Abstract
Background/Aim: The monoclonal antibody bevacizumab is a standard drug used in combination with oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) based chemotherapy in the first or second-line treatment of metastatic colorectal cancer (mCRC). Our previous study identified and subsequently validated 4 microRNAs in a small group of patients as predictors of the therapeutic response to bevacizumab combined with chemotherapy. The aim of this follow-up study is to confirm the predictive ability of these tissue miRNAs in a larger independent cohort of mCRC patients. Patients and Methods: The retrospective study included 92 patients with generalized-radically inoperable tumors treated with the combined therapy of bevacizumab/FOLFOX in a standard regimen. Results: Expression levels of candidate miRNA biomarkers (miR-92b-3p, miR-3156-5p, miR-10a-5p and miR-125a-5p) were determined in tumor tissue specimens and statistically evaluated. MiR-92b-3p and miR-125a-5p were confirmed to be associated with radiological response according to RECIST criteria (p=0.005 and 0.05, respectively) and to be up-regulated in responders to bevacizumab/FOLFOX therapy. Higher levels of miR-92b-3p were also significantly associated with extended progression-free survival (p=0.024). Conclusion: We have successfully confirmed miR-92b-3p to be up-regulated in tumor tissue of mCRC patients with good response to bevacizumab/FOLFOX therapy.
Keywords: Bevacizumab, metastatic colorectal cancer, microRNA, progression-free survival, predictive biomarker, validation
Colorectal cancer (CRC) is the most common cancer of the gastrointestinal tract and the third most common cancer after breast and lung (1). Despite significant efforts dedicated to early diagnosis of asymptomatic disease (screening), more than 20% of patients are still diagnosed with primarily metastatic disease. Even in the case of localized disease, approximately 50-60% subsequently develop a metastatic disease and in most cases this is initially unresectable. Patients with primarily unresectable metastatic disease are candidates for systemic treatment.
The cornerstone of systemic treatment of metastatic colorectal cancer are combined regimens based on fluoropyrimidines (fluorouracil/capecitabine) combined with oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) (2,3). The spectrum of treatment toxicities and the patient’s preferences are always an integral part of therapeutic planning. The effectiveness of both regimens is the same, differing in the spectrum of side effects. If the first-line oxaliplatin-based regimen is used, the irinotecan-based regimen may be used after its second-line failure and vice versa.
Chemotherapy is used in combination with targeted therapy. In metastatic colorectal cancer, the epidermal growth factor inhibitor (EGFR) is a druggable target with the monoclonal antibodies cetuximab and panitumumab available to target this receptor. Their indication is conditioned by the exclusion of the activation mutations in the oncogenes KRAS/NRAS. Another option is to use an anti-angiogenic strategy and combine chemotherapy with the monoclonal antibody bevacizumab targeting the vascular endothelial growth factor (VEGF). The indication for the combination of chemotherapy with bevacizumab is independent on RAS mutational status. Unfortunately, the clinical benefit is still limited and we are not able to predict the therapeutic response and identify patients who will benefit from the therapy.
MicroRNAs are small, non-coding RNAs that regulate gene expression at the mRNA level. They are well known to play important roles in carcinogenesis, whereas some of them have oncogenic functions while other act as tumor suppressors. MicroRNAs play important roles in the regulation of a wide range of cellular processes. They can functionally affect the cell cycle, apoptosis, migration, invasion as well as angiogenesis (4-6).
They are relatively resistant to degradation and contrary to mRNAs, they could be identified and quantified even in formalin-fixed paraffin-embedded (FFPE) tissues. This feature makes microRNAs potential biomarkers in metastatic colorectal cancer, including their possible usage as predictors of therapeutic response to bevacizumab (7-9).
