Abstract
Background/Aim: To evaluate the robustness of radiotherapy treatment planning optimization for respiratory-moving breast cancer using fixed-angle beams planning TomoDirect™ intensity-modulated radiotherapy (IMRT). Materials and Methods: A minimax optimisation algorithm was applied to 10 breast cancer patients. Two sets of treatment plans with or without robust techniques were prepared considering anterior-posterior and head-tail movements due to respiration. Parameters were compared between treatment plans: 95% planned target volume (PTV) dose, conformal index and homogeneity index (HI), and organs at risk (OAR) parameters including the lung volume receiving 20 Gy or more (V20) and 5 Gy (V5). Results: Robust planning significantly improved parameters of 95% PTV dose and HI, without deteriorating V20 or V5 in the anterior-posterior movement, while it slightly improved 95% PTV and slightly deteriorated V20 in the head-tail movement. Conclusion: Robust treatment planning improves coverage of targets moving because of respiration in the treatment of breast cancer using TomoDirect; however, normal lung doses should be cautiously evaluated on a case-by-case basis.
Keywords: Breast cancer, intensity-modulated radiotherapy, robust treatment planning
Helical intensity-modulated RT (IMRT), Radixact™ (Accuray Co. Ltd, Sunnyvale, CA, USA), provides radiation beams from any angle during gantry rotation with concomitant movements of multi-leaf collimators and a treatment couch (1). This is beneficial for the treatment of irregular-shaped targets because the angle of the beam can be changed according to the head-tail coordinates, unlike several radiation ports that are used in standard linear accelerators. In addition, TomoDirect™, consisting of non-rotational fixed beams, can be employed with Radixact, based on treatment planning. In this study, because of the target shape, we used TomoDirect™, which allows seamless dose distributions, regardless of the target shape or size.
Radixact™ continuously releases radiation during respiratory movement. When target volumes or organs at risk (OARs) move due to respiration, the administered doses are actually different from the planned ones (2). Recently, a software enabling the creation of a robust plan using minimax optimisation methods (3) has been implemented in the treatment planning machine (RayStation, RaySearch Lab, Stockholm, Sweden) (4). The minimax method optimises the worst-case scenario; it does not minimise the worst of all possible scenarios, but minimize them within some interval. The minimax optimisation methods can provide robust target coverage in addition to satisfying several constraints in each clinically relevant scenario (5). The target volume of breast cancer, which moves according to respiration, is relatively large and is close to the lung, also a critical organ. To investigate the effectiveness of robust planning during respiratory movements with TomoDirect™ IMRT, we explored the robustness of treatment planning while dealing with irregular-shaped large breast cancer targets (6).
Materials and Methods
The appropriate institutional review board approved the study (approval number: TGE01575-024). Written informed consent was obtained from all the patients. Helical IMRT Radixact has a 6 megavolt (MV) linear accelerator, apertures, a set of multi-leaf collimators, and an MV imager at the opposite side in a CT shaped gantry (6). The piled collimators are made up of 64 collimators, each of which is 5 cm long in the head-tail direction and 6.25 mm wide. They independently repeat on and off actions at a very high speed during gantry rotation according to IMRT planning. While treating breast cancer, instead of the conventional rotational beams, we used TomoDirect™ planning to reduce the dose on the lungs based on previous studies (7).
The system requires megavolt computed tomography (MVCT) examination around the planned target volume (PTV) to adjust the positioning to that of the original treatment plan before every session. The set-up errors in the therapy are then minimised. Regarding the uncertainties due to respiratory movements, no adjustment tools are available. Hence, we hypothesised the physiology of respiration involving two driving forces, chest wall movement and diaphragmatic movement. Both movements were simulated from the planning CT by deformable registration of the installed software (8).
