Abstract
Background
Screening strategies for high-risk human papillomavirus (hrHPV)-associated anal cancer are evolving. Herein, we compare anal cytology to hrHPV DNA testing and 2 novel cytology/hrHPV cotesting algorithms among 3 high-risk populations.
Methods
Anal cytology, hrHPV DNA testing, and high-resolution anoscopy (HRA)-guided biopsy results were analyzed from 1837 participants (1504 HIV-infected men who have sex with men (MSM), 155 HIV-uninfected MSM, and 178 HIV-infected women). Performance to detect histological high-grade squamous intraepithelial lesions (HSIL)/cancer was compared between 4 strategies with distinct HRA referral thresholds: cytology (atypical squamous cells of undetermined significance, ASCUS); hrHPV testing (any hrHPV positive); algorithm A (benign cytology/HPV16/18 positive or ASCUS/hrHPV positive); and algorithm B (benign or ASCUS/hrHPV positive).
Results
Histological HSIL/cancer was detected in 756 (41%) participants. Cytology had the lowest sensitivity (0.76–0.89) but highest specificity (0.33–0.36) overall and for each subgroup. Algorithm B was the most sensitive strategy overall (0.97) and for MSM (HIV-infected 0.97; HIV-uninfected 1.00). For women, hrHPV testing and both algorithms yielded higher sensitivity than cytology (0.96, 0.98, and 0.96). Specificity was low for all strategies/subgroups (range, 0.16–0.36).
Conclusions
Screening algorithms that incorporate cytology and hrHPV testing significantly increased sensitivity but decreased specificity to detect anal precancer/cancer among high-risk populations.
Keywords: anal cancer screening, high-grade squamous intraepithelial lesion, human immunodeficiency virus, human papillomavirus, HPV DNA testing
Screening strategies for high-risk human papillomavirus (hrHPV)-associated anal cancer are evolving. This study compares the screening performance of anal cytology to hrHPV DNA testing and 2 novel cotesting algorithms among 3 high-risk populations: HIV-positive MSM, HIV-negative MSM, and HIV-positive women.
Analogous to cervical cancer, high-risk human papillomavirus (hrHPV)-associated anal cancer is thought to be preventable if effective screening and treatment algorithms are implemented [1–3]. However, the incidence of anal cancer has continued to rise in the United States with a large and growing proportion of cases diagnosed at an advanced stage [4, 5]. Anal cancer mortality has increased over 3% annually, making it one of the fastest growing causes of cancer death [4]. People living with human immunodeficiency virus (PWH) and men who have sex with men (MSM) carry a significantly higher anal cancer risk than the general population [6, 7]. The highest incidence rates are reported in HIV-infected MSM (85 cases per 100 000 person-years) followed by HIV-infected women (22 per 100 000) and HIV-uninfected MSM (19 per 100 000) [8]. To develop evidence-based and practical screening strategies for anal cancer, it is critical to assess their effectiveness in high-risk populations overall as well as within subgroups for whom disease prevalence and test performance characteristics may differ.
In the absence of binding consensus guidelines endorsed by national entities such as the United States Preventive Services Taskforce, anal cancer screening programs often follow heterogeneous practice approaches based on local infrastructure and expertise. Several professional organizations have recommended cytology as the primary screening tool for anal cancer and precancers (high-grade squamous intraepithelial lesions, HSIL) [9, 10]. The performance of anal cytology has been inconsistent, as demonstrated by its wide range of sensitivity (61%–93%) and specificity (32%–67%) [11–13]. As with all morphology-based tests, cytology is hampered by substantial interobserver variability and poor reproducibility, underscoring its deficiency as a standalone screening test [14, 15]. To compensate for these inadequacies, the current practice is to refer all high-risk individuals for high-resolution anoscopy (HRA) except those with benign cytology results [16].
Several HPV assays have been validated for anal cytology samples; however, they are currently not Food and Drug Administration (FDA)-approved for anal cancer screening. In a study comparing HPV-related biomarkers, hrHPV DNA testing had the highest sensitivity for predicting anal HSIL (100%; 95% confidence interval [CI], 95.6%–100%), followed by cytology with p16/Ki-67 dual-staining, HPV E6/E7 mRNA testing, and HPV16/18 genotyping. The high anal HPV prevalence among study participants (HIV-infected MSM), however, led to a low specificity of hrHPV DNA testing (27.7%; 95% CI, 21.9%–34.3%), thereby limiting its utility [17].
