Abstract
Background
Only few data are available on treatment-associated behavior of distinct rare CNS embryonal tumor entities previously treated as “CNS-primitive neuroectodermal tumors” (CNS-PNET). Respective data on specific entities, including CNS neuroblastoma, FOXR2 activated (CNS NB-FOXR2), and embryonal tumors with multilayered rosettes (ETMR) are needed for development of differentiated treatment strategies.
Methods
Within this retrospective, international study, tumor samples of clinically well-annotated patients with the original diagnosis of CNS-PNET were analyzed using DNA methylation arrays (n = 307). Additional cases (n = 66) with DNA methylation pattern of CNS NB-FOXR2 were included irrespective of initial histological diagnosis. Pooled clinical data (n = 292) were descriptively analyzed.
Results
DNA methylation profiling of “CNS-PNET” classified 58 (19%) cases as ETMR, 57 (19%) as high-grade glioma (HGG), 36 (12%) as CNS NB-FOXR2, and 89(29%) cases were classified into 18 other entities. Sixty-seven (22%) cases did not show DNA methylation patterns similar to established CNS tumor reference classes. Best treatment results were achieved for CNS NB-FOXR2 patients (5-year PFS: 63% ± 7%, OS: 85% ± 5%, n = 63), with 35/42 progression-free survivors after upfront craniospinal irradiation (CSI) and chemotherapy. The worst outcome was seen for ETMR and HGG patients with 5-year PFS of 18% ± 6% and 22% ± 7%, and 5-year OS of 24% ± 6% and 25% ± 7%, respectively.
Conclusion
The historically reported poor outcome of CNS-PNET patients becomes highly variable when tumors are molecularly classified based on DNA methylation profiling. Patients with CNS NB-FOXR2 responded well to current treatments and a standard-risk CSI-based regimen may be prospectively evaluated. The poor outcome of ETMR across applied treatment strategies substantiates the necessity for evaluation of novel treatments.
Keywords: CNS embryonal tumor, CNS NB-FOXR2, CNS-PNET, DNA methylation profiling, ETMR
Key Points.
Molecular diagnostic differentiation of rare CNS embryonal tumors is clinically relevant.
Observed favorable outcome for CNS NB-FOXR2 is likely based on the use of CSI.
Frequent progressions of ETMR occurred irrespective of treatment strategy.
Importance of the Study.
This retrospective study is a comprehensive analysis on the clinical behavior and treatment-associated outcome of patients with rare CNS embryonal tumors. Central diagnostic re-evaluation was performed, and structured clinical data were pooled and analyzed with regard to the result of the DNA methylation-based classification. Our data show that beside high-grade gliomas also ETMR contribute to the poor outcome of historic “CNS-PNET” cohorts. In contrast to ETMR, patients with CNS NB-FOXR2 had a much better 5-year overall survival of 85%. Our data suggest that the use of craniospinal irradiation is most likely an important premise for favorable survival in patients with CNS NB-FOXR2. Patients with ETMR presented at a younger age and the majority of patients developed treatment-refractory progressions irrespective of treatment modality. The data confirm the importance of molecular diagnostic differentiation of rare CNS embryonal tumors and substantiate the necessity for development of entity-specific prospective clinical trials.
Classification of rare embryonal tumors of the central nervous system (CNS) has been a long-standing challenge that has undergone modifications over the last years. The term CNS-primitive neuroectodermal tumor (CNS-PNET), removed from the WHO classification of CNS tumors within the 2016 revision, was based on diagnostic criteria of limited specificity, and inaccurate diagnoses of other entities as “CNS-PNET” were frequent.1,2 In a previous CNS-PNET cohort, re-evaluated by DNA methylation analysis, many tumors could be epigenetically annotated to specific entities.3 Known entities comprised embryonal tumors with multilayered rosettes (ETMR), high-grade gliomas (HGG), and multiple other diagnoses. Additionally, 4 new entities were delineated based on specific DNA methylation profiles and genetic alterations.3 One of these new entities showed morphological similarity with CNS neuroblastoma and harbored chromosomal rearrangements leading to an increased expression of the forkhead box R2 (FOXR2) gene, based on which it was termed CNS neuroblastoma, FOXR2 activated (CNS NB-FOXR2).3 Nearly all CNS NB-FOXR2 samples in this series were historically diagnosed as CNS-PNET, while the other newly defined entities were histologically more diverse and were also resolved from other diagnoses.3 Besides CNS NB-FOXR2, only ETMR uniquely presented as CNS embryonal tumors on morphological evaluation in this and other series.3–6
The term ETMR has been introduced as a unifying diagnosis for tumors with diverse histological designations such as ependymoblastoma, embryonal tumor with abundant neuropil and true rosettes (ETANTR), and medulloepithelioma.1,7,8 The characteristic molecular hallmark of this entity, amplification of the microRNA cluster on chromosome 19 (C19MC), is present in ~90% of the ETMR cases, while tumors lacking the C19MC amplification frequently harbor bi-allelic DICER1 mutations, of which the first hit is generally present in the germline of the patients.8–12
Historically, patients with a diagnosis of CNS-PNET have mostly been treated similar to high-risk medulloblastoma patients with the few prospective clinical trials often including pineoblastoma patients.13–18 Reported outcome rates were poor for both, for young children treated with chemotherapy (CT)-based regimens, and for older children who underwent combined intensified radiotherapy (RT) and CT. However, after identification of the molecular heterogeneity of the histologically diagnosed CNS-PNET cohorts, previously published data on treatment and outcome of patients with CNS-PNET have to be interpreted with caution. Indeed, retrospective molecular evaluation of tumors from a cohort of patients prospectively treated as CNS-PNET patients identified a high fraction of HGG with poor prognosis, while survival rates for patients with molecularly confirmed CNS embryonal tumors were superior compared to historic series, despite the heterogeneity within this group.18
Clinical data published in the literature on patients with retrospective molecularly characterized rare CNS embryonal tumors are scarce.3 Therefore, we analyzed tumor samples of clinically well-annotated patients with a historic histopathological diagnosis of CNS-PNET to evaluate the clinical behavior within molecularly well-defined groups, with a special focus on the CNS embryonal tumor entities CNS NB-FOXR2 and ETMR.
Methods
Study Design and Participants
Twenty national groups or single institutions participated in this retrospective study and provided original tumor material and link-anonymized or anonymized clinical data of patients diagnosed previously with CNS-PNET and treated within a prospective trial or on an institutional basis. The study has been evaluated and approved by the ethics board of the coordinating institution and by local ethics boards of participating groups where required according to initial consent and trial approval.
Eligibility for evaluation within the retrospective study was based on initial local or national central histopathological diagnosis of CNS-PNET (for cases diagnosed after implementation of the 2007 WHO classification of CNS tumors), or diagnosis of supratentorial PNET (for cases with earlier diagnoses).19 At the time of study initiation, availability of sufficient archival formalin-fixed, paraffin-embedded (FFPE) material for diagnostic re-evaluation was required for inclusion. By amendment, the eligibility criteria were later adapted to include clinical information on patients based on the availability of a DNA methylation profile classified as one of the newly described, molecularly defined entities,3 of which only data from patients with CNS NB-FOXR2 are included in the amended cohort of this manuscript.
Histological and Molecular Re-Evaluation
Histological and molecular evaluation of tumor samples included an independent neuropathological review by a panel of expert neuropathologists and a DNA methylation-based classification using a random forest class prediction algorithm (version 11b4; www.molecularneuropathology.org) as described previously.20 In addition, DNA methylation data were clustered with reference samples using t-distributed stochastic neighbor embedding (t-SNE) dimensionality reduction in order to verify the results of the methylation classifier. Diagnoses were assigned based on a calibrated score of >0.9, or a lower score and clustering within the respective cohort. Copy number profiles were created from the DNA methylation data using the conumee package (v.1.3.0). Results of the neuropathological panel review will be reported separately.