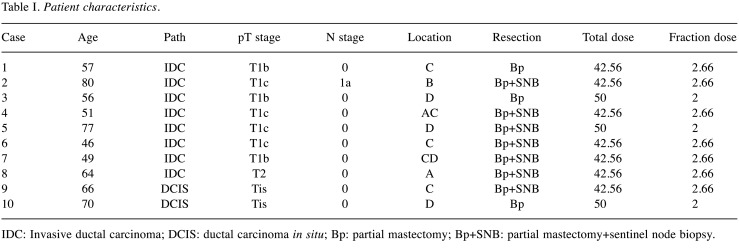
To estimate the uncertainty due to respiration, the robustness of planning was investigated. Breast cancer is the most common cancer in women and the incidence of this disease is on the rise. Whole breast Radiation Therapy (RT) is demonstrated to reduce recurrence risk and eventually decrease death rates (4). However, RT affects a wide area close to the moving target of the lungs; therefore, to investigate RT for breast cancer is a good way to measure the effectiveness of helical IMRT. To assess the method, 10 consecutive patients with stage I or II breast cancer were enrolled (9). The patients received whole breast RT following partial tumour resection with or without sentinel lymph node resection. The characteristics of the patients are summarised in Table I. In the 10 patients, the clinical target volume was determined to be the whole breast. It was delineated by two radiation oncologists, one of whom delineated the target and the other approved it. As the breast is located within a wide area and adjacent to the lungs, a considerable high treatment volume is necessary (10).
Table I. Patient characteristics.
IDC: Invasive ductal carcinoma; DCIS: ductal carcinoma in situ; Bp: partial mastectomy; Bp+SNB: partial mastectomy+sentinel node biopsy.
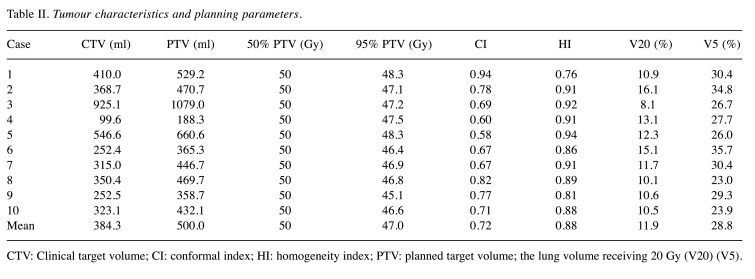
In the treatment planning, a prescribed dose of 50 Gy in 25 fractions or 42.56 Gy in 16 fractions was set to cover 50% of PTV, and 95% of PVT (95% PTV) was desired for more than 95% of the prescribed dose. The dose distribution and dose-volume histograms (DVHs) were created to mathematically evaluate the effectiveness of several sets of treatment planning. Next, several tumour parameters such as 95% PTV, conformal index (CI), and homogeneity index (HI) were calculated from DVHs as follows: the 95% isodose area was calculated as a reference isodose volume (RIV), and the CI was determined to be the RIV divided by PTV (10). Similarly, the HI was defined to be a maximal dose in the PTV divided by the reference of the prescribed dose. Factors related to OARs, such as percentage lung volume of ≥20 Gy (V20) and ≥5 Gy (V5), were calculated. To evaluate the treatment planning, parameters associated with the tumour, such as 95% PTV, CI, and HI, and those associated with OARs, such as V20 and V5, were used. The treatment parameters used in the actual treatment are shown in Table II.
Table II. Tumour characteristics and planning parameters.
CTV: Clinical target volume; CI: conformal index; HI: homogeneity index; PTV: planned target volume; the lung volume receiving 20 Gy (V20) (V5).
Respiratory movements during breast cancer treatment occur mainly in the anterior-posterior and head-tail directions (11). The respiratory motion was simulated using biomechanical deformable registration tools (RayStation) based on a model mesh deformable image registration algorithm. In this method, the boundary conditions were derived from the model-based segmentation point-point surface correspondence. This method was previously validated by clinical data from a large cohort (8). We calculated the dose distributions when the chest wall moved up by 1.5 cm in the anterior-posterior direction and the diaphragm moved in the head-tail direction due to respiration. Then, calculation was performed using the robustness software (minimax optimisation software) under the same conditions. The plans were finally compared with each other in terms of 95% PTV, CI, and HI as tumour factors and V20 and V5 as OAR factors.
