ABSTRACT
Background
Obese and malabsorptive patients have difficulty increasing serum 25-hydroxyvitamin D [25(OH)D] after taking vitamin D supplementation. Since 25(OH)D is more hydrophilic than vitamin D, we hypothesized that oral 25(OH)D supplementation is more effective in increasing serum 25(OH)D concentrations in these patients.
Objectives
We aimed to investigate the pharmacokinetics of oral 25-hydroxyvitamin D3 [25(OH)D3] and oral vitamin D3 in healthy participants with differing BMI and malabsorptive patients.
Methods
A randomized, double-blind crossover trial was performed in 6 malabsorptive patients and 10 healthy participants who were given 900 µg of either vitamin D3 or 25(OH)D3 orally followed by a pharmacokinetic study (PKS). After ≥28 d from the first dosing, each participant returned to receive the other form of vitamin D and undergo another PKS. For each PKS, serum vitamin D3 and 25(OH)D3 were measured at baseline and at 2, 4, 6, 8, and 12 h and days 1, 2, 3, 7, and 14. Pharmacokinetic parameters were calculated.
Results
Data were expressed as means ± SEMs. The PKS of 900 µg vitamin D3 revealed that malabsorptive patients had 64% lower AUC than healthy participants (1177 ± 425 vs. 3258 ± 496 ng · h/mL; P < 0.05). AUCs of 900 µg 25(OH)D3 were not significantly different between the 2 groups (P = 0.540). The 10 healthy participants were ranked by BMI and categorized into higher/lower BMI groups (5/group). The PKS of 900 µg vitamin D3 showed that the higher BMI group had 53% lower AUC than the lower BMI group (2089 ± 490 vs. 4427 ± 313 ng · h/mL; P < 0.05), whereas AUCs of 900 µg 25(OH)D3 were not significantly different between the 2 groups (P = 0.500).
Conclusions
Oral 25(OH)D3 may be a good choice for managing vitamin D deficiency in malabsorption and obesity. This trial was registered at clinicaltrials.gov as (NCT03401541.
Keywords: vitamin D, 25-hydroxyvitamin D, bioavailability, obesity, intestinal malabsorption, clinical trial
See corresponding editorial on page 831.
Introduction
Vitamin D plays an important role in regulating calcium and phosphate metabolism (1). Vitamin D is fat-soluble and when ingested is incorporated into micelles, which enter the enterocytes to form chylomicrons. Chylomicrons are then transported into the lymphatic system and subsequently into the venous circulation. Approximately 60% of the absorbed vitamin D is bound to circulating vitamin D–binding protein and 40% is rapidly cleared in the lipoprotein bound fraction (2). Once entering the circulation, vitamin D is either bound by the vitamin D–binding protein, distributed into the adipose tissue, or metabolized by the liver to 25-hydroxyvitamin D [25(OH)D], which is then metabolized by 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) in the kidneys to the active form of 1,25-dihydroxyvitamin D [1,25(OH)2D] (1). Measurement of serum 25(OH)D concentration is generally used for determining vitamin D status (1, 3). Serum 25(OH)D concentrations are inversely correlated with serum parathyroid hormone (PTH) and are associated with a multitude of skeletal and nonskeletal health outcomes (1, 3, 4).
Patients with intestinal malabsorption of vitamin D, such as those with cystic fibrosis, inflammatory bowel diseases, gastric bypass surgery, and intestinal lymphangiectasia, are unable to efficiently absorb vitamin D (5–8). Consequently, they are at an increased risk for vitamin D deficiency and therefore higher risk for osteoporosis and osteomalacia (9, 10). Patients with obesity are also susceptible to vitamin D deficiency as vitamin D derived from intestinal absorption and cutaneous synthesis is diluted in a larger body pool of fat (7, 11). In addition, patients with obesity may also have reduced liver 25-hydroxylation of vitamin D, which is thought to be due to obesity-associated fatty liver (12). Therefore, the Endocrine Society Clinical Practice Guideline for Vitamin D recommended that dosage of vitamin D therapy in obesity [BMI (kg/m2) ≥30] should be increased 2–3 times compared with those without obesity (3).
Studies have suggested that 25-hydroxyvitamin D3 [25(OH)D3] is more bioavailable than vitamin D3, causing a more rapid and sustained increase in serum 25(OH)D3 concentrations (13–21). One of the explanations is that 25(OH)D3 is more hydrophilic than vitamin D3; therefore, it could be absorbed directly into the portal system without being cleared in the lymphatic system (20). 25(OH)D is also thought to be distributed into the circulation without being diluted into the adipose tissue (16). Thus, theoretically, 25(OH)D absorption and distribution would be less affected in patients with malabsorption and obesity, respectively (16, 22). We aimed to investigate the possibility of using orally administered 25(OH)D3 as a replacement for oral vitamin D3 supplementation in patients with intestinal malabsorption of vitamin D by evaluating the pharmacokinetic parameters and safety profile of orally administered 25(OH)D3 and vitamin D3 from the corresponding change in serum concentration-time curves in adults with a history of intestinal malabsorption in comparison to healthy adults of differing BMI.
Methods
This study was a randomized, double-blind, crossover, single-center trial. The study protocol was approved by the Boston University Medical Campus Institutional Review Board (H-37167) and was registered at ClinicalTrials.gov (NCT03401541). Written informed consent was obtained from all participants. This study was conducted at the Boston University Medical Campus at a latitude of 42.2°N during wintertime (November 2018 to March 2019) when cutaneous vitamin D3 synthesis is absent or minimal (23).
Recruitment
Healthy adults and patients with intestinal malabsorption of vitamin D were enrolled in the study. Participants who were eligible for this study must have met all of the following inclusion criteria: 18 y of age or older; healthy male or female adults for healthy participants, and adult male or female patients with a history of intestinal malabsorption of vitamin D at the Boston University Medical Campus; absence of conditions known to directly affect vitamin D and calcium metabolism including history of hypercalcemia, primary or tertiary hyperparathyroidism, liver diseases, chronic kidney disease, current use of certain medications such as anticonvulsant, corticosteroid, antiretroviral medications, and use of a tanning bed in the past week prior to study enrollment; and no history of adverse reaction to orally administered vitamin D or 25(OH)D. Participants taking any forms of vitamin D supplement of >2000 IU (50 µg)/d must be willing and able to discontinue use of vitamin D supplements throughout the study and allow for at least a 14-d washout prior to enrollment. Women must be on birth control and not pregnant based on a negative pregnancy test at baseline for each of the pharmacokinetic studies. Participants were prescreened for serum total 25(OH)D concentrations, and only those serum 25(OH)D <30 ng/mL were included into the study.
Study intervention
All participants were randomly assigned by a computer randomization chart in a double-blinded manner (to the investigators and participants) to ingest 2 oral doses of 450 µg taken together (total 900 µg; in soft gel capsules) of either vitamin D3 or 25(OH)D3 diluted in medium-chain fatty acids (Carbogen Amcis BV; a company belonging to the Dishman Group) that were formulated in an identical manner and to undergo a cycle of pharmacokinetic study. The contents of vitamin D3 and 25(OH)D3 were verified by HPLC. Each participant was advised to ingest the capsules with plain water under direct observation. Blood samples of 15 mL were taken at baseline and at 2, 4, 6, 8, and 12 h and days 1, 2, 3, 7, and 14 for measurement of serum vitamin D (vitamins D2 and D3) and 25(OH)D [25-hydroxyvitamin D2 (25(OH)D2) and 25(OH)D3]. After at least 14 d of a washout period (28 d from the first dosing), each participant was asked to return on any available day to receive orally either 900 µg (36,000 IU) of vitamin D3 or 900 µg of 25(OH)D3 (depending on which one was taken in the randomization) and undergo another cycle of pharmacokinetic study. Safety was also evaluated by measurement of serum concentrations of calcium, phosphorus, albumin, creatinine, and intact PTH at baseline and at day 14 for each cycle of pharmacokinetic study. All participants were informed to refrain from taking any vitamin D supplements and avoid other sources of UVB exposure including UV tanning beds and traveling to sunny areas at a lower latitude throughout the study period.