Cohort Description and Clinical Data
Overall, 307 CNS-PNET samples were submitted. Date of diagnosis ranged from 1988 to 2017. For 197/307 (64%) samples, both DNA methylation profile and corresponding results of the neuropathological panel review were available. For 109 (36%) samples, only DNA methylation data were generated. Of the patients included after amendment, 66 patients with DNA methylation profile of CNS NB-FOXR2 were identified. Tumor material had been analysed, and results were partly published before for 74/307 samples of the re-evaluation cohort3,12,21 and 44/66 samples of the amended cohort.3,18 These include samples of 22 of 35 ETMR patients, and 14 of 20 CNS NB-FOXR2 patients clinically described in recent series.22,23
Structured clinical information was submitted for 292 patients (Figure 1A), of whom 204 were treated within or according to trials that recruited CNS-PNET patients.14–16,18,24–36 Information on staging was acquired according to the Chang-classification.37 Information on response to treatment was based on the institutional or group-specific response criteria. Data were linked to acquired results of the diagnostic re-evaluation and pooled for analyses. Detailed plausibility control and descriptive analysis of treatment were performed for patients with molecularly confirmed CNS embryonal tumor entities ETMR and CNS NB-FOXR2.
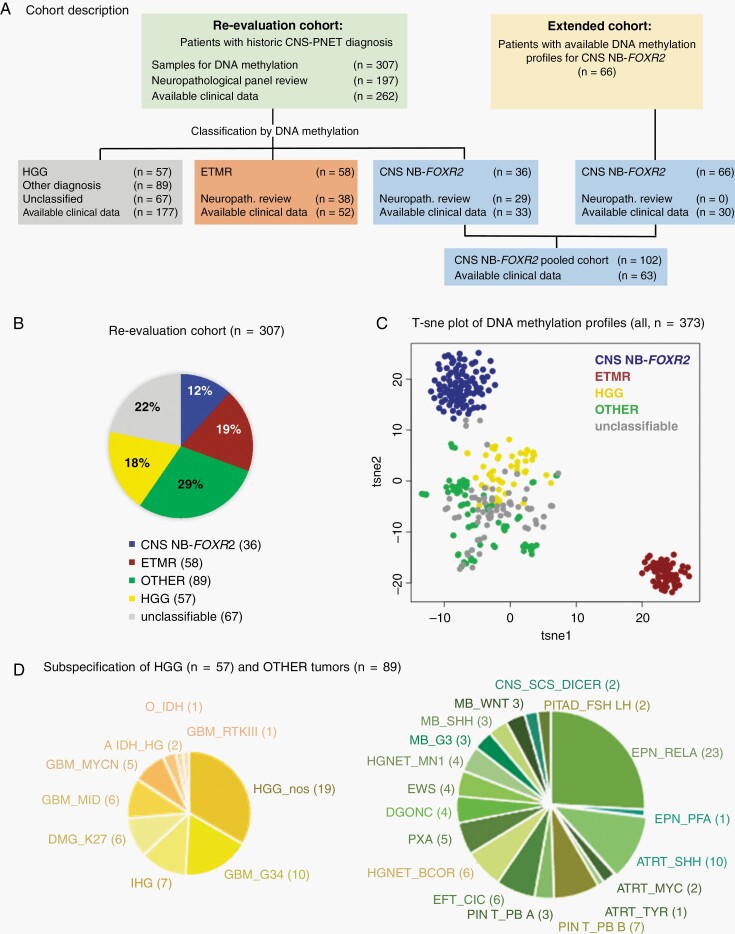
Fig. 1.
Cohort description. A. Patients were included either based on a historical diagnosis of CNS-PNET (CNS-PNET re-evaluation cohort) or on the results of DNA methylation profiles according to CNS NB-FOXR2 (extended cohort). B. Pie chart of entities diagnosed by DNA methylation profiling within the CNS-PNET re-evaluation cohort. C. t-SNE analysis of DNA methylation profiles from all included. D. Further subspecification of DNA methylation profiles of HGG and other diagnoses. Abbreviations: CNS-PNET, CNS-primitive neuroectodermal tumors; HGG, high-grade gliomas; t-SNE, t-distributed stochastic neighbor embedding.
Statistical Analysis
Data analysis focused on entity-specific description of presentation, treatment, pattern of relapse, progression-free survival (PFS), and overall survival (OS). Descriptive analyses were performed by Kaplan-Meier estimates and log-rank tests for PFS and OS rates (±standard errors). All P values were considered as explorative and no significance level was fixed. Analyses were performed using SPSS software (IBM), version 22.
Results
Molecular Evaluation of CNS-PNET Cohort
DNA methylation-based classification of the 307 “CNS-PNETs” confirmed a molecular profile matching CNS embryonal tumors of interest for 94 (31%) patients: 58 (19%) patients with ETMR and 36 (12%) patients with CNS NB-FOXR2 (Figure 1B). The DNA methylation class for these 2 entities was in accordance with parallel and blinded histopathological re-evaluation for all samples with FFPE sections (ETMR, n = 38; CNS NB-FOXR2, n = 29). Based on the DNA methylation pattern, another 57 (19%) tumors were classified as HGG, including distinct subtypes such as “glioblastoma, H3.3 G34 mutant” (GBM-G34, n = 10),38 “infantile hemispheric glioma” (IHG, n = 7),39 “diffuse midline glioma H3K27M mutant” (DMG-K27, n = 6), “glioblastoma, IDH wildtype, subclass midline” (GBM-MID, n = 6),40 “glioblastoma, subclass MYCN” (GBM-MYCN, n = 5),41 and other not further specified HGGs (HGG-NOS, n = 19) (Figure 1D). Blinded neuropathological review confirmed the diagnosis of HGG in 30/39 (77%) evaluated samples. The remaining CNS-PNET samples (n = 156) were either classified as other known entities (n = 89; 29%), including ependymoma with RELA fusion (n = 23; 7%), atypical rhabdoid/teratoid tumors (ATRT, n = 13; 4%), pineoblastoma (n = 10; 3%), medulloblastoma (n = 9; 3%), CNS HGNET-BCOR (n = 6; 2%),3 CNS CIC-rearranged sarcoma (n = 6; 2%),3 CNS HGNET-MN1 (n = 4; 1%),3 diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC, n = 4; 1%),42 CNS sarcoma with DICER1 mutation (n = 2; 0.6%),43 or were not classifiable (n = 67; 22%) by DNA methylation-based analyses at the time of evaluation (Figure 1B; Supplementary Figure S1). Overall t-SNE analysis and visualization of the DNA methylation profiles of all 373 samples showed that ETMRs and CNS NB-FOXR2 cases formed 2 distinct clusters. All other samples formed one large cluster with smaller clusters therein representing distinct entities mentioned above. Only some separation between HGGs and other tumors was observed within this cluster (Figure 1C). The distribution of entities identified by DNA methylation profiling was in line with previous reports on CNS-PNET.3 Based on these findings, we grouped the CNS-PNET cohort into 5 categories for subsequent analyses: CNS NB-FOXR2, ETMR, HGG, other, and unclassified (Figure 1B, C).
