Abstract
Background
Posterior fossa syndrome (PFS) is a known consequence of medulloblastoma resection. Our aim was to clinically define PFS, its evolution over time, and ascertain risk factors for its development and poor recovery.
Methods
Children with medulloblastoma treated at St Jude Children’s Research Hospital from 6/2013 to 7/2019 received standardized neurological examinations, before and periodically after radiation therapy. Most (98.3%) were enrolled on the ongoing multi-institutional protocol (SJMB12; NCT 01878617).
Results
Sixty (34%) of 178 evaluated children had PFS. Forty (23%) had complete mutism (PFS1) and 20 (11%) had diminished speech (PFS2). All children with PFS had severe ataxia and 42.5% of PFS1 had movement disorders. By multivariable analysis, younger age (P = .0005) and surgery in a low-volume surgery center (P = .0146) increased PFS risk, while Sonic Hedgehog tumors had reduced risk (P = .0025). Speech and gait returned in PFS1/PFS2 children at a median of 2.3/0.7 and 2.1/1.5 months, respectively, however, 12 (44.4%) of 27 PFS1 children with 12 months of follow-up were nonambulatory at 1 year. Movement disorder (P = .037) and high ataxia score (P < .0001) were associated with delayed speech recovery. Older age (P = .0147) and high ataxia score (P < .0001) were associated with delayed gait return. Symptoms improved in all children but no child with PFS had normal neurologic examination at a median of 23 months after surgery.
Conclusions
Categorizing PFS into types 1 and 2 has prognostic relevance. Almost half of the children with PFS1 with 12-month follow-up were nonambulatory. Surgical experience was a major modifiable contributor to the development of PFS.
Keywords: cerebellar mutism, medulloblastoma, outcome, posterior fossa syndrome, risk factors
Key Points.
Surgical experience contributes to incidence and severity of posterior fossa syndrome.
Many children with complete mutism remain nonambulatory at 12-month post-surgery.
High ataxia score and older age at injury predict slow and incomplete recovery.
Importance of the Study.
Posterior fossa syndrome (PFS), also known as cerebellar mutism syndrome, is a complication of surgery for medulloblastoma. While the syndrome is well known, its cause, features, risk factors, and manifestations are poorly understood. This prospective evaluation of 178 patients gives an unprecedented look at the clinical characteristics of the syndrome and defines new features such as movement disorders and opsoclonus-myoclonus syndrome. We categorize patients into PFS1 with complete mutism and PFS2 with partial mutism. Younger age, non-Sonic Hedgehog tumors, and less experienced surgeon were risk factors for the development of PFS; latter also correlated with more severe cases. Surgical skill is a modifiable risk factor and rates as low as 10% are possible in experienced hands. Our data will help clinicians prognosticate recovery as older age and more severe neurological injury predicted incomplete and slower recovery. We strongly recommend that children with medulloblastoma are operated by experienced surgeons.
Posterior fossa syndrome (PFS), also known as cerebellar mutism syndrome, may develop after posterior fossa tumor resection, especially medulloblastoma.1–3 Commonly described as being transient, recent reports demonstrate that PFS is more than a fleeting language impairment. Impairment of language with or without mutism is observed in all patients with PFS, while ataxia, dysmetria, irritability, and emotional lability are often present.1–5 Most afflicted children have persistent neurologic and neurocognitive dysfunction in excess of what is expected from their medulloblastoma therapy,1,5,6 but underlying etiology, recovery, and risk factors for chronicity remain poorly understood. Moreover, a comprehensive understanding of PFS is lacking since most of the research has been retrospective which tends to capture only the more severe cases, and the only prospective study, reported by the Children’s Oncology Group, was questionnaire-based without systematic neurologic examinations.1
The reported incidence of PFS ranges widely from 10% to 40%, and the only reproducible risk factors are midline tumor location and medulloblastoma pathology.1–7 Surgical approach with vermian dissection and surgical experience has been suggested but not validated.1–3 Tumor size, invasiveness, brainstem compression, and preexisting language impairment are also reported as risk factors,1,8,9 and recently, a study of 370 medulloblastomas identified tumor molecular subgroup as an important predictor.10 One emerging theory proposes that PFS develops because of injury to the deep cerebellar nuclei and their efferent pathways within the superior cerebellar peduncles.11–13 How this focal injury affects the child so globally is unknown, but it is postulated that the disruption of cerebellar nuclei outflow to the cerebral cortex produces transient cortical hypoperfusion as a manifestation of crossed cerebro-cerebellar diaschisis and this may contribute to global neurologic dysfunction.14–18
To better understand this entity, we present our prospectively acquired findings from serial neurologic examinations by neurologists on a cohort of children seen at our institution. The primary aims of this study were to clinically define PFS, characterize long-term outcomes of neurologic deficits, and study risk factors for PFS and poor neurologic recovery.
Methods
Study Cohort, Primary Outcomes, and Variables of Interest
Between June 1, 2013 and July 1, 2019, 195 newly diagnosed medulloblastoma patients with age ≥3 years were seen at our institution, and 178 (91.3%) participated in this study. Of the 178 children evaluated, 175 were enrolled on the institutional protocol (SJMB12; ClinicalTrials.gov identifier: NCT 01878617). Study of PFS is among the objectives of this trial. The protocol was approved by the St Jude Institutional Review Board and all 175 patients and/or legal guardians provided written informed consent before study entry. Institutional review board’s approval for exempt status was obtained for the 3 participants that were treated off protocol. All study participants were examined by a neurologist on arrival at our institution after tumor resection, after completion of craniospinal radiation therapy, and every 3-6 months thereafter. Evaluation of immediate postoperative status was based on parental reports. Eligibility criteria for the SJMB12 study include age ≥3years, no prior chemoradiotherapy, start of treatment within 36 days of definitive surgery, Lansky or Karnofsky performance score of ≥30 (except for PFS), and signing of informed consent by parents or patient if age ≥18 years.
