Key Points
Question
In adults with acute hypoxemic respiratory failure receiving mechanical ventilation, does further reduction in tidal volumes, facilitated by extracorporeal carbon dioxide removal, improve 90-day mortality compared with conventional low tidal volume ventilation?
Findings
In this randomized clinical trial that included 412 adults, 90-day mortality was 41.5% in the extracorporeal carbon dioxide removal group and 39.5% in the standard care group, a difference that was not statistically significant.
Meaning
Among patients with acute hypoxemic respiratory failure, the use of extracorporeal carbon dioxide removal to facilitate lower tidal volume ventilation, compared with conventional low tidal volume ventilation, did not significantly reduce 90-day mortality.
Abstract
Importance
In patients who require mechanical ventilation for acute hypoxemic respiratory failure, further reduction in tidal volumes, compared with conventional low tidal volume ventilation, may improve outcomes.
Objective
To determine whether lower tidal volume mechanical ventilation using extracorporeal carbon dioxide removal improves outcomes in patients with acute hypoxemic respiratory failure.
Design, Setting, and Participants
This multicenter, randomized, allocation-concealed, open-label, pragmatic clinical trial enrolled 412 adult patients receiving mechanical ventilation for acute hypoxemic respiratory failure, of a planned sample size of 1120, between May 2016 and December 2019 from 51 intensive care units in the UK. Follow-up ended on March 11, 2020.
Interventions
Participants were randomized to receive lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal for at least 48 hours (n = 202) or standard care with conventional low tidal volume ventilation (n = 210).
Main Outcomes and Measures
The primary outcome was all-cause mortality 90 days after randomization. Prespecified secondary outcomes included ventilator-free days at day 28 and adverse event rates.
Results
Among 412 patients who were randomized (mean age, 59 years; 143 [35%] women), 405 (98%) completed the trial. The trial was stopped early because of futility and feasibility following recommendations from the data monitoring and ethics committee. The 90-day mortality rate was 41.5% in the lower tidal volume ventilation with extracorporeal carbon dioxide removal group vs 39.5% in the standard care group (risk ratio, 1.05 [95% CI, 0.83-1.33]; difference, 2.0% [95% CI, −7.6% to 11.5%]; P = .68). There were significantly fewer mean ventilator-free days in the extracorporeal carbon dioxide removal group compared with the standard care group (7.1 [95% CI, 5.9-8.3] vs 9.2 [95% CI, 7.9-10.4] days; mean difference, −2.1 [95% CI, −3.8 to −0.3]; P = .02). Serious adverse events were reported for 62 patients (31%) in the extracorporeal carbon dioxide removal group and 18 (9%) in the standard care group, including intracranial hemorrhage in 9 patients (4.5%) vs 0 (0%) and bleeding at other sites in 6 (3.0%) vs 1 (0.5%) in the extracorporeal carbon dioxide removal group vs the control group. Overall, 21 patients experienced 22 serious adverse events related to the study device.
Conclusions and Relevance
Among patients with acute hypoxemic respiratory failure, the use of extracorporeal carbon dioxide removal to facilitate lower tidal volume mechanical ventilation, compared with conventional low tidal volume mechanical ventilation, did not significantly reduce 90-day mortality. However, due to early termination, the study may have been underpowered to detect a clinically important difference.
Trial Registration
ClinicalTrials.gov Identifier: NCT02654327
This randomized clinical trial examines whether lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal, compared with standard care, will decrease mortality 90 days after randomization in patients with acute hypoxemic respiratory failure.
Introduction
Acute hypoxemic respiratory failure is a leading cause of admission to intensive care units (ICUs) and is associated with significant mortality and long-term morbidity for survivors, as well as considerable resource implications for health care systems.1 A significant proportion of patients affected by acute hypoxemic respiratory failure will meet the diagnostic criteria for acute respiratory distress syndrome (ARDS).2 Invasive mechanical ventilation after tracheal intubation is often used as a life-saving intervention to maintain adequate gas exchange, but is known to contribute to the overall morbidity and mortality of this condition.3
One of the few interventions shown to reduce mortality in patients with acute hypoxemic respiratory failure and ARDS is ventilation with a lung-protective strategy aiming for a tidal volume of 6 mL/kg predicted body weight and a plateau pressure less than or equal to 30 cm H2O in patients.4 However, even when using lung-protective invasive mechanical ventilation, lung hyperinflation and injury can still occur.5 Reducing tidal volumes further may result in respiratory acidosis, which can cause further adverse effects, such as pulmonary hypertension and altered cardiac function. Extracorporeal gas exchange, including extracorporeal carbon dioxide removal (ECCO2R), can facilitate mechanical ventilation with even lower tidal volumes because it supports the removal of carbon dioxide that accumulates in this setting.6,7 The feasibility of ECCO2R in patients with acute hypoxemic respiratory failure due to ARDS has recently been demonstrated.8
The primary objective of the REST trial was to determine whether lower tidal volume ventilation facilitated by ECCO2R compared with standard care in patients with acute hypoxemic respiratory failure decreased mortality 90 days after randomization.9
Methods
Trial Design and Oversight
This was a multicenter, randomized, allocation-concealed, open-label, pragmatic clinical trial. After randomization, patients, clinical care clinicians, and researchers were unblinded due to the complex nature of the intervention. The trial was coordinated by the Northern Ireland Clinical Trials Unit and was sponsored by Belfast Health and Social Care Trust. The study design has been published9 and the trial protocol and statistical analysis plan are provided in Supplement 1 and Supplement 2. The protocol was approved by research ethics committees in England, Wales, Northern Ireland (16/SC/089), and Scotland (16/SS/048). The National Institute for Health Research in the UK convened an independently chaired (and majority independent) trial steering committee and an independent data monitoring and ethics committee. The study was conducted in accordance with Good Clinical Practice guidelines, local regulations, and the ethical principles described in the Declaration of Helsinki. Written informed consent from patients or agreement from their surrogates was obtained, keeping with regional regulations.
Sites and Patients
The trial was conducted in 51 adult, general ICUs within the National Health Service across the UK. Patients 16 years or older who were admitted to a participating ICU were eligible for inclusion if they had an acute and potentially reversible cause of moderate to severe hypoxemic respiratory; were receiving invasive mechanical ventilation using at least 5 cm H2O of positive end-expiratory pressure (PEEP); and were within 48 hours of onset of hypoxemia, defined as a ratio of the partial pressure of oxygen in arterial blood to the fractional inspired concentration of oxygen (Pao2/Fio2) of less than 150 mm Hg. Exclusion criteria included receiving invasive mechanical ventilation for more than 7 days, contraindication to limited systemic anticoagulation with heparin, untreated pulmonary embolism, pleural effusion or pneumothorax, or acute respiratory failure fully explained by left ventricular failure or fluid overload. Other reasons for exclusion are detailed in the trial protocol in Supplement 1.
