Abstract
Background
Telomerase reverse transcriptase (TERT) is essential for tumor proliferation, including in low-grade oligodendrogliomas (LGOGs). Since TERT is silenced in normal cells, it is also a therapeutic target. Therefore, noninvasive methods of imaging TERT are needed. Here, we examined the link between TERT expression and metabolism in LGOGs, with the goal of leveraging this information for noninvasive magnetic resonance spectroscopy (MRS)-based metabolic imaging of LGOGs.
Methods
Immortalized normal human astrocytes with doxycycline-inducible TERT silencing, patient-derived LGOG cells, orthotopic tumors, and LGOG patient biopsies were studied to determine the mechanistic link between TERT expression and glucose metabolism. The ability of hyperpolarized [U-13C, U-2H]-glucose to noninvasively assess TERT expression was tested in live cells and orthotopic tumors.
Results
TERT expression was associated with elevated glucose flux through the pentose phosphate pathway (PPP), elevated NADPH, which is a major product of the PPP, and elevated glutathione, which is maintained in a reduced state by NADPH. Importantly, hyperpolarized [U-13C, U-2H]-glucose metabolism via the PPP noninvasively reported on TERT expression and response to TERT inhibition in patient-derived LGOG cells and orthotopic tumors. Mechanistically, TERT acted via the sirtuin SIRT2 to upregulate the glucose transporter GLUT1 and the rate-limiting PPP enzyme glucose-6-phosphate dehydrogenase.
Conclusions
We have, for the first time, leveraged a mechanistic understanding of TERT-associated metabolic reprogramming for noninvasive imaging of LGOGs using hyperpolarized [U-13C, U-2H]-glucose. Our findings provide a novel way of imaging a hallmark of tumor immortality and have the potential to improve diagnosis and treatment response assessment for LGOG patients.
Keywords: gliomas, glucose metabolism, hyperpolarized 13C, magnetic resonance spectroscopy, telomerase
Key Points.
TERT expression mechanistically reprograms glucose metabolism via the pentose phosphate pathway.
TERT-linked metabolic reprogramming can be leveraged for noninvasive glioma imaging using hyperpolarized 13C-glucose.
Importance of the Study.
MRI is the mainstay of glioma imaging. However, MRI fails to distinguish true tumor from morphologically similar areas of gliosis, edema, and necrosis. MRI also does not distinguish tumor recurrence from treatment-induced effects such as pseudoprogression and pseudoresponse. Therefore, there is a need to identify noninvasive methods that track molecular hallmarks of tumor proliferation. Telomere maintenance is one such hallmark and most cancers, including LGOGs, maintain telomere length via TERT expression. Here, we show that TERT acts via the sirtuin SIRT2 to reprogram glucose metabolism through the PPP, resulting in elevated levels of the redox metabolites NADPH and glutathione in LGOGs. Importantly, we have leveraged this information for noninvasive hyperpolarized [U-13C, U-2H]-glucose-based imaging of TERT expression in patient-derived LGOG models in vivo. Our study enables noninvasive visualization of a hallmark of tumor proliferation and has the potential to improve longitudinal analysis of tumor burden and treatment response in LGOG patients.
Telomere maintenance is a fundamental hallmark of cancer.1 Telomeres are specialized cap-like nucleoprotein structures that protect chromosomal ends from DNA damage.2 Progressive telomere shortening during cell division leads to growth arrest and constitutes a barrier to uncontrolled proliferation.1,2 Tumor cells, therefore, need a mechanism of maintaining telomere length. Most tumors maintain telomere length by reactivating the expression of telomerase reverse transcriptase (TERT), which is the catalytic component of the enzyme telomerase that synthesizes telomeric DNA.2,3 TERT expression is silenced at birth in normal somatic cells, with the exception of stem cells, and is reactivated in tumor cells via TERT promoter mutations.3–5 Importantly, TERT promoter mutations are a prerequisite for rapid glioma growth in vivo,6 further underscoring the crucial role of TERT in glioma proliferation.
