Abstract
We previously showed that the rate of hepatocyte proliferation in livers from newborn C/EBPα knockout mice was increased. An examination of cell cycle-related proteins showed that the cyclin-dependent kinase (CDK) inhibitor p21 level was reduced in the knockout animals compared to that in wild-type littermates. Here we show additional cell cycle-associated proteins that are affected by C/EBPα. We have observed that C/EBPα controls the composition of E2F complexes through interaction with the retinoblastoma (Rb)-like protein, p107, during prenatal liver development. S-phase-specific E2F complexes containing E2F, DP, cdk2, cyclin A, and p107 are observed in the developing liver. In wild-type animals these complexes disappear by day 18 of gestation and are no longer present in the newborn animals. In the C/EBPα mutant, the S-phase-specific complexes do not diminish and persist to birth. The elevation of levels of the S-phase-specific E2F-p107 complexes in C/EBPα knockout mice correlates with the increased expression of several E2F-dependent genes such as those that encode cyclin A, proliferating cell nuclear antigen, and p107. The C/EBPα-mediated regulation of E2F binding is specific, since the deletion of another C/EBP family member, C/EBPβ, does not change the pattern of E2F binding during prenatal liver development. The addition of bacterially expressed, purified His-C/EBPα to the E2F binding reaction resulted in the disruption of E2F complexes containing p107 in nuclear extracts from C/EBPα knockout mouse livers. Ectopic expression of C/EBPα in cultured cells also leads to a reduction of E2F complexes containing Rb family proteins. Coimmunoprecipitation analyses revealed an interaction of C/EBPα with p107 but none with cdk2, E2F1, or cyclin A. A region of C/EBPα that has sequence similarity to E2F is sufficient for the disruption of the E2F-p107 complexes. Despite its role as a DNA binding protein, C/EBPα brings about a change in E2F complex composition through a protein-protein interaction. The disruption of E2F-p107 complexes correlates with C/EBPα-mediated growth arrest of hepatocytes in newborn animals.
Hepatocyte proliferation has been studied extensively in various models of liver regeneration, yet the molecular mechanisms controlling hepatocyte proliferation have only recently been elucidated. The transcription factor C/EBPα is a key protein in the inhibition of liver proliferation (11, 34). C/EBPα belongs to the C/EBP family of proteins that is characterized by the presence of a basic region and leucine zipper motif in the C-terminal region of the molecule (22, 23). C/EBPα binds to DNA as homo- or heterodimers with other family members and activates the transcription of target genes (2, 21). C/EBPα has been shown to play a significant role in adipocyte differentiation (12, 25, 37) and in the regulation of both liver- and adipocyte-specific genes (13, 44). The generation of C/EBPα knockout mice showed a central role for C/EBPα in energy metabolism (38). The study of the effect of C/EBPα on the proliferation of cells in culture clearly has shown that C/EBPα inhibited cell proliferation in transient transfection experiments (15), as well as in stable clones conditionally expressing C/EBPα (36). Although the growth-inhibitory role of C/EBPα is well established in vitro and in vivo (9, 11, 34, 36), the molecular pathways of C/EBPα-mediated growth arrest are unknown. One pathway operating in cultured cells has been suggested by the study of C/EBPα growth arrest in human fibrosarcoma HT1 cells (36). In these cells, C/EBPα brings about growth arrest via the elevation of p21-CIP-1-WAF-1-SDI-1 protein levels (36). It has been shown that C/EBPα up-regulates p21 mRNA transiently, but protein level elevation occurs primarily via the stabilization of p21 protein (36). It has been recently shown that C/EBPα can also up-regulate p21 protein in hepatoma cells (3, 5). In the liver, C/EBPα regulates p21 protein levels presumably through a protein-protein interaction (34). Serfas et al. have observed high levels of p21 protein in mouse hepatocytes that expressed C/EBPα but not in those that did not have C/EBPα (29). Taken together, these observations suggest that p21 is regulated by C/EBPα in the liver. The p21 protein is a strong inhibitor of cell proliferation in culture when it is overexpressed (30); however, contradictory observations have been made regarding its inhibitory role in vivo. p21 knockout mice develop normally and do not develop tumors (7), but the overexpression of p21 in the livers of p21 transgenic mice leads to a strong inhibition of hepatocyte proliferation during prenatal development and after partial hepatectomy (42). C/EBPα-mediated growth arrest in newborn mice involves the elevation of p21 protein levels and, presumably, the regulation of additional proteins that control cell cycle progression. In this paper, we present evidence that C/EBPα regulates the formation of E2F transcription complexes in vitro and in cell cultures, through direct interaction with retinoblastoma (Rb) and Rb-like proteins and that this pathway is likely to be involved in growth arrest.
The E2F transcription factors bind and activate promoters of several genes whose products are involved in DNA synthesis and mitosis, such as those that encode dihydrofolate reductase, b-myb, cdc2, proliferating cell nuclear antigen (PCNA), c-myc, and DNA polymerase α (6, 43). E2F binds to DNA as a heterodimer with DP proteins. At the present time, six E2F and two DP (DP1 and DP2) proteins have been identified (39, 40). E2F-dependent transcription is regulated by several pathways. The E2F1 promoter contains an E2F binding site and might be autoactivated during cell cycle progression (19, 27). However, the major pathway of E2F regulation includes the physical association of E2F-DP with Rb and the Rb-like proteins p107 and p130, and this association is controlled by the phosphorylation of Rb proteins by cyclin-dependent kinases (16, 18, 39). The investigation of Rb-E2F complexes is complicated since Rb proteins can substitute for each other. It has been shown that Rb preferentially associates with E2F1, E2F2, and E2F3; E2F5 binds to p130, and E2F4 can associate with all three Rb family members (39). Although preferential associations are well documented, their significance has not been shown. Differences in the biological functions of various Rb-E2F complexes might be proposed, based on the cell cycle points where the complexes are abundant. Rb and p130-E2F complexes predominate in quiescent cells (32), while p107-E2F complexes are found primarily in dividing cells, preferentially during S phase (8, 20). Despite the universal role of Rb-like proteins in cell cycle regulation, p107 and p130 knockout mice showed no abnormalities (24). However, double-knockout mice, deficient in both p107 and 130, die within several hours after birth (17), indicating overlapping functions for these proteins. Study of the E2F target genes in cultured p107−/− p130−/− cells from double-knockout animals confirmed the overlapping functions of p107 and p130 (17).
In this paper, we present evidence that the formation of E2F-p107 complexes during prenatal liver development in mice is dependent on C/EBPα. The E2F complexes that contain p107 are prevalent in the developing liver at day 16 of gestation but are dramatically reduced in wild-type animals before birth. In contrast, E2F-p107 complexes remain high in C/EBPα knockout mouse livers throughout development. The effect of C/EBPα on E2F complexes is specific, since mice lacking p21 or C/EBPβ show E2F binding identical to that of genetically normal littermates. In vitro experiments show that C/EBPα disrupts E2F-p107 complexes by a direct interaction of C/EBPα with p107. The E2F homology region of C/EBPα is sufficient for the interaction with p107 and for disruption of p107-E2F complexes.
MATERIALS AND METHODS
Animals.
Since C/EBPα knockout mice die within several hours after birth, C/EBPα knockout mice and genetically normal littermates were sacrificed immediately after birth. Livers were collected, frozen in liquid nitrogen, and kept at −80°C. For the study of prenatal development, C/EBPα and C/EBPβ littermates were collected at 14, 16, and 18 days of gestation. Livers were frozen in liquid nitrogen. Proteins and total RNA were isolated from two to three livers from mice of the same genotype as described below.
RNA isolation and Northern blot analysis.