Biochemical analysis
Serum samples for vitamin D (vitamins D2 and D3) and 25(OH)D [25(OH)D2 and 25(OH)D3] were measured using LC–tandem MS by Quest Diagnostic Laboratory (San Juan Capistrano, CA). Serum intact PTH was measured using a chemiluminescent immunoassay by Quest Diagnostics Laboratory. Measurement of serum calcium, phosphorus, albumin, creatinine, and intact PTH was also performed by Quest Diagnostics.
Determination of sample size
The sample size of this crossover study was based on the changes in serum vitamin D3 and 25(OH)D3 concentrations where the goals were to detect 1) a significant difference in bioavailability of vitamin D3 and 25(OH)D3 between patients and healthy controls and 2) a significant difference of the change in serum 25(OH)D concentrations between the treatment of vitamin D3 and 25(OH)D3 in the participants.
The sample size for goal 1 was based on data from the previous study (5). The means ± SDs of the AUC from 0 to 72 h (AUC0–72) for serum vitamin D3 in the healthy participants and malabsorptive patients were 2880 ± 821 ng · h/mL and 518 ± 189 ng · h/mL, respectively. Given the large difference in variance, the pooled variance was calculated and used for determining the sample size (24). Based on these results and assuming a 2-sided α value of 0.05, we calculated, using the t test for independent variables, that enrollment of at least 6 participants in each study arm would provide 95% power to demonstrate a significant difference in AUC0–72 for vitamin D3 and 25(OH)D3 between malabsorptive patients and healthy controls.
For goal 2, based on previous results (17), the mean ± SD AUC0–24 values of serum 25(OH)D after the oral administration of 20 μg vitamin D3 and 20 μg 25(OH)D3 were 764 ± 17 ng · h/mL and 1704 ± 18 ng · h/mL, respectively. Based on these results and assuming a 2-sided α value of 0.05, we calculated, using the paired t test, that enrollment of at least 6 participants in each study arm would provide 95% power to demonstrate a significant difference in AUC0–24 for 25(OH)D3 between 2 treatments [25(OH)D3 and vitamin D3] in each of the participant groups.
Pharmacokinetic and statistical analysis
Continuous outcome variables are reported as arithmetic means with SEMs. Changes (Δ) in serum vitamin D3 concentration (Δvitamin D3) and changes in serum 25(OH)D3 concentration [Δ25(OH)D3] from baseline at different time points were calculated and plotted to acquire change in concentration-time curve for each participant. Area under the change in concentration-time curve (AUC) of Δvitamin D3 and Δ25(OH)D3 from 0 to 336 h was manually calculated using the trapezoidal method. Maximum observed Δvitamin D3 and Δ25(OH)D3 (Cmax), time to Cmax (Tmax), elimination half-life (T1/2), and trough levels of Δvitamin D3 and Δ25(OH)D3 at day 14 (Ctrough) were determined. AUC, Cmax, Tmax, T1/2, and Ctrough were summarized within groups by arithmetic means and SEMs.
We performed univariate and multivariate analysis to compare pharmacokinetic parameters between groups, including malabsorptive patients versus healthy participants and vitamin D3 versus 25(OH)D3 arms. In addition, with the aim to investigate the association between BMI and absorption of vitamin D3 and 25(OH)D3, healthy participants were ranked by BMI and were categorized into higher BMI and lower BMI groups. Statistical analysis was also performed to compare pharmacokinetic parameters between the 2 groups.
For univariate comparisons of pharmacokinetic parameters (AUC, Cmax, Tmax, T1/2, and Ctrough) and baseline characteristics between groups (malabsorptive patients vs. healthy participants; healthy participants with higher BMI vs. lower BMI), independent-samples t test was used for normally distributed data and Mann-Whitney U test was used for non–normally distributed data. Chi-square and Fisher's exact tests were used to determine differences in categorical variables between groups. ANCOVA was used to compare the pharmacokinetic parameters between groups. Covariates to be included in the models included variables that tended to be different between groups (P < 0.1) and variables with biological plausibility to be confounders. For univariate comparisons of AUC of change in 25(OH)D3 concentration between the 2 arms [vitamin D3 vs. 25(OH)D3], a paired t test was used for normally distributed data and Wilcoxon signed-rank test was used for non–normally distributed data. In addition, the generalized estimating equation linear regression model was used to determine the combined effects of treatment arm [vitamin D3 vs. 25(OH)D3], participant group (malabsorptive patients vs. healthy participants), treatment arm and participant group interaction, and other potential confounders on AUC of change in 25(OH)D3 concentration. Statistical significance was defined as P < 0.05. SPSS version 23 (IBM Corp, Armonk, NY, USA) was used to perform data analyses.
Results
Baseline characteristics of the participants
A total of 20 healthy adult participants and 8 patients with malabsorption who met the eligibility criteria were enrolled for screening of serum 25(OH)D concentrations. All malabsorptive patients and none of the healthy participants were taking at least 2000 IU/d of a vitamin D supplement and stopped taking the vitamin D supplements for at least 2 wk prior to the study entry. Eight healthy participants and 1 patient with intestinal malabsorption of vitamin D had serum 25(OH)D >30 ng/mL and were excluded from the study, leaving 12 healthy participants and 7 patients with intestinal malabsorption of vitamin D eligible for the pharmacokinetic studies. During the pharmacokinetic studies, 1 healthy participant and 1 patient with malabsorption dropped out due to unwillingness to continue to participate in the study. Another healthy participant dropped out due to positive urine pregnancy test before the second-cycle pharmacokinetic study. Finally, 10 healthy participants and 6 patients with intestinal malabsorption of vitamin D completed both pharmacokinetic studies (Figure 1). Patients with a history of intestinal malabsorption included 3 with Roux-en-Y gastric bypass surgery, 1 with sleeve gastrectomy, 1 with lymphangiectasia, and 1 with ulcerative colitis. The patient with intestinal lymphangiectasia had a history of inability to absorb vitamin D demonstrated by the clinical vitamin D2 absorption test. We included a patient with ulcerative colitis because we previously showed that patients with ulcerative colitis tended to have decreased absorption of vitamin D despite the absence of small bowel involvement and dietary fat malabsorption (6).
FIGURE 1.