Primary outcomes of interest included: categorization of PFS symptoms, risk factors for development of PFS, rate of neurologic recovery, and risk factors for delayed recovery. Variables considered for association with PFS risk are provided in Figure 1. Molecular subgroups were assigned by DNA methylation.19,20 Tumor volume (height × width × anteroposterior dimension), ratio of maximum cranial cavity width and frontal horn width on axial imaging (Evans’ index), and any procedure for cerebrospinal fluid (CSF) diversion were noted. Tumor location was characterized as either midline or lateral by location on MRI. Extent of resection (EOR) was defined as gross total resection (GTR) if all radiologically visible tumor was resected, near total resection (NTR) when the residual tumor size is ≤1.5 cm2, subtotal resection (STR) when the residual tumor size is >1.5 cm2, and biopsy if <25% of the tumor was removed. M0 was defined as no evidence of metastatic disease and M+ when the tumor was metastatic. High risk was defined as metastatic or <NTR at the start of therapy. For those with <NTR after primary resection, a second neurosurgical resection was encouraged to achieve GTR/NTR prior to therapy. For patients with PFS who had two or more surgeries, the EOR from when PFS occurred was used for analysis. A low-volume surgical center was defined as a facility located outside the United States in a resource-limited country, or with an estimated cumulative annual pediatric case volume of <500 (pediatric case volume was ascertained from information available on www.acpnf.org), or where the resection was performed by an adult surgeon.21 Centers with an accredited pediatric neurosurgery fellowship were considered “high-volume” irrespective of their caseloads. Studied risk factors for delayed return of speech and gait are provided in Figure 2.
Fig. 1.
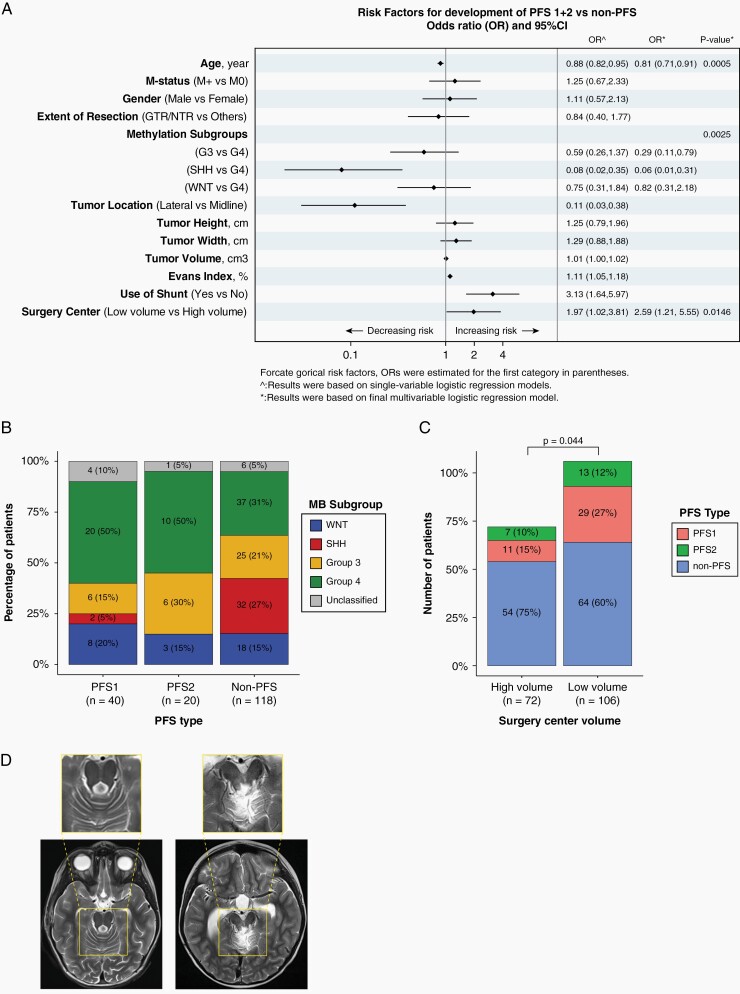
(A) Risk factors for the development of posterior fossa syndrome. (B) Methylation tumor subgroups. Much larger representation of Sonic Hedgehog group in the children without posterior fossa syndrome. (C) Distribution of posterior fossa types 1 + 2 among low- and high-volume surgery centers. P value represents Cochran-Armitage exact trend test. (D) MRI comparison of a child with almost normal neurologic examination post tumor resection (left image) and a child who did not regain speech and sitting balance 12 months after tumor resection (right image). Note severe injury to dorsal midbrain and bilateral superior cerebellar peduncles. Both had midline fourth ventricular tumor.
Fig. 2.
