Abstract
Anaplastic oligodendroglioma (AO) is rare in children. Treatment typically consists of varying combinations of surgery, chemotherapy, and radiotherapy. We present a pediatric case of frontal lobe AO with periventricular subcallosal extension and local leptomeningeal involvement. The isocitrate dehydrogenase (IDH) wild-type tumor was MGMT methylated and contained an ATRX mutation, BRAF alteration, and 1p/19q co-deletion; a combination of alterations mostly encountered in pediatric oligodendrogliomas. The patient underwent a near total resection and had a complete, durable response to temozolomide alone, suggesting that conservative management without radiation may be appropriate in some cases. We review the literature of this uncommon subtype of glioma in children.
Keywords: 1p19q, children, codeletion, oligodendroglioma, pediatric, temozolomide
1 |. INTRODUCTION
Anaplastic oligodendroglioma (AO) is a central nervous system (CNS) tumor that is uncommon in children and seen primarily in adults.1 In adults, AO is molecularly defined according to the World Health Organization (WHO) 2016 classification scheme by codeletion of the short arm of chromosome 1 and the long arm of chromosome 19 (1p/19q loss) in combination with IDH1 or IDH2 mutation. This is frequently accompanied by mutations in the TERT promoter and CIC or FUBP1.2 However, 1p/19q codeletion is less common in children, where it is most frequently encountered in older adolescents in combination with IDH1 or IDH2 mutation.3 The genetics of pediatric diffuse gliomas are distinct from their adult counterparts, with most pediatric oligodendrogliomas lacking IDH1/2 mutation and 1p/19q co-deletion, and instead harboring BRAF or FGFR1 mutation or rearrangement.4,5
Clinical outcomes and optimal treatment strategies for pediatric oligodendrogliomas, based on the underlying genetic alterations, have not been thoroughly studied. 1p/19q codeletion has a strong prognostic role in isocitrate dehydrogenase (IDH) mutant oligodendroglioma in adults; in a phase III adult trial, patients with diffuse gliomas harboring 1p/19q loss had a significantly longer survival time, lower hazard ratio for death, and lower risk of tumor progression when compared to those without the codeletion.6 Current standard of care for AO in adults consists of maximum surgical resection and radiotherapy. In contrast, the prognostic significance of 1p/19q codeletion in the absence of IDH1/2 mutation in children is unknown. Treatment regimens for pediatric oligodendroglioma vary based on the age of the patient and tumor location. A >90% resection improves both progression free survival and overall survival, but these studies were not correlated with tumor genetic markers.7 We present a case of an AO in a child with a 1p/19q codeletion but IDH wild-type status, who had a complete, prolonged response to surgery and temozolomide (TMZ) alone, suggesting that conservative management should be considered in pediatric IDH wild-type oligodendrogliomas.
2 |. RESULTS
An 8-year-old male child presented with a 1-week history of headache, nausea, and vomiting. Magnetic resonance imaging (MRI) showed a left frontal lobe tumor that extended posteriorly to the inferior genu and subcallosal area reaching the left frontal horn with evidence of hemorrhage (Fig. 1). He underwent a near total resection and pathology was consistent with AO, WHO grade III (Fig. 2), based on a mitotic index of up to eight of ten high-power fields, Ki-67 labeling index of 15%, and presence of both microvascular proliferation and palisading necrosis. Microscopic examination demonstrated leptomeningeal extension. The tumor was negative for IDH1 R132H mutant protein expression by immunohistochemistry. FISH demonstrated 1p/19q codeletion, and MGMT gene promoter methylation was confirmed by Sanger sequencing of bisulfite-treated tumor DNA. Spinal MRI and cerebrospinal fluid cytology specimens were negative. Based on the favorable cytogenetics (but prior to the IDH sequencing, which was only available later), conservative management with TMZ 200 mg/m2 daily for 5 days every 28 days for 12 cycles was employed. Therapy was well tolerated with one delay for thrombocytopenia and minor nausea relieved with ondansetron. At most recent follow-up, 42 months off-therapy, the patient had no evidence of disease (Fig. 1) and a Karnofsky score of 100.
