Abstract
Aim: There is very little literature reporting the association of matrix metalloproteinase-1 (MMP1) with personal susceptibility to bladder cancer. In the current study, we carried out the first examination of the contribution of MMP1 rs1799750 to bladder cancer risk in Taiwanese. Materials and Methods: A total of 375 bladder cancer cases and 375 healthy controls were genotyped for MMP1 rs1799750 via polymerase chain reaction-restriction fragment length polymorphism methodology and this was evaluated for association with clinicopathological factors. Results: The frequencies of MMP1 rs1799750 2G/2G, 1G/2G, and 1G/1G genotypes were 35.7%, 44.8% and 19.5% in the group with bladder cancer and 32.5%, 46.4%, and 21.1% in the healthy control group (p for trend=0.6362). The odds ratios (ORs) for bladder cancer risk after adjusting for age and gender for those carrying 1G/2G and 1G/1G genotypes at MMP1 rs1799750 were 0.88 (95% CI=0.62-1.24, p=0.4357) and 0.83 (95% CI=0.61-1.26, p=0.3990), respectively, compared with the wild-type 2G/2G genotype. In allelic frequency analysis, the adjusted OR for those carrying the 1G allele at MMP1 rs1799750 was 0.87 (95% CI=0.71-1.23, p=0.3479) compared to those people carrying a 2G allele. Conclusion: Our findings indicated that the genotypes at MMP1 rs1799750 appear to play little role in determining personal susceptibility to bladder cancer for Taiwanese.
Keywords: Genotype, MMP1, polymorphism, bladder cancer, Taiwanese
Bladder cancer is the 11th most common cancer, accounting for about 3% of total cancer deaths globally estimated in 2018 (1). In Taiwan, although the incidence rates of bladder cancer have decreased in recent years, the rapid aging of the population means bladder cancer remains a serious public burden, which has an impact on patients and their relatives, resulting in a massive financial problem for society overall (2,3). From the epidemiological viewpoint, tobacco smoking is believed to be the most critical risk factor for bladder cancer (4,5), in addition to diabetes, exposure to chemicals, contamination in drinking water by arsenic, overuse of traditional Chinese herbs and genetic variations (6-9). A better understanding about genetic predictive markers of bladder cancer is useful for personalized medication and therapy, and reducing the incidence of and death rate by bladder cancer.
In literature, mounting evidence has shown that genetic polymorphisms of matrix metalloproteinases (MMPs) may contribute to the development of bladder cancer (10). MMPs, also named as matrixins, are a large protein family controlling metabolism of the extracellular matrix (11-13). MMPs are closely related to a series of cell behaviors such as proliferation, inflammation, apoptosis, invasion, migration and angiogenesis (12,13). In literature, much evidence has shown that polymorphic variants of MMPs may be associated with personal susceptibility to several types of cancer (14-19).
In 2010, Chuang and colleagues examined the expression of MMPs in 30 bladder neoplasms, finding all were MMP1-positive, while other MMPs such as MMP2 and MMP9 were not so highly expressed (20). In addition, the overexpression of MMP1 was not correlated with tumor staging nor grading (20). The genomic role of MMP1 remains unclear. In literature, the most commonly studied polymorphic site of MMP1 is rs1799750 which located at −1,607 of the promoter of the MMP1 gene. The variants at this polymorphic site consist of the insertion (2G) polymorphism, which in mice was reported to lead to higher transcriptional activity of MMP1, potentially higher levels or rates of collagen breakdown, and higher levels of MMP1 in serum than in mice with 1G/1G genotype (21). A meta-analysis published in 2012 investigating about 10,000 cancer cases concluded that those MMP1 carrying the rs1799750 2G/2G genotype may have a slightly higher overall metastasis rate (22). In light of all the above, the aim of the current study was to examine the contribution of MMP1 rs1799750 genotype at the promoter region to the risk of bladder cancer in Taiwanese.