The clinical relevance of TERT arises from its diagnostic, prognostic, and therapeutic potential. Among low-grade gliomas defined by mutations in isocitrate dehydrogenase (IDHmut),7 TERT promoter mutations occur frequently in low-grade oligodendrogliomas (LGOGs) but rarely in low-grade astrocytomas,7,8 which use a TERT-independent telomere maintenance mechanism known as the alternative lengthening of telomeres (ALT) pathway.9,10 As a result, assessment of TERT status is a valuable addition to glioma classification.11 Research also suggests that clinically meaningful patient prognosis can be achieved on the basis of three molecular markers, ie, IDHmut, 1p19q co-deletion, and TERT promoter mutations.8 TERT is also an attractive therapeutic target since TERT expression is silenced in normal cells or expressed in a tightly regulated manner from the wild-type TERT promoter in stem cells.2,3 Since TERT promoter mutations are specific to tumor cells,3 disrupting TERT expression from the mutant TERT promoter has therapeutic potential.5
Previous studies have linked TERT expression to metabolic reprogramming in cancer.12 TERT inhibition in melanoma cells downregulates glucose transport and glycolysis.13 In primary glioblastoma cells, pharmacological or genetic inhibition of TERT abrogates glucose flux through the pentose phosphate pathway (PPP)14 and represses fatty acid synthesis.15 TERT has also been linked to altered cellular redox via modulation of reduced glutathione (GSH).16,17
Magnetic resonance spectroscopy (MRS) is a safe, non-radioactive, noninvasive method of imaging metabolism.18 Thermally polarized 13C-MRS following administration of 13C-labeled precursors traces metabolic fluxes, but its inherently low sensitivity limits its translational value.19 However, hyperpolarization enhances the signal-to-noise ratio (SNR) of 13C-MRS by >10000-fold and provides a noninvasive method of imaging metabolic fluxes.18 Importantly, hyperpolarized 13C-MRS is a translational method that is in clinical trials,18 and its feasibility has been established in glioma patients.19,20
Due to its inherent link to tumor proliferation, TERT has the potential to serve as a biomarker of tumor burden and response to therapy. The objective of this study was to examine the link between TERT expression and glucose metabolism in LGOGs with the ultimate goal of leveraging this information for noninvasive MRS-based metabolic imaging. Using genetically engineered and patient-derived LGOG models, we show that TERT expression is mechanistically associated with elevated PPP flux and higher antioxidant capacity. Importantly, we show that hyperpolarized [U-13C, U-2H]-glucose noninvasively reports on TERT expression in LGOG models in vivo.
Materials and Methods
Detailed experimental procedures are provided in the Supplementary material.
Cell Culture
Immortalized IDHmut-expressing p53/pRb-deficient normal human astrocytes (NHACONTROL) have been previously described.21 The doxycycline-inducible NHATERT+ model was generated by engineering NHACONTROL cells such that TERT expression was regulated from a tetracycline (TET)-off promoter and could be silenced upon doxycycline addition (0.4 µg/mL). RNA interference was carried out using two non-overlapping siRNA pools (Dharmacon). Human SIRT2 (Addgene) was overexpressed in NHATERT− cells by transient transfection with polyethyleneimine. NHAs that lack TERT and use the ALT pathway (NHAALT) have been previously described.22 The ALT status of NHAALT cells was confirmed by measuring c-circles using telomeric qPCR with and without amplification by φ29 polymerase as described.9,23 BT54 neurospheres and SF10417 cells were derived from LGOG patients and are grown in serum-free media.24–27 Cell lines were routinely tested for mycoplasma contamination, authenticated by short tandem repeat fingerprinting, and assayed within 6 months.
Patient Biopsies
Biopsies from LGOG or astrocytoma patients or gliosis biopsies from epileptic patients were obtained from the UCSF Brain Tumor Center Biorepository in compliance with the informed consent policy.