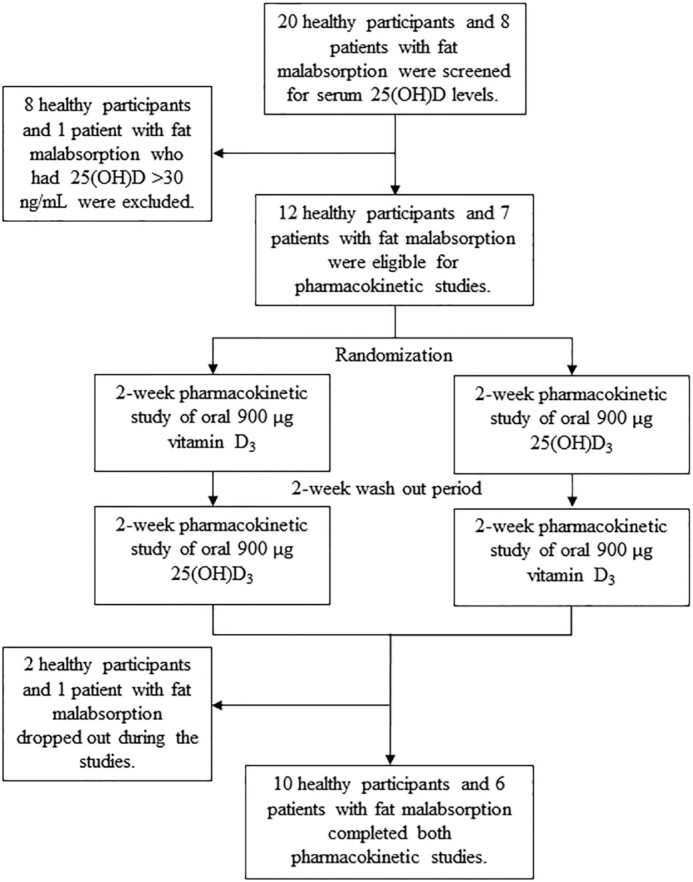
Schematic representation of the study showing the number of healthy participants and patients with intestinal malabsorption of vitamin D screened and randomly assigned in the 2 arms of the study. 25(OH)D, 25-hydroxyvitamin D; 25(OH)D3, 25-hydroxyvitamin D3.
Baseline characteristics including demographic data and serum chemistries of healthy participants and patients with intestinal malabsorption of vitamin D are shown in Table 1. Patients with intestinal malabsorption of vitamin D had significantly older age (46.5 ± 4.1 vs. 32.3 ± 2.7 y old) and serum alkaline phosphatase (88.5 ± 17.3 vs. 54.5 ± 4.8 U/L) and lower serum albumin (4.1 ± 0.05 vs. 4.4 ± 0.08 g/dL) than healthy participants (all P < 0.05).
TABLE 1.
Baseline characteristics of healthy participants and patients with intestinal malabsorption of vitamin D1
| Healthy participants (n = 10) | Patients with intestinal malabsorption of vitamin D (n = 6) | P | |
|---|---|---|---|
| Age, y | 32.3 ± 2.7 | 46.5 ± 4.1 | 0.0102* |
| Female participants, n (%) | 8 (80) | 6 (100) | 0.5003 |
| BMI, kg/m2 | 27.0 ± 2.1 | 32.7 ± 4.1 | 0.1922 |
| Ethnicity, n (%) | |||
| Caucasian | 5 (50) | 4 (67) | 0.5073 |
| Hispanic | 0 (0) | 1 (17) | |
| Asian | 2 (20) | 0 (0) | |
| Black | 3 (30) | 1 (17) | |
| Serum chemistry | |||
| Vitamin D2, ng/mL | 0.0 ± 0.0 | 0.0 ± 0.0 | — |
| Vitamin D3, ng/mL | 0.0 ± 0.0 | 1.6 ± 1.0 | 0.3134 |
| Total 25-hydroxyvitamin D, ng/mL | 17.1 ± 2.3 | 14.7 ± 3.4 | 0.5542 |
| 25-Hydroxyvitamin D2, ng/mL | 0.4 ± 0.4 | 4.2 ± 3.1 | 0.4284 |
| 25-Hydroxyvitamin D3, ng/mL | 16.7 ± 2.1 | 10.5 ± 4.2 | 0.2282 |
| Intact PTH, pg/mL | 41.5 ± 5.4 | 74.0 ± 16.9 | 0.0732 |
| Total calcium, mg/dL | 9.4 ± 0.1 | 9.4 ± 0.1 | 0.7962 |
| Phosphate, mg/dL | 3.9 ± 0.3 | 4.0 ± 0.3 | 0.7362 |
| Creatinine, mg/dL | 0.8 ± 0.03 | 0.7 ± 0.04 | 0.1032 |
| eGFR, mL ⸱ min ⸱ 1.73 m | 106.8 ± 4.7 | 104.0 ± 6.7 | 0.7332 |
| Glucose, mg/dL | 83.1 ± 7.5 | 89.8 ± 8.8 | 0.2634 |
| Albumin, g/dL | 4.4 ± 0.08 | 4.1 ± 0.05 | 0.0224* |
| Total protein, g/dL | 6.9 ± 0.08 | 6.8 ± 0.2 | 0.4852 |
| Total bilirubin, mg/dL | 0.5 ± 0.09 | 0.5 ± 0.05 | 0.7924 |
| Alkaline phosphatase, U/L | 54.5 ± 4.8 | 88.5 ± 17.3 | 0.0224* |
| Aspartate aminotransferase, U/L | 18.4 ± 1.6 | 18.7 ± 2.5 | 0.9252 |
| Alanine aminotransferase, U/L | 15.6 ± 1.0 | 16.7 ± 4.1 | 0.8092 |
1Values are means ± SEMs unless otherwise indicated. *Significant difference between groups (P < 0.05). eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone.
2P value was determined using the independent-samples t test for comparison of normally distributed data.
3P value was determined using the Fisher's exact test for comparison of categorical data.
4P value was determined using the Mann-Whitney U test for comparison of non–normally distributed data.
Pharmacokinetic studies of a single dose of orally administered 900 µg of vitamin D3 and 900 µg 25(OH)D3 in healthy participants in comparison with patients with intestinal malabsorption of vitamin D
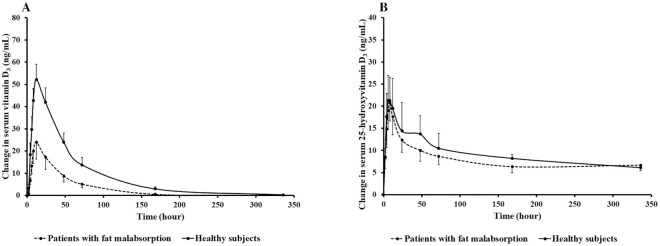
As shown in Figure 2A and Table 2, the pharmacokinetic study of orally administered 900 µg vitamin D3 demonstrated that healthy participants had an ∼2.8-fold higher AUC and 2.2-fold higher Cmax of serum vitamin D3 than patients with intestinal malabsorption of vitamin D (AUC: 3258 ± 496 vs. 1177 ± 425 ng · h/mL; Cmax: 53.5 ± 6.0 vs. 24.25 ± 8.36 ng/mL; both P < 0.05). Thus, the malabsorptive patients had an ∼64% lower AUC than the healthy participants. The difference in AUC between the 2 groups remained statistically significant in the ANCOVA model after adjustment for potential confounders, including age, BMI, and baseline serum total 25(OH)D, albumin, alkaline phosphatase, and intact PTH concentrations (both P < 0.05; Table 2). The AUC and Cmax in the pharmacokinetic study of orally administered 900 µg 25(OH)D3 demonstrated no significant difference between the 2 groups, as shown in Figure 2B and Table 2. In addition, univariate analysis showed that healthy participants had significantly higher Tmax than patients with intestinal malabsorption of vitamin D after orally receiving 900 µg 25(OH)D3 (11.20 ± 4.10 vs. 5.33 ± 0.71 h; P = 0.031). Serum concentrations of vitamin D3 and vitamin D2 as well as 25(OH)D2 were undetectable in both healthy participants and patients with intestinal malabsorption of vitamin D at baseline and at all times after orally receiving 900 µg 25(OH)D3 (data not shown).
FIGURE 2.