Randomization
After consent was obtained, eligible patients were randomized. Randomization concealment was achieved by use of an automated online or telephone-centralized 24-hour randomization facility. Patients were randomized to receive lower tidal volume ventilation with ECCO2R or lung protective ventilation alone in a 1:1 ratio using a computer-generated schedule with variable block sizes of 4, 6, and 8, stratified by recruitment center. If randomized to the ECCO2R group, it was recommended to commence within 8 hours of randomization.
Interventions
In patients assigned to receive ECCO2R, a dual-lumen catheter was inserted percutaneously into a central vein using ultrasonography guidance. Venovenous ECCO2R was then commenced using intravenous heparin as systemic anticoagulation to prevent circuit thrombosis. The pump speed was increased to achieve the maximum possible blood flow (typically 350-450 mL/min), sweep gas flow was increased to 10 L/min to maximize carbon dioxide removal, and concomitantly tidal volumes were reduced incrementally, aiming for a tidal volume less than or equal to 3 mL/kg predicted body weight. The intervention was continued for at least 48 hours, after which patients were weaned from ECCO2R, as per the trial manual provided in Supplement 3, when patients demonstrated signs of clinical improvement and improvement in the degree of hypoxemia. ECCO2R was to be used for a maximum of 7 days as part of the study protocol. An online educational package for catheter insertion and device management was provided to all sites.
For patients randomized to receive standard care, it was recommended that patients received mechanical ventilation using a tidal volume of 6 mL/kg predicted body weight with PEEP set based on the ARDSNetwork trial.4 In addition, in keeping with UK guidelines,10 patients in both the intervention and control groups could receive neuromuscular-blocking drugs,11 prone positioning,12 or referral for consideration of extracorporeal membrane oxygenation (ECMO).13
Outcome Measures
The primary outcome was all-cause mortality 90 days after randomization. Secondary clinical outcome measures were tidal volume at day 2 and day 3, ventilator-free days at day 28, duration of invasive mechanical ventilation in survivors, need for ECMO up to day 7, mortality at day 28, and adverse event rate. All outcomes were reported from the time of randomization. Prespecified clinical outcome measures are listed in eTable 1 in Supplement 4. The outcomes not reported in this article will be reported separately. A cost-effectiveness analysis is also planned, as described in the protocol in Supplement 1. Duration of critical care and hospital length of stay were defined as outcomes for the cost-effectiveness analysis. Data on physiological parameters by treatment group were also collected up to day 7.
Statistical Analysis
A sample size of 1120 patients was determined to provide 90% power to show an absolute difference of 9% in 90-day mortality, assuming a control group mortality rate of 41%.14 This postulated effect size was estimated from a previous trial on the use of lung protective ventilation,4 which demonstrated a 9% reduction in mortality in patients with hypoxaemic respiratory failure secondary to ARDS with a 50% reduction in tidal volume (from 12 to 6 mL/kg predicted body weight).15 Therefore, we hypothesized that a similar relative reduction in tidal volume would result in a 9% difference in mortality. The sample size calculation did not take a group sequential trial design into account.
Patients were analyzed according to their randomization group. For the primary outcome and other dichotomous outcomes, risk ratios and percent point differences with 95% CIs were calculated. The primary outcome of 90-day mortality was analyzed using a χ2 test and a secondary analysis using a log-binomial regression adjusted for age, sequential organ failure assessment (SOFA)16 score, and baseline Pao2/Fio2 ratio was also carried out. Plateau pressure was planned to be included as a variable in the adjusted analysis, but because it was missing in a substantial number of patients this was not possible. A post hoc sensitivity analysis using generalized estimating equations was used to account for possible clustering of observations within participating centers. There was no imputation for missing data. Continuous outcomes were compared between the 2 groups using analysis of variance/analysis of covariance, adjusting for other covariates where appropriate. Time-to-event outcomes were analyzed by survival methods and reported as hazard ratios with 95% CIs. The proportionality assumption was tested using the Schoenfeld test. Length of stay outcomes were compared using the Wilcoxon rank sum test. A prespecified sensitivity analysis was also performed for the primary outcome excluding the first 2 intervention group patients at each site to address potential learning effects. A per-protocol analysis was carried out for the secondary outcome of tidal volume at days 2 and 3 (ie, including those who were receiving ECCO2R on day 2 and 3 in the intervention group). We performed prespecified subgroup analyses using 99% CIs. Log-binomial regression was used with interaction terms (treatment group × subgroup).
One interim analysis for the primary outcome was planned before the recruitment of 560 patients. A post hoc conditional power analysis was carried out estimating the power given the observed data up to termination and then assuming varying differences between 2% and 10% for the remainder of the data that were to be observed. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Analysis was conducted using Stata/SE, version 15.1 (StataCorp). Statistical significance was defined using a 2-sided test with α = .05.
Trial Termination
During a recruitment pause for investigation of a serious adverse event (SAE) (fatal intracranial hemorrhage), the planned interim analysis was undertaken and the independent data monitoring and ethics committee recommended that the trial be stopped due to futility (given that even under optimistic assumptions the trial was unlikely to demonstrate a significant benefit for the intervention) and subsequent feasibility to continue the trial. There were no formal stopping rules for futility and the decision to stop the study was not based on a formal calculation of futility. The decision to stop was based on the opinion of the data monitoring and ethics committee based on all available information, including data from the interim analysis, feasibility of future recruitment, and a conditional power analysis. The conditional power analysis to detect a difference between the groups, assuming the patients remaining to be recruited to achieve the planned sample size met the assumptions of the original sample size, was 44%. Safety was not cited by the data monitoring and ethics committee as a reason for stopping the trial. This decision was accepted by the trial steering committee and agreed by the study sponsor and the trial was stopped on February 11, 2020.