Gene Expression and Activity
Steady-state metabolite levels (NADPH, NADP+, GSH, oxidized glutathione [GSSG], 6-phosphogluconate, lactate, reactive oxygen species [ROS]) and enzyme activities (telomerase, glucose-6-phosphate dehydrogenase [G6PDH], phosphoglycerate kinase 1 [PGK1], hexokinase) were determined using commercial kits. γH2AX and GLUT1 protein levels were measured by ELISA. Gene expression was assessed by quantitative RT-PCR and normalized to β-actin (Supplementary Methods).
13C-MRS of Cell Extracts
Cells were cultured in a medium containing 5 mM [2-13C]-glucose for 48 hours and metabolites extracted by the methanol-chloroform method.26,2713C-MR (30° flip angle, 3 second relaxation delay) spectra were obtained using a 11.7T spectrometer and peak integrals quantified to obtain metabolite concentrations. PPP flux was measured by quantifying the isotopomers of glutamate generated after PPP and glycolytic metabolism of [2-13C]-glucose.6 Glucose levels in media samples were quantified and uptake calculated as the difference in normalized fmol (Δfmol/cell) between 0 and 24 hours.
Hyperpolarized 13C-MRS of Live Cells
Hyperpolarized [U-13C, U-2H]-glucose prepared as described28 was dissolved in isotonic buffer (50 mM Tris-HCl, 13.3 mM MgCl2 in D2O, pH 8) and added to live cells23 at a concentration of 12 mM. 13C spectra were acquired on a 14T Bruker spectrometer (13° flip angle every 3 seconds for 300 seconds), and the area under the curve (AUC) for product was normalized to AUC of substrate and to cell number.
MRI
Animal studies were conducted in accordance with UCSF Institutional Animal Care and Use Committee guidelines. Doxycycline-inducible NHATERT+, NHAALT, or SF10417 cells (3 × 105 cells/10 µL) were intracranially injected into athymic nude rats (male, rnu/rnu homozygous).23,26,27 T2-weighted MRI was performed on a 3T Bruker scanner using a spin-echo TurboRARE sequence (TE/TR = 64/3484 ms, FOV = 43 × 43 mm,2 256 × 256, slice thickness = 1 mm, NA = 6).23 Tumor volume was determined using in-house MATLAB codes (https://github.com/ViswanathLab/EPSI).23 For doxycycline-mediated TERT silencing in vivo, doxycycline was administered in the feed ad libitum once tumors reached a volume of ~50 mm3 as previously described.29
Hyperpolarized 13C-MRS in vivo
Studies were performed on a Bruker 3T spectrometer. Following tumor implantation, tumor volume was monitored longitudinally by MRI and 13C studies were performed once tumors reached a volume of ~50 mm.3 For slab studies, dynamic 13C spectra were acquired from a 12 mm axial slab through the brain every 3 seconds using a flyback spectral-spatial radiofrequency pulse30 following the intravenous injection of 150 mM hyperpolarized glucose. For imaging, a 2D flyback spectral-spatial echo-planar spectroscopic imaging (EPSI) pulse30 was used with a final hyperpolarized glucose concentration of 250 mM. 13C slab spectra were analyzed using Mnova. 2D EPSI data were analyzed using in-house MATLAB codes (https://github.com/ViswanathLab/EPSI).23
Statistical Analysis
All experiments were performed on a minimum of 3 samples (n ≥ 3) and results presented as mean ± standard deviation. Statistical significance was assessed using an unpaired Student’s t test or ordinary 1-way ANOVA with P < .05 considered significant. *P < .05, **P < .01, ***P < .005, ****P < .0001, ns = non-significant.
Results
TERT Expression Is Associated With Higher Glucose Flux via the PPP in LGOGs
To begin with, we studied genetically engineered immortalized normal human astrocytes (NHAs) that express IDHmut, which is characteristic of low-grade gliomas7 and examined NHAs that lacked TERT (NHACONTROL) and those in which TERT expression was placed under the control of a doxycycline-inducible promoter. TERT expression and telomerase activity were observed in these cells in the absence of doxycycline (NHATERT+), significantly reduced upon doxycycline addition (NHATERT−) and restored following doxycycline removal (NHATERT+ rescue; Supplementary Figure S1A, B). As additional controls, we also examined NHAs that do not express TERT, but instead use the ALT pathway (NHAALT) for telomere maintenance.22,23 The ALT phenotype of NHAALT cells has previously been verified via quantification of c-circles, which are extrachromosomal DNA circles characteristic of the ALT pathway22,23 (Supplementary Figure S1C).