CNS NB-FOXR2
In total, DNA methylation profiles were available for 102 CNS NB-FOXR2 tumors. The calibrated score based on the brain tumor classifier version 11b4 was >0.9 for 94 samples. Clustering within the cohort was confirmed for the remaining 8 samples, while no major differences were observed in comparison to the samples with high scores. The samples which could be re-evaluated neuropathologically (n = 29) showed histological features with predominant neuroblastic/neurocytic differentiation corresponding to the WHO diagnosis “CNS neuroblastoma” but also very undifferentiated neuroepithelial phenotypes. DNA copy number profiles derived from DNA methylation data showed gain of chromosome 1q in nearly all samples (94%). Other chromosomal gains or losses frequently (≥20%) observed in these tumors included gain of 3q (21%), 8p (21%), 8q (28%), and 17q (49%) as well as loss of 3p (34%), 6q (24%), 10q (25%), and 16q (56%) (Figure 2A) and are in line with previous findings in this tumor group.3
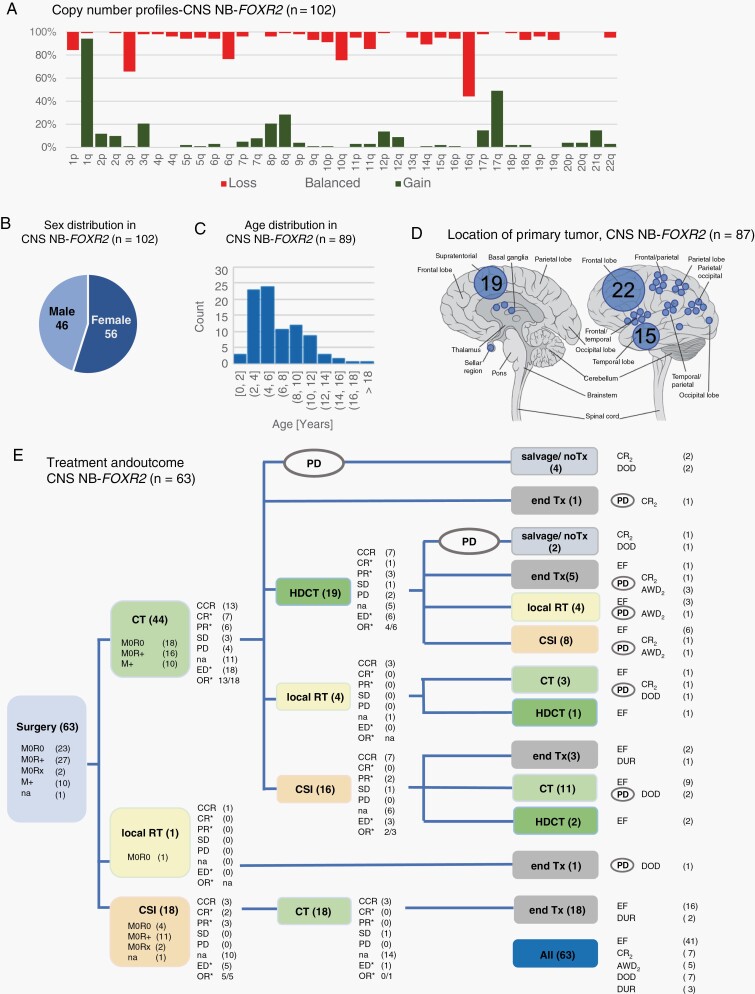
Fig. 2.
CNS NB-FOXR2: Molecular characteristics, clinical information, and treatment. A. Overview of copy number profiles of CNS NB-FOXR2. Bars indicating gain, balanced, or loss add up to 100% for each chromosome arm. B. The pie chart depicts the nearly balanced sex ratio. C. The bar chart shows the age distribution of age at first diagnosis (information available for 89/102 patients). D. Tumor location is specified for 87 patients with CNS NB-FOXR2, with each dot corresponding to one single tumor, or multiple tumors with the respective number of cases given. E. Overview of applied treatment and documented response for patients with CNS NB-FOXR2. Overall, 41 of 63 patients remained event-free (EF). Of 22 patients with event, 12 patients were alive at last follow-up with evidence of disease (AWD2), or in second complete remission (CR2). Numbers indicated with the symbol * refer to patients with previous evidence of disease (ED) and documented response to treatment. Abbreviations: CCR, continuous complete response; CR, complete response; CSI, craniospinal irradiation; CT, chemotherapy; DOD, death of disease; DUR, death of unknown reason; HDCT, high-dose chemotherapy; M+, metastatic disease; M0, localized disease, M0R0, with complete resection, M0R+, with incomplete resection, and M0Rx, with unknown resection status; na, not annotated; OR, objective response; PD, relapse or progression; PR, partial response; RT, radiotherapy; SD, stable disease; Tx, treatment.
Median age at diagnosis was 5.0 years (range 1.0-20.0 years), and sex ratio was nearly balanced with 56/102 females (55%) (Figure 2B, C). Tumor location was supratentorial in all 87 cases with available information (Figure 2D). Clinical data were available for 63 patients (Figure 1A). For 10/63 (16%) patients, macroscopic CNS metastases detected on MR-imaging were reported at diagnosis (Table 1).
Table 1.
Clinical Characteristics of Patients With CNS NB-FOXR2 and ETMR and Available Clinical Data
| CNS NB-FOXR2 (n = 63) | ETMR (n = 52) | |||
|---|---|---|---|---|
| No. | (%) | No. | (%) | |
| Age at diagnosis | ||||
| <3 years | 11 | (17) | 34 | (66) |
| ≥3 years | 52 | (83) | 18 | (34) |
| Sex | ||||
| Female | 36 | (57) | 29 | (56) |
| Male | 27 | (43) | 23 | (44) |
| Location of primary tumor | ||||
| Supratentorial | 62 | (98) | 44 | (84) |
| Infratentorial | 5 | (10) | ||
| Supra- and infratentorial | 3 | (6) | ||
| Not known | 1 | (2) | ||
| Staging at diagnosis | ||||
| Localized with complete resection (M0R0)a | 23 | (37) | 20 | (38) |
| Localized with incomplete resection (M0R+)a | 27 | (41) | 17 | (33) |
| Second surgery within primary treatment | 6/27 | 9/17 | ||
| GTR at second surgery | 5/6 | 1/9 | ||
| Localized resection status unknown (M0Rx)a | 2 | (3) | 1 | (2) |
| Microscopic spread to CSF only (M1) | 0 | 3 | (6) | |
| Intracranial/spinal leptomeningeal metastases (M2/3) | 10 | (16) | 10 | (19) |
| Not known | 1 | (3) | 1 | (2) |
| Treatment | ||||
| Within or according to a CNS-ET trial | 53 | (84) | 43 | (83) |
| Individual treatment | 9 | (14) | 6 | (11) |
| Treatment based on protocol for other entityb | 1 | (2) | 0 | |
| No antitumor treatment (early death after surgery) | 0 | 3 | (6) |
Abbreviations: CNS-ET, CNS embryonal tumor; CSF, cerebrospinal fluid; ETMR, embryonal tumor with multilayered rosettes; GTR, gross total resection; M0, localized disease, no metastases; M1, microscopic metastases to CSF; M2/3 intracranial or spinal leptomeningeal metastases visible on MRI, according to the Chang-classification.37
aInformation on CSF staging is missing for n = 6 ETMR patients with localized disease.
bn = 1 patient with CNS NB-FOXR2 was treated according to an ependymoma protocol.
Forty-four patients underwent postoperative CT, and 19 patients started treatment with craniospinal irradiation (CSI 18, local RT 1) (Figure 2E). For patients who received postoperative CT, objective response was documented in 13/18 (72%) patients with evaluable disease. High-dose CT was applied to 22 patients (after induction CT, n = 19; after irradiation, n = 3), with objective response documented in 4/6 patients with evaluable disease (Figure 2E).