Results
Patients
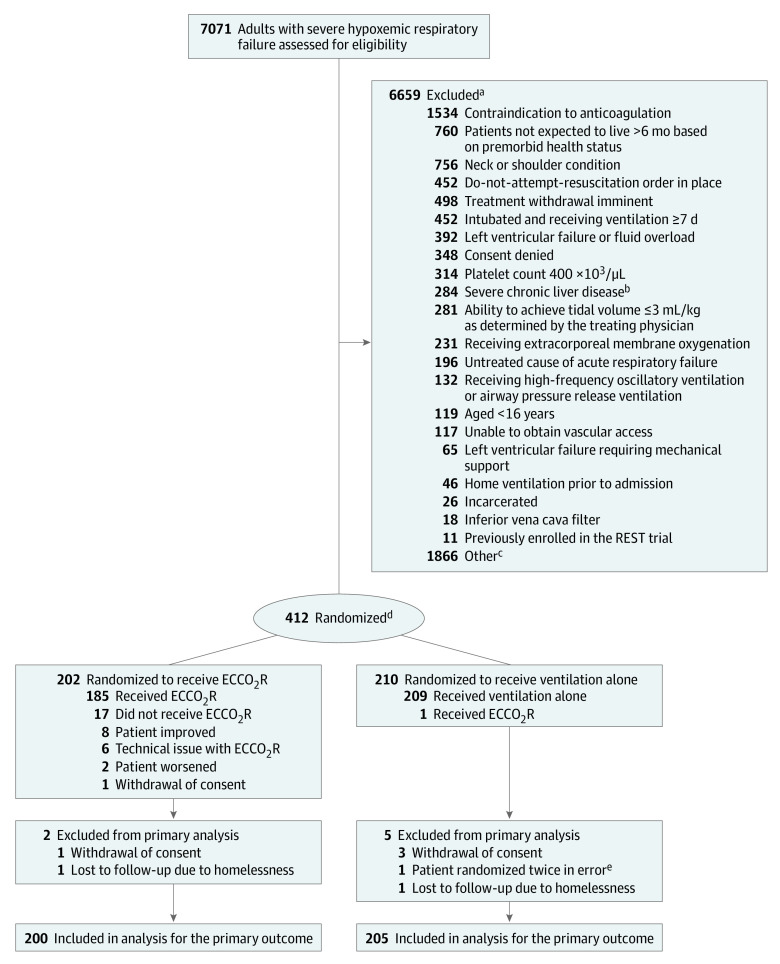
From May 2016 to December 2019, a total of 7071 patients from 51 centers were screened for eligibility and, after applying the exclusion criteria, 412 (6%) participants were recruited (eTable 2 in Supplement 4). The patients were followed up until March 11, 2020. One patient was randomized twice in error, 2 patients were lost to follow-up, and 4 patients withdrew consent for confirmation of vital status. As a result, 405 participants (200 in the intervention group and 205 in the standard care group) were included in the final analysis of the primary outcome (Figure 1). The most common reasons for exclusion were contraindication to systemic anticoagulation, a do-not-attempt-resuscitate order in place, imminent treatment withdrawal, and invasive mechanical ventilation for more than 7 days. A total of 1866 patients (28%) who were screened were excluded for other reasons, for which the most common reason was either the patient’s clinical condition rapidly improved or deteriorated. The baseline characteristics of the 2 groups were well balanced prior to randomization and typical of patients with moderate to severe acute hypoxemic respiratory failure requiring ICU care (Table 1).
Figure 1. Flow of Patients in a Study of Lower Tidal Volume Facilitated by Extracorporeal Carbon Dioxide Removal in Patients With Acute Hypoxemic Respiratory Failure.
aPatients could meet more than 1 ineligibility criterion.
bChild-Pugh score >11.
cOther was used when the reason for a patient’s exclusion was not among those predefined in the protocol; the most commonly specified free-text explanations included rapid improvement or deterioration in clinical status.
dRandomization was stratified by site.
eThis patient was randomized twice in error and was randomized to the same group.
Table 1. Baseline Characteristics in a Study of Lower Tidal Volume Facilitated by Extracorporeal Carbon Dioxide Removal in Patients With Acute Hypoxemic Respiratory Failurea.
| Characteristic | No. (%) | |
|---|---|---|
| ECCO2R (n = 202) | Ventilation alone (n = 210) | |
| Age, median (IQR), y | 60.2 (50.6-69.0) | 61.8 (50.2-69.7) |
| Sex | ||
| Men | 138 (68) | 131 (62) |
| Women | 64 (32) | 79 (38) |
| Dependency prior to hospital admission | ||
| Able to live without assistance in daily activities | 152 (87) | 160 (88) |
| Minor assistance with some daily activities | 19 (11) | 19 (10) |
| Major assistance with majority of/all daily activities | 2 (1) | 3 (2) |
| Total assistance with all daily activities | 1 (1) | 0 |
| PBW, median (IQR), kgb | 66.1 (57.0-73.3) | 66.0 (56.9-71.5) |
| ICU admission diagnostic categoryc | ||
| Respiratory | 175 (88) | 178 (85) |
| Sepsis | 86 (43) | 102 (49) |
| Cardiovascular | 45 (23) | 49 (23) |
| Kidney | 39 (20) | 42 (20) |
| Gastrointestinal | 27 (14) | 31 (15) |
| Central nervous system | 17 (9) | 14 (7) |
| Other | 14 (7) | 10 (5) |
| Toxicology | 10 (5) | 10 (5) |
| Hematology | 7 (4) | 10 (5) |
| Orthopedic | 4 (2) | 8 (4) |
| ARDS present at enrollmentd | 118 (59) [n = 199] | 130 (63) [n = 207] |
| Etiology of ARDSc | 118 | 130 |
| Pneumonia | 96 (81) | 103 (80) |
| Sepsis | 54 (46) | 66 (51) |
| Gastric content aspiration | 8 (7) | 14 (11) |
| Other | 8 (7) | 10 (8) |
| Pancreatitis | 5 (4) | 5 (4) |
| Thoracic trauma | 2 (2) | 3 (2) |
| Smoke/toxin inhalation | 2 (2) | 2 (2) |
| APACHE II score at ICU admission, median (IQR)e | 19 (15-23) | 20 (16-23) |
| SOFA score, median (IQR)f | 10 (7-12) [n = 195] | 10 (7-12) [n = 198] |
| Mode of ventilation | ||
| Mandatory | 158 (78) | 163 (78) |
| Mandatory and spontaneous breaths | 37 (18) | 31 (15) |
| Spontaneous | 6 (3) | 15 (7) |
| Adjunctive ventilatory therapies | ||
| Neuromuscular-blocking drugs | 103 (51) | 102 (49) |
| Prone positioning | 22 (11) | 23 (11) |
| Inhaled nitric oxide | 6 (3) | 4 (2) |
| Nebulized epoprostenol | 3 (1) | 5 (2) |
| Tidal volume, median (IQR), mL/kg PBW | 6.3 (5.8-7.0) [n = 201] | 6.4 (5.8-7.1) [n = 208] |
| Respiratory rate, median (IQR), breaths/min | 24 (20-28) [n = 201] | 24 (20-28) [n = 209] |
| PEEP, median (IQR), cm H2O | 10 (8-12) [n = 200] | 10 (8-12) [n = 208] |
| Plateau pressure | ||
| Median (IQR), cm H2O | 26 (23.5-30) n = [160] | 26 (23-30) [n = 163] |
| >28 cm H2O | 50 (31.3) | 58 (35.6) |
| Driving pressureg | ||
| Median (IQR), cm H2O | 15 (12-19) [n = 159] | 16 (12.5-19) [n = 163] |
| <15 cm H2O | 79 (49.7) | 69 (42.3) |
| Pao2/Fio2 ratio, median (IQR), mm Hgh | 118.1 (96.0-134.3) [n = 198] | 115.5 (93.8-132.8) [n = 203] |
| Paco2, median (IQR), mm Hg | 53.8 (47.3-62.7) [n = 198] | 54.6 (48.0-62.3) [n = 203] |
| pH level, median (IQR) | 7.30 (7.25-7.37) [n = 198] | 7.30 (7.24-7.37) [n = 202] |
Abbreviations: ECCO2R, extracorporeal carbon dioxide removal; Fio2, fraction of inspired oxygen; ICU, intensive care unit; Paco2, partial pressure of arterial carbon dioxide; Pao2, partial pressure of arterial oxygen.