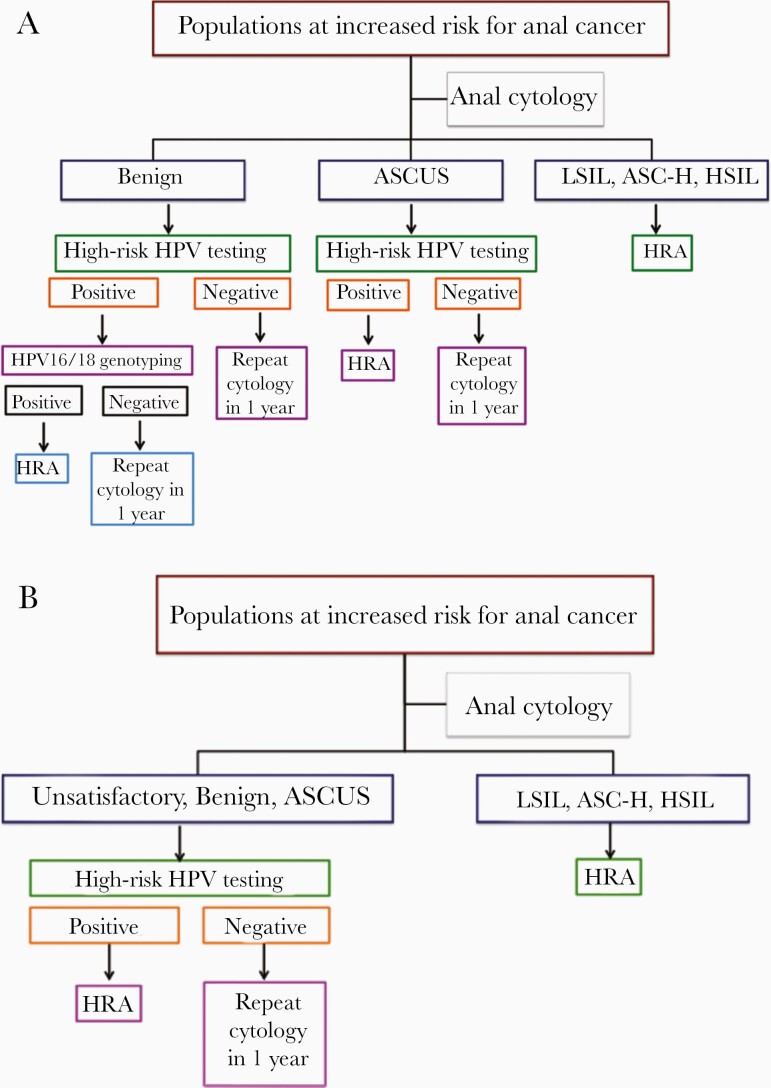
A novel cytology and hrHPV cotesting algorithm was proposed by Sambursky et al wherein hrHPV DNA testing is used as an adjunctive reflex test for the atypical squamous cells of undetermined significance (ASCUS) cytological category and HPV16/18 genotyping for the benign cytological category (hereafter, algorithm A; Figure 1A) [18]. Individuals with benign cytology/HPV16/18-positive, ASCUS/hrHPV-positive, and any higher-grade cytological abnormalities are referred for HRA. In a cohort of 894 subjects (MSM 92%, PWH 42%, anal HSIL prevalence 14.8%), the authors demonstrated that algorithm A significantly improved both sensitivity and specificity in predicting anal HSIL compared to cytology alone (96%/61% vs 89%/51%).
Figure 1.
A and B, Proposed anal cancer screening algorithms A and B. Abbreviations: ASC-H, atypical squamous cells, cannot exclude HSIL; ASCUS, atypical squamous cells of undetermined significance; HPV, human papillomavirus; HRA, high-resolution anoscopy; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion. Algorithm A: modified with permissions from Sambursky et al. 2018.
We propose a similar but simplified algorithm whereby the unsatisfactory, benign, and ASCUS cytological categories are tested reflexively for hrHPV; if positive, HRA is pursued without additional HPV16/18 genotyping (hereafter, algorithm B; Figure 1B). In this retrospective, cross-sectional cohort study we compare the screening performance of anal cytology alone, hrHPV DNA testing alone, and algorithms A and B in a cohort of 1837 high-risk individuals, including 3 distinct subgroups: HIV-infected MSM, HIV-uninfected MSM, and HIV-infected women.
METHODS
Patient Selection
The Institutional Review Board of the Icahn School of Medicine approved this research. The Mount Sinai Anal Dysplasia Program is a major referral center for the diagnosis and treatment of HPV-associated anal precancer and cancer [19]. We follow the recently updated screening recommendations from the New York State Department of Health AIDS Institute and offer anal cytology annually to all PWH ≥ 35 years of age and those < 35 presenting with symptoms suggestive of anal dysplasia [10]. Given reported high prevalence rates of anal HSIL in HIV-uninfected MSM, our program elected to also offer screening to this population [20].
We searched the HRA database between January 2015 and January 2019 for individuals who had undergone anal cytology screening, hrHPV DNA testing, and HRA examination either concurrently or within 3 months of screening cytology. For individuals with multiple visits, only results from the initial visit were included in the analysis. Individuals with prior treatment of anal HSIL or cancer were excluded. The following demographic and clinical factors were extracted from the electronic medical record: age, gender, race/ethnicity, history of receptive anal intercourse, smoking history, HIV status, CD4+ T-cell count, and HIV-1 RNA level most closely associated with the screening visit.