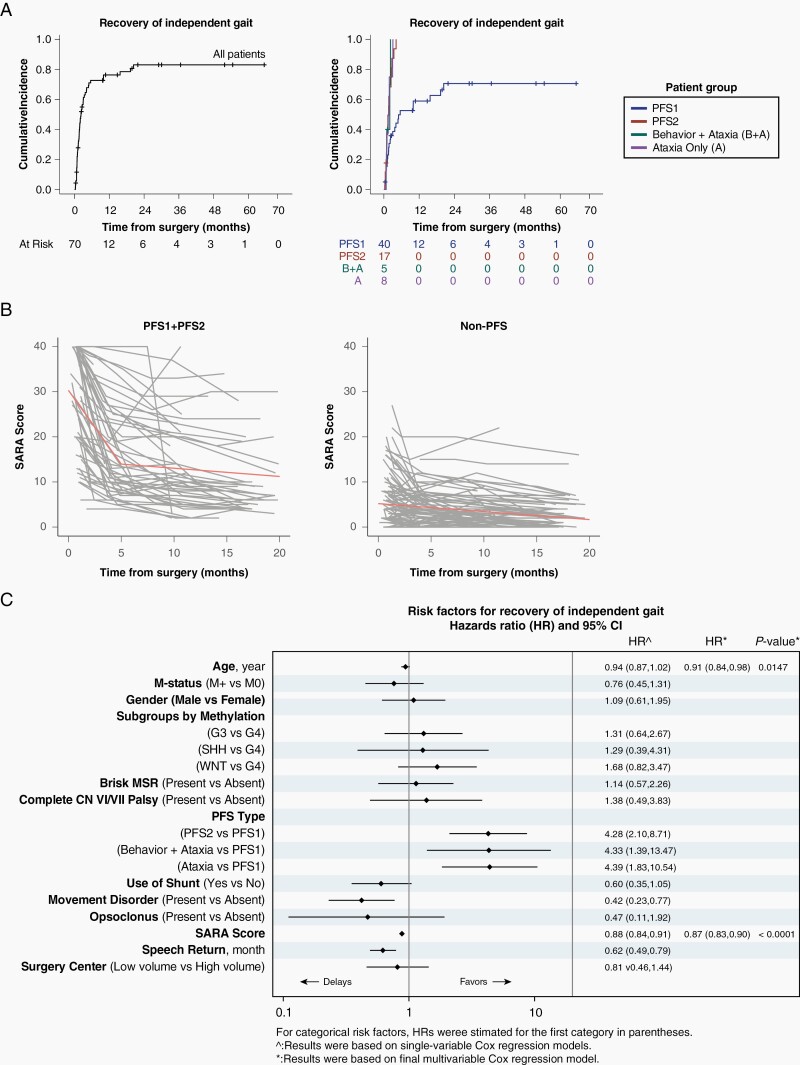
(A) Cumulative incidence plots showing recovery of independent gait in all 178 children and by different clinical subgroups. (B) Profile plots of scale for the assessment and rating of ataxia (SARA) scores in posterior fossa syndrome PFS1 + 2 cohort (n = 60) and non-PFS cohort (n = 118). A score of 0 is normal and 40 is inability to sit, speak, stand, and reach. Spikes represent children who developed treatment-related complications such as radiation necrosis. Orange straight lines represent fitted lines based on change-point linear mixed-effects model. (C) Risk factors for delayed recovery of independent gait.
Neurologic Assessments and Definitions
Every child received a standardized examination where neurologic deficits were scored as either present or absent (such as nystagmus, opsoclonus), while others were scored 1-3: 1 being normal; 2 when deficit was recognized with or without impairment of function but no need for instrumentation (brace, cane, etc.); 3 when there was severe functional impairment and need for an assistive device or complete loss of function. A modified scale for assessment and rating of ataxia (SARA) score was calculated at each visit.22 SARA score ranged from 0 to 40 with 0 being without ataxia, dysmetria, dysdiadochokinesia, and dysarthria, and 40 being unable to sit, stand, walk, speak, and reach. Tested domains on SARA included gait, stance, sitting balance, speech, dysmetria, and dysdiadochokinesia. Gait ataxia was also scored according to common terminology criteria for adverse events version 5.0: grade 1 when asymptomatic; grade 2, when some limitation on function was observed, but the patient remained able to walk without support; and grade 3 when patient was unable to stand, or walk only with support.
PFS diagnosis required impaired ability to verbalize after surgery: complete absence of vocalization (mutism) was defined as PFS1 and paucity of speech with inability to string 3-word sentences as PFS2. This (PFS1 vs PFS2) was based on the notion that patients with complete mutism are more severely affected than those with milder language impairments. The idea that language impairment exists on a spectrum and that paucity of language (not complete mutism) is an acceptable criterion for PFS diagnosis are supported in the consensus statement by an international group, as well as in a recent survey of experts.4,5 Involuntary movements, ocular fixation (involuntary target-seeking mini saccades), nystagmus, eye bobbing, and opsoclonus (multiplanar spontaneous saccades) were documented. Apraxia is difficult to assess in an irritable child but was considered present if a child could produce spontaneous involuntary movements but not to command. Return of speech was defined as ability to produce a 3-word sentence and was determined by parent recall and speech therapist’s notes. Return of gait was defined as ability to walk 10 steps (approximately 15 feet) without support based on parent recall and physical therapy notes.
Statistical Methods
Associations were investigated by Spearman correlations, Cochran-Armitage exact trend test, Fisher’s exact test, and Kruskal-Wallis test as applicable. Binary logistic regression models were used to assess associations between patient characteristics or clinical features and dichotomized PFS variable (PFS1 + 2 vs Others). Cumulative incidence plots and Cox proportional hazards models were used to study factors associated with delayed recovery of independent gait. Backward selection approach was applied to eliminate variables in multivariable models. Linear mixed-effects models with a prespecified change point were employed to model longitudinal SARA scores. A significance threshold of 0.05 was used throughout without adjusting for multiplicity.
Results
General Demographics
Demographics are provided in Table 1. Median follow-up was 19.6 months and median time from surgery to first neurologic evaluation was 28 days (range 3-276 days). Median follow-up was 23.1 months for the 60 children with PFS. There were 84 low-volume surgery centers contributing 106 participants and 29 high-volume centers contributing 72 participants (28 from St. Jude). Twenty-eight children underwent second resection prior to starting therapy (25 at our institution); 2 developed PFS, one PFS1 (complete resection) and the other PFS2 (STR).