Baseline clinical data were collected in the 24 hours prior to randomization unless stated otherwise. If more than 1 value was available for this 24-hour period, the value closest, but prior to, the time of randomization was recorded.
The predicted body weight (PBW), used to determine tidal volume, of male patients was calculated as 50 + 0.91 (centimeters of height − 152.4) and of female patients as 45.5 + 0.91 (centimeters of height − 152.4). Actual body weight was not collected.
Patients may have had more than 1 admission diagnostic category or cause of acute respiratory distress syndrome (ARDS) identified.
The presence of ARDS was assessed by the treating physician.
Scores on the Acute Physiology and Chronic Health Evaluation (APACHE) II range from 0 to 71, with higher scores indicating greater severity of illness.
Scores on the Sequential Organ Failure Assessment (SOFA) scale range from 0 to 24, with higher scores indicating greater severity of disease.
Driving pressure is equal to plateau pressure minus partial end-expiratory pressure (PEEP).
Second qualifying Pao2/Fio2 ratio.
Primary Outcome
There was no significant difference in mortality between the groups. The 90-day mortality rate was 41.5% (83 of 200) in the intervention group and 39.5% (81 of 205) in the standard care group (risk ratio [RR], 1.05 [95% CI, 0.83-1.33]; difference, 2.0% [95% CI, −7.6% to 11.5%]) (Table 2 and Figure 2). The RR was similar after adjustment for age, SOFA score, and Pao2/Fio2 ratio (RR, 1.12 [95% CI, 0.90-1.40]) and in a per-protocol analysis for the primary outcome of the group who initiated ECCO2R (Table 2). To address a potential learning effect with the intervention, a sensitivity analysis was performed excluding the first 2 patients randomized to receive the intervention at each site (Table 2). These findings were consistent with those of the primary analysis. Treatment × subgroup interactions were not significant with respect to the presence of ARDS, requirement for vasopressors, severity of hypoxemia or hypercapnia, plateau and driving pressures, Acute Physiology and Chronic Health Evaluation II score, and volume of ECCO2R by site (eFigure 1 in Supplement 4). The percentage of missing data for the primary analysis of the primary outcome was 1.7%.
Table 2. Primary, Secondary, and Other Clinical Outcomes in a Study of Lower Tidal Volume Facilitated by Extracorporeal Carbon Dioxide Removal in Patients With Acute Hypoxemic Respiratory Failure.
| Outcome | No. (%) | Difference (95% CI) | Risk ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| ECCO2R | Ventilation alone | ||||
| Primary | |||||
| 90-d mortality | 83 (41.5) [n = 200] | 81 (39.5) [n = 205] | 2.0% (−7.6% to 11.5%) | 1.05 (0.83 to 1.33) | .68 |
| Adjusted analysisa | 1.12 (0.90 to 1.40) | .29 | |||
| Sensitivity analysis to adjust for site effectb | 1.8% (−7.7% to 11.3%) | 1.04 (0.83 to 1.31) | .72 | ||
| 90-d mortality in cohort who initiated ECCO2Rc | 80 (43.5) [n = 184] | 80 (39.2) [n = 204] | 4.3% (−5.5% to 14.1%) | 1.11 (0.87 to 1.41) | .39 |
| 90-d mortality excluding the first 2 patients at each site who initiated ECCO2Rd | 48 (37.8) [n = 127] | 81 (39.5) [n = 205] | −1.7% (−12.5% to 9.0%) | 0.96 (0.72 to 1.27) | .76 |
| Secondary | |||||
| Ventilator-free days from randomization to day 28, mean (SD)e | 7.1 (8.8) [n = 199] | 9.2 (9.3) [n = 206] | −2.1 (−3.8 to 0.3) | .02 | |
| Duration receiving ventilation in survivors, mean (SD), df | 18.0 (13.6) [n = 121] | 17.4 (31.3) [n = 137] | 0.7 (−5.4 to 6.7) | .83 | |
| Need for ECMO to day 7 | 12 (6) [n = 202] | 6 (3) [n = 210] | 3.1% (−0.9% to 7.0%) | 2.08 (0.80 to 5.43) | .13 |
| 28-d mortality | 76 (38) [n = 200] | 74 (36) [n = 207] | 2.3% (−7.1% to 11.6%) | 1.06 (0.82 to 1.37) | .64 |
| ICU length of stay to death or discharge, median (IQR), dg,h | 14 (7 to 26) [n = 202] | 13 (7 to 22) [n = 210] | .67 | ||
| Hospital length of stay to death or discharge, median (IQR), dg,h | 22 (8 to 39) [n = 193] | 18 (9 to 35) [n = 201] | .65 | ||
Abbreviations: ECCO2R, extracorporeal carbon dioxide removal; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range.
Adjusted for age, qualifying partial pressure of arterial oxygen/fraction of inspired oxygen ratio and baseline Sequential Organ Failure Assessment score (risk ratio estimated from a log-binomial regression; model to estimate the percentage point difference would not converge.
Generalized estimating equations were used to account for possible clustering of observations within participating centers.
Per-protocol analysis excluded the 17 patients who did not commence ECCO2R and the 1 patient who received ECCO2R in the standard care group.
Sensitivity analysis performed for the primary outcome excluding the first 2 patients randomized to the intervention group at each site to address potential learning effects.
Ventilator-free days were defined as the number of days from the time of initiating unassisted breathing to day 28 after randomization (see the study protocol). Patients who died before day 28 were assigned 0 ventilator-free days.
Mean (SD) for treatment groups and mean difference and 95% CI presented. Survivors were defined as patients who achieved unassisted breathing, but could have subsequently died prior to day 90.
Median (IQR) with P value from Wilcoxon rank sum test are presented.
Length of stay in ICU and hospital were not secondary outcomes, but were clinical outcomes collected as part of the health economic analysis.
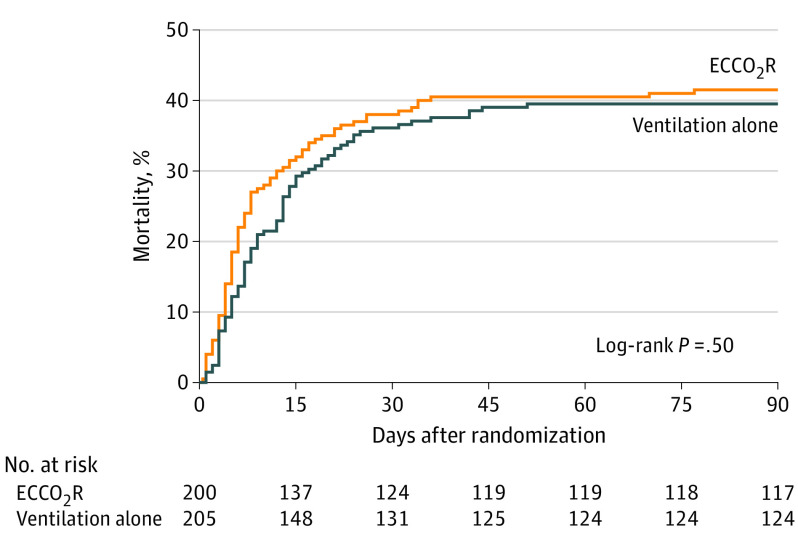
Figure 2. Kaplan-Meier Curve of the Time to Death in a Study of Lower Tidal Volume Facilitated by Extracorporeal Carbon Dioxide Removal in Patients With Acute Hypoxemic Respiratory Failure.