FIGURE 1.
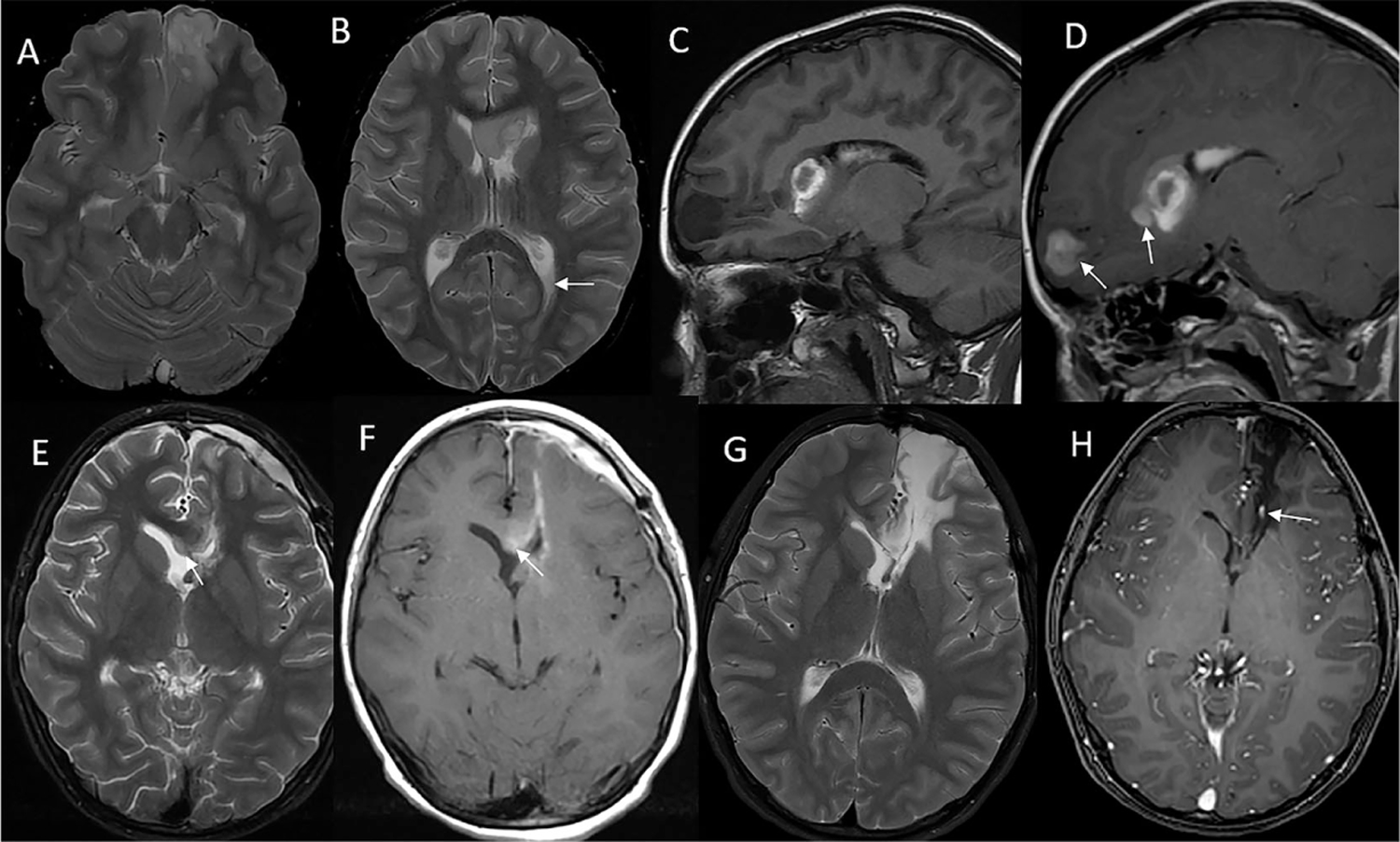
Magnetic resonance imaging of the brain. (A and B) T2-weighted axial images at diagnosis show a heterogeneous, hyperintense mass in the left inferomedial frontal lobe that extends to the left periventricular subcallosal region. Fluid level in the left occipital horn from hemorrhage (arrow). (C) Precontrast T1 sagittal image at diagnosis shows areas of T1 hyperintensity in the mass and in the left ventricle from hemorrhage. (D) Postcontrast T1 axial image shows enhancement in the solid components of the mass, separate from areas of hemorrhage (arrows). (E) T2 axial image 3-week posttumor resection shows residual hyperintense mass in the splenium of corpus callosum (arrow). (F) T1 axial postcontrast image at 3-week postresection shows enhancement in the residual mass in the genu of corpus callosum (arrow). The enhancement in the margin of the resection cavity and in the pachymeninges is postsurgical reactive change. (G) T2 axial image at most recent follow-up 42 months off therapy shows gliosis in the genu of corpus callosum at the site of residual tumor and around the resection cavity reaching the ventricular margin. (H) Postcontrast T1 axial image at most recent follow-up shows hypointensity in the same area with no solid enhancement. The linear enhancement is in a vein (arrow)
FIGURE 2.