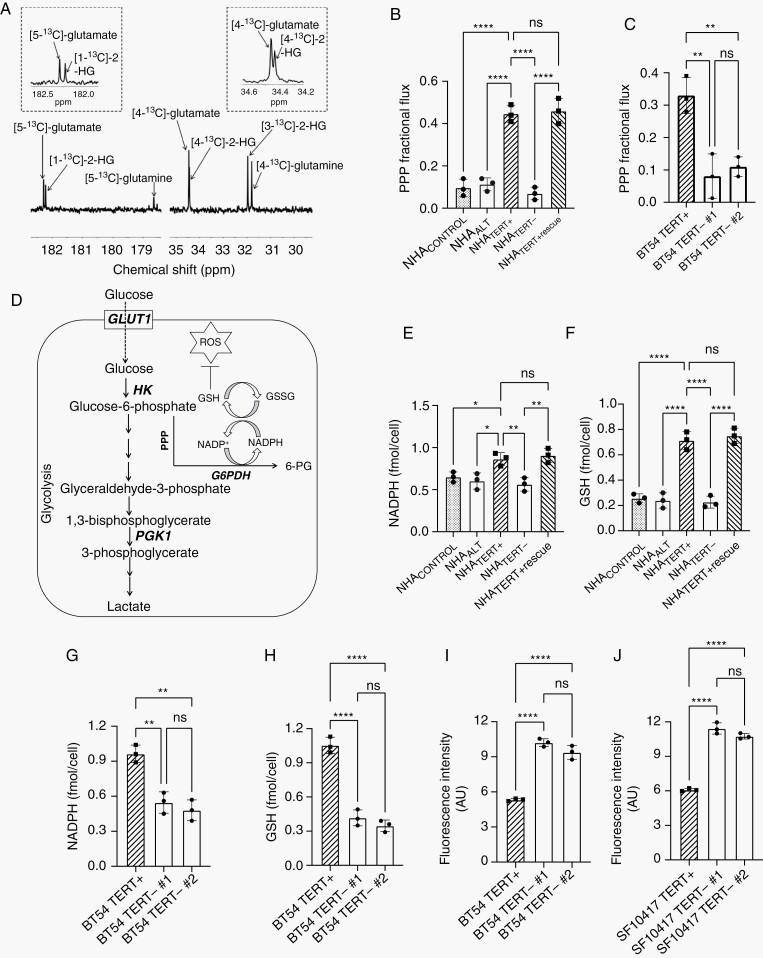
Thermally polarized 13C-MRS following administration of [2-13C]-glucose can inform on flux via the PPP, which produces [4-13C]-glutamate, and via glycolysis which yields [5-13C]-glutamate (Figure 1A).31 PPP flux was significantly higher in NHATERT+ cells relative to NHACONTROL and NHAALT (Figure 1B). Importantly, doxycycline-induced TERT silencing reduced PPP flux in NHATERT− cells to levels observed in NHACONTROL and NHAALT, an effect that was reversed in NHATERT+ rescue cells. In order to confirm the clinical relevance of these results, we examined PPP flux in the patient-derived BT54 and SF10417 models.25–27 We confirmed elevated TERT expression and telomerase activity in BT54 and SF10417 cells relative to NHACONTROL (Supplementary Figure S1D, E). Silencing TERT in BT54 and SF10417 cells (Supplementary Figure S1F–I) significantly reduced PPP flux (Figure 1C and Supplementary Figure S1J), pointing to a causal link between TERT expression and PPP flux in LGOGs.
Fig. 1.