(A) Mean ± SEM change in serum vitamin D3 concentration versus time curve after a single dose of oral 900 µg vitamin D3 in healthy participants (n = 10) and patients with intestinal malabsorption of vitamin D (n = 6). Using the Mann-Whitney U test, healthy participants had a statistically significantly higher AUC of serum vitamin D3 after ingesting 900 µg vitamin D3 compared with malabsorptive patients (mean ± SEM AUC: 3258 ± 496 vs. 1177 ± 425 ng · h/mL; P = 0.022). The difference remained significant in the ANCOVA model after adjustment for potential confounders, including age, BMI, and baseline serum total 25-hydroxyvitamin D, albumin, alkaline phosphatase, and intact parathyroid hormone concentrations (P = 0.029). (B) Mean ± SEM change in serum 25-hydroxyvitamin D3 concentration versus time curve after a single dose of oral 900 µg 25-hydroxyvitamin D3 in healthy participants (n = 10) and patients with intestinal malabsorption of vitamin D (n = 6). Using the Mann-Whitney U test, there was no statistically significant difference in AUC of serum 25-hydroxyvitamin D3 after ingesting 900 µg vitamin D3 between healthy participants and malabsorptive patients (mean ± SEM AUC: 3128 ± 545 vs. 2667 ± 735 ng · h/mL; P = 0.562). The difference remained nonsignificant in the ANCOVA model after adjustment for potential confounders, including age, BMI, and baseline serum total 25-hydroxyvitamin D, albumin, alkaline phosphatase, and intact parathyroid hormone concentrations (P = 0.540). Reproduced with permission; copyright Holick, 2021.
TABLE 2.
Pharmacokinetic parameters of oral 900 µg vitamin D3 and oral 900 µg 25-hydroxyvitamin D3 in healthy participants and patients with intestinal malabsorption of vitamin D1
| Healthy participants (n = 10) | Patients with intestinal malabsorption of vitamin D (n = 6) | P 2 | P 3 | |
|---|---|---|---|---|
| 900 µg Vitamin D3 arm | ||||
| AUC, ng · h/mL | 3258 ± 496 | 1177 ± 425 | 0.022* | 0.029* |
| Cmax, ng/mL | 53.5 ± 6.0 | 24.3 ± 8.4 | 0.016* | 0.063 |
| Tmax, h | 10.4 ± 0.7 | 11.3 ± 0.7 | 0.345 | 0.516 |
| T1/2, h | 31.4 ± 3.3 | 28.7 ± 1.5 | 0.713 | 0.318 |
| Ctrough, ng/mL | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.220 | 0.549 |
| 900 µg 25-Hydroxyvitamin D3 arm | ||||
| AUC, ng · h/mL | 3128 ± 545 | 2667 ± 735 | 0.562 | 0.540 |
| Cmax, ng/mL | 23.1 ± 4.6 | 23.2 ± 6.8 | 1.000 | 0.833 |
| Tmax, h | 11.2 ± 4.1 | 5.3 ± 0.7 | 0.031* | 0.464 |
| T1/2, h | 60.6 ± 7.9 | 65.7 ± 29.9 | 0.313 | 0.628 |
| Ctrough, ng/mL | 6.1 ± 1.3 | 6.7 ± 1.5 | 0.875 | 0.534 |
1Values are means ± SEMs. *Significant difference between groups (P < 0.05). Cmax, maximal concentration; Ctrough, trough level at day 14; T1/2, elimination half-life; Tmax, time to maximal concentration.
2Determined using the Mann-Whitney U test.
3Determined using ANCOVA test with age, BMI, and baseline total 25-hydroxyvitamin D, albumin, alkaline phosphatase, and intact parathyroid hormone concentrations as covariates.
Comparison between healthy participants with higher and lower BMI
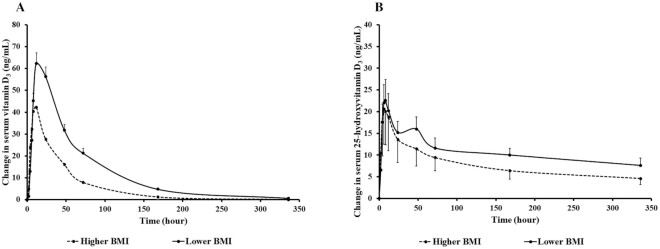
In order to investigate the relation between BMI and bioavailability of orally administered 900 µg vitamin D3 and 900 µg 25(OH)D3, the 10 healthy participants were ranked by BMI and were categorized into higher BMI and lower BMI groups (mean ± SEM BMI: 31.4 ± 2.6 vs. 22.6 ± 1.7) with 5 participants in each group. A pharmacokinetic study of orally administered 900 µg vitamin D3 showed that the higher BMI group had a 53% lower AUC and lower T1/2 than the lower BMI group (AUC: 2089 ± 490 vs. 4427 ± 313 ng · h/mL; T1/2: 23.8 ± 2.7 vs. 39.0 ± 3.1; both P < 0.05) as shown in Figure 3A and Table 3, although no significant difference in AUC between groups was observed after adjusting for baseline serum total 25(OH)D concentration and BMI in the ANCOVA model (P = 0.244). In addition, compared with the higher BMI group, the lower BMI group had significantly longer T1/2 for both vitamin D3 and 25(OH)D3, after adjusting for baseline total 25(OH)D and albumin concentrations in the ANCOVA model (both P < 0.05; Table 3). There were no significant differences in the pharmacokinetic parameters (AUC, Cmax, Tmax, and Ctrough) of orally administered 900 µg 25(OH)D3 between the 2 groups as shown in Figure 3B and Table 3.
FIGURE 3.
(A) Mean ± SEM change in serum vitamin D3 concentration versus time curve after a single dose of oral 900 µg vitamin D3 in healthy participants with higher BMI (n = 5) and lower BMI (n = 5). Using the Mann-Whitney U test, healthy participants with a higher BMI [mean ± SEM (kg/m2): 31.4 ± 2.6] had a statistically significantly lower AUC of serum vitamin D3 after ingesting 900 µg vitamin D3 compared with those with a lower BMI (mean ± SEM: 22.6 ± 1.7; mean ± SEM AUC: 2089 ± 490 vs. 4427 ± 313 ng · h/mL; P = 0.016). However, there was no significant difference between the 2 groups in the ANCOVA model after adjustment for potential confounders, including age, BMI, and baseline serum total 25-hydroxyvitamin D, albumin, alkaline phosphatase, and intact parathyroid hormone concentrations (P = 0.244). (B) Mean ± SEM change in serum 25-hydroxyvitamin D3 concentration versus time curve after a single dose of oral 900 µg 25-hydroxyvitamin D3 in healthy participants with a higher BMI (n = 5) and lower BMI (n = 5). Using the Mann-Whitney U test, there was no significant difference in AUC of serum 25-hydroxyvitamin D3 after ingesting 900 µg vitamin D3 between healthy participants with a lower BMI (mean ± SEM: 22.6 ± 1.7) and those with a higher BMI (mean ± SEM: 31.4 ± 2.6; mean ± SEM AUC: 2621 ± 765 vs. 3633 ± 616 ng · h/mL; P = 0.421). The difference remained nonsignificant in the ANCOVA model after adjustment for potential confounders, including age, BMI, and baseline serum total 25-hydroxyvitamin D, albumin, alkaline phosphatase, and intact parathyroid hormone concentrations (P = 0.500). Reproduced with permission; copyright Holick, 2021.
TABLE 3.