Materials and Methods
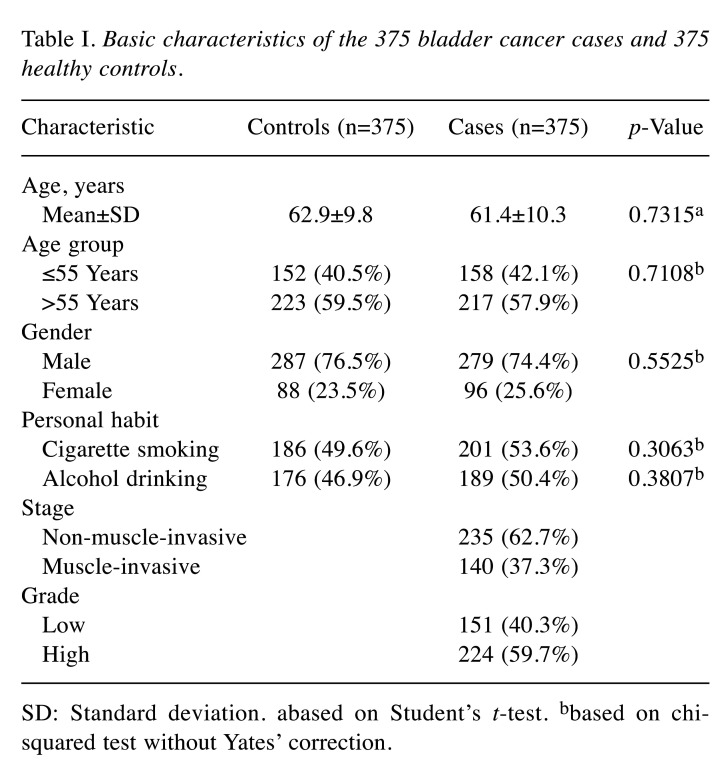
Patients with bladder cancer and matched controls. Totally, 375 cases diagnosed with bladder cancer were collected at the China Medical University Hospital in central Taiwan. At the same time, 375 controls matched for age and gender were selected from the Health Examination Cohort. The exclusion criteria for the controls were any previous malignancy, metastasized cancer, and any known familial or genetic diseases. The study design was approved by the Institutional Review Board of the China Medical University Hospital (DMR104-IRB-158). Selective demographic characteristics are summarized in Table I.
Table I. Basic characteristics of the 375 bladder cancer cases and 375 healthy controls.
SD: Standard deviation. abased on Student’s t-test. bbased on chisquared test without Yates’ correction.
Methodology for determination of MMP1 rs1799750 genotype. Genomic DNA from the blood of each participant was extracted, aliquoted and stored at −80˚C as routinely conducted (23-25). The MMP1 genotyping methodology was the same as in our recently published article (26). The polymerase chain reaction (PCR) conditions set at My Cycler (Biorad, Hercules, CA, USA) for MMP1 genotyping were initially one cycle at 94˚C for 5 min; followed by 35 cycles of 94˚C for 30 s, 57˚C for 30 s and 72˚C for 30 s, and a final extension at 72˚C for 10 min.
Methodology for statistical analysis and determination of significance. The descriptive statistics of bladder cancer cases and healthy controls are shown as the mean and standard deviation (SD) or as percentages. In Table I, Student’s t-test was adopted for the comparison of ages between the two groups. In Tables I-III, Pearson’s chi-square test without Yates’ correction was used to compare the distribution of the subgroups. In addition, associations were evaluated using adjusted odds ratios (aORs) with their corresponding 95% confidence intervals (CIs) with adjustment for age, gender, and personal habits. Differences were identified as being significant when the p-value was less than 0.05.
Results
Comparison of characteristics among the patient and the healthy control groups. Characteristics such as age, gender, personal habits, tumor stage and grades for the 375 control and 375 patients with bladder cancer are shown in Table I. Firstly, there was no difference found in the distribution of age and gender between the patient and healthy control groups since these frequencies were matched during recruiting of the non-cancer healthy controls. Secondly, neither smokers nor alcohol drinkers were found to be more frequent in the case group than in the control group (both p>0.05). It showed there was no difference in the frequency of smokers/drinkers between the two groups. Lastly, bladder cancer was mostly of non-muscle-invasive type (62.7%) and high clinical stage (59.7%).