TERT expression is associated with elevated PPP flux in LGOG cells. (A) Representative 13C-MR spectrum from NHATERT+ cells incubated with [2-13C]-glucose. Peaks for glutamate, glutamine, and 2-hydroxyglutarate (2-HG; product of the IDHmut enzyme26,27) are labeled. Insets show an expansion of the regions corresponding to [5-13C]-glutamate (left) and [4-13C]-glutamate (right). (B) PPP flux in NHACONTROL, NHAALT, NHATERT+, NHATERT−, and NHATERT+ rescue cells. (C) Effect of TERT silencing on PPP flux in BT54 neurospheres. (D) Schematic illustration of glucose metabolism via the PPP and glycolysis. NADPH (E) and GSH (F) in NHACONTROL, NHAALT, NHATERT+, NHATERT−, and NHATERT+ rescue cells. Effect of TERT silencing on NADPH (G), GSH (H), and ROS (I) in BT54 neurospheres. Effect of TERT silencing on ROS (J) in SF10417 cells. Abbreviations: GSH, glutathione; LGOG, low-grade oligodendrogliomas; PPP, pentose phosphate pathway; ROS, reactive oxygen species; TERT, telomerase reverse transcriptase.
TERT Expression Is Associated With Higher Antioxidant Capacity in LGOGs
Glucose flux through the PPP is the largest NADPH-producing pathway.32 NADPH, in turn, is essential for maintaining GSH in the reduced state32 (see schematic in Figure 1D). Since our results linked TERT to higher PPP flux, we examined whether there was a concomitant change in NADPH and GSH. In line with PPP flux, NADPH and GSH were elevated in NHATERT+ cells relative to NHACONTROL and NHAALT (Figure 1E, F). Doxycycline-induced TERT silencing reduced NADPH and GSH, an effect that was rescued by doxycycline removal (Figure 1E, F). Importantly, TERT silencing significantly reduced NADPH and GSH in the BT54 (Figure 1G, H) and SF10417 (Supplementary Figure S2A, B) models. There was no change in NADP+ or GSSG in our models (Supplementary Figure S2C–H).
NADPH and GSH mitigate oxidative stress via suppression of ROS (see Figure 1D). TERT silencing significantly increased ROS in BT54 and SF10417 cells (Figure 1I, J). Conversely, TERT expression was associated with reduced ROS in the NHATERT+ model (Supplementary Figure S2I). Taken together, these results link TERT expression to higher redox capacity and reduced oxidative stress in LGOGs.
Hyperpolarized [U-13C, U-2H]-Glucose Noninvasively Monitors TERT Expression in LGOG Cells
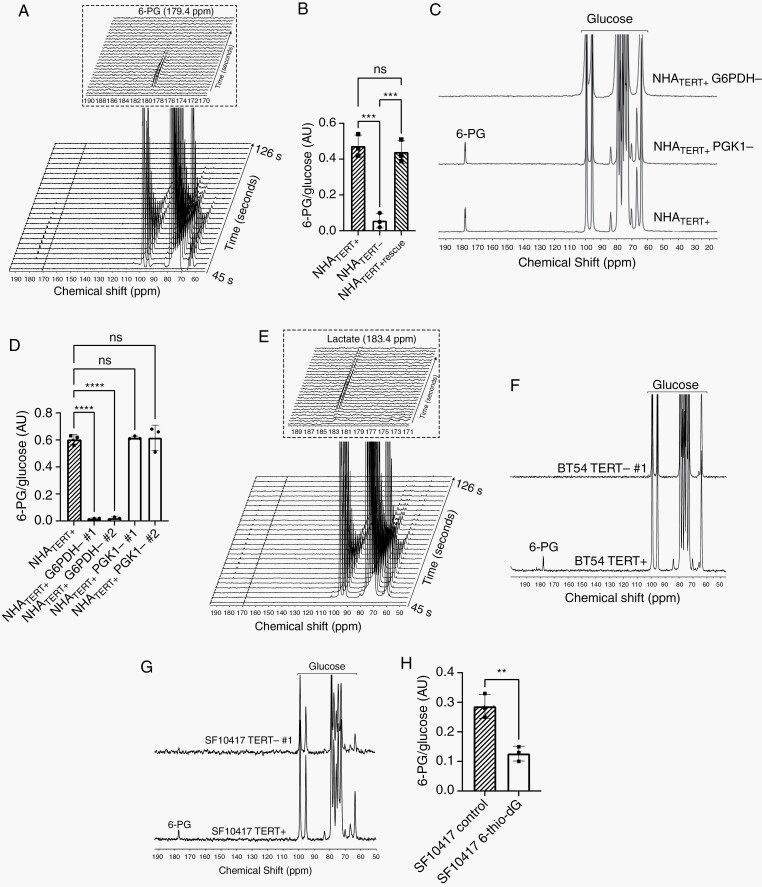