Upfront RT was administered to 51 patients (CSI and boost for 42, and local irradiation for 9 patients). Of those 51 patients, 19 received RT postoperatively, while the other 32 received RT after CT. Median CSI dose was 35 Gy (range: 23.4-41.0 Gy; dose ≤ 24 Gy: n = 12; dose > 24 Gy: n = 27; dose unknown: n = 3). Median boost dose was 55.0 Gy (range: 49.6-72.0 Gy). Objective response to treatment with CSI and boost was documented for 7/8 (88%) patients with evaluable disease.
Relapse, progression, or death of unknown reason was observed in 22/63 (35%) patients, with 11 RT-naïve at time of relapse and 6 of whom had received high-dose CT before relapse/progression (Figure 2E). For patients who had received upfront irradiation, relapse/progression or death occurred in 7/42 after CSI (2/12 with dose ≤ 24 Gy; 5/27 with dose > 24 Gy, Fisher exact test, P = 1.0) and in 4/9 after local RT. Location of relapse was local for 7/11 RT-naïve patients, whereas after local irradiation 4/4 distant events occurred (Fisher exact test, P = .08) (Table 2). Late relapses were observed (7/22 events occurred later than 2 years after diagnosis) with the latest relapse documented 5.9 years after diagnosis. Overall, 10/63 (16%) patients died within the observation time. Thus, the data show an overall high rate of survival after combined CSI and CT treatment, but prolonged period of risk for development of relapse and disease associated death.
Table 2.
Outcome According to Treatment for Patients With ETMR or CNS NB-FOXR2
| CNS NB-FOXR2 (n = 63) | ETMR (n = 49) | |||||
|---|---|---|---|---|---|---|
| Observed events | ||||||
| Relapse/progressiona | 22 (35%) | 40 (82%) | ||||
| Death | 10 (16%) | 36 (73%) | ||||
| Timing of first event | ||||||
| On treatment | 6 (27%) | 38 (95%) | ||||
| After treatment | 16 (73%) | 2 (5%) | ||||
| Outcome according to treatment with HDCT | ||||||
| HDCT applied before first event or last status | No HDCT (n = 41) | HDCT (n = 22) | No HDCT (n = 32) | HDCT (n = 17) | ||
| No. of patients with event | 13 (32%) | 9 (41%) | 29 (91%) | 11 (65%) | ||
| Outcome according to treatment with RT | ||||||
| RT applied before first event or last status | No RT (n = 12) | CSI (n = 42) | Local RT (n = 9) | No RT (n = 35) | CSI (n = 10) | Local RT (n = 4) |
| No. of patients with event | 11 (92%) | 7 (17%) | 4 (44%) | 34 (97%) | 5 (50%) | 1 (25%) |
| Local relapse/progression | 7 | 3 | - | 27 | 2 | 1 |
| Distant or combined relapse/progression | 4 | 1 | 4 | 5 | 3 | - |
| Relapse/progression NOS | - | 2 | ||||
| Death for unknown reasona | 3 | |||||
| Outcome after first event according to previous RT | ||||||
| Occurrence of first event | Before irradiation (n = 11) | After irradiation (n = 11) | Before irradiation (n = 34) | After irradiation (n = 6) | ||
| Death | 3 (27%) | 7 (64%) | 30 (88%) | 6 (100%) | ||
| Alive | 8 (73%) | 4 (36%) | 4 (12%) | 0 | ||
| PFS | ||||||
| Median PFS in years (95% CI) | 8.4 (1.1-34.1) | 0.6 (0.5-0.7) | ||||
| 5-year PFS (SE) | 63% (±8%) | 18% (±6%) | ||||
| PFS according to staging | ||||||
| M0R0, 5-year PFS (SE) | 55% (±11%) | 25% (±10%) | ||||
| M0R+, 5-year PFS (SE, log-rank Pb) | 77% (±8%, 0.57) | 19% (±10%, 0.65) | ||||
| M+, 5-year PFS (SE, log-rank Pb) | 48% (±16%, 0.54) | 8% (±7%, 0.08) | ||||
| OS | ||||||
| Median OS in years (95% CI) | 17.6 (15.5-19.7) | 1.2 (0.7-1.6) | ||||
| 5-year OS (SE) | 85% (±5%) | 24% (±6%) | ||||
| OS according to staging | ||||||
| M0R0, 5-year OS (SE) | 85% (±8%) | 34% (±11%) | ||||
| M0R+/Rx, 5-year OS (SE, log- rank Pb) | 90% (±7%, 0.59) | 23% (±12%, 0.60) | ||||
| M+, 5-year OS (SE, log-rank Pb) | 70% (±15%, 0.11) | 8% (±7%, 0.007) | ||||
| Duration of follow-up of surviving patients | ||||||
| median follow-up in years (range) | 5.7 (0.3-19.5) | 3.9 (1.0-18.5) |
Abbreviations: CI, confidence interval; CSI, craniospinal irradiation; ETMR, embryonal tumor with multilayered rosettes; HDCT, high-dose chemotherapy; M+, metastatic disease; M0, localized disease, M0R0, with complete resection, M0R+, with incomplete resection, M0Rx, with unknown resection status; NOS, not otherwise specified; OS, overall survival; PD, relapse or progression; PFS, progression-free survival; RT, radiotherapy; SE, standard error.
Patients without treatment are excluded (ETMR, n = 3).
aThree patients with CNS NB-FOXR2 and death for unknown reasons are included in these counts.
bGiven values for log-rank P refer to comparison to M0R0.
ETMR
Fifty-eight tumors were classified as ETMR according to methylation profiling (54 with calibrated scores >0.9 based on classifier version 11b4). The samples that could be re-evaluated neuropathologically showed histological features of an immature small cell tumor with multilayered (ependymoblastic) rosettes or medulloepithelioma-like trabecular structures, varying amounts of synaptophysin positive neuropil, and characteristic LIN28A expression. Copy number profiles derived from the methylation data identified the characteristic C19MC amplification in 55/58 (95%) of cases and gain of chromosome 2 in 47/58 (81%) (Figure 3A, B). The 4 samples with calibrated scores of the DNA methylation profile <0.9 clustered at the edges of the ETMR cohort, with 3 of them being C19MC-negative as described within a previous large series of ETMR patients.12
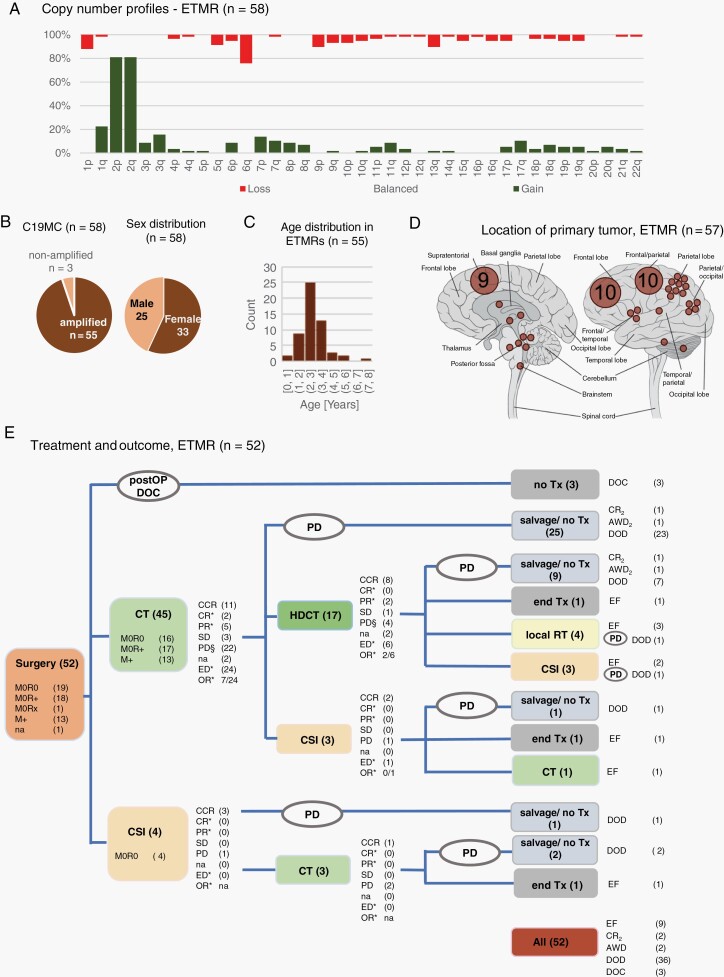
Fig. 3.