In our previous study, we used a high-throughput approach and identified 4 candidate miRNAs (miR-92b-3p, miR-3156-5p, miR-10a-5p and miR-125a-5p) enabling us to predict the therapeutic response to bevacizumab/FOLFOX regimen in mCRC (7). Here, in this follow-up validation study, we aim to confirm their predictive potential in a larger independent cohort of mCRC patients treated with the bevacizumab/FOLFOX regimen.
Patients and Methods
Patients and study design. The study included 92 patients with generalized, radically inoperable metastatic colorectal cancer (mCRC) of the same ethnicity (Caucasian) treated with the combined therapy of bevacizumab/FOLFOX, in a standard regimen as the first-line treatment. All patients were treated in the Masaryk Memorial Cancer Institute from 2012 to 2016. Patient clinicopathological characteristics are summarized in Table I. The response to the Bevacizumab/FOLFOX treatment was assessed according to Response Evaluation Criteria in Solid Tumours (RECIST) criteria and duration of progression-free survival (PFS). Patients were stratified into good responders (complete or partial response i.e. responders) and a group with poor response (stable and/or progressive disease i.e. non-responders). The study protocol was approved by the local ethical board and written informed consent was obtained from all patients enrolled in the study.
Table I. Patient characteristics.
PFS: Progression-free survival; IQR: interquartile range.
RNA isolation and microRNA quantification. Total RNA enriched for small RNA was isolated from formalin-fixed paraffin-embedded (FFPE) samples by xylene deparaffinization, followed by purification by the mirVana™ miRNA Isolation Kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. The concentration and purity of RNA were determined by UV spectrophotometry using Nanodrop ND-1000 (Thermo Fisher Scientific, Waltham, MA, USA). Specific primers for miRNAs were used in reverse transcription and qPCR according to the TaqMan Advanced miRNA Assays (Thermo Fisher Scientific Inc.). qRT-PCR was performed on the QuantStudio 12K Flex Real-Time PCR System (Thermo Fisher Scientific Inc.). PCR reactions were run in triplicates and the average threshold cycle and standard deviation (SD) values were calculated.
Statistical analysis. Average expression levels of candidate miRNAs in qRT-PCR quantification were normalized to the expression of miR-6098 as endogenous control - selected based on our previous results (7). Normalized expression data were evaluated by the Mann-Whitney test, ROC analysis to identify cut-off values and Kaplan-Meier analysis followed by a long-rank test using GraphPad Prism v5.0 (GraphPad Software, La Jolla, CA, USA). p-Values lower than 0.05 were considered statistically significant.
Results
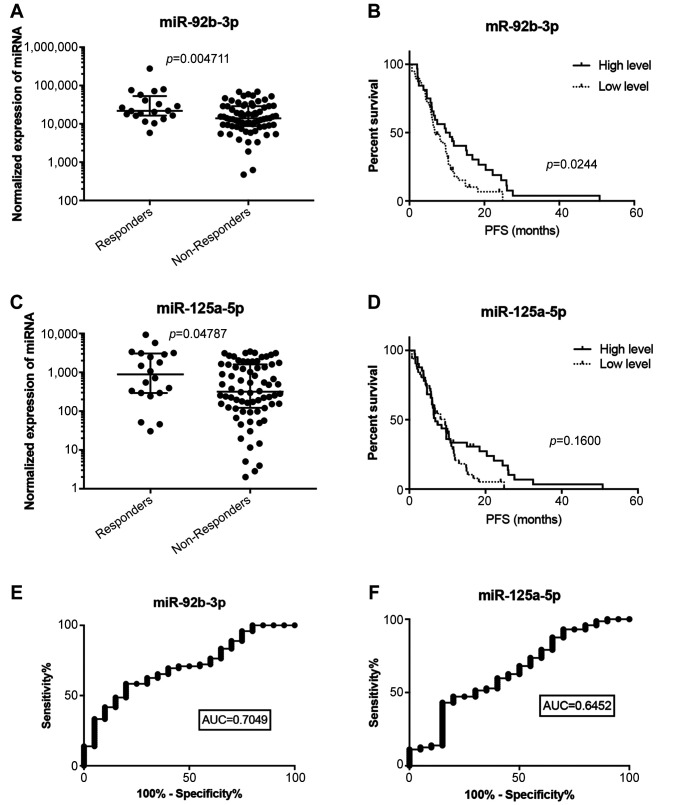
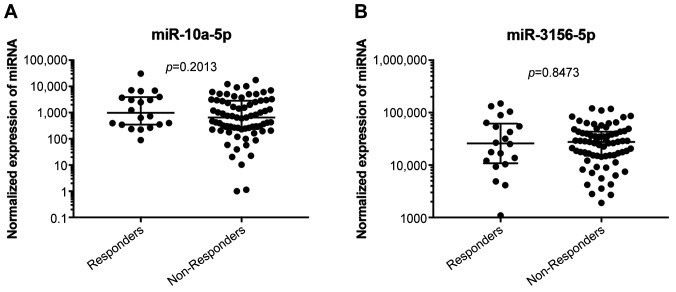