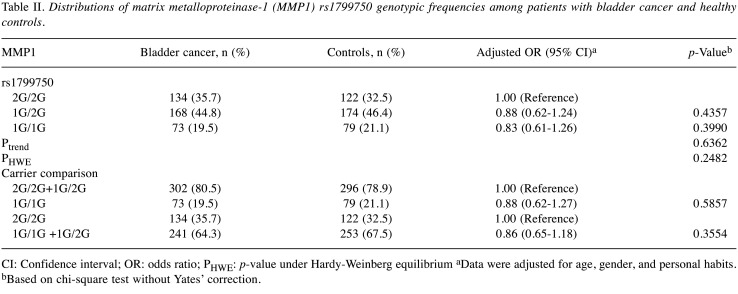
Association of MMP1 rs1799750 genotypes and bladder cancer risk. The genotypic analysis for the MMP1 rs1799750 among the controls and the patients with bladder cancer is shown in Table II. The MMP1 rs1799750 genotypic frequency distributions were not significantly different between the control and the case groups (p for trend=0.6362) (Table II). In detail, 1G/2G and 1G/1G variants at MMP1 rs1799750 seemed not to be associated with an elevated bladder cancer risk [aOR=0.88 (95% CI=0.62-1.24) and 0.83 (95% CI=0.61-1.26); p=0.4357 and 0.3990, respectively; Table II]. Compared with those carrying a 2G allele (2G/2G+1G/2G), homozygosity for the 1G allele at MMP1 rs1799750 conferred no risk for bladder cancer (aOR=0.88, 95% CI=0.62-1.27; p=0.5857) (Table II). Lastly, compared with those homozygous for the 2G allele, the analytic results still showed that carrying a 1G allele (1G/1G+1G/2G) at MMP1 rs1799750 did not alter bladder cancer risk (aOR=0.86, 95% CI=0.65-1.18; p=0.3554) (Table II). Overall, MMP1 rs1799750 seemed to have no direct contribution to the risk of bladder cancer.
Table II. Distributions of matrix metalloproteinase-1 (MMP1) rs1799750 genotypic frequencies among patients with bladder cancer and healthy controls.
CI: Confidence interval; OR: odds ratio; PHWE: p-value under Hardy-Weinberg equilibrium aData were adjusted for age, gender, and personal habits. bBased on chi-square test without Yates’ correction.
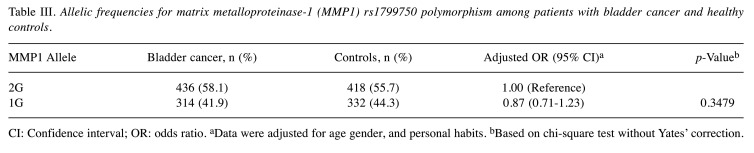
Bladder cancer risk according to MMP1 promoter allelic frequencies. The distributions of alleles for MMP1 rs1799750 among the patients with bladder cancer and healthy controls are shown in Table III. Consistent with the findings in Table II, analysis of allelic frequencies showed that the 1G allele at MMP1 rs1799750 was not significantly associated with the risk of bladder cancer in Taiwan (p=0.3479, adjusted OR=0.87, 95% CI=0.71-1.23) (Table III). In detail, the percentages of variant allelic frequencies in the groups of cases and controls were 41.9% and 44.3%, respectively (Table III). The results of analysis of MMP1 rs1799750 genotypes after stratifying by age, gender, smoking and alcohol consumption status showed no associations at all (all p>0.05, data not shown). Clinically, there was no significant difference in distributions between different stages and grades (both p>0.05, data not shown).
Table III. Allelic frequencies for matrix metalloproteinase-1 (MMP1) rs1799750 polymorphism among patients with bladder cancer and healthy controls.
CI: Confidence interval; OR: odds ratio. aData were adjusted for age gender, and personal habits. bBased on chi-square test without Yates’ correction.
Discussion
One of the major components of the extracellular matrix is collagen, the enzymatic substrate of MMP1. In esophageal and colorectal cancer tissues, MMP1 has been shown to be overexpressed and associated with higher risk of death (27,28). As early as 1979, a pilot study showed that muscle-invasive bladder cancer tissues have higher collagenase activity as their non-invasive counterparts (29). Later MMP1 was detected in the urine of patients with bladder cancer for the first time, and its concentration was found to be significantly increased in urine from patients with higher stage and grade (30). Elevated MMP1 has also been associated with poor survival of patients with bladder cancer (31). Notably, it has been reported that MMP1 was overexpressed in all tissues of fresh-prepared samples from patients with bladder cancer (n=30), while the overexpression of MMP1 was not correlated with tumor staging nor grading (20). It is hypothesized that inhered genomic variations may determine personal susceptibility to bladder cancer initiation, progression, invasion and metastasis.