ETMR: Molecular characteristics, clinical information, and treatment. A. Overview of copy number profiles of ETMR. Bars indicating gain, balanced, or loss add up to 100% for each chromosome arm. B. Pie charts depict that 55/58 ETMR carry the characteristic C19MC amplification. The sex ratio was nearly balanced. C. The bar chart shows the age distribution of age at first diagnosis (information available for 55/58 patients). D. Tumor location is specified with each dot corresponding to one single tumor, or multiple tumors with the respective number of cases given. E. Overview of applied treatment and documented response for patients with ETMR. Overall, 9 of 52 patients remained event-free (EF). Of 43 patients with event, 3 died postoperatively, 38 progressed/relapsed while on treatment, and 2 relapsed after the end of treatment. The symbol § indicates the number of patients with relapse or progression (PD) as first documented response, which differs from overall number of PD after the respective treatment element due to initial continuous complete response (CCR), complete response (CR), partial response (PR), or stable disease (SD) and later PD before the onset of next treatment element; Further explanations and abbreviations: see Figure 2. Abbreviations: ETMR, embryonal tumor with multilayered rosettes; OR, objective response; SD, stable disease.
Median age at diagnosis was 2.5 years (range 0.8-7.6 years), and sex ratio was nearly balanced with 33/58 females (57%) (Figure 3B, C). Most tumors (n = 48, 83%) were located supratentorially, with a potential sampling bias towards supratentorial lesions, as only tumors with an initial diagnosis of CNS-PNET or stPNET were collected. Still, in 9 (16%) patients, an infratentorial (n = 6) or extended infra-/supratentorial (n = 3) location was documented (location unknown, n = 1). Detailed clinical data were available for 52/58 patients (Figure 1A). Initial metastatic presentation was reported in 13 (25%) patients (Table 1).
Of the 49 patients who received postoperative treatment, treatment was started with CT for most patients (n = 45; 92%) (Figure 3E). Objective response was documented in 7/24 (29%) patients with evaluable disease, whereas 22/45 (49%) showed treatment-refractory relapse or progression, and another 3 patients progressed after initial response or stable disease. Seventeen patients underwent high-dose CT with autologous stem cell rescue after conventional CT, with objective response documented in 2/6 (33%) patients with evaluable disease. Immediate and delayed relapse/progression to high-dose CT was documented in 9/16 (56%) patients. Upfront irradiation was applied either as CSI (n = 10) or local irradiation (n = 4) in combination with CT ± high-dose CT treatment. Median CSI dose was 24 Gy (range: 23.4-35.2 Gy), median local boost dose was 55.0 Gy (range: 54.0-59.7 Gy).
Relapse or progression occurred in 40/49 (82%) treated patients, with 38/40 (95%) progressing/relapsing while on treatment. Relapse or progression occurred in 34 RT-naïve patients. After irradiation, relapse/progression occurred in 5/10 patients after CSI, and in 1/4 patients after local irradiation and high-dose CT (Table 2). All 6 patients who relapsed after RT died. Of 34 patients who relapsed before irradiation, 22 patients received RT as salvage treatment, and 4 were alive at last follow-up (Table 2). There was no first event documented later than 1.4 years after diagnosis. Overall, 36/49 (73%) patients died within the observation time, with the latest death documented 2.4 years after diagnosis. Of 13 survivors at last follow-up, 10 patients were alive longer than 2.5 years after diagnosis. Of the latter patients, all had supratentorially located tumors, 6/10 were females, 9/10 were not metastasized, gross total resection was achieved for 7/10 by first or second resection, and all were irradiated within primary (n = 8), or salvage (n = 2) treatment. The data show the highly aggressive and treatment-refractory behavior of most ETMR, while prolonged survival has been observed for a subset of ETMR patients. There were no molecular features detected that were associated with a favorable outcome based on the here performed analyses.
Survival Analyses
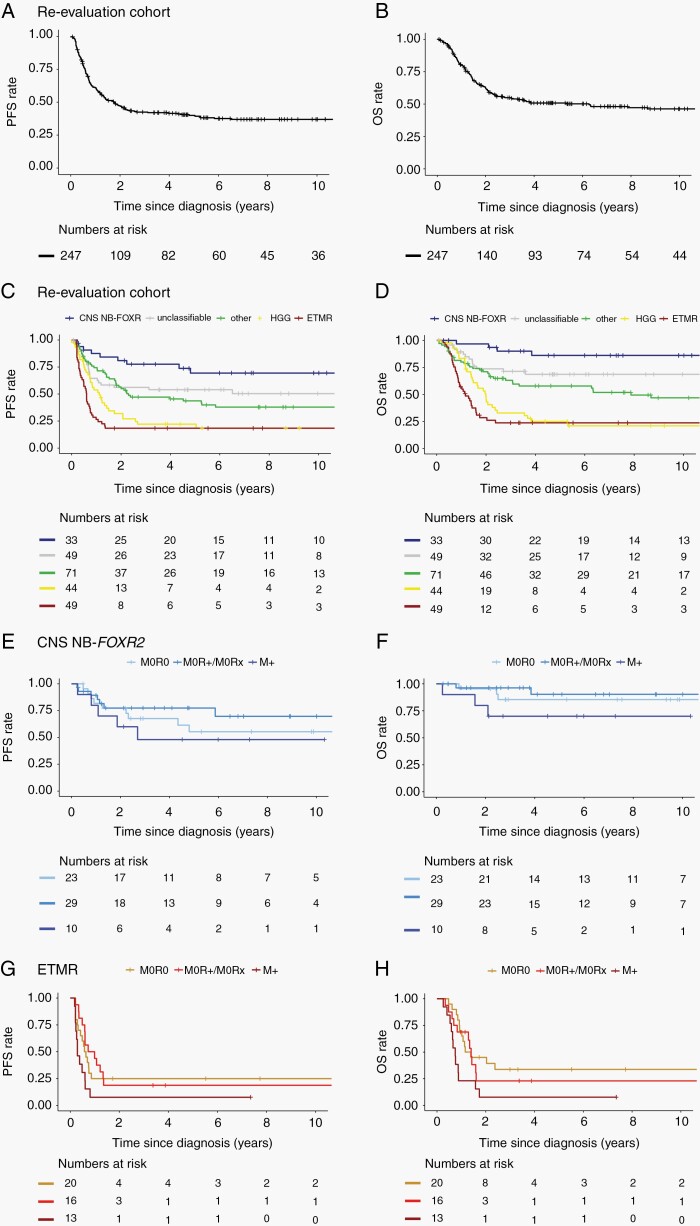
In the CNS-PNET re-evaluation cohort, the overall 5-year PFS and OS were 40% ± 3% and 51% ± 3%, respectively (Figure 4A, B). Survival markedly differed for the patients with different molecularly informed diagnoses. Best survival rates were observed for CNS NB-FOXR2 patients (5-year PFS and OS: 69% ± 9%; 86% ± 7%) (Figure 4C, D). Similar survival rates were observed for the extended cohort of CNS NB-FOXR2 patients (5-year PFS and OS: 57% ± 10%; 85% ± 5%). Combining both CNS NB-FOXR2 cohorts, 5-year PFS and OS were 63% ± 7% and 85% ± 5%, respectively. No statistically relevant differences in survival according to initial staging were observed in this cohort (Table 2, Figure 4E, F). The worst survival rates within the CNS-PNET re-evaluation cohort were observed for ETMR patients (5-year PFS and OS: 18% ± 6%; 24% ± 6%) and HGG patients (5-year PFS and OS: 22%% ± 7%; 25% ± 7%) (Figure 4C, D). Presentation with metastases, but not postoperative residual tumor had a negative impact on outcome for patients with ETMR, with 5-year PFS and OS of 25% ± 10%, and 34% ± 11% for 20 nonmetastatic patients with complete resection, compared to 8% ± 7% for both PFS (log-rank P = .08) and OS (log-rank P = .007) for 13 patients with metastases at presentation (Table 2, Figure 4G, H).