Expression levels of four candidate miRNAs identified and validated in the small validation cohort in our previous study (7) were selected for this independent validation study. Expression levels of these miRNAs (miR-92b-3p, miR-3156-5p, miR-10a-5p, and miR-125a-5p) were successfully determined in tumor tissue specimens of 92 patients with mCRC. MiR-92b-3p and miR-125a-5p were confirmed to be associated with radiological response according to the RECIST criteria [p=0.005, area under curve (AUC)=0.705 and p=0.05, AUC=0.645, respectively] (Figure 1A, C, E, F) and to be up-regulated in responders to bevacizumab/FOLFOX therapy. Expression levels of miR-3156-5p and miR-10a-5p did not differ significantly between the groups of patients with poor and good responses (Figure 2A, B). Kaplan-Meier analysis showed that only higher levels of miR-92b-3p are significantly associated with extended PFS (p=0.024) (Figure 1B). Association of miR-125a-5p and PFS did not reach statistical significance (Figure 1D).
Figure 1. MiRNAs associated with response to bevacizumab/FOLFOX therapy. Normalized expression of (A) miR-92b-3p and (C) miR-125a-5p in patients responding and non-responding to bevacizumab/FOLFOX therapy. Kaplan-Meier survival curves estimating time to progression in bevacizumab/FOLFOX-treated patients with metastatic colorectal cancer according to (B) miR-92b-3p and (D) miR-125a-5p. Receiver operating characteric (ROC) analysis of the use of (E) miR-92b-3p and (F) miR-125a-5p in discriminating between responders and non-responders to bevacizumab/FOLFOX therapy. PFS, Progression-free survival; AUC, area under the curve.
Figure 2. MiRNAs which are not associated with response to the bevacizumab/FOLFOX therapy. Normalized expression of (A) miR-10a-5p and (B) miR-3156-5p in patients responding and non-responding to bevacizumab/FOLFOX therapy.
Discussion
This retrospective validation study aimed to validate the ability of selected microRNAs to predict therapeutic response to bevacizumab treatment in combination with chemotherapy in the FOLFOX regimen. This is a standard treatment option for metastatic colorectal cancer. The addition of bevacizumab to FOLFOX chemotherapy resulted in a prolongation of time to progression (PFS) and overall patient survival of 1.4 months (2). Unfortunately, significant differences in the efficacy of this combined treatment in patients with metastatic colorectal cancer have been repeatedly observed (2). In our previous published study, we compared microRNAs in tumor tissue samples from patients with very good responses, i.e. those who achieved complete or partial remission with tumor tissue samples from patients with rapid disease progression on bevacizumab/FOLFOX. The results of our previous research indicated that there are four microRNAs showing a promising predictive potential in this setting (7). The aim of this follow-up study was to confirm the predictive ability of these tissue microRNAs in a larger independent cohort of metastatic colorectal cancer patients.