Anal Cytology and hrHPV DNA Testing
Anal swab samples were collected using a Dacron swab or cytobrush for both cytological diagnosis and hrHPV DNA testing. Swabs/brushes were inserted into the anal canal to above the squamocolumnar junction, withdrawn with circular motion while applying peripheral pressure to open mucosal folds and survey mucosal surfaces, and then vigorously rinsed in liquid medium for the ThinPrep Pap Test (Cytyc Corporation). ThinPrep slides were prepared following standard protocol and diagnosed by cytopathologists from the Mount Sinai Hospital in accordance with the 2001 Bethesda criteria: unsatisfactory (ie, less than 2000 to 3000 nucleated squamous cells), benign, ASCUS, low-grade squamous intraepithelial lesions (LSIL), atypical squamous cells cannot exclude HSIL (ASC-H), HSIL, or cancer [21].
Currently, hrHPV DNA testing has not been FDA approved for anal cancer screening. Our clinical laboratory has validated the Cobas 4800 system (Roche Diagnostics) with anal cytology specimens. Regardless of cytology result, ThinPrep medium aliquots were tested for hrHPV DNA using the Cobas 4800 system following manufacturer instructions. The assay reports presence or absence of HPV16 and 18 separately. Twelve additional high-risk types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) are reported as a pooled result referred to as others.
HRA, Biopsy, and Histological Diagnosis
HRA examination and biopsies were performed in an ambulatory setting following previously described techniques [22]. Briefly, after treatment with 5% acetic acid and Lugol’s iodine, the perianal region, distal anal canal, and squamocolumnar junction were examined using a high-resolution colposcope at 15-fold magnification. Biopsies were taken from areas with mucosal changes suspicious for HSIL or cancer. If no suspicious areas were identified, biopsy was not performed and the HRA result was recorded as benign. Random biopsies of nondysplastic-appearing tissue were not performed. Each subject underwent a mean of 3 biopsies (range, 0–9).
After biopsy samples were processed using standard histological protocols, hematoxylin and eosin slides were prepared. Following the Lower Anogenital Squamous Terminology recommendations, surgical pathologists from the Mount Sinai Hospital diagnosed all biopsies as negative for dysplasia, LSIL (synonymous with anal intraepithelial neoplasia 1, AIN 1), or HSIL (synonymous with AIN 2/3) [23]. The designation of HSIL required abnormal cells with nuclear enlargement, coarse chromatin, and irregular nuclear membrane occupying the middle third (AIN 2) or upper third (AIN 3) of the squamous epithelium.
Statistical Analysis
Using biopsy-confirmed HSIL as reference, we calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each screening strategy for the entire cohort and each subgroup. HRA referral thresholds were: (1) cytology: ASCUS, (2) standalone hrHPV DNA testing: any hrHPV positive, (3) algorithm A: benign cytology/HPV16/18 positive or ASCUS/hrHPV positive, and (4) algorithm B: benign or ASCUS cytology/hrHPV positive. Using binomial distribution, we calculated 95% CI for each test characteristic. Risk ratios were also calculated, comparing the risk of HSIL for persons who meet the specified test thresholds to those with normal cytology and negative hrHPV DNA testing.
RESULTS
Patient Characteristics
From January 2015 to January 2019, 1837 individuals underwent anal cytology, hrHPV DNA testing, and HRA examination either concurrently or within 3 months. The cohort comprised 3 subgroups: 1504 (82%) HIV-infected MSM, 155 (8%) HIV-uninfected MSM, and 178 (10%) HIV-infected women. Table 1 details patient demographic characteristics. The 3 subgroups showed significant differences in age, race/ethnicity, current smoking status, and history of receptive anal intercourse (P < .001).
Table 1.
Patient Characteristics for the Entire Cohort and 3 Subgroups
| Characteristics | Entire Cohort (n = 1837) | Subgroups | P | ||
|---|---|---|---|---|---|
| HIV-Infected MSM (n = 1504) |
HIV-Uninfected MSM (n = 155) |
HIV-Infected Women (n = 178) |
|||
| Age, y, median (IQR) | 45 (34–54) | 45 (34–54) | 37 (31–48) | 52 (44–57) | <.001 |
| Race/ethnicity | |||||
| White | 691 (38) | 586 (39) | 90 (58) | 15 (8) | <.001 |
| African American | 293 (16) | 222 (14) | 10 (7) | 71 (40) | |
| Hispanic | 357 (19) | 302 (20) | 9 (6) | 46 (26) | |
| Other | 252 (14) | 210 (14) | 26 (17) | 16 (9) | |
| Unknown | 244 (13) | 193 (13) | 20 (13) | 30 (17) | |
| Current smoker | 394 (21) | 310 (21) | 19 (12) | 65 (37) | <.001 |
| HIV RNA < 100 copies/mL | 1510 (92) | 1363 (92) | … | 147 (85) | .003 |
| CD4+ T-cell count ≥ 500 cells/mL | 1136 (71) | 1025 (72) | … | 111 (66) | .11 |
| Receptive anal intercourse | 1773 (97) | 1502 (99) | 155 (100) | 116 (65) | <.001 |
| Anal cytology | |||||
| Unsatisfactory | 66 (4) | 52 (3) | 7 (5) | 7 (4) | .19 |
| Benign | 410 (22) | 325 (22) | 42 (27) | 43 (24) | |
| ASCUS | 716 (39) | 577 (38) | 62 (40) | 77 (43) | |
| LSIL | 525 (29) | 443 (30) | 40 (26) | 42 (24) | |
| ASC-H | 44 (2) | 42 (3) | 1 (1) | 1 (1) | |
| HSIL | 76 (4) | 65 (4) | 3 (2) | 8 (4) | |
| Anal hrHPV DNA testing | |||||
| Any hrHPV positive | 1514 (82) | 1248 (83) | 130 (84) | 136 (76) | .08 |
| HPV16/18 positive | 679 (37) | 550 (37) | 63 (41) | 66 (37) | .61 |
| Others positivea | 1420 (77) | 1186 (79) | 115 (74) | 119 (67) | .001 |
| Biopsy-proven HSIL and cancer | 756 (41)b | 646 (43) | 55 (36) | 55 (31) | .003 |
Data are No. of cases (%) unless otherwise indicated.