Table 1.
Cohort Demographics, n = 178 patients
| Variable | Number (percentage) |
|---|---|
| Gender | |
| Female | 62 (34.8%) |
| Male | 116 (65.2%) |
| Mean age at surgery | 10.2 years (SD 5.0) |
| Median follow-up | |
| All patients (n = 178) PFS patients (n = 60) |
19.6 months (0.4-72.8) 23.1 months (0.4-70.7) |
| Tumor location | |
| Midline | 137 (77%) |
| Lateral | 41 (23%) |
| Tumor subgroups | |
| WNT | 29 (16.3%) |
| SHH | 34 (19.1%) |
| Group 3 | 37 (20.8%) |
| Group 4 | 67 (37.6%) |
| Unclassified | 11 (6.2%) |
| CSF diversion | |
| Yes | 80 (44.9%) |
| No | 98 (55.1%) |
| Surgical center | |
| High volume | 72 (40.4%) |
| Low volume | 106 (59.6%) |
| Clinical subgroups | |
| PFS1 | 40 (22.5%) |
| PFS2 | 20 (11.2%) |
| Ataxia (grade 3) + behaviora | 6 (3.4%) |
| Ataxia (grade 3) | 11 (6.2%) |
| Ataxia (grade 1-2) | 78 (43.8%) |
| Minor deficit without ataxia | 2 (1%) |
| Normal | 21 (11.8%) |
Abbreviations: CSF, cerebrospinal fluid; PFS1, posterior fossa syndrome with complete mutism; PFS2, posterior fossa syndrome with partial mutism; SHH, Sonic Hedgehog; WNT, wingless-related integration site.
aInability to walk and significant emotional lability and irritability.
Categorization of PFS
We identified 60 (34%) children with PFS: PFS1 (complete mutism) in 40 (23%) and PFS2 (partial mutism) in 20 (11%). Of the remaining 118 patients, all but 21 children had some neurologic deficits at initial presentation. The list below provides the main neurologic deficits observed at initial presentation and their relationship to PFS1 and PFS2 (Table 2).
Table 2.
Frequency of Clinical Neurologic Symptoms and Signs at Baseline and Comparisons Between PFS and Non-PFS Groups
| Variable at Baseline | PFS Type 1 (n = 40) | PFS Type 2 (n = 20) | Others (n = 118) | PFS1 vs PFS2 vs Others | PFS1 vs Others | PFS2 vs Others | PFS1 vs PFS2 | PFS1 + 2 vs Others |
|---|---|---|---|---|---|---|---|---|
| Cochran-Armitage Exact Trend TestP value | Fisher’s ExactP value | Fisher’s ExactP value | Fisher’s ExactP value | Fisher’s ExactP value | ||||
| Normal/brisk MSR | 82.5% | 80.0% | 87.3% | .4481 | .4386 | .4795 | 1.0000 | .3706 |
| Focal weakness | 55.9% | 45.0% | 6.8% | <.0001 | <.0001 | <.0001 | .5743 | <.0001 |
| Opsoclonus | 7.5% | 5.0% | 0.8% | .0280 | .0503 | .2698 | 1.0000 | .0448 |
| Eye bobbing | 7.5% | 5.0% | 0.0% | .0071 | .0153 | .1449 | 1.0000 | .0121 |
| Square waves | 30.0% | 30.0% | 7.6% | .0003 | .0008 | .0094 | 1.0000 | .0002 |
| Movement disorder | 42.5% | 20.0% | 6.0% | <.0001 | <.0001 | .0559 | .1500 | <.0001 |
| Speech issues | 97.5% | 95.0% | 0.0% | <.0001 | <.0001 | <.0001 | 1.0000 | <.0001 |
| Irritability issues | 82.5% | 70.0% | 8.5% | <.0001 | <.0001 | <.0001 | .3258 | <.0001 |
| Apraxia | 80.0% | 40.0% | 0.8% | <.0001 | <.0001 | <.0001 | .0034 | <.0001 |
| Anesthesia | 97.5% | 84.2% | 54.0% | <.0001 | <.0001 | .0216 | .0937 | <.0001 |
| NGT/GT | 55.0% | 30.0% | 9.6% | <.0001 | <.0001 | .0213 | .0999 | <.0001 |
| Swallowing problems | 67.5% | 25.0% | 5.2% | <.0001 | <.0001 | .0112 | .0026 | <.0001 |
| Spearman CorrelationP value | WMWP value | WMWP value | WMWP value | WMWP value | ||||
| CTCAE ataxia | ||||||||
| Grade 0 | 0.0% | 0.0% | 22.0% | .0001a | <.0001 | <.0001 | .5512 | <.0001 |
| Grade 1 | 10.0% | 10.0% | 54.2% | |||||
| Grade 2 | 7.5% | 15.0% | 10.2% | |||||
| Grade 3 | 82.5% | 75.0% | 13.6% | |||||
| SARA scoreMean ± standard deviation | 30.8 ± 11.7 | 20.8 ± 7.4 | 5.6 ± 6.1 | <.0001b | <.0001 | <.0001 | .0005 | <.0001 |
Abbreviations: CTCAE, common terminology criteria for adverse events; MSR, muscle stretch reflexes; NGT/GT, Nasogastric tube or gastric tube; PFS1, posterior fossa syndrome with complete mutism; PFS2, posterior fossa syndrome with partial mutism; SARA, scale for assessment and rating of ataxia; WMW, Wilcoxon-Mann-Whitney test.
aSpearman correlation coefficient was estimated to be 0.64. bSpearman correlation coefficient was estimated to be 0.75.