Pharmacokinetic parameters of oral 900 µg vitamin D3 and oral 900 µg 25-hydroxyvitamin D3 in healthy participants with higher and lower BMI1
| Healthy participants with higher BMI (n = 5) | Healthy participants with lower BMI (n = 5) | P 2 | P 3 | |
|---|---|---|---|---|
| BMI, kg/m2 | 31.4 ± 2.6 | 22.6 ± 1.7 | <0.001 | — |
| 900 µg Vitamin D3 arm | ||||
| AUC, ng · h/mL | 2089 ± 490 | 4427 ± 313 | 0.016* | 0.244 |
| Cmax, ng/mL | 44.4 ± 8.3 | 62.6 ± 5.1 | 0.310 | 0.926 |
| Tmax, h | 9.6 ± 0.9 | 11.2 ± 0.7 | 0.310 | 0.931 |
| T1/2, h | 23.8 ± 2.7 | 39.0 ± 3.1 | 0.010* | 0.038* |
| Ctrough, ng/mL | 0.0 ± 0.0 | 0.6 ± 0.6 | 0.690 | 0.931 |
| 900 µg 25-Hydroxyvitamin D3 arm | ||||
| AUC, ng · h/mL | 2621 ± 765 | 3633 ± 616 | 0.421 | 0.500 |
| Cmax, ng/mL | 21.4 ± 7.2 | 24.8 ± 4.9 | 0.548 | 0.584 |
| Tmax, h | 15.2 ± 7.3 | 7.2 ± 0.4 | 0.841 | 0.918 |
| T1/2, h | 55.0 ± 9.6 | 66.2 ± 11.0 | 0.476 | 0.011* |
| Ctrough, ng/mL | 4.6 ± 1.3 | 7.6 ± 1.7 | 0.421 | 0.918 |
1Values are means ± SEMs. *Significant difference between groups (P < 0.05). Cmax, maximal concentration; Ctrough, trough level at day 14; T1/2, elimination half-life; Tmax, time to maximal concentration.
2Determined using the Mann-Whitney U test.
3Determined using ANCOVA test with baseline total 25-hydroxyvitamin D and albumin concentrations as covariates.
Serum 25(OH)D concentration after a single dose of orally administered 900 µg vitamin D3 and 900 µg 25(OH)D3 in healthy participants and patients with intestinal malabsorption of vitamin D
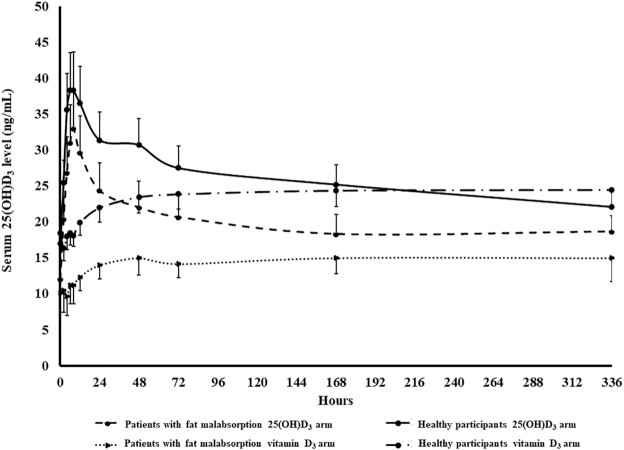
As shown in Figure 4, after a single dose of oral 900 µg 25(OH)D3, serum 25(OH)D3 concentration increased from baseline prior to ingestion of 25(OH)D3 (healthy participants: 17.0 ± 1.7 ng/mL; patients with intestinal malabsorption of vitamin D: 12.0 ± 3.6 ng/mL) to the maximum concentration (healthy participants: 38.3 ± 5.4 ng/mL; patients with intestinal malabsorption of vitamin D: 33.0 ± 3.3 ng/mL) at 8 h after dosing, and then decreased and reached a plateau at ∼5 ng/mL higher than baseline (healthy participants: 22.1 ± 2.2 ng/mL; patients with intestinal malabsorption of vitamin D: 18.7 ± 2.7 ng/mL) at day 14. On the other hand, giving a single dose of 900 µg vitamin D3 did not show a peak-and-trough 25(OH)D3 concentration pattern, but serum 25(OH)D3 concentrations gradually increased from baseline prior to ingestion of vitamin D3 (healthy participants: 18.5 ± 2.0 ng/mL; patients with intestinal malabsorption of vitamin D: 10.1 ± 2.8 ng/mL) by ∼5 ng/mL, reached a plateau at day 3, and the concentrations stayed stable until day 14 (healthy participants: 24.5 ± 2.2 ng/mL; patients with intestinal malabsorption of vitamin D: 15.0 ± 3.3 ng/mL). Using the Wilcoxon signed-rank test, the AUC of 25(OH)D3 for the 25(OH)D3 arm was statistically significantly higher than the AUC of 25(OH)D3 for vitamin D3 arm in both healthy participants (3128 ± 545 vs. 1463 ± 331 ng · h/mL; P < 0.001) and malabsorptive patients (2667 ± 735 vs. 1491 ± 473 ng · h/mL; P < 0.001). Using the generalized estimating equation linear regression model, the AUC of 25(OH)D3 was significantly associated with treatment arm (P < 0.001) and baseline serum albumin (P = 0.036), after adjusting for participant group and other potential confounders (Table 4). There was no statistically significant interaction between treatment arm [vitamin D3 vs. 25(OH)D3] and participant group (malabsorptive patients vs. healthy participants; P = 0.547).
FIGURE 4.
Mean ± SEM serum 25-hydroxyvitamin D3 concentration versus time curve after a single dose of oral 900 µg vitamin D3 or 25-hydroxyvitamin D3 in healthy participants (n = 10) and patients with intestinal malabsorption of vitamin D (n = 6). Using the Wilcoxon signed-rank test, the AUC of 25(OH)D3 for the 25(OH)D3 arm was statistically significantly higher than the AUC of 25(OH)D3 for the vitamin D3 arm in both healthy participants (mean ± SEM: 3128 ± 545 vs. 1463 ± 331 ng · h/mL; P < 0.001) and malabsorptive patients (mean ± SEM: 2667 ± 735 vs. 1491 ± 473 ng · h/mL; P < 0.001). 25(OH)D3, 25-hydroxyvitamin D3. Reproduced with permission; copyright Holick, 2021.
TABLE 4.
Effects of treatment arms and baseline characteristics of participants on AUC of 25-hydroxyvitamin D3 by generalized estimating equation1
| Variable | Wald chi-square | P |
|---|---|---|
| Treatment arm | ||
| 900 µg 25-hydroxyvitamin D3 | Reference | — |
| 900 µg vitamin D3 | 15.32 | <0.001* |
| Participant group | ||
| Healthy participants | Reference | — |
| Patients with intestinal malabsorption of vitamin D | 0.03 | 0.869 |
| Treatment arm × participant group interaction | 0.36 | 0.547 |
| Participant characteristics | ||
| Age | 1.52 | 0.218 |
| BMI | 0.11 | 0.737 |
| Baseline 25-hydroxyvitamin D concentration | 0.90 | 0.344 |
| Baseline intact parathyroid hormone concentration | 0.24 | 0.119 |
| Baseline alkaline phosphatase concentration | 0.06 | 0.806 |
| Baseline albumin concentration | 4.39 | 0.036* |
1P values were determined using the generalized estimating equation linear regression model. *Significant independent association with AUC of 25-hydroxyvitamin D3 (P < 0.05).
Comparison of Tmax between vitamin D3 and 25(OH)D3
As shown in Figure 5, the change in concentration-time curve of mean ± SEM serum vitamin D3 and 25(OH)D3 in all participants revealed that the mean serum 25(OH)D3 concentration reached its maximal level at ∼8 h, which was 4 h earlier than the mean serum vitamin D3 concentrations after the oral administration of 900 µg 25(OH)D3 and 900 µg vitamin D3, respectively. This result was consistent with the Wilcoxon signed-rank test that revealed a statistically significant difference in Tmax of vitamin D3 compared with Tmax of 25(OH)D3 for all participants (9.0 ± 2.6 vs. 10.8 ± 4.5 h; P = 0.015).