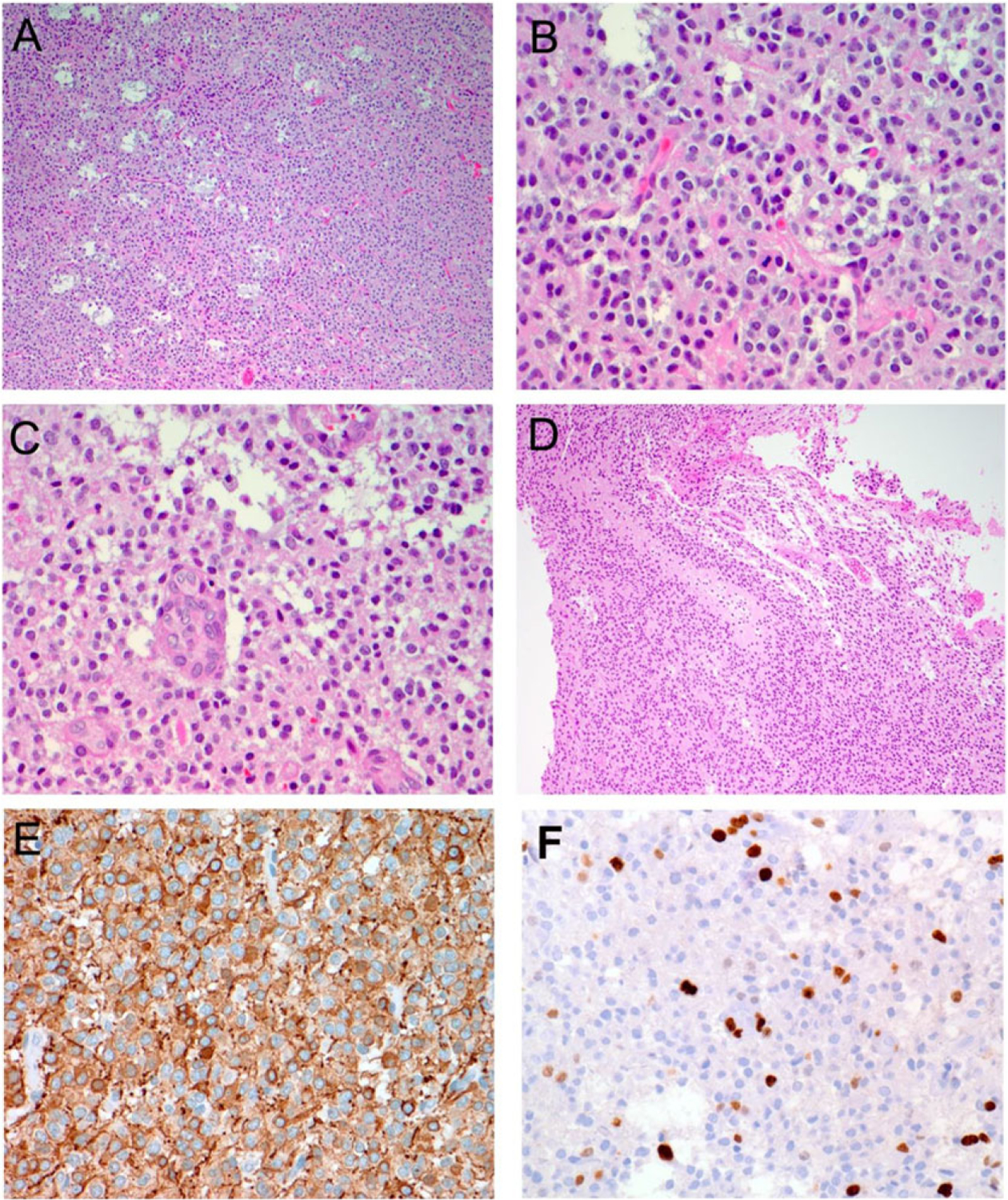
Histopathology of pediatric anaplastic oligodendroglioma. Hematoxylin and eosin stain (A–D) as well as immunohistochemistry for GFAP (E) and MIB-1 (F). Microscopic examination demonstrates a glial neoplasm composed of cells having relatively uniform, round nuclei and prominent perinuclear halo (A and B). Occasional mitotic figures (B), focal microvascular proliferation (C), and foci of pseudopalisading necrosis (D) are identified
To characterize the tumor further, next-generation sequencing was recently performed as previously described.8 Analysis confirmed 1p/19q codeletion and identified ATRX mutation, BRAF gene rearrangement, and CHEK1 frameshift mutation (Supplementary Fig. S1). No mutations were found in IDH1, IDH2, TERT promoter, CIC, or FUBP1.
3 |. DISCUSSION
Oligodendroglioma is a rare childhood tumor representing approximately 1% of primary pediatric CNS tumors.9 Most are low grade (WHO grade II) and exhibit a low frequency of histologic progression; in a series of 50 patients, approximately 25% of tumors were anaplastic (WHO grade III) and only one of eight low-grade tumors sequentially surveyed progressed to anaplasia.10 Unlike their adult counterparts, pediatric oligodendroglioma is poorly understood due to sparse molecular studies reported to date. In adults, the molecular profile of oligodendroglioma may predict survival and therapy response. While adult data can be used to guide pediatric treatment, the classic codeletion of 1p/19q seen in adult oligodendroglioma is not commonly seen in children. In a retrospective review of 28 pediatric oligodendrogliomas (20 grade II and eight grade III), only three had whole arm 1p/19q codeletion.11 One of the three cases was anaplastic. More cytogenetic abnormalities were seen in the anaplastic cases (n = 5). In a separate retrospective review of 40 oligodendrogliomas in patients ≤ 20 years