Apraxia
Apraxia was only present in children with PFS and was more frequent in severe cases: 32/40 (80%) PFS1 vs 8/20 (40%) PFS2 (P = .0034).
Ataxia
Postoperative grade-3 ataxia was reported by parents in all PFS children as compared to 17/118 (14%) without PFS (P = .0001). A higher SARA score at enrollment correlated with increasing severity of PFS: PFS1 = 30.8 ± 11.7 vs PFS2 = 20.8 ± 7.4 vs non-PFS = 5.6 ± 6.1 (P < .0001).
Behavior change
Irritability and emotional lability were present in 47/60 (78%) children with PFS: 33 (82.5%) PFS1 and 14 (70%) PFS2 (P = .326); and 6/118 (5%) without PFS (P < .0001). Additionally, 10 (16.7%) of children with PFS displayed spontaneous giggling (6 PFS1; 4 PFS2). All 6 non-PFS children with irritability also displayed grade-3 ataxia.
Movement disorders
Involuntary movements were present in 28 (16%) children with PFS: 17 (42.5%) PFS1 and 4 (20%) PFS2 (P = .15); and 7/118 (6%) without PFS (P < .0001). These included multifocal myoclonus in 11, focal dystonia in 14, limb tremor in 6, ballism in 5, dyskinesia in 2, and vocal cord tremor in 1; 11 children had more than one abnormal movement.
Ocular abnormalities
Impaired ocular fixation was seen in 18 (30%) of the PFS1 + 2 group and 9 (8%) of the rest (P = .0002). Opsoclonus and accompanying multifocal myoclonus were seen in 5 children; 4 of whom had PFS1.
Other symptoms
Focal weakness was difficult to assess because of apraxia but was likely present in 34 (57%) of children with PFS and 8 (7%) of the others (P < .0001). Cranial nerve deficits, dysarthria, swallowing impairment, need for tube feeding, and other deficits are depicted in Table 2 and Figures 1 and 3. Swallowing impairment was more prevalent in children with PFS than those without (P < .0001) and more common in PFS1 vs PFS2 (P = .0026). Tube feeding was needed postoperatively and during the radiation phase of therapy in 20 (50%) of 40 children with PFS1, 5 (25%) with PFS2, 4 (24%) in children with grade-3 ataxia, and 6 (6%) of 101 others. The indication for a feeding tube during this time was due to swallowing issues (n = 27/35; 77%) and less frequently for nutritional support (n = 8/35; 13%).
Fig. 3.
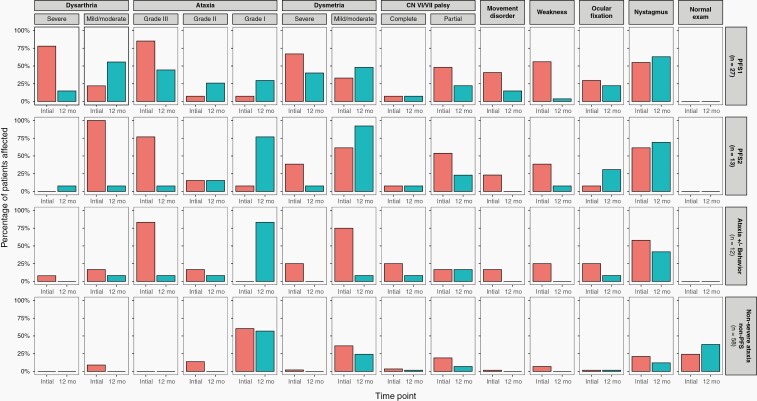
Proportions of children affected with neurologic deficits at baseline and 12 months after surgery in posterior fossa syndrome type 1 (PFS1), posterior fossa syndrome type 2 (PFS2), severe ataxia group with or without behavior change, and those with normal language and without severe ataxia (others).
Risk Factors for Development of PFS
Younger age, surgery in a low-volume center, larger ventricles, and use of CSF diversion associated with increased PFS risk on univariable analysis (Figure 1A), while lateral tumor location and Sonic Hedgehog (SHH) subgroup tumors associated with reduced risk (Figure 1A, B). Younger children were more likely to have larger ventricles (Evans’ index, P < .0001), but there was no difference in Evans’ index, tumor size, extent of tumor resection, and age distribution among children treated at low or high-volume surgery centers (P = .80, .12, .14, and .21, respectively), CSF diversion procedures were more common at low-volume centers (P = .002). On multivariable analysis, younger age at diagnosis (P = .0005), surgery in a low-volume center (P = .0146), and non-SHH subgroups (P = .0025) remained associated with PFS1 + 2 (Figure 1A). Surgery in a low-volume center also resulted in more severe, ie, PFS1 cases (P = .044, Figure 1C, D). Risk group (average vs high) did not associate with PFS or its severity (P = .53 and .42), and neither did M status (P = .53 and .42). EOR was also not predicative of PFS development (odds ratio 0.84, confidence interval 0.40, 1.77) (Figure 1A).
Rate of Recovery
Twelve-month follow-up was completed by 110 children: 27 PFS1; 13 PFS2; 12 grade-3 ataxia with or without behavior impairment; and 58 others (14 normal; 44 mild-to-moderate ataxia Figure 3). None of the children with PFS1 + 2 had a normal neurological examination at 12-month follow-up. By comparison, normal neurologic examination increased from 24.1% to 37.9% in children without PFS or severe ataxia (Figure 3). The list below describes the rate of recovery for the main neurologic deficits and how each relates to PFS1 and PFS2.