Total RNA was isolated as described previously (36). Total RNA (25 μg) was loaded on a 1% agarose–2.2 M formaldehyde gel, transferred onto a membrane, and hybridized with specific probes. Each filter was hybridized sequentially with C/EBPα-, p21-, C/EBPβ-, and 18S rRNA-specific probes as described previously (36). Quantitation of Northern blots was performed by using phosphorimaging. The levels of C/EBPα, C/EBPβ, and p21 mRNAs were normalized to the 18S rRNA control.
Protein isolation and Western blot analysis.
The isolation of nuclear proteins from livers has been described in our previous publications (34, 36). Briefly, the livers were homogenized in buffer A containing 25 mM Tris-HCl (pH 7.5), 50 mM KCl, 2 mM MgCl2, 1 mM EDTA, and 5 mM dithiothreitol (DTT). Nuclei were pelleted by centrifugation at 1,710 × g for 10 min and washed with buffer A. The supernatant (cytoplasm) was frozen. High-salt extraction of nuclear proteins was performed by incubation of nuclei with buffer B (25 mM Tris-HCl [pH 7.5], 0.42 M NaCl, 1.5 mM MgCl2, 1 mM DTT, 0.5 mM EDTA, and 25% sucrose) for 30 min on ice. After centrifugation, the supernatant (nuclear extract) was divided into small fractions and kept at −80°C. Western blot analysis was carried out as described previously (36). Briefly, 50 to 100 μg of nuclear proteins was loaded on a 12% polyacrylamide–0.1% sodium dodecyl sulfate gel. After separation, proteins were transferred onto membranes (Bio-Rad) by electroblotting. To equalize the protein loading, a preliminary filter was stained with Coomassie blue to verify the measured protein concentration. After the detection of specific proteins, each filter was reprobed with antibodies to β-actin (Sigma) to verify protein loading. Filters were blocked with 10% dry milk–2% bovine serum albumin prepared on TTBS (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 0.05% Tween 20) saline buffer. Incubations with primary and secondary antibodies were carried out according to recommendations for each antibody. Dry milk (0.5%) was added to TTBS, and this solution was used for incubation with antibodies. Immunoreactive proteins were detected by using the ECL protocol (Amersham). Antibodies to human C/EBPα are described in our earlier publication (36). Antibodies to E2F1 (C-20 and KH95), E2F2 (C-20), E2F3 (N-20), E2F4 (C-20), E2E5 (E-19), cyclin A, cyclin E, DP1 (K-20), cdk2, cdk4, Rb (C-15 and IF8), p107 (SD9 and C-18), p130 (C-20), and PCNA were from Santa Cruz Biotechnology.
Analysis of E2F binding in HT1 cells.
The conditions for the culturing of HT1 cells have been described previously (36). The HT1 cells were induced by (isopropyl-β-d-thiogalactopyranoside) (IPTG) (for C/EBPα induction) or glucose (control). Twenty-four hours after plating and IPTG addition, cytoplasm and nuclear extracts were isolated as described previously (36). E2F binding activity was analyzed by band shift assay as described below.
Electrophoretical mobility shift assay.
E2F binding activity was investigated by using the band shift assay. Single-stranded dihydrofolate reductase-E2F oligonucleotides (nucleotide sequence of the upper chain, 5′-CTAGTGCAATTTCGCGCCAAACTTG-3′) were synthesized, purified by gel fractionation, and annealed. After purification by gel electrophoresis, the double-stranded oligonucleotide was labeled with Klenow enzyme in a fill-in reaction with P32 dCTP. Protein extracts (5 μg) were incubated in a binding buffer containing 20 mM Tris-HCl [pH 7.5], 100 mM KCl, 5 mM DTT, 2 mM MgCl2, 10% glycerol, 0.5 μg of salmon sperm DNA per 10 μl, and 50 to 100,000 cpm of probe. Incubations were carried out at 4°C for 30 min. Samples were loaded on a 5% native polyacrylamide gel and run for 3 to 4 h at 4°C. For the detection of proteins that are involved in E2F complexes, specific antibodies were added to the binding reaction mixture before probe addition. For p107 supershift, monoclonal antibody SD9 (Santa Cruz Biotechnology) was used. In experiments with a Y-DLF peptide, the increasing amounts of the Y-DLF peptide were preincubated with nuclear extracts for 15 min at room temperature and added to the binding reaction mixture.
IP-bandshift assay and IP-Western blot analysis.
The interaction of C/EBPα and p107 in rat livers was studied by the immunoprecipitation (IP)-bandshift assay and by IP-Western blot analysis. A total of 500 μg of nuclear extracts from regenerating rat livers (24 h after partial hepatectomy) was incubated with antibodies to p107, cdk2, and Rb for 1 h and with protein A-agarose overnight. After being washed with phosphate-buffered saline (four times), immunoprecipitates were incubated with 0.5% deoxycholate and Nonidet P-40 as described previously (8). Samples were centrifuged, and the supernatant was added to the binding reaction mixture containing the bZIP probe. Conditions for the gel shift assay were described earlier (33, 36). For IP-Western blotting, C/EBPα was immunoprecipitated from rat livers as described above, and p107 was detected with an antibody specific to p107. Two types of antibodies were used for these studies: monoclonal SD9 and polyclonal C-18 (Santa Cruz Biotechnology).
Interaction of p107 with E2F homology region of C/EBPα.
A short peptide containing the E2F homology region (see Fig. 6) was synthesized at Baylor College of Medicine. The sequence of the Y-DLF peptide (corresponding to amino acids 67 to 81 of C/EBPα) is as follows: YIDPAAFNDEFLADLF. The Y-DLF peptide was purified by high-pressure liquid chromatography and analyzed by gel electrophoresis. Y-DLF peptide was covalently attached to Sepharose (Pharmacia) as described in the manufacturer’s protocol. Since p107 expression is induced in S phase, we used nuclear extracts from rat livers 24 h after partial hepatectomy (peak of DNA synthesis) (34). A control peptide with a random composition of the amino acids (C-pept.) was linked to Sepharose and used as the control for specific interaction. Nuclear proteins were incubated with Y-DLF-Sepharose or with C-pept.-Sepharose overnight at 4°C, washed four times with phosphate-buffered saline, and analyzed by Western blotting with a monoclonal antibody to p107.
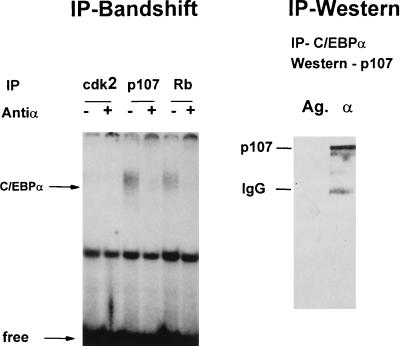
FIG. 6.
C/EBPα interacts with p107. (Left) Rb, p107, and cdk2 proteins were immunoprecipitated from rat liver nuclear extracts isolated 24 h after partial hepatectomy. The presence of C/EBPα in IPs was analyzed by gel shift assay after release with deoxycholate and Nonidet P-40 treatment as described in Materials and Methods. Antibody to C/EBPα was added to the binding reaction mixture before the probe addition. (Right) C/EBPα was immunoprecipitated from rat liver nuclear extract. The presence of p107 was determined by Western blot analysis with monoclonal antibodies. Immunoprecipitate with agarose (Ag.) serves as the control on nonspecific absorption. IgG, immunoglobulin G.
RESULTS
C/EBPα and p21 expression is induced before birth.