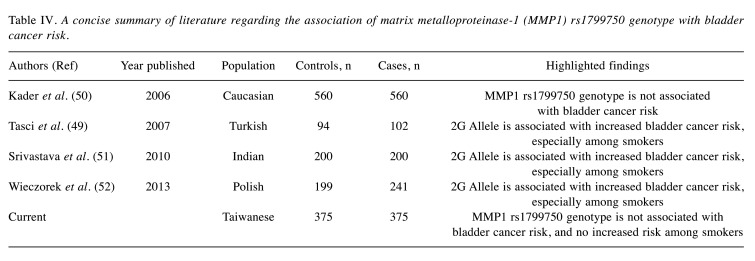
In the literature, associations of MMP1 genotypes have been examined among many types of cancer, such as lung (32,33), breast (34-36), oral (37), colorectal (38,39), gastric (40-42), endometrial (43), cervical (44,45), prostate (17,46), and ovarian (47,48), in addition to bladder cancer (49-52). Although there were several studies investigating the association of MMP1 genotypes with bladder cancer, the results were inconclusive and the total sample size is far from convincing, which we discuss later.
In the present study, it was found that the 1G allele of MMP1 rs1799750 was not significantly associated with risk of bladder cancer (Tables II and III). In addition, MMP1 rs1799750 genotypes were not associated with age, gender, nor smoking and alcohol consumption behaviors (data not shown). Compared with previous studies, our samples are more genetically conserved (all Taiwanese) and representative (375 patients with bladder cancer and 375 healthy controls). We have summarized previous articles on MMP1 in bladder cancer in Table IV. Our findings are consistent with that reported by Kader and colleagues (50) but not with others with relatively limited samples (49-52). There may be ethnic variations among the reports, and more investigations are needed. Another limitation of the study is that we did not measure the phenotypic status of the participants, and have no understanding the correlation of 1G allele at MMP1 rs1799750 and transcriptional activity. In addition, the associations between the expression level of MMP1 with other parameters, such as age, gender, smoking or alcohol consumption status, or tumor stage and grade, are not available. Chuang and colleagues analyzed 30 samples, finding that MMP2 was correlated with high-grade tumors and MMP9 was correlated with advanced tumor stage. However, the expression of MMP1, MMP3, and tissue inhibitor of metalloproteinase-1 and -2 was not correlated with either tumor staging or grading (20). Their data are valuable, however, there is no genotype–phenotype correlation.
Table IV. A concise summary of literature regarding the association of matrix metalloproteinase-1 (MMP1) rs1799750 genotype with bladder cancer risk.
In conclusion, this study examined the genotypic patterns of MMP1 rs1799750 among Taiwanese people. Neither MMP1 1G nor 2G allele appeared to contribute to susceptibility of bladder cancer. Some other markers, for instance single nucleotide polymorphisms on other MMPs may play more critical roles than MMP1, and further investigations are needed to elucidate their role in bladder cancer etiology.
Conflicts of Interest
The Authors have declared no conflicts of interest.
Authors’ Contributions
Research design: Liao CH, Tsai CW and Chang WS; patient and questionnaire summaries: Liao CH, Wu HC and Wang BR; experimental work: Gong CL, Chang WS and Wang ZH; statistical analysis: Hsu SW, Huang WC and Shen TC; article writing: Tsai CW and Bau DT; review and revision: Bau DT.
Acknowledgements
The Authors thank Tissue-bank of China Medical University Hospital for technical assistance in data and sample collection. This study was supported by the Taichung Armed Forces General Hospital to Dr. Liao (TCAFGH-D-109021) and by Asia University and China Medical University Hospital to Drs. Shen and Gong (CMU109-ASIA-04).