Speech recovery
Median time to speech recovery in the 40 PFS1 children was 2.3 months (range 0.3-14.5) with one child experiencing no speech recovery 12 months from surgery. Median time to speech recovery in 20 PFS2 children was 0.7 months (range 0.3-3.6, P = .059). Severe dysarthria at 12 months in children with PFS1 and PFS2 were present in 14.8% and 7.7%, respectively (Figure 3).
Apraxia recovery
Apraxia recovered in 1.6 months (range 0.2-14.7) in PFS1 vs 1.1 months (range 0.5-1.7) in children with PFS2 (P = .0168).
Ataxia and dysmetria recovery
Of the 40 evaluable children with PFS1 for walking recovery (Figure 2A), 25 (62.5%) regained independent walking by a median of 2.1 months (range 0.3-20.4). Of the remaining 15 at a median follow-up of 19.8 months (range 0.4-65.4), 9 remain nonambulatory at more than 12 months of follow-up and 6 have not yet reached the 12-month mark. In 27 PFS1 subjects that completed 12-month follow-up, the rate of grade-3 ataxia (nonambulatory) dropped from 85.2% at initial evaluation to 44.4% and severe dysmetria from 67% to 14.1%; grade-2 ataxia was present in 25.9%, and grade-1 in the rest (Figure 3). By comparison, all the 17 evaluable patients with PFS2 for walking recovery regained ambulation (P < .0001) at a median of 1.47 months (range 0.3-4.2); two did not because of radiation-induced brainstem inflammation and one decided to receive treatment closer to home. In 13 subjects with PFS2 that completed 12-month follow-up, the rate of grade-3 ataxia dropped from 76.9% at baseline to 7.7% at 12 months and severe dysmetria from 38.5% to 7.7% (nonambulatory patient had radiation-induced brainstem inflammation). Of the 13 evaluable non-PFS ataxic patients, 12 (88.2%) regained independent walking at a median of 1.65 months (range 0.7-3.1), and one who did not was paraplegic because of spinal tumor; 2 children were nonambulatory because of radiation-induced brainstem inflammation and two had missing data.
The mean SARA score, for children with PFS1 + 2 improved from 28 at baseline to 10 at 12-month follow-up. For children with severe ataxia without mutism, the mean SARA score improved from 15 at baseline to 7.5 at 12-month follow-up. Based on change-point linear mixed-effects model (Figure 2B), SARA scores dropped in PFS1 + 2 cohort at a rate of 3.27 units per month during the first 5 months after surgery and slowed to 0.18 units monthly decline after 5 months. For non-PFS1 + 2 cohort, SARA scores steadily dropped at a monthly rate of 0.18 units without a change point. We did not find a difference in the slope of SARA scores among children treated at low- and high-volume surgery centers (P = .373).
Behavior recovery
Irritability resolved in a median time of 1.4 months (range 0.2-14.7) in PFS1 and 1.1 months (range 0.3-2.4) in PFS2 (P = .071).
Ocular abnormalities and movement disorder recovery
Nystagmus and ocular fixation remained unchanged. Ballism, dyskinesia, and dystonia were short-lasting, while myoclonus persisted at 12-month follow-up in 4 children with PFS1 and vocal cord tremor in one.
Independence from tube feeding
Of the 20 PFS1 children needing tube feeding after surgery, 10 (50%) still needed it 12 months after surgery, as well as 2 (40%) of 5 with PFS2, and 1 (17%) of 6 others.
Risk Factors for Delayed Recovery
Delayed speech recovery
Having PFS1 (P = .006), movement disorder (P = .034), and high SARA score (P < .0001) associated with delayed speech return on univariable analysis. Movement disorder (P = .037) and high SARA score (P < .0001) remained significant on multivariable analysis.
Delayed gait recovery
PFS1 (P = .0002), movement disorder (P = .005), delayed speech return (P = .0001), and high SARA score (P = .0001) associated with delayed gait recovery on univariable analysis. Older age (P = .0147) and high SARA score (P < .0001) were significant on multivariable analysis (Figure 2C).
Discussion
This is the first study with standardized prospective neurologic assessments to examine clinical features of PFS and their outcome. Herein, we confirm already described features of severe apraxia, irritability, emotional lability, and severe ataxia in children with PFS. In addition, we identified, previously unreported, movement disorders and eye movement abnormalities and show that presence of any movement disorder, including opsoclonus-myoclonus syndrome, correlated with PFS and incomplete neurologic recovery. We also replicated the recent finding that SHH subgroup tumors have a lower risk of PFS,10 and we believe that this reflects the association between SHH tumors and lateral cerebellar hemispheric location since midline location increased PFS risk by univariable analysis.
We divided study participants into different categories: PFS was diagnosed solely based on verbal output and was subdivided into PFS1 (complete mutism) and PFS2 (paucity of speech); and those without impairment of verbal output, who were categorized by ataxia into “ataxia-behavior” group, “ataxia only” group, and “others” (non-severe ataxia, non-PFS). PFS1 patients additionally had apraxia, very high SARA score, and severe irritability; PFS2 had significant irritability, and a high SARA score (but lower than PFS1); ataxia-behavior patients had significant irritability and grade-3 ataxia; ataxia only patients had normal behavior with grade-3 ataxia; and others had mild to moderate ataxia or normal examinations. The presence of these severe ataxia groups suggests that PFS exists on a spectrum and in the future one could imagine an additional re-classification of severe ataxia groups as PFS3 and PFS4.