C/EBPα expression is induced in the liver before birth (2) and correlates with the inhibition of hepatocyte proliferation in newborn mice (11, 34). p21 protein is coordinately expressed in the liver (5, 29, 34) and is likely to be controlled by C/EBPα through the stabilization of the p21 protein (34). Overexpression of p21 inhibited liver proliferation during prenatal development (42); however, the expression of endogenous p21 during prenatal development has not been described. To examine the expression of p21 during gestation, total RNA was isolated from livers at different times during fetal development. Northern blot analysis showed low levels of p21 mRNA present before birth (14 and 16 days of gestation) (Fig. 1A). However, p21 mRNA levels are induced at day 18 and in newborn mice. The levels of p21 mRNA calculated as the ratio to 18S rRNA are 15-fold higher in newborn animals than in mice at 16 days of gestation. This pattern of induction suggests that p21 may play a role in the inhibition of hepatocyte proliferation in newborn mice and is also consistent with the role of p21 during liver development described by Wu et al. (42). C/EBPα mRNA was also induced before birth as was C/EBPβ, in agreement with previously published observations (2). It has been previously reported that p21 knockout mice survive in expected Mendelian frequencies and do not show tumor formation (7), suggesting that hepatocyte proliferation was not altered by the absence of p21 protein. We determined the rate of proliferation in newborn p21 knockout animals by measuring the levels of the S-phase-specific protein PCNA. Western blot analysis of two newborn p21 knockout mice and two heterozygous littermates shows no difference in the levels of PCNA (Fig. 1C). Thus, despite the induction of p21 expression in genetically normal newborn mice (Fig. 1A), the deletion of the p21 gene does not cause a change in the levels of PCNA. Based on these data and on the previously published observations (7), we conclude that the proliferation of hepatocytes is not significantly different between heterozygous and p21 knockout newborn animals.
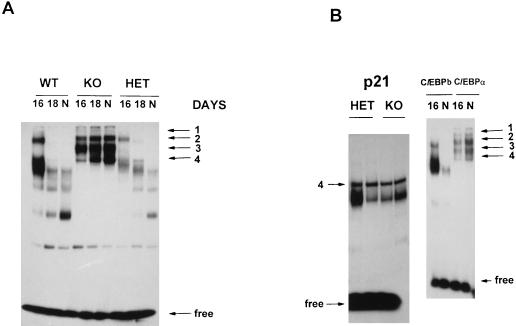
FIG. 1.
Expression of p21 in the liver is induced before birth. (A) Northern blot analysis of mRNA expression in mouse livers during prenatal liver development. Total RNA was isolated from two to three livers harvested at different stages of gestation (14, 16, and 18 days) and from newborn (N) mice and analyzed with probes to p21, C/EBPα, and C/EBPβ and 18S rRNA as a loading control. (B) Levels of p21, C/EBPα, and C/EBPβ mRNAs were calculated as the ratio to 18S RNA by using phosphorimaging. (C) Levels of the S-phase-specific protein PCNA are not changed in p21 knockout newborn mice. Nuclear extracts from two heterozygous (HET) and two p21 knockout (KO) mouse livers were analyzed with antibodies to PCNA as described in Materials and Methods.
The composition of E2F complexes during prenatal development.
The observation that hepatocyte division was increased in C/EBPα knockout mice prompted us to measure the levels and activity of several cell cycle-related proteins that might be involved in the C/EBPα-mediated control of hepatocyte proliferation. E2F binding during prenatal liver development in C/EBPα knockout, wild-type, and heterozygous mice was examined by the electrophoretic mobility shift assay. At day 16 of gestation, multiple E2F complexes are observed in mice of all genotypes (Fig. 2A). However, the pattern of E2F binding at that stage of development is significantly different between livers that lack C/EBPα and those expressing C/EBPα. Dramatic differences in E2F binding are seen at 18 days of gestation and in newborn mice (20 days). At day 18, C/EBPα expression is maximal (Fig. 1A), and E2F binding is dramatically reduced in wild-type and heterozygous animals but is not changed in C/EBPα knockout mice. In wild-type newborn mice, E2F binding shifted to low-molecular-weight complexes. Because p21 has been shown to disrupt cdk2-E2F complexes (31) and p21 levels are reduced in C/EBPα knockout mice, we also examined E2F binding in p21 knockout mouse livers. Figure 2B shows that there is no detectable difference in either the composition or the intensity of the E2F complexes between heterozygous and p21 knockout mouse livers. This observation suggests that C/EBPα regulates E2F complexes through a p21-independent mechanism(s). The pattern of E2F complexes in the C/EBPα homozygous mutants is specific, as another member of the C/EBP family, C/EBPβ, has no effect on the pattern of E2F binding activity. In C/EBPβ knockout mice, the pattern of E2F binding is similar to that observed in wild-type animals (Fig. 2B), showing that C/EBPβ is not involved in the regulation of E2F complexes. Thus, although the expression of p21 and C/EBPβ is induced during prenatal liver development (Fig. 1A), alterations in E2F binding are observed only in C/EBPα mutant animals. Our data demonstrate that C/EBPα, but not C/EBPβ, directly or indirectly down-regulates the formation of the E2F complexes and that the E2F regulation by C/EBPα is p21 independent.
FIG. 2.
E2F binding is altered in C/EBPα knockout animals during prenatal development. (A) Gel shift assay of nuclear extracts isolated from livers of mice at different stages of gestation (16 and 18 days) and of newborn (N) animals. The 16-day points of all genotypes represent isolates from three livers from mice of the same genotype. Four E2F complexes specific to C/EBPα knockout (KO) mouse livers are shown by arrows. (B) E2F binding in p21 knockout and C/EBPβ and C/EBPα and knockout newborn mice. Nuclear extracts were isolated from livers of newborn p21 knockout (KO) or heterozygous (HET) littermates and analyzed by gel shift assay.
The S-phase-specific E2F complex: cdk2-DP1-E2F-p107-cyclin A is expressed in C/EBPα knockout mouse livers during prenatal development.
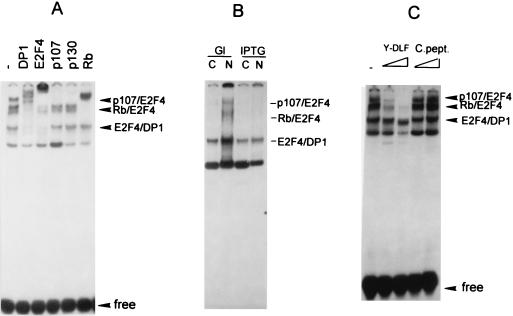
E2F proteins have been shown to associate with different Rb and Rb-like proteins, and this association is cell cycle specific (1, 32, 39). Therefore, we examined the composition of the E2F complexes in newborn C/EBPα knockout mouse livers by using gel shift-supershift assays (Fig. 3A). Specific antibodies to DP1, E2F1, E2F2, cdk2, cdk4, p107, Rb, and cyclins A, E, and D1 were individually added to the binding reaction mixtures. Antibodies to DP1-supershifted complexes 1, 2, and 3, indicating that those are formed by E2F-DP1 heterodimers. Complex 2 was also shifted by antibodies to p107 and therefore contains E2F-DP1-p107. We conclude that the upper E2F complex is formed by cdk2, DP1, E2F, p107, and cyclin A, as this complex is completely supershifted by each of these antibodies. Antibodies to DP2, E2F1, E2F2, E2F3, E2F4, and E2F5 did not supershift E2F complexes under our experimental conditions (for DP2, E2F3, E2F4, and E2F5, data not shown). We suggest that the E2F protein present in C/EBPα knockout mouse livers might be an unknown member of the E2F family. However, it might also be possible that the antibodies used in these studies do not supershift the E2F in complexes in C/EBPα knockout mouse liver extracts. In any event, gel shift analysis indicates that the pattern of E2F binding in mouse livers lacking C/EBPα is different from E2F binding in the livers of genetically normal littermates and that the E2F complex, cdk2-DP1-E2F-p107-cyclin A, is observed only in C/EBPα-null livers. Figure 3 shows the supershift analysis with newborn C/EBPα knockout mouse livers. Similar results were obtained with C/EBPα knockout mouse livers after 16 and 18 days of gestation. The cdk2-DP1-p107-cyclin A complex has been previously characterized by several laboratories as an S-phase-specific E2F complex (8, 20, 26). Elevated levels of this complex in C/EBPα knockout mice correlate with the increased rate of hepatocyte proliferation in these animals (34). As C/EBPα is a transcription factor, the formation of the cdk2-E2F-p107 complexes could be controlled by C/EBPα through the regulation of the genes whose protein products are components of the E2F-p107 complex. We measured the levels of cyclin A, cdk2, and DP1 in the livers of C/EBPα knockout mice and in those of genetically normal littermates. The membrane was reprobed with β-actin as a loading control after removal of the previous antibodies, as described in the legend to Fig. 3. p107 protein was not detected by Western blotting under the conditions of this experiment; however, its expression was detected by using much higher amounts of protein (see below). The levels of DP1 and cdk2 proteins are unchanged during liver development in all three genotypes; however, the expression of cyclin A is dramatically reduced before and at birth in genetically normal animals and heterozygous mice. Increased levels of cyclin A in the knockout mice may contribute to the increased proliferation through promotion of the formation of E2F-p107-cyclin A complexes. The mechanism by which cyclin A is elevated is currently under investigation. However, in this paper we report that C/EBPα has a role in preventing the formation of E2F complexes and that C/EBPα directly disrupts the E2F complexes containing p107 (see below). In the absence of C/EBPα, the E2F complexes associated with the S phase persist in the livers of the mutant animals, consistent with increased hepatocyte cell division.