Fig. 4.
Kaplan-Meier plots of survival. PFS (A) and OS (B) for the re-evaluation cohort. Respective PFS (C) and OS (D) for this same cohort with patients grouped according to the result of DNA methylation profiles: CNS NB-FOXR2, ETMR, HGG, other, and unclassified. Survival according to postoperative staging for the pooled cohort of patients with CNS NB-FOXR2: PFS (E) and OS (F) (n = 1 patient with missing information on initial staging is not regarded for this analysis), and for patients with ETMR: PFS (G) and OS (H). Patients without postoperative treatment were excluded from survival analyses. Abbreviations: ETMR, embryonal tumor with multilayered rosettes; HGG, high-grade gliomas; OS, overall survival; PFS, progression-free survival.
Five-year PFS and OS for the pooled group of patients with tumors classified as other known entities were 44% ± 6% and 58% ± 6%, respectively. The mixed cohort of patients with tumors unclassifiable by DNA methylation at the time of analysis showed 5-year PFS and OS of 54% ± 7% and 69% ± 7%, respectively (Figure 4C, D).
Discussion
The heterogeneity of the historical cohorts of tumors diagnosed as “CNS-PNET” as well as the emergence of molecularly defined entities has led to diagnostic and therapeutic uncertainty.1–3,18 Historically, patients with CNS-PNET were treated with age-dependent intensive multimodal treatment strategies resulting in high rates of toxicity and poor survival rates.13–18,25,44–46 Survival rates of the current CNS-PNET re-evaluation cohort are comparable to previously published series. Crucially, our cohort confirms that only a minor proportion of patients included in historical CNS-PNET cohorts can be considered as having CNS embryonal tumors according to current diagnostic methods and criteria.2,3,18 In the present re-evaluation cohort, 19% of tumors were classified as ETMR, and 12% were classified as CNS NB-FOXR2. Reliable diagnosis of these tumors is clinically relevant, as they demonstrate a particular clinical behavior. In contrast to ETMR, patients with CNS NB-FOXR2 present at an older age, have exclusively supratentorial tumors, and show higher response as well as superior survival rates. In the ACNS0332 trial, which included patients older than 3 years of age with CNS-PNET, patients with supratentorial embryonal tumors and pineoblastomas had a favorable outcome after excluding other entities, mainly HGG. However, the majority of the patients in the favorable prognostic group in this series were diagnosed with pineoblastoma. A separate analysis of patients with CNS NB-FOXR2 was not possible due to the limited number of cases.18 Further data on CNS neuroblastoma are rare. Since the initial description of the respective histopathological diagnosis, only very few cases have been published. In the early series, the reported survival was poor, while the few additional reports, some lacking molecular annotation, indicate a superior survival.21,22,47–49
While the favorable survival for patients with CNS NB-FOXR2 is clearly confirmed in our series, our data show a higher rate of recurrences in nonirradiated and locally irradiated patients. Despite the small numbers and data quality limitations of retrospective analysis, the observation of distant metastases after local irradiation may indicate a treatment-induced shift of recurrences to distant sites, as it has been shown in medulloblastoma patients.50,51 The applied CSI doses in our series were variable. Since only 7/42 patients relapsed or died after CSI, the impact of radiation dose on treatment benefit cannot be assessed. While these findings need prospective confirmation, a “medulloblastoma”-like treatment with combination of (medulloblastoma) standard dose CSI and maintenance CT may be a reasonable treatment choice for older children with localized disease. The benefit of local irradiation for younger children is controversial, with salvage-CSI at the time of relapse as a therapeutic alternative. Given the rarity of the disease, an international registry with prospective clinical data collection may provide a reliable interim source of information until a cooperative, prospective clinical trial can be launched. The observation of frequent late relapses points to the necessity of long-term follow-up.
In agreement with previous publications, survival rates for ETMR were very poor despite intensive multimodal treatment.7,8,52 Response to CT was documented for a subset of patients of our cohort, but most patients showed early treatment-refractory progressions on initial treatment with CT as well as after initial postoperative RT. Frequent early progression and poor OS despite intensive multimodal treatment indicate that innovative treatment approaches are required to improve the outcome of this devastating disease. Therefore, the goal is a prospective evaluation of rationally designed targeted therapies that are based on potential effective drug combinations, identified by molecular sequencing and preclinical analyses.12,23,53–56
The role of upfront RT for treatment of ETMR cannot be determined based on this retrospective series. However, in agreement with further series, most survivors were irradiated.57 The majority of these patients have received CSI, but survivors were also documented within the small group of children, who received local irradiation in combination with dose-intense CT. This may indicate that similar to ATRT addition of upfront local irradiation may be a reasonable treatment for this entity which mainly presents at a very young age.58 According to other publications that describe long-term survival for ETMR patients, 10/50 treated patients in our series were survivors with prolonged follow-up23,59,60. In our series, this was, however, rather influenced by the absence of early progression and not associated with a specific treatment.
Within the heterogeneous group of HGG and other entities, detected in this and previous CNS-PNET re-evaluation series, there are several rare entities with idiosyncratic driving mutations or pathways.38–41,61 Collaborative efforts will be needed to assess the clinical behavior and response to conventional or targeted treatment of these entities. Furthermore, there are still tumors that cannot be reliably classified according to the current DNA methylation-based class prediction algorithm (version 11b4).20 This cohort likely represents a mix of rare and heterogenous neuroepithelial and mesenchymal tumors. Survival of the respective patients was moderate and superior to previously reported CNS-PNET series, which is likely explained by exclusion of poor-prognostic HGG of this cohort. Further improvement of diagnostic classification is required for appropriate assignment of treatment strategy.
The evidence of our data is limited by the retrospective design of the study, the long period of initial patient diagnosis, and the variability in applied treatment. However, due to the novelty of the diagnostic delineation of these entities, there are no prospective data within this scope available.
Our data proof the relevance of molecular diagnostic differentiation and development of entity-specific, prospective trials for CNS NB-FOXR2 and ETMR. Our data may serve as baseline data required for the setup of respective trials.
Supplementary Material
Acknowledgment
The authors thank all clinicians and involved data managers for the contribution of data as well as all pathologists who have sent the cases for central review and molecular analysis.
Funding
The work has been funded by the German Childhood Cancer Foundation (Deutsche Kinderkrebsstiftung) and KINDerLEBEN e.V. Berlin.
