L, de Groot K, Savola S, Van Roy N, Van Dorpe J, Flucke U. “Atypical” pleomorphic lipomatous tumor: A clinicopathologic, immunohistochemical and molecular study of 21 cases, emphasizing its relationship to atypical spindle cell lipomatous tumor and suggesting a morphologic spectrum (Atypical spindle cell/pleomorphic lipomatous tumor) Am J Surg Pathol. 2017;41(11):1443–1455. doi: 10.1097/PAS.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 15.Endo M, Lin PP. Surgical margins in the management of extremity soft tissue sarcoma. Chin Clin Oncol. 2018;7(4):37. doi: 10.21037/cco.2018.08.10. [DOI] [PubMed] [Google Scholar]
- 16.Smith CA, Martinez SR, Tseng WH, Tamurian RM, Bold RJ, Borys D, Canter RJ. Predicting survival for well-differentiated liposarcoma: the importance of tumor location. J Surg Res. 2012;175(1):12–17. doi: 10.1016/j.jss.2011.07.024. [DOI] [PubMed] [Google Scholar]