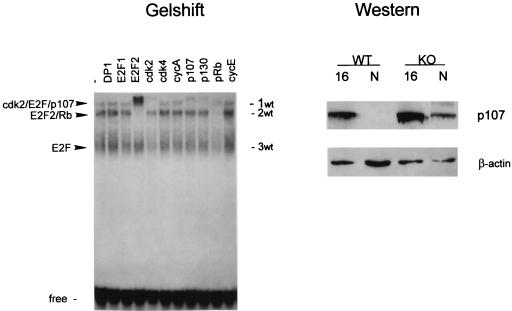
FIG. 3.
(A) C/EBPα knockout mice contain an S-phase-specific complex: cdk2-E2F-DP1-p107-cyclin A. Antibodies to putative components of the E2F complex (indicated at the top) were added to binding reaction mixtures before the addition of nuclear extracts. To better resolve the E2F complexes, electrophoresis was run twice as long. (B) Expression of the components of E2F-p107 complexes during mouse liver development. Nuclear extracts (50 μg) from mice at 16 or 18 days of gestation or from newborn (N) mice were loaded on a 12% polyacrylamide–0.1% sodium dodecyl sulfate gel and analyzed by Western blotting. The same filter was probed sequentially with antibodies to cyclin A, DP1, cdk2, PCNA, and β-actin after removal of the previous antibodies. The results were obtained with nuclear proteins isolated from the animals (wild type [WT], knockout [KO], or heterozygous [HET]) that were analyzed in Fig. 1 and Fig. 2A.
Composition of E2F complexes at day 16 of gestation in wild-type mice differs from that in C/EBPα knockout littermates.
An analysis of E2F binding in wild-type and heterozygous animals showed the presence of low-mobility E2F complexes at day 16 of gestation (Fig. 2). To determine the composition of these complexes, gel shift-supershift analysis was performed with nuclear extracts from livers at 16 days of gestation. Two pools of three animals each were used for these studies. Figure 4 shows that antibodies to DP1 do not supershift E2F complexes in wild-type livers. Slow-migrating E2F complex 1wt consists of cdk2, E2F, and p107, as antibodies to these proteins supershifted the complex. Cyclin A was not detectable in the E2F complexes in livers after 16 days of gestation. Antibodies to p130 used in these studies partially cross-react with p107 and show a weak supershift of the E2F-p107 complex. Complex 2wt is supershifted with antibodies to E2F2 and Rb. Complex 3wt contains free E2F, since antibodies to Rb family members did not react with this complex. Thus, analysis of E2F complexes in wild-type livers at 16 days of gestation showed quite a different composition of E2F complexes than that observed in C/EBPα knockout littermates. Wild-type animals contain cdk2-E2F-p107 and E2F2-Rb complexes, while E2F2-Rb complexes are not detectable in C/EBPα knockout mouse livers. The cdk2-E2F-p107 complex is present in both genotypes at 16 days of gestation. Since the cdk2-E2F-p107 complex disappeared at later stages of development, we examined the expression of p107 under conditions that allowed us to detect this protein by Western blot assay. We found that p107 can be detected if up to 200 μg of protein is loaded on the gel (the usual loading amount is 50 μg of nuclear extract/lane). The results of Western blot analysis of p107 expression with 200 μg of total protein are shown in Fig. 4. At day 16, livers from mice of both genotypes contain p107 protein. In contrast, this protein is not detectable in livers from mice at the newborn stage, but in C/EBPα knockout mouse livers, p107 levels are relatively high. Reprobing the same filter with β-actin antibodies indicated equal protein loading. Thus, these data show that p107 expression is also regulated during prenatal liver development and might contribute to the change in E2F complexes. Our data suggest that the pathway of the regulation for E2F complexes involves the disruption of E2F-Rb family complexes by C/EBPα via a direct interaction with p107.
FIG. 4.
Composition of E2F complexes in wild-type livers at day 16 of gestation. Nuclear proteins (5 μg) isolated from three livers were incubated with the E2F probe in the presence of antibodies (indicated at the top) and analyzed by gel shift assay. Nuclear proteins (200 μg) from mouse livers after 16 days of gestation and newborn (N) mouse livers were loaded on a 6% polyacrylamide gel and probed with polyclonal (C-18) antibodies to p107. After being stripped, the membrane was reprobed with antibodies to β-actin as a loading control.
C/EBPα disrupts the E2F-p107 complexes in nuclear extracts from newborn mice.
To examine the effect of the nuclear proteins from wild-type animals on E2F-p107 complexes, the nuclear extracts from mice of both genotypes were added to the binding reaction mixture. The addition of proteins from wild-type livers to null extracts resulted in a reduction of the E2F complexes (Fig. 5A). These data suggest that nuclear extracts from wild-type animals contain some factors that disrupt the E2F complexes through a direct or indirect interaction with the complex. Because p21 has been reported to disrupt E2F complexes, we examined the effect of both C/EBPα and p21 proteins on the E2F complexes. Figure 5B shows that bacterially expressed, gel-purified histidine-tagged C/EBPα disrupts the p107-E2F complexes in nuclear extracts from C/EBPα knockout mice. This result is consistent with the suggestion that in wild-type animals, increased C/EBPα levels disrupt the E2F complexes during prenatal liver development. In the complete absence of C/EBPα, a new cdk2-E2F-p107 complex can be formed. To examine the possibility that C/EBPα regulates cdk2-E2F-p107 complexes in wild-type liver extracts, His-C/EBPα was incorporated into the binding reaction with nuclear extracts from livers of wild-type animals at 16 days of gestation. Figure 5C shows that C/EBPα also disrupts E2F-p107 and E2F-Rb complexes in wild-type livers. This observation is consistent with the suggestion that the induction of C/EBPα at day 18 of gestation (Fig. 1) leads to the disruption of E2F-p107 and E2F-Rb complexes. It is interesting that the E2F binding pattern after the neutralization of the E2F-p107 complexes by C/EBPα in knockout mouse livers (Fig. 5B, lanes 2 and 3) is similar to that observed after the addition of anti-p107 (lane 8). This correlation suggested that C/EBPα destroys E2F-p107 complexes through p107 (see below). In this in vitro assay, the addition of high concentrations of C/EBPα led to the appearance of a new larger complex (Fig. 5B, lane 3) that contains C/EBPα, as shown by the incorporation of antisera specific to C/EBPα into the binding reaction mixture (data not shown). However, we were not able to detect the large complex in cultured cells overexpressing C/EBPα, nor were these complexes formed when lower concentrations of C/EBPα were added.