Out of 4 evaluated miRNAs (miR-92b-3p, miR-3156-5p, miR-10a-5p, and miR-125a-5p) only miR-92b-3p and miR-125a-5p were confirmed to have differential expression in tumors of mCRC patients with good and poor response to bevacizumab/FOLFOX therapy. MiR-125a-5p and also the miR-125a-3p were described as down-regulated in lung cancer cells, where they were shown to induce apoptosis by activating p53 (10,11). In breast cancer, miR-125a-5p is a tumor suppressor that targets HDAC4 and indicates prognostic functioning (12).
When tested in survival analysis for correlation with progression-free survival, only miR-92b-3p was confirmed to be up-regulated in tumor tissue of patients with good response to therapy. Taken together, miR-92b-3p was confirmed in an independent cohort of mCRC patients treated with bevacizumab/FOLFOX therapy to be associated with therapeutic response. This observation is in full agreement with the observation from our previous study (7). MiR-92b-3p is one of the miRNAs included in the recently developed tissue-derived miRNA profile to predict clinical benefit from chemotherapy evaluated in tissue biopsies and serum from patients with mCRC (9). Deregulated expression of miR-92b-3p has been reported in a variety of cancers; however, the results in terms of its biological role still remain controversial. Tumor suppressive activity of miR-92b-3p has been shown in esophageal (13) and pancreatic cancer (14). Higher levels of miR-92b-3p were associated with the absence of metastasis and good prognosis of esophageal cancer patients (13). In pancreatic cancer, the substitution of miR-92b-3p inhibited cell proliferation, migration and invasion (14). MiR-92b-3p inhibits proliferation and migration of murine-derived C2C12 myoblasts (15).
On the other hand, oncogenic functions of miR-92b-3p were described in glioblastoma cells, where it recovered the proliferative capacity of glioblastoma cells via the transforming growth factor-β signaling pathway (16). Similarly, in colorectal cancer, miR-92b-3p was shown to facilitate colorectal cancer cell proliferation, migration, and invasion (17). A recent study reported that miR-92b-3p is functionally linked to lncRNA SNHG14 and plays an essential role in cell proliferation, migration and invasion of CRC cells (18). As per the data above and the possible miR-92b-3p oncogenic role in CRC, its positive association with a good therapeutic response could be interpreted as counterintuitive. However, miR-92b-3p’s positive role in anti-angiogenic therapy is probably based on its functional link to hypoxia and angiogenesis. MiR-92b-3p was shown to act as a hypoxia-induced miRNA (hypoxamir) in the regulation of the mTOR signaling pathway (19). This regulatory mechanism also explains the role of miR-92b-3p in the pathological vascular smooth muscle cells and pulmonary artery smooth muscle cells proliferative response under hypoxia (20). MiR-92b-3p levels in tumor tissue could possibly reflect the extend of tumor neo-angiogenesis, high angiogenic density, and as a consequence, the response to anti-angiogenic treatment. Moreover, very recently, the potential of exosomes with overexpressed miR-92b-3p was shown to be effective in antiangiogenic therapy of an experimental model of ovarian cancer, confirming its functional involvement in the process of angiogenesis (21). From the diagnostic point of view, circulating miR-92b-3p was observed to be a promising marker in the monitoring of the chemoresistance and prognosis of small cell lung cancer (22) and synovial sarcoma (23).
Nevertheless, our validation study has several limitations and there is still a number of issues that need to be addressed in order to establish miR-92b-3p as a predictive biomarker to be used in the clinical setting. Our study is a single-center study based on the patients of the same ethnicity. To confirm the clinical benefit of our experimental biomarker, a prospective, multi-centric study based on larger independent patient cohorts has to be performed. The analytical performance of miR-92b-3p (AUC=0.705) is not sufficient for usage as a single biomarker; therefore, we expect this biomarker to be used in combination with other biomarkers or predictive scores.