References
- 1.Cai Z, Liu Q. Understanding the Global Cancer Statistics 2018: implications for cancer control. Sci China Life Sci. 2021;64(6):1017–1020. doi: 10.1007/s11427-019-9816-1. [DOI] [PubMed] [Google Scholar]
- 2.Chang WS, Tsai CW, Ji HX, Wu HC, Chang YT, Lien CS, Liao WL, Shen WC, Tsai CH, Bau DT. Associations of cyclooxygenase 2 polymorphic genotypes with bladder cancer risk in Taiwan. Anticancer Res. 2013;33(12):5401–5405. [PubMed] [Google Scholar]
- 3.Chang WS, Liao CH, Tsai CW, Hu PS, Wu HC, Hsu SW, Hsiao CL, Hsu CH, Hung YW, Bau DT. Association of enhancer of Zeste 2 (EZH2) genotypes with bladder cancer risk in Taiwan. Anticancer Res. 2016;36(9):4509–4514. doi: 10.21873/anticanres.10997. [DOI] [PubMed] [Google Scholar]
- 4.Cumberbatch MG, Rota M, Catto JW, La Vecchia C. The role of tobacco smoke in bladder and kidney carcinogenesis: A comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016;70(3):458–466. doi: 10.1016/j.eururo.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 5.van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP. Quantified relations between exposure to tobacco smoking and bladder cancer risk: a meta-analysis of 89 observational studies. Int J Epidemiol. 2016;45(3):857–870. doi: 10.1093/ije/dyw044. [DOI] [PubMed] [Google Scholar]
- 6.Lai MN, Wang SM, Chen PC, Chen YY, Wang JD. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst. 2010;102(3):179–186. doi: 10.1093/jnci/djp467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang HY, Wang JD, Lo TC, Chen PC. Occupational exposure to herbs containing aristolochic acids increases the risk of urothelial carcinoma in Chinese herbalists. J Urol. 2013;189(1):48–52. doi: 10.1016/j.juro.2012.08.090. [DOI] [PubMed] [Google Scholar]
- 8.Poon SL, Huang MN, Choo Y, McPherson JR, Yu W, Heng HL, Gan A, Myint SS, Siew EY, Ler LD, Ng LG, Weng WH, Chuang CK, Yuen JS, Pang ST, Tan P, Teh BT, Rozen SG. Mutation signatures implicate aristolochic acid in bladder cancer development. Genome Med. 2015;7(1):38. doi: 10.1186/s13073-015-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jhuang JR, Chiang CJ, Su SY, Yang YW, Lee WC. Reduction in the incidence of urological cancers after the ban on Chinese herbal products containing aristolochic acid: An interrupted time-series analysis. Sci Rep. 2019;9(1):19860. doi: 10.1038/s41598-019-56394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kader AK, Liu J, Shao L, Dinney CP, Lin J, Wang Y, Gu J, Grossman HB, Wu X. Matrix metalloproteinase polymorphisms are associated with bladder cancer invasiveness. Clin Cancer Res. 2007;13(9):2614–2620. doi: 10.1158/1078-0432.CCR-06-1187. [DOI] [PubMed] [Google Scholar]
- 11.de Souza AP, Trevilatto PC, Scarel-Caminaga RM, Brito RB, Line SR. MMP-1 promoter polymorphism: association with chronic periodontitis severity in a Brazilian population. J Clin Periodontol. 2003;30(2):154–158. doi: 10.1034/j.1600-051x.2003.300202.x. [DOI] [PubMed] [Google Scholar]
- 12.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 13.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82(6):1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CW, Chang WS, Gong CL, Shih LC, Chen LY, Lin EY, Li HT, Yen ST, Wu CN, Bau DT. Contribution of matrix metallopeptidase-1 genotypes, smoking, alcohol drinking and areca chewing to nasopharyngeal carcinoma susceptibility. Anticancer Res. 2016;36(7):3335–3340. [PubMed] [Google Scholar]