FIGURE 5.
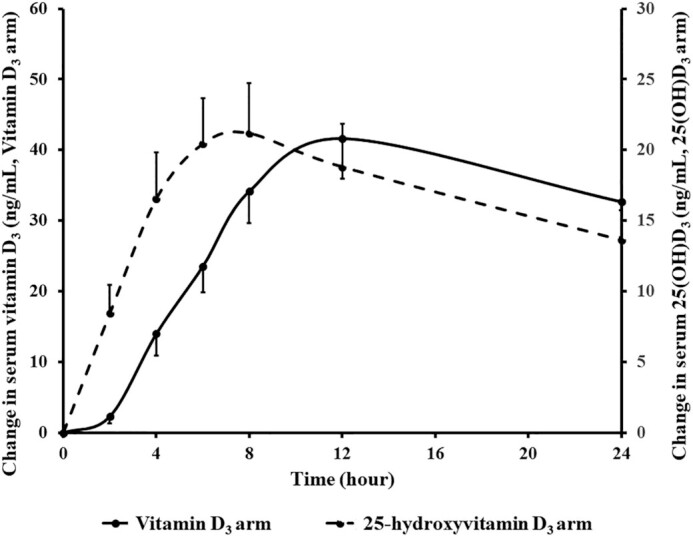
Mean ± SEM serum concentrations of 25(OH)D3 and vitamin D3 in all participants (n = 16) after oral administration of 900 µg 25-hydroxyvitamin D3 and 900 µg vitamin D3. The mean serum 25(OH)D3 concentration reached its maximal level at ∼8 h, which was 4 h earlier than the mean serum vitamin D3 concentrations after the oral administration of 900 µg 25(OH)D3 and 900 µg vitamin D3, respectively. Using the Wilcoxon signed-rank test, there was a significant difference in Tmax of vitamin D3 compared with Tmax of 25(OH)D3 for all participants (9.0 ± 2.6 vs. 10.8 ± 4.5 h; P = 0.015). Tmax, time to maximal concentration; 25(OH)D3, 25-hydroxyvitamin D3. Reproduced with permission; copyright Holick, 2021.
Safety profile of 900 µg vitamin D3 and 900 µg 25(OH)D3
No adverse reactions or signs of vitamin D toxicity (e.g., hypercalcemia, acute renal failure, polyuria, and kidney stones) were observed in any of the participants throughout the study. Serum calcium, phosphorus, intact PTH, albumin, and creatinine did not change significantly from baseline after completion of each pharmacokinetic study of 900 µg vitamin D3 and 900 µg 25(OH)D3 in both groups (Supplemental Table 1).
Discussion
We observed that the bioavailability of a single oral dose of 900 µg 25(OH)D3 was not significantly different between the healthy participants and the malabsorptive patients unlike the significantly decreased bioavailability after the oral ingestion of 900 µg vitamin D3 in the healthy participants with a higher BMI and in the patients with intestinal malabsorption syndromes (Figure 2). Moreover, serum 25(OH)D3 reached its maximal concentration earlier than serum vitamin D3 concentrations after the oral administration of 900 µg 25(OH)D3 and 900 µg vitamin D3, respectively (Figure 5). We also performed an analysis in healthy participants to determine the bioavailability of vitamin D3 and 25(OH)D3 in those with higher BMI and lower BMI. We found that 900 µg of orally administered vitamin D3 was less bioavailable in those with a higher BMI as compared with those with a lower BMI (Figure 3). The bioavailability of 25(OH)D3 was found to be the same in the healthy participants independent of their BMI. This suggests that 25(OH)D3, once ingested, enters in the circulation without being diluted in the adipose tissue. Based on our results, it can be concluded that orally administered 25(OH)D3 may be an effective choice for treatment and prevention of vitamin D deficiency in patients with intestinal malabsorption and obesity. Further larger clinical trials are required to support our findings.
Our results on serum 25(OH)D3 concentrations after oral administration of 900 µg vitamin D3 and 900 µg 25(OH)D3 (Figure 4) also give some insight into the pharmacokinetics of vitamin D3 and 25(OH)D3. The gradual increase in serum 25(OH)D3 after ingestion of vitamin D3 suggests that, once ingested, not all of the vitamin D3 is metabolized by liver 25-hydroxylase(s) to 25(OH)D3 at once. Rather, it equilibrates into the body fat where it is slowly released into the circulation and gets metabolized into 25(OH)D3 (25–27). It is of particular interest based on the change in concentration-time curve of 25(OH)D3 that serum 25(OH)D3 increased to the maximum concentration at 8 h, and then gradually decreased and becomes almost stable at 14 d at ∼5 ng/mL above baseline. This suggests that, once entering the circulation, 25(OH)D3 is not only metabolized by the 25-hydroxyvitamin D-24-hydroxylase (CYP24A1) but also is likely distributed into different types of tissues, such as macrophages, breast, skin, prostate, colon, etc., which have the capacity to metabolize 25(OH)D3 to 1,25(OH)2D3 (1, 28–30). It has been demonstrated that that adipocytes and pre-adipocytes also have the capacity to metabolize 25(OH)D3 to 1,25(OH)2D3 and therefore 25(OH)D3 may enter these cells (31). Furthermore, skeletal muscle has been shown to play a role in storage of 25(OH)D by incorporating the vitamin D–binding protein (DBP) from the blood into the myocytes where it binds to cytoplasmic actin (32). In addition, when 25(OH)D3 is produced in the liver, it is likely bound to DBP as it exits the hepatocyte before or immediately after it enters the circulation. By giving 25(OH)D3 orally, it directly enters the liver via the venous portal system. It is possible that less 25(OH)D3 is bound to DBP and therefore free concentrations are higher and distributed into tissues and metabolized differently. Further studies with radioactive 25(OH)D3 and vitamin D3 and measures of their blood and tissue concentrations would help provide an insight into the tissue distribution and handling of orally administered vitamin D3 and 25(OH)D3.
Patients with intestinal malabsorption tend to have difficulty increasing serum 25(OH)D despite receiving high-dose vitamin D supplementation (5–10). This was first observed by Thompson et al. (8) that patients with celiac disease, biliary obstruction, and pancreatic insufficiency could not absorb orally administered tritiated vitamin D3. Lo et al. (5) demonstrated that patients with intestinal malabsorption of vitamin D could not increase their serum vitamin D2 concentrations above 10 ng/mL after receiving 50,000 IU vitamin D2 as compared with normal controls who increased their serum vitamin D2 concentrations to a peak of >50 ng/mL by 12 h. Farraye et al. (6) developed a vitamin D bioavailability test by giving a single dose of orally administered 50,000 IU vitamin D2 to subjects and measuring serum vitamin D2 12 h later. They showed that patients with inflammatory bowel diseases, although being well treated and quiescent, had an ∼30% decrease in the absorption of vitamin D2 when compared with normal controls (6).