To our knowledge, this is the first systematic study of prognostic factors for delayed and poor neurologic recovery. Here it is important to note that our criteria for recovery did not mean return of normal function, but rather return of language and gait were measured by ability to speak 3-word sentences and walking 10 steps without support. PFS1, movement disorder, and high baseline SARA score predicted delayed return of language on univariable analysis. Movement disorder and high baseline SARA scores remained significant on multivariable analysis whereas PFS1 likely lost significance because of strong correlation with high SARA score. Similarly, PFS1, movement disorder, delayed speech recovery, and high baseline SARA score associated with delayed return of independent gait on univariable analysis. Elevated baseline SARA score and older age remained significant on multivariable analysis. These findings were substantiated by all nonambulatory children at 12 months having a baseline SARA score of ≥30. Also, neuroplasticity becomes less efficient with age,23 and this may explain why older age delays recovery.
Importantly, we observed that children operated in low-volume centers had a higher risk of developing PFS1 + 2 and proportionally more PFS1 than seen in high-volume centers. Surgical ability is challenging to define but evidence suggests that surgical volume in neurosurgery is associated with better outcomes.24–27 Our definition of experience was conservative and reasonable but ultimately subjective. In the absence of comprehensive data from the low-volume centers, it is not possible to be definitive about these inferences. However, the incidence of PFS was significantly lower for high-volume centers as compared to low. At our institution, the rate was 11% (7% for PFS1) and other academic centers have reported similar numbers.5,24,28 We found no difference between low- and high-volume centers in age distribution, ventricular size, EOR, and tumor size, thus supporting importance of surgical experience and skill in preventing development of PFS. However, we did find that children operated at low-volume centers were more likely to receive CSF diversion which may increase the risk of neurologic injury.29–31 Therefore, it stands to reason that surgical technique and experience play a role in the development and severity of PFS. Like Cobourn et al,28 surgeons at our facility minimize cerebellar retraction, particularly to the lateral walls of the fourth ventricle and cerebellar hemispheres. They never use fixed retractors, avoid manipulation of floor of the fourth ventricle and cerebellar peduncles, limit or avoid a vermian incision by releasing and retracting the cerebellar tonsils with a telovelar approach, and resect the tumor by central debulking which allows the periphery to collapse into the surgical field.
While most children improved their neurologic deficits over 12 months, those with PFS fared worse and recovered more slowly. For example, normal neurologic examination increased from 24.1% to 37.9% in 58 non-PFS non-severe ataxia children whereas no child with PFS1 + 2 or grade-3 ataxia had normal neurologic examination at 12 months. Dysmetria, dysarthria, and weakness improved in most; however, a significant minority of children with PFS1 still had severe dysarthria and dysmetria at 12 months (Figure 3). Complete cranial nerve palsies did not improve in the PFS1 + 2 group suggesting an early intervention with nerve grafting may be considered in these children.
All children with physical and swallowing impairment received physical, occupational, and speech therapy. Thus, we could not do a meaningful analysis of role of these interventions on recovery from their deficits. Except for few anecdotal reports, there is no known pharmacologic intervention that helps these children and we do not offer these routinely. We occasionally use benzodiazepines, selective serotonin reuptake inhibitors, and atypical anti-psychotics to control behavior and treat anxiety or depression.
The strengths of this study included a large cohort and prospective data gathering. One weakness was the lack of comprehensive speech and language assessment that may have missed children with minor impairments. As a result, we may have underestimated PFS2 since some children may have impaired language but were able to string 3-word sentences. Also, immediate pre- and postoperative evaluations were not done in many as most patients were referred to us from other facilities. However, we believe risk of poor recall by parents is low as most of these children came within few weeks of original surgery. Because of variability in the quality of preoperative scans from referring centers, we could not meaningfully study brainstem and cerebellar nuclei invasion, and instead used size of the tumor as a surrogate. As explained earlier, we did not study interventions to improve PFS symptoms as all received physical, occupational, and speech therapy. Our future endeavors will include reporting on the neuropsychological outcomes and correlations of imaging and neurological syndromes of this population.
Conclusion
Too many children continue to develop postoperative PFS though rates as low as 10% are achievable. PFS has differing grades of severity and categorizing it will help clinicians prognosticate recovery and help researchers better study neuroanatomical and neuropsychological correlations and investigate pathophysiology. While neurologic deficits improve in most, a significant number, especially with PFS1, are left with severe deficits and functional dependency. Our results suggest that patients operated at high-volume pediatric neurosurgical centers are less likely to suffer PFS and PFS1. We strongly recommend that children with medulloblastoma are operated on by experienced neurosurgeons.
Funding
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC) and the National Cancer Institute grant (P30 CA021765) (St. Jude Cancer Center Support Grant). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
The authors thank Aksana Vasilyeva, PhD from the St. Jude Division of Neuro-Oncology for coordination of study samples, Emily Walker from the Hartwell Center for assistance with methylation profiling of our tumor samples, Ruth Tatevossian, MD, PhD, and Sujuan Jia, PhD, from the Diagnostic Biomarkers Shared Resource for performing nucleic acids extraction, and Jessica Smith, RN, from Division of Neurology for scheduling neurology clinic visits.
Conflict of interest statement. The authors have no conflict to report with this study.
Authorship statement. Dr. R.B.K., G.W.R., Z.P., and A.G. were involved in the concept and design of the study. Dr. R.B.K. is the principal neurologist and wrote the first draft. Dr. G.W.R. is the senior author, co-corresponding author, and PI of the medulloblastoma study. Dr. A.O.-T. and Ms. J.H. are the study biostatisticians. All authors reviewed and edited the manuscript.