FIG. 5.
(A) Mixing of wild-type (W) and C/EBPα knockout (K) nuclear extracts results in the disruption of E2F complexes. A mixture of nuclear extracts from wild-type and C/EBPα knockout mouse livers was preincubated on ice for 15 min and added to the E2F binding reaction mixture. (B) C/EBPα disrupts E2F-p107 complexes in nuclear extracts from C/EBPα knockout mouse livers. Increasing amounts of bacterially expressed, purified C/EBPα and p21 (100 and 200 ng, respectively) were added to E2F binding reaction mixtures before the addition of nuclear extract from C/EBPα knockout mouse livers. Antibody to p107 (SD9) was added before the probe addition. (C) C/EBPα disrupts E2F-p107 and E2F-Rb complexes in livers at day 16 of gestation from wild-type animals. C/EBPα was incorporated into the binding reaction mixture with nuclear extracts from wild-type livers at day 16 of gestation as described above. NS, nonspecific binding; free, unbound oligonucleotide.
C/EBPα interacts with p107 through the E2F homology region.
To determine which protein components of the E2F-p107 complexes interact with C/EBPα, IP-band shift assays were carried out. Because C/EBPα is highly expressed in the liver, nuclear extracts from rat livers were used as the source of C/EBPα protein. Rat liver nuclear proteins (24 h after partial hepatectomy) were incubated with antibodies to E2F1, DP1, cdk2, p107, and cyclin A and protein A-agarose. This protein extract was chosen because for rats at this time after partial hepatectomy, p107 protein is expressed at high levels correlating with the peak of DNA synthesis in regenerating livers (14). After being washed, immunoprecipitates were analyzed by the electrophoretic mobility shift assay with the bZIP oligonucleotide containing a C/EBP consensus binding site (41). C/EBPα-specific binding activity is detectable only in p107 and in Rb immunoprecipitates and not in cdk2 immunoprecipitates (Fig. 6). We were not able to detect C/EBPα in IPs with DP1, cyclin A, and E2F1 antibodies (data not shown). To confirm the interaction of C/EBPα with p107, C/EBPα was precipitated from liver nuclear extracts and the presence of p107 was examined by Western blot analysis with anti-p107 antibodies. An immunoreactive protein with the correct molecular weight (107) is observed coprecipitating with C/EBPα (Fig. 6). Immunoreactive p107 protein was detected in C/EBPα immunoprecipitates with both monoclonal and polyclonal specific antibodies. Thus, two independent methods showed that C/EBPα interacts with p107, a component of the S-phase-specific E2F-p107 complex.
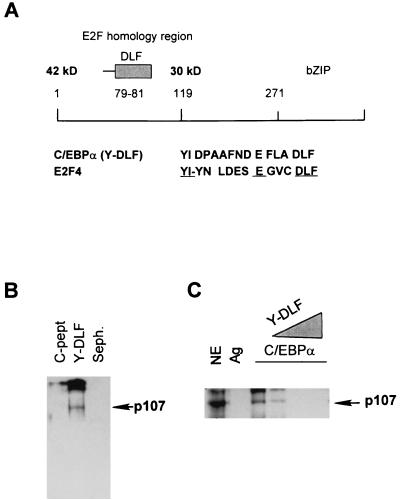
Chen et al. reported that C/EBPα and C/EBPβ proteins directly interact with Rb (4). The putative C/EBPα region of interaction includes a sequence that is homologous to the E2F-like region that interacts with the Rb pocket. Figure 7A shows the location of this region within C/EBPα and its sequence similarity with the corresponding E2F region that is necessary for the interaction with pocket proteins. To examine whether this region of C/EBPα interacted with p107 protein, a short peptide, YIDPAAFNDEFLADLF (Y-DLF peptide; Fig. 7A), was synthesized and linked to Sepharose. A control peptide with an unrelated composition of 18 amino acids (C-pept.) was used. Sepharose-Y-DLF and Sepharose-C-pept. were incubated with nuclear proteins from rat livers isolated 24 h after partial hepatectomy (S phase). Western blot analysis with a monoclonal p107 antibody (Fig. 7B) showed that immunoreactive p107 protein is associated with Y-DLF but not with the control peptide. To examine whether the Y-DLF region of C/EBPα was sufficient for the interaction with p107, increasing amounts of the Y-DLF peptide were preincubated with nuclear extracts and C/EBPα was immunoprecipitated. Western blot analysis of p107 in C/EBPα IPs shows that the Y-DLF peptide competes for the binding of p107 and, at high concentrations, blocks the binding (Fig. 7C). Thus, the E2F homology region of C/EBPα is involved in the interaction of C/EBPα with the p107 protein.
FIG. 7.
(A) Diagram showing localization of the E2F homology region within the C/EBPα molecule. The homology of C/EBPα and E2F4 is shown below. (B) Y-DLF region of C/EBPα interacts with p107 protein. The Y-DLF peptide was linked to Sepharose and incubated with nuclear extracts from dividing rat livers (24 h after partial hepatectomy) overnight. After being washed, samples were analyzed by Western blotting with p107 antibodies. C-pept. was used as the control for specific binding. (C) E2F homology region of C/EBPα is sufficient for the interaction with p107. Nuclear extracts (NE) were preincubated with increasing amounts of Y-DLF peptide. C/EBPα was immunoprecipitated, and p107 protein was detected in IPs by Western blotting with a monoclonal antibody. Ag, agarose.
The E2F homology region of C/EBPα is sufficient to disrupt E2F-p107 complexes in C/EBPα knockout mouse livers.
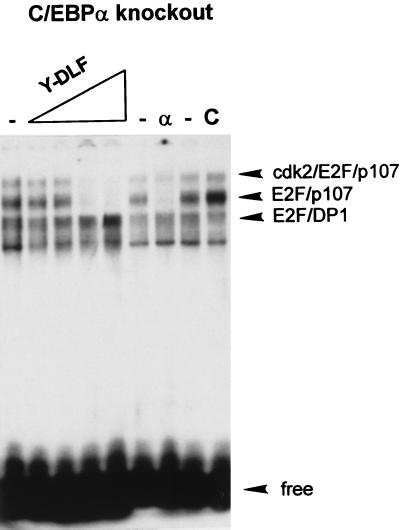
Because the Y-DLF peptide of C/EBPα interacts with the p107 protein, we propose that this region may compete with p107 for the binding to E2F. Therefore, we investigated whether the Y-DLF region is involved in the disruption of the E2F-p107 complexes. Figure 8 shows the effect of the Y-DLF peptide on E2F binding in nuclear extracts from C/EBPα knockout mice. Increasing amounts of Y-DLF peptide (1, 10, 50, and 100 ng) were incorporated into the binding reaction mixture. The control peptide was used at the highest concentration (100 ng). As can be seen, the Y-DLF peptide, but not the control peptide, disrupts both E2F-p107 and E2F-p107-cyclin A complexes. This effect is specific to E2F-p107 complexes, since the E2F-DP1 complex is not affected by Y-DLF under the same conditions. Upon the addition of the Y-DLF peptide, one can observe a slight increase in the E2F-DP1 complex. Thus, the E2F homology region of C/EBPα is sufficient to bring about the disruption of E2F complexes that contain p107 in liver nuclear extracts of C/EBPα knockout mice.
FIG. 8.
E2F homology region of C/EBPα is sufficient to disrupt E2F-p107 complexes. Nuclear extract from C/EBPα knockout mouse livers was incubated with increasing amounts of Y-DLF peptide (5, 10, 50, and 100 ng) and analyzed by gel shift assay. The control peptide (C) (100 ng) shows no effect on the E2F complexes.