old, only 10 (25%) had the codeletion, and eight of the 10 were ≥15 years old.10 Similarly, Suri et al. found 1p19q codeletion in four of seven patients, 19–25 years old, and no codeletions in children <15 years old.3 The sequencing data from our patient’s tumor support a pediatric rather than adult-type diffuse glioma due to the BRAF alteration, which typically correlates with oligodendroglial morphology, and the lack of IDH1/IDH2 mutation.4,5
Standard of care for AO treatment in adults has traditionally consisted of gross total resection and radiation; however, more recently, adjuvant chemotherapy was proven efficacious. Two randomized trials, EORTC 26951 and RTOG 9402, demonstrated that newly diagnosed patients with AO and 1p/19q codeletion benefitted from adding procarbazine, lomustine, and vincristine (PCV) to radiation.12,13 There were significant toxicities seen with this regimen as nearly two-thirds of patients had grade III or IV toxicities most frequently hematologic, neurologic (cognitive or mood change and peripheral or autonomic neuropathy), gastrointestinal (nausea and vomiting), hepatic, or dermatologic.6 This led to studies exploring less toxic agents including TMZ. In a prospective adult study of AO recurrent after surgery and radiotherapy, 39 patients were treated with TMZ 150–200 mg/m2 for 5 days every 28-day cycle. Treatment was well tolerated, and the response rate was 61.5%. A positive correlation existed between the response rate and 1p/19q deletion, and MGMT promoter methylation and presence of the codeletion, indicating that single agent TMZ was active in recurrent AO.14 Furthermore, a phase II trial of primary TMZ in grade III oligodendroglial tumors, including 11 AO, demonstrated that TMZ was active and well tolerated.15 All patients with AO showed MGMT methylation. Our patient’s tumor was MGMT methylated, consistent with his excellent response to TMZ.