Conflict of interest statement. There is no conflict of interest declared by any of the authors.
Authorship statement. Study concept: K.v.H., C.Haberler, B.P., E.I.H., C.D., S.R., S.M.P., D.C., T.P., and M.K. Study coordination and manuscript writing: K.v.H., C.Haberler, T.P., and M.K. Figures: K.v.H., F.S.H., M.W., and M.K. DNA methylation profiling, classification and interpretation: M.K., F.S.H., K.O., A.v.D., and D.C. Neuropathology panel review: C.Haberler., F.G., C.Hawkins., D.F.B., C.E., P.B., M.Gessi, A.K., T.S.J., and T.P. Provision of samples, clinical data, and clinical interpretation: E.S., T.d.R., S.J., M.Z., D.Sumerauer, M.P.P., C.D., D.v.V., I.S., J.G., J.C.P., N.U.G., M.Massimino, M.J.G.C., M.Garami, E.K., A.S., D.Scheie, O.C., L.M., J.C., B.Z., N.B., M.G., D.A., M.S., O.Z., A.G., M.Mynarek, B.O.J., S.R., U.S., P.B., B.v.Z., E.I.H., E.J.R., M.R., P.H., M.Ł., and P.W. Curation of data: K.v.H. and M.K. Statistical analyses: K.v.H., F.K., M.W., and R.K. All authors contributed and reviewed the final manuscript.
References
- 1.Louis DN, Ohgaki H, Wiestler O, et al. WHO Classification of Tumours of the Central Nervous System. Revised 4th ed. Lyon: International Agency for Research on Cancer; 2016. [Google Scholar]
- 2.Schwalbe EC, Hayden JT, Rogers HA, et al. Histologically defined central nervous system primitive neuro-ectodermal tumours (CNS-PNETs) display heterogeneous DNA methylation profiles and show relationships to other paediatric brain tumour types. Acta Neuropathol. 2013;126(6):943–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturm D, Orr BA, Toprak UH, et al. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell. 2016;164(5):1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris SP, Velazquez Vega J, Aboian M, et al. High-grade neuroepithelial tumor with BCOR exon 15 internal tandem duplication-a comprehensive clinical, radiographic, pathologic, and genomic analysis. Brain Pathol. 2020;30(1):46–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tauziède-Espariat A, Pagès M, Roux A, et al. ; RENOCLIP-LOC . Pediatric methylation class HGNET-MN1: unresolved issues with terminology and grading. Acta Neuropathol Commun. 2019;7(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Łastowska M, Trubicka J, Sobocińska A, et al. Molecular identification of CNS NB-FOXR2, CNS EFT-CIC, CNS HGNET-MN1 and CNS HGNET-BCOR pediatric brain tumors using tumor-specific signature genes. Acta Neuropathol Commun. 2020;8(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korshunov A, Sturm D, Ryzhova M, et al. Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol. 2014;128(2):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spence T, Sin-Chan P, Picard D, et al. CNS-PNETs with C19MC amplification and/or LIN28 expression comprise a distinct histogenetic diagnostic and therapeutic entity. Acta Neuropathol. 2014;128(2):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfister S, Remke M, Castoldi M, et al. Novel genomic amplification targeting the microRNA cluster at 19q13.42 in a pediatric embryonal tumor with abundant neuropil and true rosettes. Acta Neuropathol. 2009;117(4):457–464. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Lee KF, Lu Y, et al. Frequent amplification of a chr19q13.41 microRNA polycistron in aggressive primitive neuroectodermal brain tumors. Cancer Cell. 2009;16(6):533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uro-Coste E, Masliah-Planchon J, Siegfried A, et al. ETMR-like infantile cerebellar embryonal tumors in the extended morphologic spectrum of DICER1-related tumors. Acta Neuropathol. 2019;137(1):175–177. [DOI] [PubMed] [Google Scholar]
- 12.Lambo S, Gröbner SN, Rausch T, et al. The molecular landscape of ETMR at diagnosis and relapse. Nature. 2019;576(7786):274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizer BL, Weston CL, Robinson KJ, et al. Analysis of patients with supratentorial primitive neuro-ectodermal tumours entered into the SIOP/UKCCSG PNET 3 study. Eur J Cancer. 2006;42(8):1120–1128. [DOI] [PubMed] [Google Scholar]
- 14.Massimino M, Gandola L, Spreafico F, et al. Supratentorial primitive neuroectodermal tumors (S-PNET) in children: a prospective experience with adjuvant intensive chemotherapy and hyperfractionated accelerated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64(4):1031–1037. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich C, von Bueren AO, von Hoff K, et al. Treatment of young children with CNS-primitive neuroectodermal tumors/pineoblastomas in the prospective multicenter trial HIT 2000 using different chemotherapy regimens and radiotherapy. Neuro Oncol. 2013;15(2):224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerber NU, von Hoff K, Resch A, et al. Treatment of children with central nervous system primitive neuroectodermal tumors/pinealoblastomas in the prospective multicentric trial HIT 2000 using hyperfractionated radiation therapy followed by maintenance chemotherapy. Int J Radiat Oncol Biol Phys. 2014;89(4):863–871. [DOI] [PubMed] [Google Scholar]
- 17.Jakacki RI, Burger PC, Kocak M, et al. Outcome and prognostic factors for children with supratentorial primitive neuroectodermal tumors treated with carboplatin during radiotherapy: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62(5):776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang EI, Kool M, Burger PC, et al. Extensive molecular and clinical heterogeneity in patients with histologically diagnosed CNS-PNET treated as a single entity: a report from the children’s oncology group randomized ACNS0332 trial. J Clin Oncol. 2018;36(34):3388–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis DN, Ohgaki H, Wiestler O, Cavenee WK, eds. WHO Classification of Tumours of the Central Nervous System. Lyon: IARC; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holsten T, Lubieniecki F, Spohn M, et al. Detailed clinical and histopathological description of 8 cases of molecularly defined CNS neuroblastomas. J Neuropathol Exp Neurol. 2021;80(1):52–59. [DOI] [PubMed] [Google Scholar]
- 22.Korshunov A, Okonechnikov K, Schmitt-Hoffner F, et al. Molecular analysis of pediatric CNS-PNET revealed nosologic heterogeneity and potent diagnostic markers for CNS neuroblastoma with FOXR2-activation. Acta Neuropathol Commun. 2021;9(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juhnke BO, Gessi M, Gerber NU, et al. Treatment of embryonal tumours with multilayered rosettes with carboplatin/etoposide induction and high-dose chemotherapy within the prospective P-HIT Trial [published online ahead of print April 28, 2021]. Neuro Oncol. doi: 10.1093/neuonc/noab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aridgides PD, Kang G, Mazewski C, Merchant TE. Outcomes after radiation therapy for very young children with high-risk medulloblastoma or supratentorial primitive neuroectodermal tumor treated on COG ACNS0334. Int J Radiat Oncol Biol Phys. 2019;105(1):S109. [Google Scholar]
- 25.Dufour C, Kieffer V, Varlet P, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk medulloblastoma or supratentorial primitive neuro-ectodermic tumors. Pediatr Blood Cancer. 2014;61(8):1398–1402. [DOI] [PubMed] [Google Scholar]