Median (interquartile range) time to death was 6 (4-14) days in the ECCO2R group and 9 (5-16) days in the ventilation alone group. The unadjusted hazard ratio for death at 90 days in the ECCO2R group was 1.1 (95% CI, 0.8-1.5). The proportionality P = .40, suggesting that the proportionality assumption was met.
Secondary Outcomes
The secondary outcomes are presented in Table 2. There were significantly fewer ventilator-free days at day 28 in the intervention group (7.1 [95% CI, 5.9-8.3] vs 9.2 [95% CI, 7.9-10.4] days; mean difference, −2.1 days [95% CI, −3.8 to −0.3]; P = .02). There was no significant between-group difference in duration of ventilation, need for ECMO at day 7, mortality at 28 days, or duration of ICU or hospital stay.
Additional Secondary Outcomes and Intervention Fidelity
Of the 202 patients allocated to the intervention group, 186 patients (92%) received ECCO2R after randomization, with a mean (SD) duration of ECCO2R of 4 (2) days. One patient in the standard care group received nonprotocol ECCO2R for 2 days. A total of 50 patients (28%) were successfully weaned from ECCO2R and it was stopped due to receiving 7 days of treatment in 33 patients (18%). ECCO2R was discontinued for safety reasons in 14 patients (8%), the need for ECMO in 12 patients (7%), and withdrawal of active medical treatment or death in 28 patients (16%) (eTable 3 in Supplement 4).
Patients randomized to receive ECCO2R had a lower tidal volume than those randomized to receive standard care at day 2 (4.5 [95% CI, 4.3-4.8] vs 6.5 [95% CI, 6.3-6.7] mL/kg; mean difference, 2.0 mL/kg [95% CI, 1.7-2.3]) and day 3 (4.4 [95% CI, 4.1-4.6] vs 6.7 [95% CI, 6.4-7.0] mL/kg; mean difference, 2.3 mL/kg [95% CI, 2.0-2.7]). In patients receiving ECCO2R on day 2 and 3, tidal volume was lower than in the standard care group at day 2 (4.2 [95% CI, 4.0-4.4] vs 6.5 [95% CI, 6.3-6.7] mL/kg; mean difference, 2.4 mL/kg [95% CI, 2.0-2.7]) and day 3 (3.8 [95% CI, 3.6-4.0] vs 6.7 [95% CI, 6.4-7.0] mL/kg; mean difference, 2.9 mL/kg [95% CI, 2.5-3.3]) (Figure 3A; eTable 4 in Supplement 4).
Figure 3. Physiological Parameters by Treatment Group to Day 7 in a Study of Lower Tidal Volume Facilitated by Extracorporeal Carbon Dioxide Removal in Patients With Acute Hypoxemic Respiratory Failure.
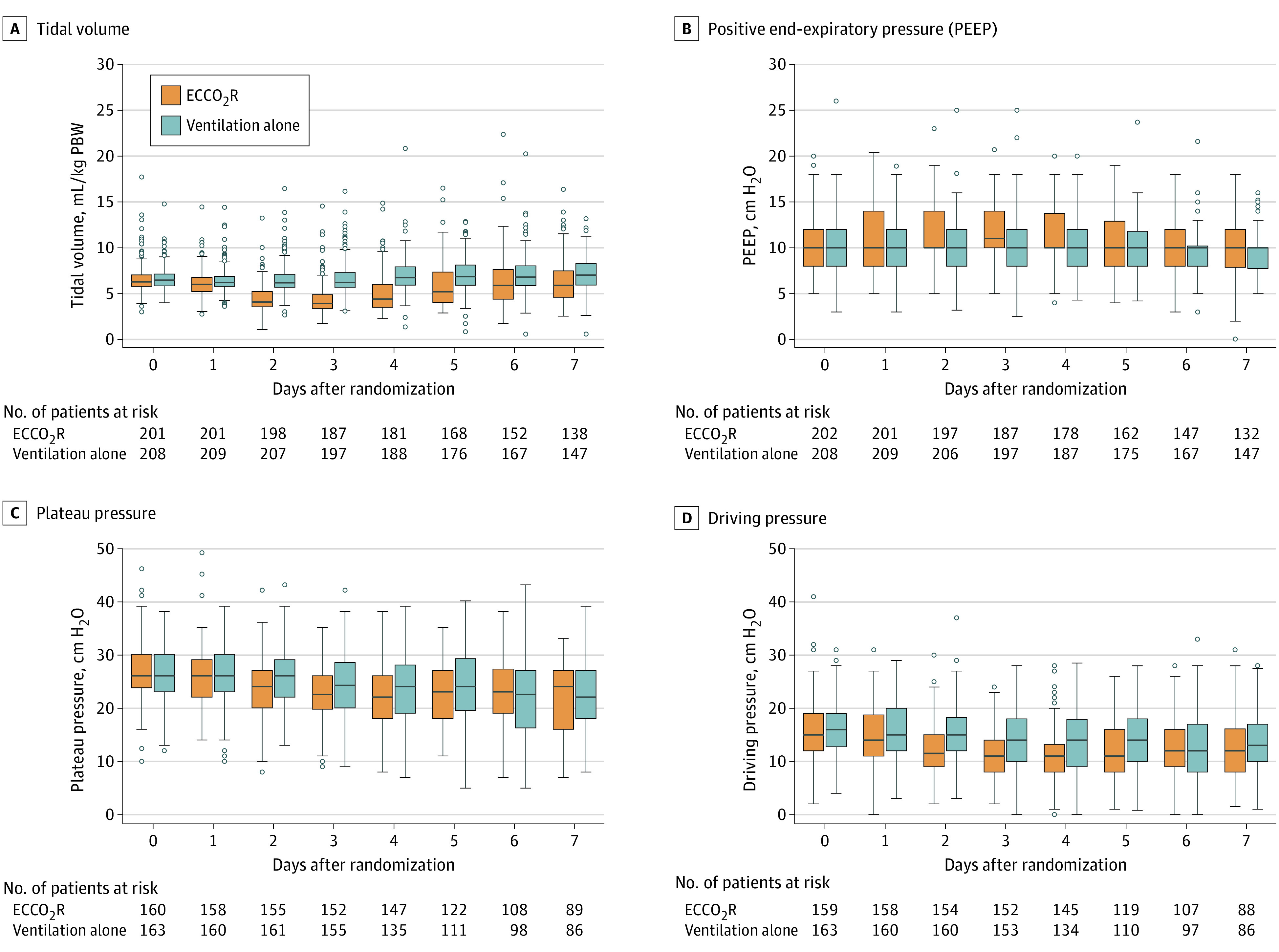
Driving pressure is equal to plateau pressure minus PEEP.