References
- 1.Robertson PL, Muraszko KM, Holmes EJ, et al. ; Children’s Oncology Group . Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg. 2006;105(6 Suppl):444–451. [DOI] [PubMed] [Google Scholar]
- 2.Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K. Cerebellar mutism: review of the literature. Childs Nerv Syst. 2011;27(3):355–363. [DOI] [PubMed] [Google Scholar]
- 3.Tamburrini G, Frassanito P, Chieffo D, Massimi L, Caldarelli M, Di Rocco C. Cerebellar mutism. Childs Nerv Syst. 2015;31(10):1841–1851. [DOI] [PubMed] [Google Scholar]
- 4.Wickenhauser ME, Khan RB, Raches D, et al. Characterizing posterior fossa syndrome: a survey of experts. Pediatr Neurol. 2020;104:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudrunardottir T, Morgan AT, Lux AL, et al. ; Iceland Delphi Group . Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst. 2016;32(7):1195–1203. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber JE, Palmer SL, Conklin HM, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro Oncol. 2017;19(12):1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Küpeli S, Yalçın B, Bilginer B, Akalan N, Haksal P, Büyükpamukçu M. Posterior fossa syndrome after posterior fossa surgery in children with brain tumors. Pediatr Blood Cancer. 2011;56(2):206–210. [DOI] [PubMed] [Google Scholar]
- 8.Korah MP, Esiashvili N, Mazewski CM, et al. Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;77(1):106–112. [DOI] [PubMed] [Google Scholar]
- 9.Di Rocco C, Chieffo D, Frassanito P, Caldarelli M, Massimi L, Tamburrini G. Heralding cerebellar mutism: evidence for pre-surgical language impairment as primary risk factor in posterior fossa surgery. Cerebellum. 2011;10(3):551–562. [DOI] [PubMed] [Google Scholar]
- 10.Jabarkheel R, Amayiri N, Yecies D, et al. Molecular correlates of cerebellar mutism syndrome in medulloblastoma. Neuro Oncol. 2020;22(2):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris EB, Phillips NS, Laningham FH, et al. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009;132(Pt 11):3087–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEvoy SD, Lee A, Poliakov A, et al. Longitudinal cerebellar diffusion tensor imaging changes in posterior fossa syndrome. Neuroimage Clin. 2016;12:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law N, Greenberg M, Bouffet E, et al. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro Oncol. 2012;14(10):1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagiuchi T, Ishii K, Aoki Y, et al. Bilateral crossed cerebello-cerebral diaschisis and mutism after surgery for cerebellar medulloblastoma. Ann Nucl Med. 2001;15(2):157–160. [DOI] [PubMed] [Google Scholar]
- 15.Germanò A, Baldari S, Caruso G, et al. Reversible cerebral perfusion alterations in children with transient mutism after posterior fossa surgery. Childs Nerv Syst. 1998;14(3):114–119. [DOI] [PubMed] [Google Scholar]
- 16.Albazron FM, Bruss J, Jones RM, et al. Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology. 2019;93(16):e1561–e1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patay Z, Enterkin J, Harreld JH, et al. MR imaging evaluation of inferior olivary nuclei: comparison of postoperative subjects with and without posterior fossa syndrome. AJNR Am J Neuroradiol. 2014;35(4):797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller NG, Reddick WE, Kocak M, et al. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am J Neuroradiol. 2010;31(2):288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovestadt V, Remke M, Kool M, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125(6):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon WE, Gienapp AJ, Khan NR, et al. Commentary: the clinical experience of a Junior resident in pediatric neurosurgery and introduction of the resident experience score. Neurosurgery. 2020;86(5):E447–E454. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz-Hübsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–1720. [DOI] [PubMed] [Google Scholar]
- 23.Cacucci F, Vargha-Khadem F. Contributions of nonhuman primate research to understanding the consequences of human brain injury during development. Proc Natl Acad Sci USA. 2019; 116(52):26204–26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renne B, Radic J, Agrawal D, et al. Cerebellar mutism after posterior fossa tumor resection in children: a multicenter international retrospective study to determine possible modifiable factors. Childs Nerv Syst. 2020;36(6):1159–1169. [DOI] [PubMed] [Google Scholar]
- 25.Smith ER, Butler WE, Barker FG 2nd. Craniotomy for resection of pediatric brain tumors in the United States, 1988 to 2000: effects of provider caseloads and progressive centralization and specialization of care. Neurosurgery. 2004;54(3):553–563; discussion 563-5. [DOI] [PubMed] [Google Scholar]
- 26.Barker FG 2nd, Curry WT Jr, Carter BS. Surgery for primary supratentorial brain tumors in the United States, 1988 to 2000: the effect of provider caseload and centralization of care. Neuro Oncol. 2005;7(1):49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies JM, Ozpinar A, Lawton MT. Volume-outcome relationships in neurosurgery. Neurosurg Clin N Am. 2015;26(2):207–218, viii. [DOI] [PubMed] [Google Scholar]
- 28.Cobourn K, Marayati F, Tsering D, et al. Cerebellar mutism syndrome: current approaches to minimize risk for CMS. Childs Nerv Syst. 2020;36(6):1171–1179. [DOI] [PubMed] [Google Scholar]
- 29.Gupta T, Sarkar C, Rajshekhar V, et al. Indian Society of Neuro-Oncology consensus guidelines for the contemporary management of medulloblastoma. Neurol India. 2017;65(2):315–332. [DOI] [PubMed] [Google Scholar]
- 30.Goel A. Whither preoperative shunts for posterior fossa tumours? Br J Neurosurg. 1993;7(4):395–399. [DOI] [PubMed] [Google Scholar]
- 31.Due-Tønnessen BJ, Helseth E. Management of hydrocephalus in children with posterior fossa tumors: role of tumor surgery. Pediatr Neurosurg. 2007;43(2):92–96. [DOI] [PubMed] [Google Scholar]