Abbreviations: ASC-H, atypical squamous cells, cannot exclude HSIL; ASCUS, atypical squamous cells of uncertain significance; HIV, human immunodeficiency virus; hrHPV, high-risk human papilloma virus; HSIL, high-grade squamous intraepithelial lesion; IQR, interquartile range; LSIL, low-grade squamous intraepithelial lesion; MSM, men who have sex with men.
aOthers include HPV31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
bFour patients were diagnosed with superficially invasive squamous cell carcinoma of the anus.
Anal Cytology, hrHPV DNA Testing, and HRA Results
Anal cytology and hrHPV DNA testing results are shown in Table 1. For the entire cohort, hrHPV and HPV16/18 prevalence was 82% (95% CI, 80%–84%) and 37% (95% CI, 35%–39%). The 3 subgroups showed comparable HPV prevalences (P = .08 and .61). Prevalence of hrHPV and HPV16/18 increased in parallel with the severity grade of cytological diagnoses (data not shown).
Biopsy results included superficially invasive squamous cell carcinoma of the anus (SISCCA, n = 4, 0.2%), HSIL (n = 752, 41%), LSIL (n = 669, 36%), and benign findings (n = 412, 22%). Patients who had a normal HRA exam without biopsy (n = 180) were included in the category of benign findings. Of HSIL cases, 526 (70%) were AIN 2 and 226 (30%) were AIN 3. Most HSIL cases (74%) were localized (ie, 1 or 2 lesions), while 26% were extensive (ie, 3 or more lesions). The prevalence of HSIL and cancer in HIV-infected MSM (43%) was significantly higher than that in HIV-uninfected MSM and HIV-infected women (36% and 31%, respectively, P = .003).
As shown in Table 2, the incidence of histological HSIL/cancer increased with the severity grade of cytological diagnoses (P < .001 for trend). In the entire cohort, as well as within the benign, ASCUS, LSIL, and ASC-H cytological categories, the incidence of histological HSIL/cancer was significantly higher among subjects who tested positive for hrHPV, particularly HPV16/18, compared to those who tested negative (P < .001; Table 2 and Table 3). The 4 patients with SISCCA were positive for HPV16 and corresponding cytology results were HSIL (2 patients), LSIL, and ASCUS.
Table 2.
Correlating HRA and Biopsy Results With Cytological Diagnosis and hrHPV Status
| Assay Result | Total No. of Cases | HRA and Biopsy Result | ||
|---|---|---|---|---|
| Benign | LSIL | HSIL/Cancera | ||
| Cytology | ||||
| Unsatisfactory | 66 | 26 (40) | 23 (35) | 17 (25) |
| Benign | 410 | 147 (36) | 166 (40) | 97 (24) |
| ASCUS | 716 | 179 (25) | 270 (38) | 267 (37) |
| LSIL | 525 | 52 (10) | 190 (36) | 283 (54) |
| ASC-H | 44 | 4 (9) | 12 (27) | 28 (64) |
| HSIL | 76 | 4 (5) | 8 (11) | 64 (84) |
| hrHPV DNA testing | ||||
| Negative | 323 | 160 (50) | 135 (42) | 28 (9) |
| Any hrHPV positive | 1514 | 252 (17) | 534 (35) | 728 (48) |
| HPV16/18 positive | 679 | 86 (13) | 190 (28) | 403 (59) |
| Others positiveb | 835 | 166 (20) | 344 (41) | 325 (39) |
Data are No. of cases (%).
Abbreviations: ASC-H, atypical squamous cells, cannot exclude HSIL; ASCUS, atypical squamous cells of undetermined significance; benign, negative for intraepithelial lesion or malignancy; hrHPV, high-risk human papillomavirus; HRA, high-resolution anoscopy; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
aProportions of histological HSIL/cancer increased with the severity grade of cytological diagnoses (P < .001 for trend).
bOthers: HPV31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68
Table 3.