Patients in the intervention group, compared with the standard care group, had a lower Pao2/Fio2 ratio on day 2 (147.8 [95% CI, 140.4-155.1] vs 161.1 [95% CI, 153.3-169.0]; mean difference, 13.3 mm Hg [95% CI, 2.6-24.1]) and on day 3 (147.9 [95% CI, 140.9-154.9] vs 167.0 [95% CI, 158.6-175.4]; mean difference, 19.1 mm Hg [95% CI, 8.2-30.1]) after randomization (eFigure 2A and eTable 4 in Supplement 4). Patients in the intervention group had higher PEEP than patients in the control group (Figure 3B; eTable 4 in Supplement 4). Plateau pressure was lower in the intervention group on day 2 (23.5 [95% CI, 22.6-24.3] vs 25.7 [95% CI, 24.9-26.6]; mean difference, 2.3 cm H2O [95% CI, 1.1-3.4]) and on day 4 (22.2 [95% CI, 21.2-23.1] vs 23.7 [95% CI, 22.6-24.8]; mean difference, 1.6 cm H2O [95% CI, 0.1-3.0]) after randomization (Figure 3C; eTable 4 in Supplement 4). Driving pressure was lower in the intervention group than in the control group from day 2 to 5 following randomization (Figure 3D; eTable 4 in Supplement 4). Total respiratory rate was higher in the intervention group than the control group from day 2 to 4 following randomization (day 2: 26.6 [95% CI, 25.8-27.3] vs 24.6 [95% CI, 23.9-25.3]; mean difference, 2.0 breaths per minute [95% CI, 0.9-3.0]; day 3: 27.8 [95% CI, 26.9-28.7] vs 24.4 [95% CI, 23.6-25.2]; mean difference, 3.4 breaths per minute [95% CI, 2.2-4.6]; day 4: 27.0 [95% CI, 26.0-28.1] vs 24.4 [95% CI, 23.5-25.4]; mean difference, 2.6 breaths per minute [95% CI, 1.2-4.0]; eFigure 2B and eTable 4 in Supplement 4). Minute ventilation was lower in the intervention group than in the control group from day 1 following randomization (eFigure 2C and eTable 4 in Supplement 4). Paco2 was higher from day 2 following randomization (eFigure 2D and eTable 4 in Supplement 4) and pH was lower in the intervention group than the standard care group following randomization (eFigure 2E and eTable 4 in Supplement 4). The rate of carbon dioxide removal is shown in eTable 4 in Supplement 4.
Patients in the intervention group were more likely to receive ventilation with a mandatory mode of ventilation to day 7 (59 [39.1%] vs 32 [18.9%]; difference, 20.1% [95% CI, 10.4-29.9]) on day 7), received more neuromuscular blockade from day 2 following randomization (110 [55.6%] vs 92 [44.2%]; difference, 11.3% [95% CI, 1.7%-21.0%] on day 2), and received ventilation less frequently in the prone position on days 1 (11 [5.5%] vs 29 [13.9%]; difference, −8.4% [95% CI, −14.0% to −2.8%]) and 2 (18 [9.1%] vs 27 [13.0%]; difference, −3.9% [95% CI, −10.0% to 2.2%]) following randomization (eTable 5 in Supplement 4).
Serious Adverse Events
Adverse event rates are presented in Table 3. Adverse events were more common in the intervention group. Eighty patients experienced SAEs (62 [31%] in the intervention group and 18 [9%] in the standard care group). Twenty-one patients experienced 22 SAEs related to the study device (eTable 6 in Supplement 4). There were 12 events defined as intracranial hemorrhage (9 of which were defined as SAEs), all of which occurred in the intervention group. Of these SAEs, 5 were considered to be at least possibly related to the intervention by the site investigator (3 patients had an intracerebral hemorrhage and 2 patients had a subarachnoid hemorrhage). An additional 4 SAEs were considered unlikely to be related to the intervention by the site investigator (1 patient had an intracerebral hemorrhage and 3 had hemorrhagic changes on brain imaging). There were 21 events defined as bleeding at other sites, 7 of which were defined as SAEs, with 6 occurring in the intervention group and 1 in the control group. Of those SAEs in the intervention group, 4 were considered to be at least possibly related to the intervention by the site investigator (airway bleeding, hemothorax in a patient with chest trauma, bleeding from a venous hemodialysis catheter, and a hematoma at an attempted vascular access site). The additional 2 SAEs were considered unlikely to be related to the intervention by the site investigator (upper gastrointestinal bleeding and pharyngeal bleeding following reintubation). The event in the control group was an episode of rectal bleeding.
Table 3. Adverse Events in a Study of Lower Tidal Volume Facilitated by Extracorporeal Carbon Dioxide Removal in Patients With Acute Hypoxemic Respiratory Failure.
| Adverse event | ECCO2R (n = 202) | Ventilation alone (n = 210) | ||
|---|---|---|---|---|
| No. of events | No. (%) of patients | No. of events | No. (%) of patients | |
| Adverse eventsa | 168 | 106 (52.5) | 61 | 48 (22.9) |
| Related to study interventiona,b | 65 | 51 (25.3) | 0 | 0 |
| Serious adverse eventsc,d | 70 | 62 (30.7) | 20 | 18 (8.6) |
| Related to study interventionb | 22 | 21 (10.4) | 0 | 0 |
| Adverse events of specific interest | ||||
| Bleeding at other site (excluding intracranial hemorrhage) | 18 | 17 (8.4) | 3 | 3 (1.4) |
| Intracranial hemorrhage | 10 | 10 (5.0) | 2 | 2 (1.0) |
| Device failure causing adverse event | 9 | 9 (4.5) | 0 | 0 |
| Bleeding at cannula site | 8 | 8 (4.0) | 0 | 0 |
| Infectious complicationse | 7 | 7 (3.5) | 1 | 1 (0.5) |
| Heparin-induced thrombocytopenia | 4 | 4 (2.0) | 0 | 0 |
| Hemolysis | 3 | 3 (1.5) | 0 | 0 |
| Ischemic stroke | 1 | 1 (0.5) | 3 | 3 (1.4) |
| Serious adverse events of specific interest f | ||||
| Bleeding at other site (excluding intracranial hemorrhage) | 6 | 6 (3.0) | 1 | 1 (0.5) |
| Intracranial hemorrhage | 9 | 9 (4.5) | 0 | 0 |
| Infectious complicationse | 5 | 5 (2.5) | 0 | 0 |
| Device failure causing serious adverse event | 2 | 2 (1.0) | 0 | 0 |
| Heparin-induced thrombocytopenia | 1 | 1 (0.5) | 0 | 0 |
| Ischemic stroke | 1 | 1 (0.5) | 3 | 3 (1.4) |
Abbreviation: ECCO2R, extracorporeal carbon dioxide removal.