The paired t-test was used to compare the factors, and a p-value ≤0.05 was considered statistically significant. All analyses were performed using STATA (STATA ver.16.0, StataCorp, TX, USA).
Results
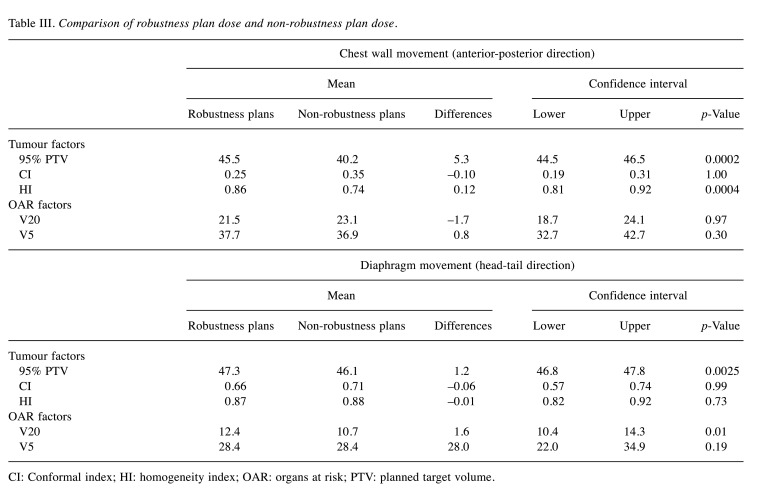
The 10 patients included in the study were successfully treated following the original planning and showed no treatment-related toxicities during follow-up. The tumour factors of PTV coverage, HI and CI, and organ at risk (OAR) factors of V20 and V5 in the planning were within the acceptable range (Table II). When the chest wall was simulated to move in the anterior-posterior direction, the robust plan improved the tumour-associated parameters of 95% PTV (40.2-45.5 Gy) and homogeneity index (HI) (0.74-0.86) compared to those following the non-robust plan (p=0.0004 and p=0.0002, respectively). However, the OAR-associated parameters of V20 and V5 were unchanged (Table III). When the diaphragm was simulated to move in the head-tail direction, the result of the robust plan was slightly better than that of the non-robust plans in terms of 95% PTV (46.1-47.3 Gy), whereas it was slightly worse in terms of V20 (10.7-12.4). The effects of robust planning were different for the anterior-posterior and head-tail respiratory movements.
Table III. Comparison of robustness plan dose and non-robustness plan dose.
CI: Conformal index; HI: homogeneity index; OAR: organs at risk; PTV: planned target volume.
Discussion
In this study, robust planning significantly improved the tumour-associated parameters of 95% PTV and HI, while it did not change the OAR parameters of V20 and V5 due to chest wall movement in the anterior-posterior direction. As for the diaphragm movement in the head-tail direction, 95% PTV slightly improved, while D20 slightly deteriorated. As respiration consists of chest wall movement and diaphragm movement, the former contributes to the anterior-posterior movement and the latter to the head-tail movement. In the simulation study, the anterior-posterior movement appears to have a bigger effect on reducing the coverage of the target, than that in the head-tail direction. Therefore, immobilisation should be performed to minimise movements in the anterior-posterior direction when the target of treatment is the breast. Hence, training patients in abdominal respiration is recommended during treatment to reduce the chest wall movement.
A major concern for radiotherapy, especially sophisticated radiotherapy such as IMRT, has been how to make robust treatment planning. Although helical IMRT is an ideal concept that makes whole-body IMRT possible, no equipment is available to control respiratory movement. Thus, we investigated whether robust treatment planning can assist to improve tumour coverage without increasing OAR irradiation exposure. In this study, the robust treatment planning improved the dose distributions when the chest wall moved in the anterior-posterior direction.