TMZ versus PCV with or without radiotherapy has not been compared. However, due to the results from EORTC 26951 and RTOG 9402, the phase II CODEL trial for the treatment of anaplastic gliomas with 1p19q codeletion has been revised. The trial is now comparing TMZ versus radiotherapy and PCV versus radiotherapy and TMZ. The endpoint of the study is progression-free survival. While TMZ is active in AO, the durability of response and the success of salvage therapy after recurrence with TMZ compared to PCV are unknown; therefore, a variety of factors should be considered when deciding which regimen to use in children such as degree of resection, tumor location, MGMT methylation status, expected toxicities, and family input.
Our patient’s complete, durable response 42 months off-therapy with TMZ alone suggests that conservative management is a promising approach. Observation alone with close follow-up may also be a reasonable strategy for well-resected pediatric IDH wild-type oligodendrogliomas. Solitary treatment with TMZ in select patients such as ours avoids upfront radiation, which is especially important in younger patients, and reserves radiotherapy if the tumor progresses. With this approach, careful radiographic follow-up for many years is imperative.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by the National Institutes of Health Director’s Early Independence Award (DP5 OD021403) to D.S.
Grant sponsor: National Institutes of Health Director’s Early Independence Award; Grant number:DP5OD021403.
Abbreviations:
- AO
anaplastic oligodendroglioma
- CNS
central nervous system
- IDH
isocitrate dehydrogenase
- MRI
magnetic resonance imaging
- PCV
procarbazine, lomustine, and vincristine
- TMZ
temozolomide
- WHO
World Health Organization
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Creach KM, Rubin JB, Leonard JR, et al. Oligodendrogliomas in children. J Neurooncol 2012;106(2):377–382. [DOI] [PubMed] [Google Scholar]
- 2.Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suri V, Jha P, Agarwal S, et al. Molecular profile of oligodendrogliomas in young patients. Neuro-oncology 2011;13(10):1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 2013;45(6):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qaddoumi I, Orisme W, Wen J, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 2016;131(6):833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: intergroup radiation therapy oncology group trial 9402. J Clin Oncol 2006;24(18):2707–2714. [DOI] [PubMed] [Google Scholar]
- 7.Wu CT, Tsay PK, Jaing TH, et al. Oligodendrogliomas in children: clinical experiences with 20 patients. J Pediatr Hematol Oncol 2016;38(7):555–558. [DOI] [PubMed] [Google Scholar]
- 8.Kline CN, Joseph NM, Grenert JP, et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro-oncology 2017;19(5):699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghavan R, Balani J, Perry A, et al. Pediatric oligodendrogliomas: a study of molecular alterations on 1p and 19q using fluorescence in situ hybridization. J Neuropathol Exp Neurol 2003;62(5):530–537. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez FJ, Tihan T, Lin D, et al. Clinicopathologic features of pediatric oligodendrogliomas: a series of 50 patients. Am J Surg Pathol 2014;38(8):1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nauen D, Haley L, Lin MT, et al. Molecular analysis of pediatric oligodendrogliomas highlights genetic differences with adult counterparts and other pediatric gliomas. Brain Pathol 2016;26(2):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 13.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandes AA, Tosoni A, Cavallo G, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: a prospective GICNO study. J Clin Oncol 2006;24(29):4746–4753. [DOI] [PubMed] [Google Scholar]
- 15.Gan HK, Rosenthal MA, Dowling A, et al. A phase II trial of primary temozolomide in patients with grade III olidenodroglial brain tumors. Neuro Oncol 2010;12(5):500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