Incidence of Histological HSIL/Cancer Stratified by Anal Cytology and hrHPV Status
| Cytological Category | Histological HSIL/Cancer | |||||
|---|---|---|---|---|---|---|
| Total | hrHPV Negative | Any hrHPV Positive | P a | HPV16/18 Positive | Others Positiveb | |
| Unsatisfactory | 26 (17/66) | 15 (3/20) | 30 (14/46) | .19 | 30 (3/10) | 31 (11/36) |
| Benign | 24 (97/410) | 5 (5/109) | 31 (92/301) | <.001 | 33 (34/103) | 29 (58/198) |
| ASCUS | 37 (267/716) | 8 (12/146) | 45 (255/570) | <.001 | 56 (130/232) | 37 (125/338) |
| LSIL | 54 (283/525) | 17 (7/41) | 57 (276/484) | <.001 | 66 (171/258) | 46 (105/226) |
| ASC-H | 64 (28/44) | 0 (0/6) | 74 (28/38) | <.001 | 80 (20/25) | 62 (8/13) |
| HSIL | 84 (64/76) | 100 (1/1) | 84 (63/75) | .70 | 88 (45/51) | 75 (18/24) |
Data are percent (n/N) where n = number of subjects with histological HSIL/cancer and N = total number of subjects with given cytological diagnosis and hrHPV status.
Abbreviations: ASC-H, atypical squamous cells, cannot exclude HSIL; ASCUS, atypical cells of undetermined significance; benign, negative for intraepithelial lesion or malignancy; hrHPV, high-risk human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
aP value for comparison of proportions of HSIL/cancer found in hrHPV negative vs any hrHPV positive.
bOthers: HPV31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
Screening Performance
Table 4 details screening performance of the 4 strategies for the entire cohort and each subgroup. Cytology alone had the lowest sensitivity overall and for each subgroup. hrHPV DNA testing alone and algorithm B yielded significantly higher sensitivity than cytology alone for the entire cohort (0.96 and 0.97 vs 0.85), for HIV-infected MSM (0.96 and 0.97 vs 0.85), and for HIV-uninfected MSM (0.96 and 1.00 vs 0.76). For HIV-infected women, all 3 strategies (A, B, and hrHPV DNA testing) tended to be more sensitive than cytology alone, albeit not to a degree of statistical significance (0.98, 0.96, and 0.96 vs 0.89). Specificity was low for all strategies and subgroups (range, 0.16–0.36). The specificity of cytology alone tended to be higher compared to the other strategies. These differences were statistically significant for the overall cohort and HIV-infected MSM, but insignificant for HIV-uninfected MSM and HIV-infected women.
Table 4.
Screening Performance of 4 Strategies for the Entire Cohort and 3 Subgroups
| Population | Strategy | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | RR (95% CI) |
|---|---|---|---|---|---|---|
| Entire cohort (n = 1837) | Cytology | 0.85 (.82–.87) | 0.33 (.31–.36) | 0.47 (.44–.50) | 0.76 (.72–.80) | 10.3 (4.4–24.3) |
| hrHPV DNA testing | 0.96 (.95–.98) | 0.27 (.25–.30) | 0.48 (.46–.51) | 0.91 (.88–.94) | 10.4 (4.4–24.7) | |
| Algorithm A | 0.89 (.87–.92) | 0.27 (.24–.30) | 0.46 (.44–.49) | 0.79 (.75–.83) | 10.1 (4.3–23.8) | |
| Algorithm B | 0.97 (.96–.98) | 0.24 (.21–.26) | 0.47 (.45–.50) | 0.93 (.89–.96) | 10.3 (4.4–24.2) | |
| HIV-infected MSM (n = 1504) | Cytology | 0.85 (.82–.88) | 0.33 (.30–.36) | 0.49 (.46–.52) | 0.75 (.70–.79) | 10.7 (4.5–25.1) |
| hrHPV DNA testing | 0.96 (.96–.98) | 0.27 (.24–.30) | 0.50 (.47–.53) | 0.91 (.86–.94) | 10.8 (4.6–25.6) | |
| Algorithm A | 0.89 (.87–.92) | 0.27 (.24–.30) | 0.48 (.45–.51) | 0.77 (.72–.81) | 10.4 (4.4–24.6) | |
| Algorithm B | 0.97 (.96–.98) | 0.24 (.21–.27) | 0.49 (.46–.52) | 0.92 (.87–.95) | 10.7 (4.5–25.2) | |
| HIV-uninfected MSM (n = 155) | Cytology | 0.76 (.63–.87) | 0.36 (.27–.46) | 0.40 (.30–.50) | 0.74 (.59–.85) | 8.6 (3.6–21.0) |
| hrHPV DNA testing | 0.96 (.88–.99) | 0.23 (.15–.33) | 0.41 (.32–.50) | 0.92 (.74–.99) | 8.9 (3.7–21.4) | |
| Algorithm A | 0.89 (.78–.96) | 0.27 (.19–.37) | 0.40 (.31–.49) | 0.81 (.65–.93) | 8.8 (3.6–21.2) | |
| Algorithm B | 1.00 (.94–1.00) | 0.16 (.09–.25) | 0.40 (.31–.48) | 1.00 (.79–1.00) | 8.6 (3.6–20.8) | |
| HIV-infected women (n = 178) | Cytology | 0.89 (.78–.96) | 0.36 (.27–.45) | 0.38 (.30–.47) | 0.88 (.76–.96) | 8.3 (3.4–20.2) |
| hrHPV DNA testing | 0.96 (.87–1.00) | 0.33 (.24–.42) | 0.39 (.31–.48) | 0.95 (.83–.99) | 8.5 (3.5–20.5) | |
| Algorithm A | 0.98 (.90–1.00) | 0.28 (.21–.37) | 0.38 (.30–.47) | 0.97 (.86–.99) | 8.3 (3.4–20.0) | |
| Algorithm B | 0.96 (.88–1.00) | 0.29 (.21–.38) | 0.38 (.30–.46) | 0.95 (.82–.99) | 8.3 (3.4–20.0) |
HRA referral threshold: (1) cytology: ASCUS, (2) hrHPV testing: any hrHPV positive, (3) algorithm A: benign cytology/HPV16/18 positive or ASCUS/hrHPV positive, and (4) algorithm B: benign or ASCUS cytology/hrHPV positive.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HRA, high-resolution anoscopy; hrHPV, high-risk human papillomavirus; MSM, men who have sex with men; NPV, negative predictive value; PPV, positive predictive value; RR, relative risk.