Since 25(OH)D is a more hydrophilic metabolite of vitamin D, it theoretically could directly enter the portal circulation bypassing the lymphatic system without being cleared in the lipoprotein-bound fraction (20). Several clinical studies have supported this theory as they reported that 25(OH)D3 is more bioavailable and can increase serum 25(OH)D3 concentration more rapidly and sustainably than vitamin D3 (13–21). Therefore, the ability to absorb 25(OH)D would be less compromised in malabsorptive patients. In fact, this concept was investigated by Stamp (33) in 1974, who performed an experiment to determine intestinal absorption of 25(OH)D3 in 20 healthy adults and 10 patients with intestinal diseases. He developed a “25(OH)D3 absorption test” by giving a single dose of oral 10 µg 25(OH)D3/kg to the participants and observed the response in their 25(OH)D concentrations. He found that 5 of the 10 patients could increase serum 25(OH)D concentrations to the same degree as healthy adults and the other 5 could not. He then concluded that the test provides either rapid initial treatment in responders or a clear indication for parenteral vitamin D administration in nonresponders (33). Davies et al. (34) gave oral [14C]-vitamin D3 and [3H]-25(OH)D3 to malabsorptive patients and normal controls and assessed peak radioactivity in their serum and feces. They observed that the degree of malabsorption of 25(OH)D3 was less severe than of vitamin D3 in malabsorptive patients. They, however, concluded that the magnitude of malabsorption of both vitamin D3 and 25(OH)D3 was relatively insignificant. They therefore concluded that vitamin D3 supplementation would be effective and 25(OH)D3 supplementation would be unnecessary in treating patients with malabsorption (34). In contrast, further studies have shown that a significant number of malabsorptive patients with various conditions could not increase their serum vitamin D and serum 25(OH)D concentrations despite receiving high doses of vitamin D2 or vitamin D3 supplementation (5–8). One of the possible explanations for the disparity in the observations is that different causes of malabsorption may respond differently to oral 25(OH)D3. Another explanation could be differences in the formulation of the 25(OH)D3. In our study, 25(OH)D3 and vitamin D3 were in a liquid gel capsule formulation. Whether this formulation has the same bioavailability in most if not all disorders of intestinal malabsorption requires further study.
The results from our study revive the concept that orally administered 25(OH)D3 can be effectively used to treat vitamin D deficiency in patients with intestinal malabsorption of vitamin D who are unable to sufficiently absorb vitamin D. However, our study carries some limitations that require further investigations before the results can be generalized to clinical practice. Since this study has a relatively small sample size, the characteristics of participants in both the malabsorptive and healthy groups may not represent the general population. Davies et al. showed that patients with celiac disease could not absorb vitamin D3 but absorbed 25(OH)D3 as effectively as healthy controls, whereas those with short bowel syndrome could not absorb either vitamin D3 or 25(OH)D3 (34). Stamp (33) reported that 5 of the 10 patients with intestinal disease also had 25(OH)D3 malabsorption. In our study, 3 of the 6 malabsorptive patients had gastric bypass surgery. In addition, in the generalized estimating equation model for the AUC of 25(OH)D3, we were unable to demonstrate statistical significance in the treatment arm and participant group interaction, possibly due to the limited statistical power as a result of the small sample size. Therefore, it cannot be concluded with certainty that, compared with oral vitamin D3, the ability of oral 25(OH)D3 to increase serum 25(OH)D3 is significantly less compromised in malabsorptive patients. Further studies should be conducted to evaluate the bioavailability of 25(OH)D3 in a larger number of patients with various types of malabsorptive conditions.
Individuals with obesity require higher doses of vitamin D to achieve sufficient serum 25(OH)D because vitamin D acquired from intestinal absorption and cutaneous synthesis is sequestered in a larger body pool of fat (3, 7, 11). This has been well demonstrated by Wortsman et al. (11) that the bioavailability of an oral dose of 50,000 IU vitamin D2 as well as vitamin D3 from cutaneous synthesis upon whole-body UVB radiation was ∼50% lower in obese individuals than those with a normal BMI. Our analysis comparing healthy participants with higher (31.4 ± 2.6) and lower (22.6 ± 1.7) BMI suggested that that dose requirement of oral 25(OH)D3 for achieving a certain concentration of serum 25(OH)D in obese individuals is comparable to nonobese individuals. This warrants further study.
In conclusion, the current randomized, double-blind crossover study demonstrated that the bioavailability of 900 µg of orally administered 25(OH)D3 was not different between malabsorptive patients and healthy participants. The same malabsorption patients in this crossover study, however, demonstrated a significant 64% decrease in their ability to absorb 900 µg of orally administered vitamin D3 compared with the healthy controls. Comparison between healthy participants with higher (31.4 ± 2.6) and lower (22.6 ± 1.7) BMI showed that 900 µg of orally administered vitamin D3 tended to be less bioavailable in those with a higher BMI, whereas the bioavailability of 900 µg of orally administered 25(OH)D3 was not significantly different between the 2 groups. Furthermore, the observation that the blood concentrations of 25(OH)D3 increased more rapidly when compared with the blood concentrations of vitamin D3 after the same participants ingested 900 µg vitamin D3 and 25(OH)D3 suggests that the more hydrophilic 25(OH)D3 was absorbed directly into the portal system and distributed into the circulation, whereas vitamin D3 was incorporated into chylomicrons and absorbed into the lymphatic system before entering the circulation. Therefore, orally administered 25(OH)D3 offers several advantages for treating and preventing recurrent vitamin D deficiency. By giving 25(OH)D3, the blood concentrations quickly increase into a sufficient range, whereas when a given dose of vitamin D3 is provided to the patient, a steady-state blood concentration of 25(OH)D3 is not reached until ∼6–8 wk later (3). Patients with intestinal malabsorption of vitamin D who have a difficult time absorbing an adequate amount of vitamin D from their diet and supplements could benefit by ingesting 25(OH)D3. Finally, it is often very problematic to try to correct vitamin D deficiency in patients with obesity because the hydrophobic vitamin D is diluted in the large fat pool in the body. In addition, patients with obesity may have reduced liver 25-hydroxylation of vitamin D secondary to obesity-associated fatty liver (12). Based on our study, it appears that the same amount of 25(OH)D3 can be prescribed to all vitamin D–deficient patients without concern about their BMI.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—TAK, AS, and MFH: conception or design of the work: NC, AS, GHY, SD, CMA, AM, and MFH: data collection; NC, AS, and MFH: data analysis and interpretation; NC: drafting of the manuscript; NC, AS, CMA, and MFH: critical revision of the manuscript; NC, TAK, AS, GHY, SD, CMA, AM, and MFH: final approval of the version to be published; MFH: guarantor; and all authors: read and approved the final manuscript. MFH was a former consultant for Quest Diagnostics, Inc., a consultant for Ontometrics, Inc., and Biogena, Inc., and on the Speaker's Bureau for Abbott, Inc. MFH shares in a patent pending with Carbogen Amcis BV. CMA reports receiving personal fees from Nutrisystem, Zafgen, Sanofi-Aventis, Orexigen, EnteroMedics, GI Dynamics, Scientific Intake, Gelesis, Novo Nordisk, SetPoint Health, Xeno Biosciences, Rhythm Pharmaceuticals, Eisai, and Takeda, outside of the funded work; reports receiving grant funding from Aspire Bariatrics, GI Dynamics, Orexigen, Takeda, the Vela Foundation, Gelesis, Energesis, Coherence Lab, and Novo Nordisk, outside of the funded work; and reports past equity interest in ScienceSmart, LLC. All other authors report no conflicts of interest.
Notes
This trial was funded in part by a grant from Carbogen Amcis BV, institutional resources and the National Center for Advancing Translational Sciences grant (UL1TR001430). NC received the institutional research training grant from the Ruth L Kirchstein National Research Service Award program from the National Institutes of Health (2 T32 DK 7201–42). CMA is supported by P30 DK046200 from the National Institute of Diabetes and Digestive and Kidney Disease. Carbogen Amcis BV, Netherlands, provided assistance and the supply of vitamin D3 and 25-hydroxyvitamin D3.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: Cmax, maximal change in concentration; Ctrough, trough level above baseline at day 14; DBP, vitamin D–binding protein; PKS, pharmacokinetic study; PTH, parathyroid hormone; T1/2, elimination half-life; Tmax, time to maximal concentration; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyvitamin D2; 25(OH)D3, 25-hydroxyvitamin D3.