Induction of C/EBPα in cultured cells leads to disruption of E2F-p107 and E2F-Rb complexes.
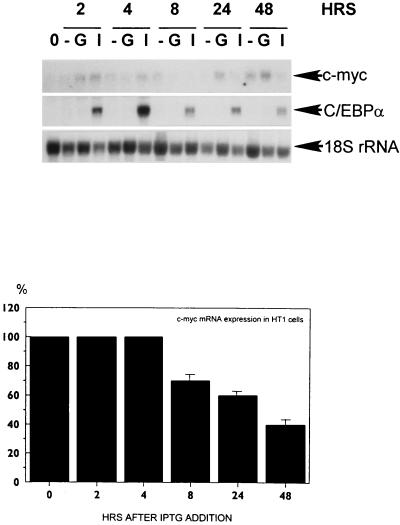
Having observed that C/EBPα regulates E2F complexes in vivo and in vitro and that this regulation correlates with an increased rate of proliferation (34), we examined E2F complex formation in a previously described cell line that contains C/EBPα under the control of an inducible promoter (36). Forced expression of C/EBPα in human HT1 fibrosarcoma cells causes growth arrest (36). Initially, we examined E2F binding in dividing HT1 cells by using Bandshift-supershift assays in order to identify the proteins that form E2F complexes in these cells. Figure 9A shows that HT1 cells contain E2F4-DP1 as the major E2F binding complex, because antibodies to E2F4 and to DP1 interact with all E2F complexes except the one with the fastest mobility. p107-E2F4 and p130-E2F4 complexes are also observed in HT1 cells. E2F1, E2F2, and E2F3 do not appear to be present in the complexes, as addition of antibodies to each of these E2F proteins did not result in supershifted E2F complexes (data not shown). To test the effect of C/EBPα on E2F complexes in cultured cells, HT1 cells synchronized by high density and serum deprivation were released to grow in the presence of glucose (control) and in the presence of 10 mM IPTG (C/EBPα inducer). Figure 9B shows that HT1 cells expressing C/EBPα do not contain detectable amounts of the E2F-p107 and E2F-Rb complexes. These data show that induction of C/EBPα in cultured cells leads to disruption of or failure to form E2F-p107 and E2F-Rb complexes. To examine whether the E2F homology region of C/EBPα is sufficient to disrupt p107 and Rb containing E2F complexes, the Y-DLF peptide was incorporated into the E2F binding reaction mixture with nuclear proteins from HT1 cells. The effect of the Y-DLF peptide on the E2F complexes that are observed in dividing HT1 cells was similar to that in liver nuclear extracts with specific disruption of the E2F4-Rb and E2F4-p107 complexes (Fig. 9C). The control peptide did not affect the E2F complexes. These data suggest that C/EBPα-dependent regulation of the E2F complexes in the liver and in cultured cells is mediated through a direct interaction with Rb and Rb-like proteins and that the region of C/EBPα containing the Y-DLF sequence is sufficient to disrupt the E2F-Rb and E2F-p107 complexes. To examine the expression of E2F-dependent genes in response to C/EBPα induction, levels of mRNA for c-myc (encoded by an E2F target gene) were determined in HT1 cells after induction of C/EBPα. Northern blot analysis and phosphorimager calculations of two independent experiments are shown in Fig. 10. The expression of c-myc mRNA is reduced in response to C/EBPα induction. The correlation between the disruption of E2F-p107 complexes and the reduction of c-myc mRNA is consistent with the suggestion that C/EBPα down-regulates E2F-dependent genes.
FIG. 9.
C/EBPα regulates E2F complexes in HT1 cultured cells. (A) Composition of E2F complexes in dividing HT1 cells. Nuclear extracts (5 μg) from HT1 cells were incubated with the E2F oligomer in the presence of antibodies (indicated at the top) and analyzed by gel shift assay. (B) Induction of C/EBPα by IPTG (36) leads to the disruption of E2F-p107 and E2F-Rb complexes. Cytoplasmic (C) and nuclear (N) proteins of HT1 cells were isolated 24 h after IPTG or glucose (Gl) (control) addition and analyzed by gel shift assay. Free probe was run off the gel. (C) The E2F homology region of C/EBPα (Y-DLF) is sufficient to disrupt E2F-p107 and E2F-Rb complexes. Y-DLF peptide and control peptide (C.pept.) were preincubated with nuclear extracts from dividing HT1 cells and added to the E2F binding reaction mixture.
FIG. 10.
Overexpression of C/EBPα results in the repression of c-myc mRNA in HT1 cells. Total RNA from HT1 cells treated with glucose (G) or IPTG (I) and from untreated cells (−) was analyzed by Northern blotting with c-myc, C/EBPα, and 18S probes. 0, prior to addition of glucose or IPTG. The bottom section shows phosphorimager analysis of two independent experiments. Levels of c-myc mRNA were calculated as a ratio to 18S rRNA in IPTG- and glucose-treated cells, and then the percentages of c-myc mRNA were calculated in IPTG-treated cells compared to those in glucose-treated cells. Error bars indicate standard deviation.
DISCUSSION
The transcription factor C/EBPα has been shown to regulate several biological processes such as adipose differentiation (12, 25, 37, 44), energy metabolism (38), and cell proliferation (9, 15, 36). The transcriptional regulation of specific genes by C/EBPα has been found to be a major mechanism in the control of adipose tissue differentiation and energy metabolism. However, the regulation of cell proliferation by C/EBPα is more complicated and involves both transcriptional control (3, 5, 36) and interaction with proteins that regulate cell cycle progression (34). The expression of C/EBPα and C/EBPβ proteins has been shown to be increased in the liver before birth (2). The generation of knockout mouse models suggested that C/EBPα is necessary to decrease hepatocyte proliferation in newborn animals (11, 34), while the deletion of C/EBPβ had no effect on liver proliferation (28). One possible pathway of C/EBPα-mediated growth arrest in newborn mice has been suggested by the observation that C/EBPα regulates the cyclin-dependent kinase inhibitor p21 (5, 34, 36). However, measurements of PCNA levels in p21 knockout mice (Fig. 1C) showed no differences in the levels of this S-phase-specific protein which serves as an indicator of proliferation. The description of p21 knockout mice (7) did not suggest any alterations in hepatocyte proliferation. These observations suggest that the reduction of p21 protein in C/EBPα knockout mouse livers is probably not sufficient to cause an increased rate of proliferation and that, in addition to p21 regulation, C/EBPα controls the expression of other proteins that regulate cell cycle progression. In this paper, we present evidence that C/EBPα controls the formation of E2F complexes in newborn mice. These data demonstrate that one pathway of C/EBPα-mediated growth arrest involves the disruption of E2F-p107 complexes. It has been stated that p21 can disrupt cdk2-E2F-p130 complexes when it is added to the binding reaction (31). However, E2F binding in p21 knockout newborn mouse livers was not different from that in p21 heterozygous mice, indicating that C/EBPα-mediated regulation of the E2F complexes in newborn mice is p21 independent. We suggest that the p21-dependent pathway of C/EBPα-mediated growth arrest involves the inhibition of cdk2 and cdk4 kinase activities as has been described by Wu et al. (42) and is shown by our data (35). In p21 knockout animals, this pathway might be rescued by other members of the p21 family, such as p27 or p57.