- 15.Sun KT, Tsai CW, Chang WS, Shih LC, Chen LY, Tsai MH, Ji HX, Hsiao CL, Liu YC, Li CY, Bau DT. The contribution of matrix metalloproteinase-1 genotype to oral cancer susceptibility in Taiwan. In Vivo. 2016;30(4):439–444. [PubMed] [Google Scholar]
- 16.Wu MH, Tzeng HE, Wu CN, Yueh TC, Peng YC, Tsai CH, Wang YC, Ke TW, Pei JS, Chang WS, Tsai CW, Bau DT. Association of matrix metalloproteinase-9 rs3918242 promoter genotypes with colorectal cancer risk. Anticancer Res. 2019;39(12):6523–6529. doi: 10.21873/anticanres.13867. [DOI] [PubMed] [Google Scholar]
- 17.Liao CH, Wu HC, Hu PS, Hsu SW, Shen TC, Hsia TC, Chang WS, Tsai CW, Bau DT. The association of matrix metalloproteinase-1 promoter polymorphisms with prostate cancer in Taiwanese patients. Anticancer Res. 2018;38(7):3907–3911. doi: 10.21873/anticanres.12675. [DOI] [PubMed] [Google Scholar]
- 18.Yueh TC, Wu CN, Hung YW, Chang WS, Fu CK, Pei JS, Wu MH, Lai YL, Lee YM, Yen ST, Li HT, Tsai CW, Bau DT. The contribution of MMP-7 genotypes to colorectal cancer susceptibility in Taiwan. Cancer Genomics Proteomics. 2018;15(3):207–212. doi: 10.21873/cgp.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen TC, Chang WS, Tsai CW, Chao CY, Lin YT, Hsiao CL, Hsu CL, Chen WC, Hsia TC, Bau DT. The contribution of matrix metalloproteinase-1 promoter genotypes in Taiwan lung cancer risk. Anticancer Res. 2018;38(1):253–257. doi: 10.21873/anticanres.12215. [DOI] [PubMed] [Google Scholar]
- 20.Chuang CK, Pang ST, Chuang TJ, Liao SK. Profiling of matrix metalloproteinases and tissue inhibitors of metalloproteinases proteins in bladder urothelial carcinoma. Oncol Lett. 2010;1(4):691–695. doi: 10.3892/ol_00000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tower GB, Coon CI, Brinckerhoff CE. The 2G single nucleotide polymorphism (SNP) in the MMP-1 promoter contributes to high levels of MMP-1 transcription in MCF-7/ADR breast cancer cells. Breast Cancer Res Treat. 2003;82(2):75–82. doi: 10.1023/B:BREA.0000003948.14026.7c. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Guo H, Li Y, Xu X, Yang K, Bai Y. Association between polymorphisms in the promoter regions of matrix metalloproteinases (MMPs) and risk of cancer metastasis: a meta-analysis. PLoS One. 2012;7(2):e31251. doi: 10.1371/journal.pone.0031251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen GL, Wang SC, Shen TC, Chang WS, Lin C, Hsia TC, Bau DT, Tsai CW. Significant association of chitinase 3-like 1 genotypes to asthma risk in Taiwan. In Vivo. 2021;35(2):799–803. doi: 10.21873/invivo.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen KY, Chien WC, Liao JM, Tsai CW, Chang WS, Su CH, Hsu SW, Wang HC, Bau DT. Contribution of interleukin-10 genotype to triple negative breast cancer risk. Anticancer Res. 2021;41(5):2451–2457. doi: 10.21873/anticanres.15020. [DOI] [PubMed] [Google Scholar]
- 25.Wu CN, Chang WS, Shih LC, Wang YC, Lee HT, Yu CC, Wang ZH, Mong MC, Hsia TC, Tsai CW, Bau DT. Interaction of DNA repair gene XPC with smoking and betel quid chewing behaviors of oral cancer. Cancer Genomics Proteomics 18(3. 2021;Suppl):441–449. doi: 10.21873/cgp.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai CB, Hsia NY, Wang YC, Wang ZH, Chin YT, Huang TL, Yu CC, Chang WS, Tsai CW, Yin MC, Bau DT. The significant association of MMP-1 genotypes with Taiwan pterygium. Anticancer Res. 2020;40(2):703–707. doi: 10.21873/anticanres.14000. [DOI] [PubMed] [Google Scholar]
- 27.Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2(4):461–462. doi: 10.1038/nm0496-461. [DOI] [PubMed] [Google Scholar]