Contributor Information
Nipith Charoenngam, Section of Endocrinology, Diabetes, Nutrition, and Weight Management, Department of Medicine, Boston University School of Medicine, Boston, MA, USA; Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Tyler A Kalajian, Section of Endocrinology, Diabetes, Nutrition, and Weight Management, Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Arash Shirvani, Section of Endocrinology, Diabetes, Nutrition, and Weight Management, Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Grace H Yoon, Section of Endocrinology, Diabetes, Nutrition, and Weight Management, Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Suveer Desai, Section of Endocrinology, Diabetes, Nutrition, and Weight Management, Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Ashley McCarthy, Section of Endocrinology, Diabetes, and Hypertension, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Caroline M Apovian, Section of Endocrinology, Diabetes, and Hypertension, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Michael F Holick, Section of Endocrinology, Diabetes, Nutrition, and Weight Management, Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Data Availability
Individual patient data will be available beginning 9 months and ending 36 months after article publication upon reasonable request to mfholick@bu.edu.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. [DOI] [PubMed] [Google Scholar]
- 2.Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91(6):2552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–6S. [DOI] [PubMed] [Google Scholar]
- 5.Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644–9. [DOI] [PubMed] [Google Scholar]
- 6.Farraye FA, Nimitphong H, Stucchi A, Dendrinos K, Boulanger AB, Vijjeswarapu A, Tanennbaum A, Biancuzzo R, Chen TC, Holick MF. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn's disease. Inflamm Bowel Dis. 2011;17(10):2116–21. [DOI] [PubMed] [Google Scholar]
- 7.Lespessailles E, Toumi H. Vitamin D alteration associated with obesity and bariatric surgery. Exp Biol Med. 2017;242(10):1086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest. 1966;45(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz S, Weinerman S. Osteoporosis and gastrointestinal disease. Gastroenterol Hepatol (NY). 2010;6(8):506–17. [PMC free article] [PubMed] [Google Scholar]
- 10.Basha B, Rao DS, Han ZH, Parfitt AM. Osteomalacia due to vitamin D depletion: a neglected consequence of intestinal malabsorption. Am J Med. 2000;108(4):296–300. [DOI] [PubMed] [Google Scholar]
- 11.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. [DOI] [PubMed] [Google Scholar]
- 12.Roizen JD, Long C, Casella A, O'Lear L, Caplan I, Lai M, Sasson I, Singh R, Makowski AJ, Simmons Ret al. . Obesity Decreases hepatic 25-hydroxylase activity causing low serum 25-hydroxyvitamin D. J Bone Miner Res. 2019;34(6):1068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8(3):222–30. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff-Ferrari HA, Dawson-Hughes B, Stocklin E, Sidelnikov E, Willett WC, Edel JO, Stahelin HB, Wolfram S, Jetter A, Schwager Jet al. . Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27(1):160–9. [DOI] [PubMed] [Google Scholar]
- 15.Cashman KD, Seamans KM, Lucey AJ, Stocklin E, Weber P, Kiely M, Hill TR. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr. 2012;95(6):1350–6. [DOI] [PubMed] [Google Scholar]
- 16.Cesareo R, Falchetti A, Attanasio R, Tabacco G, Naciu AM, Palermo A. Hypovitaminosis D: is it time to consider the use of calcifediol?. Nutrients. 2019;11(5):1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jetter A, Egli A, Dawson-Hughes B, Staehelin HB, Stoecklin E, Goessl R, Henschkowski J, Bischoff-Ferrari HA. Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone. 2014;59:14–9. [PubMed] [Google Scholar]
- 18.Navarro-Valverde C, Sosa-Henriquez M, Alhambra-Exposito MR, Quesada-Gomez JM. Vitamin D3 and calcidiol are not equipotent. J Steroid Biochem Mol Biol. 2016;164:205–8. [DOI] [PubMed] [Google Scholar]
- 19.Shieh A, Ma C, Chun RF, Witzel S, Rafison B, Contreras HTM, Wittwer-Schegg J, Swinkels L, Huijs T, Hewison Met al. . Effects of cholecalciferol vs calcifediol on total and free 25-hydroxyvitamin D and parathyroid hormone. J Clin Endocrinol Metab. 2017;102(4):1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitrin MD, Pollack KL, Bolt MJ, Rosenberg IH. Comparison of vitamin D and 25-hydroxyvitamin D absorption in the rat. Am J Physiol. 1982;242(4):G326–32. [DOI] [PubMed] [Google Scholar]
- 21.Vaes AMM, Tieland M, de Regt MF, Wittwer J, van Loon LJC, de Groot L. Dose-response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: a randomized controlled trial in older adults. Clin Nutr. 2018;37(3):808–14. [DOI] [PubMed] [Google Scholar]
- 22.Cianferotti L, Cricelli C, Kanis JA, Nuti R, Reginster JY, Ringe JD, Rizzoli R, Brandi ML. The clinical use of vitamin D metabolites and their potential developments: a position statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Endocrine. 2015;50(1):12–26. [DOI] [PubMed] [Google Scholar]
- 23.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–8. [DOI] [PubMed] [Google Scholar]
- 24.Clifton L, Birks J, Clifton DA. Comparing different ways of calculating sample size for two independent means: a worked example. Contemp Clin Trials Commun. 2019;13:100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connors MH, Sheikholislam BM, Irias JJ. Vitamin D toxicity after dieting in hypoparathyroidism. Pediatrics. 1976;57(5):794. [PubMed] [Google Scholar]
- 26.Ziaie H, Razmjou S, Jomhouri R, Jenabi A. Vitamin D toxicity: stored and released from adipose tissue?. Arch Iran Med. 2016;19(8):597–600. [PubMed] [Google Scholar]
- 27.Perticone M, Maio R, Sciacqua A, Suraci E, Pinto A, Pujia R, Zito R, Gigliotti S, Sesti G, Perticone F. Ketogenic diet-induced weight loss is associated with an increase in vitamin D levels in obese adults. Molecules. 2019;24(13):2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer D, Becker S, Cordes T, Bucker B, Diedrich K, Friedrich M, Salehin D, Thill M. Vitamin D-24-hydroxylase in benign and malignant breast tissue and cell lines. Anticancer Res. 2009;29(9):3641–5. [PubMed] [Google Scholar]
- 29.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29(12):664–73. [DOI] [PubMed] [Google Scholar]
- 30.Omdahl JL, Bobrovnikova EA, Choe S, Dwivedi PP, May BK. Overview of regulatory cytochrome P450 enzymes of the vitamin D pathway. Steroids. 2001;66(3-5):381–9. [DOI] [PubMed] [Google Scholar]
- 31.Nimitphong H, Holick MF, Fried SK, Lee M-J. 25-hydroxyvitamin D₃ and 1,25-dihydroxyvitamin D₃ promote the differentiation of human subcutaneous preadipocytes. PLoS One. 2012;7(12):e52171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rybchyn MS, Abboud M, Puglisi DA, Gordon-Thomson C, Brennan-Speranza TC, Mason RS, Fraser DR. Skeletal muscle and the maintenance of vitamin D status. Nutrients. 2020;12(11):3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamp TC. Intestinal absorption of 25-hydroxycholecalciferol. Lancet North Am Ed. 1974;304(7873):121–3. [DOI] [PubMed] [Google Scholar]
- 34.Davies M, Mawer EB, Krawitt EL. Comparative absorption of vitamin D3 and 25-hydroxyvitamin D3 in intestinal disease. Gut. 1980;21(4):287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual patient data will be available beginning 9 months and ending 36 months after article publication upon reasonable request to mfholick@bu.edu.