Multiple changes in the expression of C/EBP proteins during prenatal liver development and at birth prompted us to examine E2F complexes in C/EBPβ knockout newborn mice as well. These investigations revealed that although C/EBPβ expression is induced before birth (Fig. 1) and C/EBPβ protein interacts with Rb (4), the regulation of the E2F complexes is specific to the C/EBPα protein. C/EBPα knockout animals contain cdk2-E2F-p107 and cdk2-E2F-p107-cyclin A complexes that have been characterized as S-phase-specific complexes (8, 20, 26). Although the biological targets of the E2F-p107 complexes in the liver are not known, the induction of these complexes in dividing cells is well documented. Flodby et al. have described increased expression of c-myc mRNA in C/EBPα knockout mice (11). Both c-myc and PCNA have E2F binding sites in their promoter regions (6, 43). Data from this paper also show that in livers of C/EBPα knockout mice, two other E2F-dependent genes that encode cyclin A and p107 are induced. Taken together, these observations suggest that cdk2-E2F-p107-cyclin A complexes seen in C/EBPα knockout mouse livers may be positive regulators of the c-myc, PCNA, cyclin A gene, and p107 loci, all of which are associated with increased cell proliferation. It is interesting that the reduction of PCNA, cyclin A, and p107 at day 18 of gestation and in wild-type newborn animals correlates with a disappearance of the E2F-p107 complexes.
Under the conditions of our experiments, genetically normal newborn littermates do not contain detectable E2F-p107 complexes. This observation is unexpected, since the livers of newborn mice continue to proliferate after birth until the adult age is reached. It is likely that there are several pathways of growth control in newborn mice, only some of which are C/EBPα dependent and involve p21 and E2F complexes. The increased rate of proliferation in C/EBPα knockout mouse livers indicates that the regulation of E2F binding might be an additional, p21-independent pathway of C/EBPα-mediated growth arrest in vivo. In this paper, we present an analysis of E2F binding during later stages of prenatal liver development, between day 16 of gestation and birth. This period was chosen because C/EBPα expression is induced in wild-type animals at day 18 of gestation and in newborn animals (2) (Fig. 1). Dramatic alterations of E2F complexes in wild-type animals correlate with the kinetics of C/EBPα induction. We suggest that the C/EBPα-dependent regulation of E2F complexes also occurs earlier in gestation, because differences in the composition of E2F complexes are detected at day 16 of gestation (Fig. 2). It is interesting that two components of the S-phase-specific E2F complexes, cyclin A and p107, are also regulated by C/EBPα at the protein level. These proteins are expressed at high levels in C/EBPα knockout newborn mice but are not detectable in wild-type littermates (Fig. 4). Because both genes are regulated by E2F, it is possible that these alterations are due to repression of E2F-dependent transcription by C/EBPα. Although reduction of cyclin A and p107 could also contribute to the regulation of E2F complexes, we think that the direct interaction of C/EBPα with p107 and the disruption of the E2F-p107 complexes are a major pathway of C/EBPα-mediated control of E2F complexes.
Experiments with full-length C/EBPα and with the Y-DLF E2F homology region of C/EBPα showed that in vitro, C/EBPα disrupts E2F-p107 complexes via a direct interaction with p107 and that the E2F homology region of C/EBPα is sufficient to bring about the disruption. This pathway of E2F regulation would depend on the concentration and localization of C/EBPα but not on the transcriptional capacities of C/EBPα. Although C/EBPα has been well characterized as a transcription factor and can activate p21 transcription in cultured cells (5, 36), we could not detect transcriptional activation of p21 by C/EBPα in the liver. Protein-protein interactions with E2F-p107 complexes and with p21 (34) appear to be the major pathways of C/EBPα-mediated growth arrest. E2F complexes are important regulators of cell cycle progression that operate in all mammalian cells. C/EBPα-mediated regulation of E2F complexes suggests the potential capacity of C/EBPα to inhibit proliferation of a broad number of cells and may explain the nearly universal character of C/EBPα-mediated growth arrest in cultured cells described by many investigators. In agreement with this suggestion, we found that ectopic expression of C/EBPα in cultured HT1 cells leads to disruption (or prevention of formation) of E2F complexes containing p107 (Fig. 9). The interaction of C/EBPα and C/EBPβ with Rb was described by Chen et al. (4). The authors suggest that this interaction might be involved in the differentiation of adipocytes, because DNA binding activities of C/EBPβ and C/EBPα were increased as a result of this interaction (4). We suggest that the activation of C/EBPα binding by interaction with Rb (4) and the disruption of E2F-p107 complexes by C/EBPα might contribute to the differentiation and growth cessation of adipocytes as two tightly regulated, interdependent processes.
The effect of C/EBPα on E2F-p107 complexes is specific, because the deletion of another member of the C/EBP family, C/EBPβ, did not alter E2F complexes in newborn mice. Although C/EBPβ contains a similar DLF peptide that is likely to be involved in the interaction with Rb (4), C/EBPβ knockout mice do not show a difference in E2F binding (Fig. 2B), suggesting that in vivo, C/EBPβ does not regulate E2F complexes in newborn mice or during prenatal development. C/EBPα is highly expressed in quiescent hepatocytes and in differentiated adipocytes, cells that do not proliferate extensively in the adult animals. On the contrary, C/EBPβ expression is observed in many tissues and in dividing cultured cells. This difference in the expression of C/EBPα and C/EBPβ correlates with the functional differences of these proteins. Although both proteins have regions of high-level sequence similarity, they do not completely share functions. The interaction of C/EBPβ with Rb is involved in the differentiation of adipocytes (4), while the interaction of C/EBPα with p107 correlates with C/EBPα-mediated growth arrest and leads to the disruption of E2F complexes.
Several earlier studies suggest that p107 is an inhibitor of cell proliferation, operating through depression of E2F-dependent transcription by interaction with E2F (10). Our results do not support a growth inhibitory function of p107 during prenatal liver development. The elevation of p107 protein levels correlates with an increase of hepatocyte proliferation in newborn livers. Data from our studies suggest that cdk2-E2F-p107-cyclin A complexes may also contribute to the promotion of proliferation during prenatal liver development. We have observed that increased levels of cdk2-E2F-p107-cyclin A complexes correlate with induction of hepatocyte proliferation in newborn C/EBPα knockout mice and that the elevation of cdk2-E2F-p107-cyclin A complexes in C/EBPα knockout mice is accompanied by increased expression of several E2F targets. Based on these observations, we suggest that cdk2-E2F-p107-cyclin A complexes may play a positive role in the promotion of hepatocyte proliferation during prenatal liver development. The C/EBPα-dependent pathway of growth arrest in hepatocytes involves disruption of these cdk2-E2F-p107-cyclin A complexes.
ACKNOWLEDGMENTS
We thank T. Bilyeu and P. Iakova for excellent technical assistance and K. Faraj for the preparation of the manuscript.
This work was supported by NIH grants R01 GM55188-01 (N.A.T), K01 AG00766-01 (N.A.T), DK49285 (G.J.D), AG13663 (G.J.D), and AFAR grant A97161 (N.A.T).