Conclusion
Our previous study indicated a potential predictive value of miR-92b-3p, miR-3156-5p, miR-10a-5p and miR-125a-5p in mCRC treated with bevacizumab/FOLFOX therapy. We have successfully confirmed miR-92b-3p to be up-regulated in tumor tissue of mCRC patients with good response to this therapeutic regimen. MiR-92b-3p is a hypoxia-induced miRNA involved in angiogenesis, and, therefore, there is a possible link between the levels of this miRNA and the response to anti-angiogenic therapy in mCRC.
Conflicts of Interest
None.
Authors’ Contributions
Study design: DK, IK, MS; collection of data: TM, KS, IK, DK, MS, PF; data analysis: TM, KS, MS, PF, DK, IK; article preparation, editing, and review: DK, IK, MS, IKr.
Acknowledgements
The Authors acknowledge the CF Genomics of CEITEC supported by the NCMG research infrastructure (LM2015091 funded by MEYS CR) for their support with obtaining the scientific data presented in this paper. This study was supported by the Ministry of Health of the Czech Republic, by the Czech Health Research Council project, No. NU20-03-00127.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2018. . Available at: https://gco.iarc.fr/today. [Last accessed on June 8, 2021]
- 2.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 3.Pai SG, Fuloria J. Novel therapeutic agents in the treatment of metastatic colorectal cancer. World J Gastrointest Oncol. 2016;8(1):99–104. doi: 10.4251/wjgo.v8.i1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slaby O, Svoboda M, Michalek J, Vyzula R. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol Cancer. 2009;8:102. doi: 10.1186/1476-4598-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annese T, Tamma R, De Giorgis M, Ribatti D. microRNAs biogenesis, functions and role in tumor angiogenesis. Front Oncol. 2020;10:581007. doi: 10.3389/fonc.2020.581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goblirsch M, Richtig G, Slaby O, Berindan-Neagoe I, Gerger A, Pichler M. MicroRNAs as a tool to aid stratification of colorectal cancer patients and to guide therapy. Pharmacogenomics. 2017;18(10):1027–1038. doi: 10.2217/pgs-2017-0004. [DOI] [PubMed] [Google Scholar]
- 7.Kiss I, Mlčochová J, Součková K, Fabian P, Poprach A, Halamkova J, Svoboda M, Vyzula R, Slaby O. MicroRNAs as outcome predictors in patients with metastatic colorectal cancer treated with bevacizumab in combination with FOLFOX. Oncol Lett. 2017;14(1):743–750. doi: 10.3892/ol.2017.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiala O, Pitule P, Hosek P, Liska V, Sorejs O, Bruha J, Vycital O, Buchler T, Poprach A, Topolcan O, Finek J. The association of miR-126-3p, miR-126-5p and miR-664-3p expression profiles with outcomes of patients with metastatic colorectal cancer treated with bevacizumab. Tumour Biol. 2017;39(7):1010428317709283. doi: 10.1177/1010428317709283. [DOI] [PubMed] [Google Scholar]
- 9.Poel D, Gootjes EC, Bakkerus L, Trypsteen W, Dekker H, van der Vliet HJ, van Grieken NCT, Verhoef C, Buffart TE, Verheul HMW. A specific microRNA profile as predictive biomarker for systemic treatment in patients with metastatic colorectal cancer. Cancer Med. 2020;9(20):7558–7571. doi: 10.1002/cam4.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang L, Huang Q, Chang J, Wang E, Qiu X. MicroRNA HSA-miR-125a-5p induces apoptosis by activating p53 in lung cancer cells. Exp Lung Res. 2011;37(7):387–398. doi: 10.3109/01902148.2010.492068. [DOI] [PubMed] [Google Scholar]