Discussion
In this retrospective analysis, we compared the performance of 4 strategies in predicting anal precancer and cancer: cytology, standalone hrHPV DNA testing and 2 novel cotesting algorithms. The cohort was predominantly composed of HIV-infected MSM but also included a robust number of HIV-uninfected MSM and HIV-infected women, 2 populations at increased risk for anal cancer often underrepresented in previous studies.
Compared to cytology, we found that standalone hrHPV DNA testing or cotesting algorithms significantly increased screening sensitivity and decreased specificity for the entire cohort, as well as all 3 subgroups. Compared to the study by Sambursky et al [18], we confirmed the superior sensitivity of their cotesting algorithm (0.96 vs 0.89) but failed to replicate its superior specificity (0.61 vs 0.27). The notable drop in test specificity could be attributable to the discrepant HSIL/cancer prevalence between the 2 study cohorts (14.8% in Sambursky cohort vs 41% in ours). While high sensitivity is undoubtedly preferable for cancer screening, additional tests or steps that improve specificity could minimize the costs and harms associated with needless procedures [24, 25].
Both algorithms A and B use hrHPV DNA testing to triage cytological benign and ASCUS categories, the rationale being 2-fold. First, in contrast to LSIL and HSIL cytology, hrHPV status was more heterogeneous in the benign and ASCUS categories. Second, hrHPV was a strong predictor of histological HSIL diagnoses within the benign and ASCUS categories, providing valuable risk stratification information. For example, ASCUS/hrHPV-positive or ASCUS/HPV16/18-positive subjects carried a 5.6- and 7-fold higher HSIL risk than ASCUS/hrHPV-negative subjects (45% and 56% vs 8%). Similarly, divergent HSIL risk was observed in the benign cytology category (31% and 33% vs 5%). Sambursky et al reported an even greater impact of hrHPV and HPV16/18 positivity on HSIL risk: 16- and 31-fold increased risk in the benign category and 13- and 18-fold in the ASCUS category [18].
The key difference between algorithms A and B lies in whether subjects with benign cytology but non-16/18 hrHPV should undergo HRA. Of 198 such subjects in our cohort, 58 (29%) were found to have HSIL by HRA and biopsy, a risk comparable to their benign cytology/HPV16/18-positive counterparts (33%). Thus, we recommend HRA referral for all subjects with benign cytology but hrHPV infection, regardless of hrHPV type. Doing so improved sensitivity significantly but only slightly lowered specificity compared to algorithm A. In a study of 426 HIV-infected MSM (HSIL prevalence 38%), the SeVIHanal group reported that in the benign cytology category, hrHPV positivity had a 29.3% PPV while the lack thereof had a 90.2% NPV for histological HSIL [26]. The authors recommended HRA for HIV-infected MSM with benign cytology and any hrHPV infection, an approach directly in line with our proposed algorithm B.
Unsatisfactory cytology, often resulting in uncertainty for management and possible diagnostic delays, is not addressed in algorithm A. With unsatisfactory samples amounting to as much as 17% in some practices, current recommendations are to repeat anal cytology [27, 28]. We included 66 such cases (4% of our cohort), all of which were tested for hrHPV successfully. The HSIL prevalence was 30% among unsatisfactory cytology/hrHPV-positive subjects, supporting direct HRA referral and omitting repeat cytology in this scenario. hrHPV cotesting could therefore expedite referral for definitive tissue diagnosis and decrease the risk of loss to follow-up. A clinical trial on cervical cancer screening showed that using hrHPV to triage unsatisfactory cervical cytology is both feasible and cost-effective [29].
Of the 4 screening strategies we analyzed, standalone hrHPV DNA testing and both cotesting algorithms were more sensitive than cytology alone. This raises the question as to whether hrHPV DNA testing should play a more pivotal role in anal cancer screening akin to what is already accepted clinical practice in cervical cancer screening [30]. hrHPV DNA testing of anal swab samples has not gained wide acceptance, primarily due to the high hrHPV prevalence in screening populations. In a hypothetical population of 10 000 HIV-infected MSM (anal HSIL prevalence 25%), Clarke et al calculated screening sensitivity/specificity of 0.95/0.24 for hrHPV DNA testing and 0.81/0.53 for cytology [25]. We herein report very similar results for hrHPV DNA testing (0.96/0.27), but lower specificity for cytology (0.85/0.33), a finding that calls into question the putative advantage of cytology over hrHPV DNA testing. This discrepancy may in part be attributable to the wide range of diagnostic accuracy among cytopathologists. Conversely, hrHPV DNA testing is a fully automated real-time PCR assay that is less contingent upon human interpretation [31]. As in cervical cancer screening, the incorporation of hrHPV testing could advance anal cancer screening from a subjective and morphology-based approach to a more objective and risk-based one.