REFERENCES
- 1.Beijersbergen R L, Kerkhoven R M, Zhu L, Carlee L, Voorhoeve P M, Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–2690. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- 2.Birkenmeier E H, Gwynn B, Howard S, Jerry J, Gordon J I, Landschulz W H, McKnight S L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989;3:1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- 3.Cha H H, Cram E J, Wang E C, Huang A J, Kasler H G, Firestone G L. Glucocorticoids stimulate p21 gene expression by targeting multiple transcriptional elements within a steroid responsive region of the p21waf1/cip1 promoter in rat hepatoma cells. J Biol Chem. 1998;273:1998–2007. doi: 10.1074/jbc.273.4.1998. [DOI] [PubMed] [Google Scholar]
- 4.Chen P-L, Riley D J, Chen Y, Lee W-H. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 1996;10:2794–2804. doi: 10.1101/gad.10.21.2794. [DOI] [PubMed] [Google Scholar]
- 5.Cram E J, Ramos R A, Wang E C, Cha H H, Nishio Y, Firestone G L. Role of the CCAAT/enhancer binding protein-alpha transcription factor in the glucocorticoid stimulation of p21waf1/cip1 gene promoter activity in growth-arrested rat hepatoma cells. J Biol Chem. 1998;273:2008–2014. doi: 10.1074/jbc.273.4.2008. [DOI] [PubMed] [Google Scholar]
- 6.DeGregori J, Kowalik T, Nevins J R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis and G1/S regulatory genes. Mol Cell Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng C, Zhang P, Harper W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 8.Devoto S H, Mudryj M, Pines J, Hunter T, Nevins J R. A cyclin A-protein kinase complex possesses sequence-specific DNA binding activity: p33cdk2 is a component of the E2F-cyclin A complex. Cell. 1992;68:167–176. doi: 10.1016/0092-8674(92)90215-x. [DOI] [PubMed] [Google Scholar]
- 9.Diehl A M, Johns D C, Yang S, Lin H, Yin M, Matelist L A, Lawrence J H. Adenovirus-mediated transfer of CCAAT/enhancer-binding protein-alpha identifies a dominant antiproliferative role for this isoform in hepatocytes. J Biol Chem. 1996;271:7343–7350. doi: 10.1074/jbc.271.13.7343. [DOI] [PubMed] [Google Scholar]
- 10.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 11.Flodby P, Barlow C, Kylefjord H, Ahrlund-Richter L, Xanthopoulos K G. Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein α. J Biol Chem. 1996;271:24753–24760. doi: 10.1074/jbc.271.40.24753. [DOI] [PubMed] [Google Scholar]
- 12.Freytag S O, Paielli D L, Gilbert J D. Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994;8:1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- 13.Friedman A D, Landschulz W H, McKnight S L. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev. 1989;3:1314–1322. doi: 10.1101/gad.3.9.1314. [DOI] [PubMed] [Google Scholar]
- 14.Garriga J, Limon A, Mayol X, Rane S G, Albrecht J H, Reddy E P, Andres V, Grana X. Differential regulation of the retinoblastoma family of proteins during cell proliferation and differentiation. Biochem J. 1998;333:645–654. doi: 10.1042/bj3330645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendricks-Taylor L R, Darlington G J. Inhibition of cell proliferation by C/EBP alpha occurs in many cell types, does not require the presence of p53 or Rb, and is not affected by large T-antigen. Nucleic Acids Res. 1995;23:4726–4733. doi: 10.1093/nar/23.22.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiebert S W, Chellappan S P, Horowitz J M, Nevins J R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- 17.Hurford R K, Cobrinik D, Lee M H, Dyson N. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 1997;11:1447–1463. doi: 10.1101/gad.11.11.1447. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda M A, Jakoi L, Nevins J R. A unique role for the Rb protein in controlling E2F accumulation during cell growth and differentiation. Proc Natl Acad Sci USA. 1996;93:3215–3220. doi: 10.1073/pnas.93.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson D G, Ohtani K, Nevins J R. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994;8:1514–1525. doi: 10.1101/gad.8.13.1514. [DOI] [PubMed] [Google Scholar]
- 20.Krek W, Xu G, Livingston D M. Cyclin A-kinase regulation of E2F-1 DNA binding function underlies suppression of an S phase checkpoint. Cell. 1995;83:1149–1158. doi: 10.1016/0092-8674(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 21.Landschulz W H, Johnson P F, Adashi E Y, Graves B J, McKnight S L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988;2:786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- 22.Landschulz W H, Johnson P F, McKnight S L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 23.Landschulz W H, Johnson P F, McKnight S L. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989;243:1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- 24.Lee M H, Williams B O, Mulligan G, Mukai S, Bronson R T, Dyson N, Harlow E, Jacks T. Targeted disruption of p107: functional overlap between p107 and Rb. Genes Dev. 1996;10:1621–1632. doi: 10.1101/gad.10.13.1621. [DOI] [PubMed] [Google Scholar]
- 25.Lin F-T, Lane M D. CCAAT/enhancer binding protein α is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci USA. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mudryj M, Devoto S H, Hiebert S W, Hunter T, Pines J, Nevins J R. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991;65:1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- 27.Neuman E, Flemington E K, Sellers W R, Kaelin W G. Transcription of the E2F-1 gene is rendered cell cycle dependent by E2F DNA-binding sites within its promoter. Mol Cell Biol. 1994;14:6607–6615. doi: 10.1128/mcb.14.10.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Screpanti I, Musiani P, Bellavia D, Cappelletti M, Aiello F B, Maroder M, Frati L, Modesti A, Gulino A, Poli V. Inactivation of the IL-6 gene prevents development of multicentric Castleman’s disease in C/EBP beta-deficient mice. J Exp Med. 1996;184:1561–1566. doi: 10.1084/jem.184.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serfas M S, Goufman E, Feuerman M H, Gartel A L, Tyner A L. p53-independent induction of p21WAF1/CIP1 expression in pericentral hepatocytes following carbon tetrachloride intoxication. Cell Growth Differ. 1997;8:951–961. [PubMed] [Google Scholar]
- 30.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 31.Shiyanov P, Bagchi S, Adami G, Kokontis J, Hay N, Arroyo M, Morozov A, Raychaudhuri P. p21 disrupts the interaction between cdk2 and the E2F-p130 complex. Mol Cell Biol. 1996;16:737–744. doi: 10.1128/mcb.16.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith E J, Leone G, DeGregori J, Jakoi L, Nevins J R. The accumulation of an E2F-p130 transcriptional repressor distinguishes a G0 cell state from a G1 cell state. Mol Cell Biol. 1996;16:6965–6976. doi: 10.1128/mcb.16.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timchenko N, Wilson D R, Taylor L R, Abdelsayed S, Wilde M, Sawadogo M, Darlington G J. Autoregulation of the human C/EBPα gene by stimulation of upstream stimulatory factor binding. Mol Cell Biol. 1995;15:1192–1202. doi: 10.1128/mcb.15.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timchenko N A, Harris T E, Wilde M, Bilyeu T A, Burgess-Beusse B L, Finegold M J, Darlington G J. CCAAT/enhancer binding protein α regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol. 1997;17:7353–7361. doi: 10.1128/mcb.17.12.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timchenko N A, Wilde M, Kosai K, Heydari A R, Bilyeu T A, Finegold M J, Mohamedali K, Richardson A, Darlington G J. Regenerating livers of old rats contain high levels of C/EBPα that correlate with altered expression of cell cycle associated proteins. Nucleic Acids Res. 1998;26:3293–3299. doi: 10.1093/nar/26.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timchenko N A, Wilde M, Nakanishi M, Smith J R, Darlington G J. CCAAT/enhancer binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- 37.Umek R M, Friedman A D, McKnight S L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 38.Wang N D, Finegold M J, Bradley A, Ou C N, Abdelsayed S V, Wilde M D, Taylor L R, Wilson D R, Darlington G J. Impaired energy homeostasis in C/EBP alpha knock-out mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg C. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg R A. Tumor suppressor genes. Science. 1991;254:1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- 41.Wilson D R, Juan T S-C, Wilde M D, Fey G H, Darlington G J. A 58-base-pair region of the human C3 gene confers synergistic inducibility by interleukin-1 and interleukin-6. Mol Cell Biol. 1990;10:6181–6191. doi: 10.1128/mcb.10.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Wade M, Krall L, Grisham J, Xiong Y, Van Dyke T. Targeted in vivo expression of the cyclin-dependent kinase inhibitor p21 halts hepatocyte cell-cycle progression, postnatal liver development, and regeneration. Genes Dev. 1996;10:245–260. doi: 10.1101/gad.10.3.245. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi M, Hayashi Y, Matsukage A. Essential role of E2F recognition sites in regulation of the proliferating cell nuclear antigen gene promoter during Drosophila development. J Biol Chem. 1995;270:25159–25165. doi: 10.1074/jbc.270.42.25159. [DOI] [PubMed] [Google Scholar]
- 44.Yeh W-C, Cao Z, Classon M, McKnight S L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]