- 28.Murray GI, Duncan ME, O’Neil P, McKay JA, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998;185(3):256–261. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Wirl G, Frick J. Collagenase—a marker enzyme in human bladder cancer. Urol Res. 1979;7(2):103–108. doi: 10.1007/BF00254689. [DOI] [PubMed] [Google Scholar]
- 30.Nutt JE, Mellon JK, Qureshi K, Lunec J. Matrix metalloproteinase-1 is induced by epidermal growth factor in human bladder tumour cell lines and is detectable in urine of patients with bladder tumours. Br J Cancer. 1998;78(2):215–220. doi: 10.1038/bjc.1998.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durkan GC, Nutt JE, Rajjayabun PH, Neal DE, Lunec J, Mellon JK. Prognostic significance of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in voided urine samples from patients with transitional cell carcinoma of the bladder. Clin Cancer Res. 2001;7(11):3450–3456. [PubMed] [Google Scholar]
- 32.Liu L, Wu J, Wu C, Wang Y, Zhong R, Zhang X, Tan W, Nie S, Miao X, Lin D. A functional polymorphism (-1607 1G→2G) in the matrix metalloproteinase-1 promoter is associated with development and progression of lung cancer. Cancer. 2011;117(22):5172–5181. doi: 10.1002/cncr.26154. [DOI] [PubMed] [Google Scholar]
- 33.Heist RS, Marshall AL, Liu G, Zhou W, Su L, Neuberg D, Lynch TJ, Wain J, Christiani DC. Matrix metalloproteinase polymorphisms and survival in stage I non-small cell lung cancer. Clin Cancer Res. 2006;12(18):5448–5453. doi: 10.1158/1078-0432.CCR-06-0262. [DOI] [PubMed] [Google Scholar]
- 34.Balkhi S, Mashayekhi F, Salehzadeh A, Saedi HS. Matrix metalloproteinase (MMP)-1 and MMP-3 gene variations affect MMP-1 and -3 serum concentration and associates with breast cancer. Mol Biol Rep. 2020;47(12):9637–9644. doi: 10.1007/s11033-020-05962-x. [DOI] [PubMed] [Google Scholar]
- 35.Hsiao CL, Liu LC, Shih TC, Lai YL, Hsu SW, Wang HC, Pan SY, Shen TC, Tsai CW, Chang WS, Su CH, Way TD, Chung JG, Bau DT. The association of Matrix Metalloproteinase-1 promoter polymorphisms with breast cancer. In Vivo. 2018;32(3):487–491. doi: 10.21873/invivo.11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padala C, Tupurani MA, Puranam K, Gantala S, Shyamala N, Kondapalli MS, Gundapaneni KK, Mudigonda S, Galimudi RK, Kupsal K, Nanchari SR, Chavan U, Chinta SK, Mukta S, Satti V, Hanumanth SR. Synergistic effect of collagenase-1 (MMP1), stromelysin-1 (MMP3) and gelatinase-B (MMP9) gene polymorphisms in breast cancer. PLoS One. 2017;12(9):e0184448. doi: 10.1371/journal.pone.0184448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vairaktaris E, Serefoglou Z, Avgoustidis D, Yapijakis C, Critselis E, Vylliotis A, Spyridonidou S, Derka S, Vassiliou S, Nkenke E, Patsouris E. Gene polymorphisms related to angiogenesis, inflammation and thrombosis that influence risk for oral cancer. Oral Oncol. 2009;45(3):247–253. doi: 10.1016/j.oraloncology.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Wu MH, Yueh TC, Chang WS, Tsai CW, Fu CK, Yang MD, Yu CC, Bau DT. Contribution of Matrix Metalloproteinase-1 genotypes to colorectal cancer in Taiwan. Cancer Genomics Proteomics. 2021;18(3):245–251. doi: 10.21873/cgp.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Lima JM, de Souza LG, da Silva ID, Forones NM. E-cadherin and metalloproteinase-1 and -7 polymorphisms in colorectal cancer. Int J Biol Markers. 2009;24(2):99–106. doi: 10.1177/172460080902400206. [DOI] [PubMed] [Google Scholar]