Breast cancer is the most common cancer in women according to the National Cancer registry in Japan in 2017, and the fifth most common cause of cancer-related deaths in 2018. In the treatment of breast cancer, RT plays an important role in reducing the recurrent risk and eventually prolongs survival (9). Several studies, including randomised clinical studies and meta-analyses, have shown that RT is the standard treatment modality after breast-conserving therapy and mastectomy (12). The main target volume of breast cancer is the entire breast, which is close to the lungs and moves within a wide area due to respiration.
In the treatment of breast cancer, several methods, such as field-in-field technique (13,14) and IMRT (15), are used to provide homogenous radiation dose to target tissues. Helical IMRT is a rotational IMRT dedicated machine that is available for whole-body IMRT. The combination of gantry rotations and simultaneous treatment couch movements realise seamless IMRT dose distributions for relatively large tumours. In this study, we used TomoDirect™ planning, which provides seamless dose distributions, to reduce the dose delivered to the lungs (7). This machine does not require joints between treatment volumes, which often makes hot and/or cold spots in the radiation volumes, causing complications and/or recurrences (16).
In the treatment of helical IMRT, MVCT was employed to ensure negligible set-up errors before every treatment session, so set-up errors are considered to be minimal. In contrast, it has no mechanism to avoid the effect of respiratory movements. Respiratory movement is the main cause for the differences between actual treatment and planning. For these reasons, we attempted to reduce the effects of respiratory movement, which is a potential problem that needs to be addressed.
The quality of RT actually depends on concentrating the radiation to the target. RT planning is basically designed to provide an adequate dose to the target so as to avoid excess radiations to OARs or other unnecessary radiation exposure to non-OAR tissues. Robustness focuses on the uncertainties between the planned and actual dose distributions (4). In the era of IMRT, dose distributions are significantly improved using inverse planning methods that comprise the summation of many small beams arranged by specially programmed computers. This type of therapy does not account for internal movement of the body through respiration(intrafraction) uncertainties such as respiratory movements. Therefore, we devised a simplified respiration model to investigate the effects of robust planning.
The limitation of this study is that the deformable registration image, which is deformed according to the surrounding structures, was used instead of 4D-CT, although respiratory movement is sometimes complicated. If deformable images are replaced by actual 4D images, more reliable data could be generated. Since TomoDirect™ IMRT has no mechanism to eliminate respiratory movement, robust planning of RT will be increasing important. The mechanism of TomoDirect™ IMRT is different from that of standard treatment machines; thus, the influences of set-up errors and respiratory movements are different. Further investigation is warranted for the treatment of patients with moving targets, using TomoDirect™ IMRT (15,16).
In conclusion, robust planning using TomoDirect™ IMRT for breast cancer (17) may be useful to deliver adequate target doses while avoiding unnecessary radiation exposure to the surrounding healthy tissues.
Conflicts of Interest
None.
Authors’ Contributions
Hideyuki Hongo conceptualized the study, formulated the theory, and performed the computations. Koichi Tokuue drafted the manuscript. Misato Mase developed the theoretical formalism and performed the analytical and numerical calculations. Takeji Sakae, Motoko Omura supervised the project. All Authors helped in critically revising the manuscript.