- 11.Jiang L, Chang J, Zhang Q, Sun L, Qiu X. MicroRNA hsa-miR-125a-3p activates p53 and induces apoptosis in lung cancer cells. Cancer Invest. 2013;31(8):538–544. doi: 10.3109/07357907.2013.820314. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh TH, Hsu CY, Tsai CF, Long CY, Chai CY, Hou MF, Lee JN, Wu DC, Wang SC, Tsai EM. miR-125a-5p is a prognostic biomarker that targets HDAC4 to suppress breast tumorigenesis. Oncotarget. 2015;6(1):494–509. doi: 10.18632/oncotarget.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma G, Jing C, Li L, Huang F, Ding F, Wang B, Lin D, Luo A, Liu Z. MicroRNA-92b represses invasion-metastasis cascade of esophageal squamous cell carcinoma. Oncotarget. 2016;7(15):20209–20222. doi: 10.18632/oncotarget.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long M, Zhan M, Xu S, Yang R, Chen W, Zhang S, Shi Y, He Q, Mohan M, Liu Q, Wang J. miR-92b-3p acts as a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol Cancer. 2017;16(1):167. doi: 10.1186/s12943-017-0723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Z, Shi J, Ning Z, Hou L, Hu CY, Wang C. MiR-92b-3p inhibits proliferation and migration of C2C12 cells. Cell Cycle. 2020;19(21):2906–2917. doi: 10.1080/15384101.2020.1827511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu T, Wang H, Jiang M, Yan Y, Li W, Xu H, Huang Q, Lu Y, Chen J. The E3 ubiquitin ligase CHIP/miR-92b/PTEN regulatory network contributes to tumorigenesis of glioblastoma. Am J Cancer Res. 2017;7(2):289–300. [PMC free article] [PubMed] [Google Scholar]
- 17.Gong L, Ren M, Lv Z, Yang Y, Wang Z. miR-92b-3p promotes colorectal carcinoma cell proliferation, invasion, and migration by inhibiting FBXW7 in vitro and in vivo. DNA Cell Biol. 2018;37(5):501–511. doi: 10.1089/dna.2017.4080. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Duan W, Mo Z, Wang J, Yang W, Wu W, Li X, Lin S, Tan Y, Wei W. Upregulation of SNHG14 suppresses cell proliferation and metastasis of colorectal cancer by targeting miR-92b-3p. J Cell Biochem. 2020;121(2):1998–2008. doi: 10.1002/jcb.29434. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Heo J, Kang H. miR-92b-3p-TSC1 axis is critical for mTOR signaling-mediated vascular smooth muscle cell proliferation induced by hypoxia. Cell Death Differ. 2019;26(9):1782–1795. doi: 10.1038/s41418-018-0243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao X, Ma C, Chen S, Dang J, Cheng X, Zhu D. Reverse the down regulation of miR-92b-3p by hypoxia can suppress the proliferation of pulmonary artery smooth muscle cells by targeting USP28. Biochem Biophys Res Commun. 2018;503(4):3064–3077. doi: 10.1016/j.bbrc.2018.08.095. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Wang C, Li Y, Li M, Zhu T, Shen Z, Wang H, Lv W, Wang X, Cheng X, Xie X. Potential of peptide-engineered exosomes with overexpressed miR-92b-3p in anti-angiogenic therapy of ovarian cancer. Clin Transl Med. 2021;11(5):e425. doi: 10.1002/ctm2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Shan W, Hong B, Zou J, Li H, Han D, Zhang Y, Li L, Li D, Lin W. Circulating miR-92b and miR-375 for monitoring the chemoresistance and prognosis of small cell lung cancer. Sci Rep. 2020;10(1):12705. doi: 10.1038/s41598-020-69615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uotani K, Fujiwara T, Yoshida A, Iwata S, Morita T, Kiyono M, Yokoo S, Kunisada T, Takeda K, Hasei J, Numoto K, Nezu Y, Yonemoto T, Ishii T, Kawai A, Ochiya T, Ozaki T. Circulating MicroRNA-92b-3p as a novel biomarker for monitoring of synovial sarcoma. Sci Rep. 2017;7(1):14634. doi: 10.1038/s41598-017-12660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]