- 40.Devulapalli K, Bhayal AC, Porike SK, Macherla R, Akka J, Nallari P, Ananthapur V. Role of interstitial collagenase gene promoter polymorphism in the etiology of gastric cancer. Saudi J Gastroenterol. 2014;20(5):309–314. doi: 10.4103/1319-3767.141693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dedong H, Bin Z, Peisheng S, Hongwei X, Qinghui Y. The contribution of the genetic variations of the matrix metalloproteinase-1 gene to the genetic susceptibility of gastric cancer. Genet Test Mol Biomarkers. 2014;18(10):675–682. doi: 10.1089/gtmb.2014.0117. [DOI] [PubMed] [Google Scholar]
- 42.Song YX, Zhou X, Wang ZN, Gao P, Li AL, Liang JW, Zhu JL, Xu YY, Xu HM. The association between individual SNPs or haplotypes of matrix metalloproteinase 1 and gastric cancer susceptibility, progression and prognosis. PLoS One. 2012;7(5):e38002. doi: 10.1371/journal.pone.0038002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beeghly-Fadiel A, Xiang YB, Deming SL, Long JR, Xu WH, Cai Q, Zheng W, Shu XO. No association between matrix metalloproteinase (MMP)-1, MMP-3, and MMP-7 SNPs and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1925–1928. doi: 10.1158/1055-9965.EPI-09-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai HC, Chu CM, Lin YW, Chang CC, Nieh S, Yu MH, Chu TY. Matrix metalloproteinase 1 gene polymorphism as a prognostic predictor of invasive cervical cancer. Gynecol Oncol. 2005;96(2):314–319. doi: 10.1016/j.ygyno.2004.09.065. [DOI] [PubMed] [Google Scholar]
- 45.Ju W, Kang S, Kim JW, Park NH, Song YS, Kang SB, Lee HP. Promoter polymorphism in the matrix metalloproteinase-1 and risk of cervical cancer in Korean women. Cancer Lett. 2005;217(2):191–196. doi: 10.1016/j.canlet.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 46.Albayrak S, Cangüven O, Göktaş C, Aydemir H, Köksal V. Role of MMP-1 1G/2G promoter gene polymorphism on the development of prostate cancer in the Turkish population. Urol Int. 2007;79(4):312–315. doi: 10.1159/000109715. [DOI] [PubMed] [Google Scholar]
- 47.Ju W, Kim JW, Park NH, Song YS, Kim SC, Kang SB, Lee HP. Matrix metalloproteinase-1 promoter polymorphism and epithelial ovarian cancer: does ethnicity matter. J Obstet Gynaecol Res. 2007;33(2):155–160. doi: 10.1111/j.1447-0756.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Jin X, Kang S, Wang Y, Du H, Zhang J, Guo W, Wang N, Fang S. Polymorphisms in the promoter regions of the matrix metalloproteinases-1, -3, -7, and -9 and the risk of epithelial ovarian cancer in China. Gynecol Oncol. 2006;101(1):92–96. doi: 10.1016/j.ygyno.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 49.Tasci AI, Tugcu V, Ozbek E, Ozbay B, Simsek A, Koksal V. A single-nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances bladder cancer susceptibility. BJU Int. 2008;101(4):503–507. doi: 10.1111/j.1464-410X.2007.07315.x. [DOI] [PubMed] [Google Scholar]
- 50.Kader AK, Shao L, Dinney CP, Schabath MB, Wang Y, Liu J, Gu J, Grossman HB, Wu X. Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Res. 2006;66(24):11644–11648. doi: 10.1158/0008-5472.CAN-06-1212. [DOI] [PubMed] [Google Scholar]
- 51.Srivastava P, Gangwar R, Kapoor R, Mittal RD. Bladder cancer risk associated with genotypic polymorphism of the matrix metalloproteinase-1 and 7 in North Indian population. Dis Markers. 2010;29(1):37–46. doi: 10.3233/DMA-2010-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wieczorek E, Reszka E, Jablonowski Z, Jablonska E, Krol MB, Grzegorczyk A, Gromadzinska J, Sosnowski M, Wasowicz W. Genetic polymorphisms in matrix metalloproteinases (MMPs) and tissue inhibitors of MPs (TIMPs), and bladder cancer susceptibility. BJU Int. 2013;112(8):1207–1214. doi: 10.1111/bju.12230. [DOI] [PubMed] [Google Scholar]