Could a single screening strategy work for diverse high-risk subgroups against the backdrop of heterogeneous hrHPV prevalence and cancer risk? The answer appears to be affirmative: hrHPV DNA testing alone and algorithm B demonstrated outstanding sensitivity for the entire cohort and all 3 major high-risk subgroups. We included a robust number of HIV-uninfected MSM (n = 155) and HIV-infected women (n = 178), 2 populations often underrepresented in previous studies. hrHPV DNA testing may be of particular utility for women: studies have shown that compared to MSM, women reported higher pain scores and greater reluctance to proceed with HRA [32, 33]. Given the high NPV (0.95) we reported for female patients, hrHPV DNA testing or cotesting could decrease the number of HRA procedures, possibly prolong screening intervals, and ultimately increase screening compliance [34].
Our study has several strengths. It features the largest clinical dataset published to date capturing concomitant anal cytology, hrHPV DNA testing, and HRA results for individual patients. High-risk populations other than HIV-infected MSM are well represented. Notable limitations include its retrospective nature as well as lack of cost-effectiveness analyses that are essential for the development of screening guidelines. As our study population reflects that of a specialized program in an urban region that receives referrals for patients at risk for anal dysplasia, results may not be generalizable.
In summary, among established populations at increased risk for anal cancer, screening algorithms that incorporate cytology and hrHPV DNA testing significantly increased sensitivity and further decreased specificity to detect anal precancer/cancer compared to cytology alone (the current standard of care). These findings justify the pursuit of cost-effectiveness analysis that compares these strategies to identify the optimal approach for anal cancer screening and to inform clinical practice.
Notes
Presented in part: Conference on Retroviruses and Opportunistic Infections, 8–11 March 2020, Boston, MA.
Financial support. This work was supported by the National Cancer Institute, National Institutes of Health (grant number R01CA232888).
Potential conflicts of interest. A. A. D. received consulting fees from Merck for unrelated projects. All other authors report no potential conflicts of interest. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Revollo B, Videla S, Llibre JM, et al. . Routine screening of anal cytology in persons with human immunodeficiency virus and the impact on invasive anal cancer: a prospective cohort study. Clin Infect Dis 2020; 71:390–9. [DOI] [PubMed] [Google Scholar]
- 2.Poynten IM, Jin F, Roberts JM, et al. . The natural history of anal high-grade squamous intraepithelial lesions in gay and bisexual men [published online ahead of print 28 April 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa166. [DOI] [PubMed] [Google Scholar]
- 3.Wentzensen N, Clarke MA. From clinical epidemiology to practice recommendations: knowledge gaps and uncertainty in the management of anal precancers. Cancer 2017; 123:4530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshmukh AA, Suk R, Shiels MS, et al. . Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001–2015. J Natl Cancer Inst 2020; 112:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colón-López V, Shiels MS, Machin M, et al. . Anal cancer risk among people with HIV infection in the United States. J Clin Oncol 2018; 36:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverberg MJ, Lau B, Justice AC, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA . Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis 2012; 54:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machalek DA, Poynten M, Jin F, et al. . Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13:487–500. [DOI] [PubMed] [Google Scholar]
- 8.Clifford GM, Georges D, Shiels MS, et al. . A meta-analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int J Cancer 2021; 148:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscicki AB, Darragh TM, Berry-Lawhorn JM, et al. . Screening for anal cancer in women. J Low Genit Tract Dis 2015; 19:S27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown G.Screening for anal dysplasia and cancer in patients with HIV; Medical Care Committee of the New York State Department of Health AIDS Institute. Baltimore, MD: Johns Hopkins University, 2020. [Google Scholar]
- 11.Panther LA, Wagner K, Proper J, et al. . High resolution anoscopy findings for men who have sex with men: inaccuracy of anal cytology as a predictor of histologic high-grade anal intraepithelial neoplasia and the impact of HIV serostatus. Clin Infect Dis 2004; 38:1490–2. [DOI] [PubMed] [Google Scholar]
- 12.Gonçalves JCN, Macedo ACL, Madeira K, et al. . Accuracy of anal cytology for diagnostic of precursor lesions of anal cancer: systematic review and meta-analysis. Dis Colon Rectum 2019; 62:112–20. [DOI] [PubMed] [Google Scholar]