Adverse event totals include serious adverse events.
A list of adverse events that were defined as related to the study intervention was provided in the study protocol (Supplement 1). Events that were possibly, probably, or definitely related to the study intervention (or were not assessable) were defined as related.
Serious adverse events defined by the System Organ Class can be found in eTable 7 in Supplement 4.
A serious adverse event was defined as any adverse event that led to death or resulted in a life-threatening illness or injury, permanent impairment of a body structure or a body function, patient hospitalization or prolongation of existing hospitalization, medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment to a body structure or function, fetal distress, fetal death, or a congenital abnormality or birth defect.
Infectious complications were determined by the site investigator.
There were no episodes of hemolysis or bleeding at cannula site reported as serious adverse events.
Conditional Power Analysis
Post hoc conditional power analysis for mortality showed a conditional power of 4% for a 2% effect size, 8% for a 4% effect size, 17% for a 6% effect size, 31% for an 8% effect size, and 48% for a 10% effect size.
Discussion
In this UK multicenter, randomized clinical trial that was stopped early due to futility, tidal volume reduction during invasive mechanical ventilation facilitated by ECCO2R, compared with standard care, in patients with acute hypoxemic respiratory failure did not reduce mortality at 90 days.
The aim of supportive care with invasive mechanical ventilation in patients with acute hypoxemic respiratory failure during the past 20 years has moved away from targeting normal gas exchange to limiting ventilator-induced lung injury.3,4 A secondary analysis of the ARMA trial suggested there may be no safe threshold for tidal volumes with in vivo data providing biological plausibility for the benefit of further reduction in tidal volumes.15,17 In the study, a reduction in mean tidal volumes of 2.0 mL/kg at day 2 and 2.3 mL/kg at day 3 were achieved, from a prerandomization tidal volume of 6.6 mL/kg, with significant reduction in tidal volumes to day 7, which were associated with significant reductions in plateau and driving pressures. It was mandated that the intervention was applied for at least 48 hours to ensure an effective “dose” of lower tidal volumes, although it is possible that a longer duration of ECCO2R, with greater tidal volume reduction, may have been required to demonstrate an effect because higher intensities of invasive mechanical ventilation have been shown to be associated with increased risk of death in a time-dependent fashion.18 Duration of ECCO2R in the study was limited to less than 7 days due to regulations associated with use of the device, and the intervention was discontinued in 33 patients for this reason. It is unknown whether the results would have changed had these 18% of patients in the intervention group received longer ECCO2R treatment. The primary aim of the trial was to lower tidal volumes facilitated by extracorporeal carbon dioxide removal. Permissive hypercapnia was tolerated to enable tidal volume reduction.19 There was a lower Pao2/Fio2 ratio, higher respiratory rate, and greater hypercapnia and respiratory acidosis in the intervention group, although these effects were modest. As a result, harmful effects associated with these physiological consequences could have contributed to the lack of clinical benefit. Furthermore, that the minute ventilation was reduced indicates that the increase in respiratory rate is unlikely to have offset the reduction in ventilator-induced lung injury achieved with tidal volume reduction. The effect of a larger reduction in ventilator-induced lung injury on outcome remains unknown. Lung-protective ventilation has also been demonstrated to improve outcomes in patients with acute hypoxemic respiratory failure without ARDS,20 so the aim was to include a broad cohort that would reflect the general population of critically ill patients who may benefit. A systematic review concluded that although evidence was limited, ECCO2R was feasible and had been shown to facilitate further reduction in tidal volumes with the potential to mitigate ventilator-induced lung injury and improve outcomes in patients with more severe hypoxemia.21 This work informed the use of a Pao2/Fio2 ratio of less than 150 mm Hg as the qualifying level of hypoxemia for this study population.22
After adjustment for age, degree of hypoxia, and organ dysfunction, the primary outcome was unchanged. Furthermore, results of subgroup analyses did not suggest that the effects of the intervention were modified by any of the variables investigated. Although results of subgroup analyses showed that other baseline characteristics associated with ventilator-induced lung injury did not have an effect on outcome, it remains unknown whether a different population might benefit from ECCO2R. Enrichment strategies to identify a population that may be more likely to benefit are needed for future trials of ECCO2R.23,24
Five patients in the current study were reported to have intracranial hemorrhage related to the intervention. This incidence is comparable to data from previous trials of ECMO in severe acute respiratory failure.13,25 A review of changes in Paco2, presence of thrombocytopenia or coagulopathy, and the degree of therapeutic anticoagulation and blood pressure was performed in these patients, but it was not possible to identify a clear mechanism for these events. Patients with severe hypoxemic respiratory failure have an increased risk of intracranial hemorrhage, with recent data reporting a background rate of intracranial hemorrhage in patients with severe hypoxemic respiratory failure to be approximately 8% to 10%, although this was substantially increased in patients receiving ECMO.26,27
Limitations
The study has several limitations. First, only 6% of screened patients were included in the study, which may limit the generalizability of the results. Second, 17 patients (8.4%) did not receive the intervention as randomized, which could have diluted the effect in the intervention group, although a per-protocol analysis of patients who received the intervention did not change the outcome. Third, most of the sites were naive to the intervention before the study commenced. Although an extensive educational package and training program addressing catheter insertion and maintenance of the device was put in place at all sites, it is possible that practical inexperience with the intervention may have negatively affected the outcomes in the intervention group. Volume-outcome relationships have been previously reported with ECMO.28 In an attempt to address a potential learning effect, a sensitivity analysis excluding the first 2 patients randomized to the intervention group at each site was undertaken that showed no notable change to the primary outcome, and there was no significant difference in a subgroup analysis between sites that recruited more or fewer than 10 patients to the trial. Fourth, other aspects of care were not standardized in each group because this was a pragmatic trial, and clinicians were free to treat patients as they would normally. The use of the intervention was associated with longer use of neuromuscular-blocking drugs and less prone positioning. Although the difference in the use of neuromuscular-blocking drugs is unlikely to have modified outcome, the less frequent use of prone positioning could have affected the outcome in the intervention group, albeit the absolute difference in the use of prone positioning between the groups was relatively small.11,12 Fifth, it is possible that the trial was underpowered to detect a clinically important difference, particularly because the trial was stopped before recruitment of the planned sample size was achieved. Sixth, due to the complexity of the intervention, blinding to the clinicians or patients was not possible, which could have resulted in performance bias.