References
- 1.Mackie TR. History of tomotherapy. Phys Med Biol. 2006;51(13):R427–R453. doi: 10.1088/0031-9155/51/13/R24. [DOI] [PubMed] [Google Scholar]
- 2.Cai W, Hurwitz MH, Williams CL, Dhou S, Berbeco RI, Seco J, Mishra P, Lewis JH. 3D delivered dose assessment using a 4DCT-based motion model. Med Phys. 2015;42(6):2897–2907. doi: 10.1118/1.4921041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredriksson A, Forsgren A, Hårdemark B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med Phys. 2011;38(3):1672–1684. doi: 10.1118/1.3556559. [DOI] [PubMed] [Google Scholar]
- 4.Korevaar EW, Habraken SJM, Scandurra D, Kierkels RGJ, Unipan M, Eenink MGC, Steenbakkers RJHM, Peeters SG, Zindler JD, Hoogeman M, Langendijk JA. Practical robustness evaluation in radiotherapy - A photon and proton-proof alternative to PTV-based plan evaluation. Radiother Oncol. 2019;141:267–274. doi: 10.1016/j.radonc.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Haraldsson A, Engleson J, Bäck SÅJ, Engelholm S, Engström PE. A Helical tomotherapy as a robust low-dose treatment alternative for total skin irradiation. J Appl Clin Med Phys. 2019;20(5):44–54. doi: 10.1002/acm2.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takano S, Omura M, Suzuki R, Tayama Y, Matsui K, Hashimoto H, Hongo H, Nagata H, Tanaka K, Hata M, Inoue T. Intensity-modulated radiation therapy using TomoDirect for postoperative radiation of left-sided breast cancer including lymph node area: comparison with TomoHelical and three-dimensional conformal radiation therapy. J Radiat Res. 2019;60(5):694–704. doi: 10.1093/jrr/rrz052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velec M, Moseley JL, Svensson S, Hårdemark B, Jaffray DA, Brock KK. Validation of biomechanical deformable image registration in the abdomen, thorax, and pelvis in a commercial radiotherapy treatment planning system. Med Phys. 2017;44(7):3407–3417. doi: 10.1002/mp.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JM, Park SY, Ye SJ, Kim JH, Carlson J, Wu HG. New conformity indices based on the calculation of distances between the target volume and the volume of reference isodose. Br J Radiol. 2014;87(1043):20140342. doi: 10.1259/bjr.20140342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chopra S, Dinshaw KA, Kamble R, Sarin R. Breast movement during normal and deep breathing, respiratory training and set up errors: implications for external beam partial breast irradiation. Br J Radiol. 2006;79(945):766–773. doi: 10.1259/bjr/98024704. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) , Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier-Bidoz N, Kirova YM, Campana F, Dendale R, Fourquet A. Simplified field-in-field technique for a large-scale implementation in breast radiation treatment. Med Dosim. 2012;37(2):131–137. doi: 10.1016/j.meddos.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 13.Karpf D, Sakka M, Metzger M, Grabenbauer GG. Left breast irradiation with tangential intensity modulated radiotherapy (t-IMRT) versus tangential volumetric modulated arc therapy (t-VMAT): trade-offs between secondary cancer induction risk and optimal target coverage. Radiat Oncol. 2019;14(1):156. doi: 10.1186/s13014-019-1363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang JH, Wu XX, Lin X, Shi JT, Ma YJ, Duan S, Huang XB. Evaluation of fixed-jaw IMRT and tangential partial-VMAT radiotherapy plans for synchronous bilateral breast cancer irradiation based on a dosimetric study. J Appl Clin Med Phys. 2019;20(9):31–41. doi: 10.1002/acm2.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricotti R, Ciardo D, Fattori G, Leonardi MC, Morra A, Dicuonzo S, Rojas DP, Pansini F, Cambria R, Cattani F, Gianoli C, Spinelli C, Riboldi M, Baroni G, Orecchia R, Jereczek-Fossa BA. Intra-fraction respiratory motion and baseline drift during breast Helical Tomotherapy. Radiother Oncol. 2017;122(1):79–86. doi: 10.1016/j.radonc.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Yang YM, Geurts M, Smilowitz JB, Sterpin E, Bednarz BP. Monte Carlo simulations of patient dose perturbations in rotational-type radiotherapy due to a transverse magnetic field: a tomotherapy investigation. Med Phys. 2015;42(2):715–725. doi: 10.1118/1.4905168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannan R, Thompson RF, Chen Y, Bernstein K, Kabarriti R, Skinner W, Chen CC, Landau E, Miller E, Spierer M, Hong L, Kalnicki S. Hypofractionated whole-breast radiation therapy: does breast size matter. Int J Radiat Oncol Biol Phys. 2012;84(4):894–901. doi: 10.1016/j.ijrobp.2012.01.093. [DOI] [PubMed] [Google Scholar]