- 13.Bean SM, Chhieng DC, Roberson J, et al. . Anal-rectal cytology: correlation with human papillomavirus status and biopsy diagnoses in a population of HIV-positive patients. J Low Genit Tract Dis 2010; 14:90–6. [DOI] [PubMed] [Google Scholar]
- 14.Darragh TM, Winkler B, Souers RJ, Laucirica R, Zhao C, Moriarty AT; College of American Pathologists Cytopathology Committee . Room for improvement: initial experience with anal cytology: observations from the College of American Pathologists interlaboratory comparison program in nongynecologic cytology. Arch Pathol Lab Med 2013; 137:1550–4. [DOI] [PubMed] [Google Scholar]
- 15.Darragh TM, Tokugawa D, Castle PE, et al. . Interrater agreement of anal cytology. Cancer Cytopathol 2013; 121:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillman RJ, Cuming T, Darragh T, et al. . 2016 IANS international guidelines for practice standards in the detection of anal cancer precursors. J Low Genit Tract Dis 2016; 20:283–91. [DOI] [PubMed] [Google Scholar]
- 17.Wentzensen N, Follansbee S, Borgonovo S, et al. . Human papillomavirus genotyping, human papillomavirus mRNA expression, and p16/Ki-67 cytology to detect anal cancer precursors in HIV-infected MSM. AIDS 2012; 26:2185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambursky JA, Terlizzi JP, Goldstone SE. Testing for human papillomavirus strains 16 and 18 helps predict the presence of anal high-grade squamous intraepithelial lesions. Dis Colon Rectum 2018; 61:1364–71. [DOI] [PubMed] [Google Scholar]
- 19.Gaisa MM, Liu Y, Deshmukh AA, Stone KL, Sigel KM. Electrocautery ablation of anal high-grade squamous intraepithelial lesions: effectiveness and key factors associated with outcomes. Cancer 2020; 126:1470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palefsky JM, Holly EA, Ralston ML, Jay N, Berry JM, Darragh TM. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. AIDS 1998; 12:495–503. [DOI] [PubMed] [Google Scholar]
- 21.Nayar R, Wilbur DC.. The Bethesda system for reporting cervical cytology: definitions, criteria, and explanatory notes. Switzerland: Springer, 2015. [Google Scholar]
- 22.Jay N, Berry JM, Hogeboom CJ, Holly EA, Darragh TM, Palefsky JM. Colposcopic appearance of anal squamous intraepithelial lesions: relationship to histopathology. Dis Colon Rectum 1997; 40:919–28. [DOI] [PubMed] [Google Scholar]
- 23.Darragh TM, Colgan TJ, Thomas Cox J, et al. ; Members of the LAST Project Work Groups . The Lower Anogenital Squamous Terminology Standardization Project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol 2013; 32:76–115. [DOI] [PubMed] [Google Scholar]
- 24.Wentzensen N, Arbyn M, Berkhof J, et al. . Eurogin 2016 roadmap: how HPV knowledge is changing screening practice. Int J Cancer 2017; 140:2192–200. [DOI] [PubMed] [Google Scholar]
- 25.Clarke MA, Wentzensen N. Strategies for screening and early detection of anal cancers: A narrative and systematic review and meta-analysis of cytology, HPV testing, and other biomarkers. Cancer Cytopathol 2018; 126:447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viciana P, Milanés-Guisado Y, Fontillón M, et al. . High-risk human papilloma virus testing improves diagnostic performance to predict moderate- to high-grade anal intraepithelial neoplasia in human immunodeficiency virus-infected men who have sex with men in low-to-absent cytological abnormalities. Clin Infect Dis 2019; 69:2185–92. [DOI] [PubMed] [Google Scholar]
- 27.Morency EG, Harbert T, Fatima N, Samolcyzk J, Maniar KP, Nayar R. Anal cytology: institutional statistics, correlation with histology, and development of multidisciplinary screening program with review of the current literature. Arch Pathol Lab Med 2019; 143:23–9. [DOI] [PubMed] [Google Scholar]
- 28.Khattab R, McMeekin E, Taege AJ, et al. . Unsatisfactory exfoliative anal cytology samples, 15-year experience with histologic, cytologic, and molecular follow-up. Diagn Cytopathol 2018; 46:117–21. [DOI] [PubMed] [Google Scholar]
- 29.Giorgi Rossi P, Carozzi F, Collina G, et al. ; NTCC Working Group . HPV testing is an efficient management choice for women with inadequate liquid-based cytology in cervical cancer screening. Am J Clin Pathol 2012; 138:65–71. [DOI] [PubMed] [Google Scholar]
- 30.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320:674–86. [DOI] [PubMed] [Google Scholar]
- 31.Burd EM. Human papillomavirus laboratory testing: the changing paradigm. Clin Microbiol Rev 2016; 29:291–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stier EA, Lensing SY, Darragh TM, et al. . Prevalence of and risk factors for anal high-grade squamous intraepithelial lesions in women living with human immunodeficiency virus. Clin Infect Dis 2020; 70:1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman E, de Castro C, Williamson T, et al. ; EVVA Study Group . Acceptability of anal cancer screening tests for women living with HIV in the EVVA study. Curr Oncol 2020; 27:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Wang Y, Gaisa MM, et al. . Negative predictive value of human papillomavirus testing: implications for anal cancer screening in people living with HIV/AIDS. J Oncol 2020; 2020:6352315. [DOI] [PMC free article] [PubMed] [Google Scholar]