Conclusions
In patients requiring mechanical ventilation for acute hypoxemic respiratory failure, lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal, compared with standard care, did not result in a reduction in mortality at 90 days. However, due to early termination, the study may have been underpowered to detect a clinically important difference.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Trial protocol
Statistical analysis plan
Study manual
eFigure 1. Treatment-by-subgroup interactions
eFigure 2. Physiological parameters by treatment group to day 7; PaO2/FiO2 Ratio (A), Respiration Rate (B), Minute Ventilation (C), Partial Pressure of Arterial Carbon Dioxide (PaCO2) (D) and pH (E) to day 7
eTable 1. Pre-specified secondary endpoints
eTable 2. Enrollment by site
eTable 3. Treatment after trial entry
eTable 4. Daily mean (SD) physiological parameters to day 7
eTable 5. Mode of ventilation and use of adjunctive therapies
eTable 6. Serious Adverse Events related to the study intervention
eTable 7. Serious Adverse Events by System Organ Class
Nonauthor collaborators
Data sharing statement
References
- 1.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group . Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788-800. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 2.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. [DOI] [PubMed] [Google Scholar]
- 3.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126-2136. doi: 10.1056/NEJMra1208707 [DOI] [PubMed] [Google Scholar]
- 4.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308. doi: 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 5.Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175(2):160-166. doi: 10.1164/rccm.200607-915OC [DOI] [PubMed] [Google Scholar]
- 6.Stewart TE, Meade MO, Cook DJ, et al. ; Pressure- and Volume-Limited Ventilation Strategy Group . Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. N Engl J Med. 1998;338(6):355-361. doi: 10.1056/NEJM199802053380603 [DOI] [PubMed] [Google Scholar]
- 7.Boyle AJ, Sklar MC, McNamee JJ, et al. ; International ECMO Network (ECMONet) . Extracorporeal carbon dioxide removal for lowering the risk of mechanical ventilation: research questions and clinical potential for the future. Lancet Respir Med. 2018;6(11):874-884. doi: 10.1016/S2213-2600(18)30326-6 [DOI] [PubMed] [Google Scholar]
- 8.Combes A, Fanelli V, Pham T, Ranieri VM; European Society of Intensive Care Medicine Trials Group and the “Strategy of Ultra-Protective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to severe ARDS” (SUPERNOVA) investigators . Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med. 2019;45(5):592-600. doi: 10.1007/s00134-019-05567-4 [DOI] [PubMed] [Google Scholar]
- 9.McNamee JJ, Gillies MA, Barrett NA, et al. Protective ventilation with veno-venous lung assist in respiratory failure: a protocol for a multicentre randomised controlled trial of extracorporeal carbon dioxide removal in patients with acute hypoxaemic respiratory failure. J Intensive Care Soc. 2017;18(2):159-169. doi: 10.1177/1751143716681035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidelines on the management of acute respiratory distress syndrome. Intensive Care Society and Faculty of Intensive Care Medicine . Accessed December 4, 2020. https://ics.ac.uk/ICS/ICS/GuidelinesAndStandards/Guidelines_pg3.aspx
- 11.Papazian L, Forel JM, Gacouin A, et al. ; ACURASYS Study Investigators . Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107-1116. doi: 10.1056/NEJMoa1005372 [DOI] [PubMed] [Google Scholar]
- 12.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group . Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159-2168. doi: 10.1056/NEJMoa1214103 [DOI] [PubMed] [Google Scholar]
- 13.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration . Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-1363. doi: 10.1016/S0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 14.Young D, Lamb SE, Shah S, et al. ; OSCAR Study Group . High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368(9):806-813. doi: 10.1056/NEJMoa1215716 [DOI] [PubMed] [Google Scholar]
- 15.Hager DN, Krishnan JA, Hayden DL, Brower RG; ARDS Clinical Trials Network . Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172(10):1241-1245. doi: 10.1164/rccm.200501-048CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, et al. ; the SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 17.Frank JA, Parsons PE, Matthay MA. Pathogenetic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest. 2006;130(6):1906-1914. doi: 10.1378/chest.130.6.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madotto F, Rezoagli E, McNicholas BA, et al. ; LUNG SAFE Investigators and the ESICM Trials Group . Patterns and impact of arterial CO2 management in patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Chest. 2020;158(5):1967-1982. doi: 10.1016/j.chest.2020.05.605 [DOI] [PubMed] [Google Scholar]
- 20.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308(16):1651-1659. doi: 10.1001/jama.2012.13730 [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald M, Millar J, Blackwood B, et al. Extracorporeal carbon dioxide removal for patients with acute respiratory failure secondary to the acute respiratory distress syndrome: a systematic review. Crit Care. 2014;18(3):222. doi: 10.1186/cc13875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39(5):847-856. doi: 10.1007/s00134-012-2787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goligher EC, Amato MBP, Slutsky AS. Applying precision medicine to trial design using physiology: extracorporeal CO2 removal for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196(5):558-568. doi: 10.1164/rccm.201701-0248CP [DOI] [PubMed] [Google Scholar]
- 24.Goligher EC, Combes A, Brodie D, et al. ; SUPERNOVA investigators (European Society of Intensive Care Medicine trials group) and for the International ECMO Network (ECMONet) . Determinants of the effect of extracorporeal carbon dioxide removal in the SUPERNOVA trial: implications for trial design. Intensive Care Med. 2019;45(9):1219-1230. doi: 10.1007/s00134-019-05708-9 [DOI] [PubMed] [Google Scholar]
- 25.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet . Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965-1975. doi: 10.1056/NEJMoa1800385 [DOI] [PubMed] [Google Scholar]
- 26.Lockie CJA, Gillon SA, Barrett NA, et al. Severe respiratory failure, extracorporeal membrane oxygenation, and intracranial hemorrhage. Crit Care Med. 2017;45(10):1642-1649. doi: 10.1097/CCM.0000000000002579 [DOI] [PubMed] [Google Scholar]
- 27.Arachchillage DRJ, Passariello M, Laffan M, et al. Intracranial hemorrhage and early mortality in patients receiving extracorporeal membrane oxygenation for severe respiratory failure. Semin Thromb Hemost. 2018;44(3):276-286. doi: 10.1055/s-0038-1636840 [DOI] [PubMed] [Google Scholar]
- 28.Fan E, Brodie D. Higher volumes, better outcomes: the end or just the beginning of the story for extracorporeal membrane oxygenation? Am J Respir Crit Care Med. 2015;191(8):864-866. doi: 10.1164/rccm.201503-0459ED [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
Study manual
eFigure 1. Treatment-by-subgroup interactions
eFigure 2. Physiological parameters by treatment group to day 7; PaO2/FiO2 Ratio (A), Respiration Rate (B), Minute Ventilation (C), Partial Pressure of Arterial Carbon Dioxide (PaCO2) (D) and pH (E) to day 7
eTable 1. Pre-specified secondary endpoints
eTable 2. Enrollment by site
eTable 3. Treatment after trial entry
eTable 4. Daily mean (SD) physiological parameters to day 7
eTable 5. Mode of ventilation and use of adjunctive therapies
eTable 6. Serious Adverse Events related to the study intervention
eTable 7. Serious Adverse Events by System Organ Class
Nonauthor collaborators
Data sharing statement