- 26.Fangusaro J, Finlay J, Sposto R, et al. Intensive chemotherapy followed by consolidative myeloablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) in young children with newly diagnosed supratentorial primitive neuroectodermal tumors (sPNETs): report of the Head Start I and II experience. Pediatr Blood Cancer. 2008;50(2):312–318. [DOI] [PubMed] [Google Scholar]
- 27.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 28.Geyer JR, Sposto R, Jennings M, et al. ; Children’s Cancer Group . Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. [DOI] [PubMed] [Google Scholar]
- 29.Grundy RG, Wilne SH, Robinson KJ, et al. ; Children’s Cancer and Leukaemia Group (formerly UKCCSG) Brain Tumour Committee . Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: results of the first UKCCSG/SIOP CNS 9204 trial. Eur J Cancer. 2010;46(1):120–133. [DOI] [PubMed] [Google Scholar]
- 30.Lannering B, Rutkowski S, Doz F, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012;30(26):3187–3193. [DOI] [PubMed] [Google Scholar]
- 31.Massimino M, Gandola L, Biassoni V, et al. Evolving of therapeutic strategies for CNS-PNET. Pediatr Blood Cancer. 2013;60(12):2031–2035. [DOI] [PubMed] [Google Scholar]
- 32.Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children’s Cancer Group Study. J Clin Oncol. 1999;17(7):2127–2136. [DOI] [PubMed] [Google Scholar]
- 33.Perek D, Perek-Polnik M, Drogosiewicz M, Dembowska-Bagińska B, Barszcz S. [Treatment results of patients over 3 years of age with medulloblastoma]. Med Wieku Rozwoj. 2003;7(2):201–210. [PubMed] [Google Scholar]
- 34.Szentes A, Erős N, Kekecs Z, et al. Cognitive deficits and psychopathological symptoms among children with medulloblastoma. Eur J Cancer Care (Engl). 2018;27(6):e12912. [DOI] [PubMed] [Google Scholar]
- 35.Hoff KV, Hinkes B, Gerber NU, et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer. 2009;45(7):1209–1217. [DOI] [PubMed] [Google Scholar]
- 36.Timmermann B, Kortmann RD, Kühl J, et al. Role of radiotherapy in supratentorial primitive neuroectodermal tumor in young children: results of the German HIT-SKK87 and HIT-SKK92 trials. J Clin Oncol. 2006;24(10):1554–1560. [DOI] [PubMed] [Google Scholar]
- 37.Chang CH, Housepian EM, Herbert C Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. [DOI] [PubMed] [Google Scholar]
- 38.Korshunov A, Capper D, Reuss D, et al. Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol. 2016;131(1):137–146. [DOI] [PubMed] [Google Scholar]
- 39.Clarke M, Mackay A, Ismer B, et al. Infant high-grade gliomas comprise multiple subgroups characterized by novel targetable gene fusions and favorable outcomes. Cancer Discov. 2020;10(7):942–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reuss DE, Kratz A, Sahm F, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015;130(3):407–417. [DOI] [PubMed] [Google Scholar]
- 41.Korshunov A, Schrimpf D, Ryzhova M, et al. H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol. 2017;134(3):507–516. [DOI] [PubMed] [Google Scholar]
- 42.Deng MY, Sill M, Sturm D, et al. Diffuse glioneuronal tumour with oligodendroglioma-like features and nuclear clusters (DGONC) - a molecularly defined glioneuronal CNS tumour class displaying recurrent monosomy 14. Neuropathol Appl Neurobiol. 2020;46(5):422–430. [DOI] [PubMed] [Google Scholar]
- 43.Koelsche C, Mynarek M, Schrimpf D, et al. Primary intracranial spindle cell sarcoma with rhabdomyosarcoma-like features share a highly distinct methylation profile and DICER1 mutations. Acta Neuropathol. 2018;136(2):327–337. [DOI] [PubMed] [Google Scholar]
- 44.Stensvold E, Krossnes BK, Lundar T, et al. Outcome for children treated for medulloblastoma and supratentorial primitive neuroectodermal tumor (CNS-PNET) - a retrospective analysis spanning 40 years of treatment. Acta Oncol. 2017;56(5):698–705. [DOI] [PubMed] [Google Scholar]
- 45.Choi SH, Kim SH, Shim KW, et al. Treatment outcome and prognostic molecular markers of supratentorial primitive neuroectodermal tumors. PLoS One. 2016;11(4):e0153443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Rojas T, Bautista F, Flores M, et al. Management and outcome of children and adolescents with non-medulloblastoma CNS embryonal tumors in Spain: room for improvement in standards of care. J Neurooncol. 2018;137(1):205–213. [DOI] [PubMed] [Google Scholar]
- 47.Horten BC, Rubinstein LJ. Primary cerebral neuroblastoma. A clinicopathological study of 35 cases. Brain. 1976;99(4):735–756. [DOI] [PubMed] [Google Scholar]
- 48.Bennett JP Jr, Rubinstein LJ. The biological behavior of primary cerebral neuroblastoma: a reappraisal of the clinical course in a series of 70 cases. Ann Neurol. 1984;16(1):21–27. [DOI] [PubMed] [Google Scholar]
- 49.Bianchi F, Tamburrini G, Gessi M, Frassanito P, Massimi L, Caldarelli M. Central nervous system (CNS) neuroblastoma. A case-based update. Childs Nerv Syst. 2018;34(5):817–823. [DOI] [PubMed] [Google Scholar]
- 50.Ashley DM, Merchant TE, Strother D, et al. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s Oncology Group study P9934. J Clin Oncol. 2012;30(26):3181–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mynarek M, von Hoff K, Pietsch T, et al. Nonmetastatic medulloblastoma of early childhood: results from the prospective clinical trial HIT-2000 and an extended validation cohort. J Clin Oncol. 2020;38(18):2028–2040. [DOI] [PubMed] [Google Scholar]
- 52.Horwitz M, Dufour C, Leblond P, et al. Embryonal tumors with multilayered rosettes in children: the SFCE experience. Childs Nerv Syst. 2016;32(2):299–305. [DOI] [PubMed] [Google Scholar]
- 53.Sin-Chan P, Mumal I, Suwal T, et al. A C19MC-LIN28A-MYCN oncogenic circuit driven by hijacked super-enhancers is a distinct therapeutic vulnerability in ETMRs: a lethal brain tumor. Cancer Cell. 2019;36(1):51–67.e7. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt C, Schubert NA, Brabetz S, et al. Preclinical drug screen reveals topotecan, actinomycin D, and volasertib as potential new therapeutic candidates for ETMR brain tumor patients. Neuro Oncol. 2017;19(12):1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann JE, Wefers AK, Lambo S, et al. A mouse model for embryonal tumors with multilayered rosettes uncovers the therapeutic potential of Sonic-hedgehog inhibitors. Nat Med. 2017;23(10):1191–1202. [DOI] [PubMed] [Google Scholar]
- 56.Kleinman CL, Gerges N, Papillon-Cavanagh S, et al. Fusion of TTYH1 with the C19MC microRNA cluster drives expression of a brain-specific DNMT3B isoform in the embryonal brain tumor ETMR. Nat Genet. 2014;46(1):39–44. [DOI] [PubMed] [Google Scholar]
- 57.Jaramillo S, Grosshans DR, Philip N, et al. Radiation for ETMR: literature review and case series of patients treated with proton therapy. Clin Transl Radiat Oncol. 2019;15:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chi SN, Zimmerman MA, Yao X, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27(3):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mozes P, Hauser P, Hortobágyi T, et al. Evaluation of the good tumor response of embryonal tumor with abundant neuropil and true rosettes (ETANTR). J Neurooncol. 2016;126(1):99–105. [DOI] [PubMed] [Google Scholar]
- 60.Hanson D, Hoffman LM, Nagabushan S, et al. A modified IRS-III chemotherapy regimen leads to prolonged survival in children with embryonal tumor with multilayer rosettes. Neurooncol Adv. 2020;2(1):vdaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.