Hyperpolarized [U-13C, U-2H]-glucose has been used to probe glucose metabolism via glycolysis and the PPP in tumor cells other than gliomas.28,33–36 Since our results linked TERT to higher PPP flux, we examined hyperpolarized [U-13C, U-2H]-glucose (T1 = 14 ± 2 seconds, 24% polarization, consistent with prior publications28,33–36) metabolism in our models. As shown in Figure 2A, we observed buildup of the PPP metabolite [1-13C]-6-phosphogluconate (6-PG, 179.4 ppm; see schematic in Figure 1D) in NHATERT+ cells. We did not observe [1-13C]-lactate (183.4 ppm). Importantly, doxycycline-induced TERT silencing significantly reduced 6-PG production, and restoration of TERT expression via doxycycline removal restored 6-PG production (Figure 2B). The chemical shift of [1-13C]-6-PG (179.4 ppm) is indistinguishable from that of [1-13C]-3-phosphoglycerate (179.5 ppm) which can also be produced from glucose via glycolysis (see Figure 1D). Previous studies have disagreed on the assignment of this resonance and have variously assigned it to either 6-PG28,33,35 or 3-phosphoglycerate.34,36 In order to determine whether the resonance observed at 179.4 ppm in our experiments corresponded to 6-PG, we examined hyperpolarized [U-13C, U-2H]-glucose metabolism in NHATERT+ cells in which expression of G6PDH, the enzyme responsible for 6-PG synthesis (see Figure 1D), was silenced (Supplementary Figure S3A, B). As shown in Figure 2C, D, the resonance at 179.4 ppm was lost in NHATERT+ G6PDH− cells. In contrast, the 179.4 ppm peak was observed in NHATERT+ cells in which PGK1, the glycolytic enzyme catalyzing 3-phosphoglycerate synthesis (see Figure 1D), was silenced (Supplementary Figure S3C, D), suggesting that 179.4 ppm peak likely corresponds to 6-PG.
Fig. 2.
TERT-linked increase in PPP flux can be noninvasively monitored using hyperpolarized [U-13C, U-2H]-glucose in live LGOG cells. (A) Representative spectral array of hyperpolarized [U-13C, U-2H]-glucose metabolism to 6-PG in NHATERT+ cells. Inset shows an expansion of the region around 6-PG. (B) 6-PG/glucose ratio in the doxycycline-inducible NHATERT+ model. Representative summed 13C-MR spectra (C) and quantification of 6-PG production from hyperpolarized [U-13C, U-2H]-glucose (D) in NHATERT+ cells in which G6PDH or PGK1 has been silenced. (E) Representative spectral array of hyperpolarized [U-13C, U-2H]-glucose metabolism to lactate in NHAALT cells. Inset shows an expansion of the region around lactate. Representative summed 13C spectra showing the effect of TERT silencing on hyperpolarized [U-13C, U-2H]-glucose metabolism in the BT54 (F) or SF10417 (G) models. (H) Effect of 6-thio-2′-deoxyguanosine (6-thio-dG) on the 6-PG/glucose ratio in SF10417 cells. Abbreviations: LGOG, low-grade oligodendrogliomas; PPP, pentose phosphate pathway; TERT, telomerase reverse transcriptase.
In contrast to NHATERT+ cells, NHAALT cells produced the glycolytic metabolite lactate (183.4 ppm, Figure 2E), but not 6-PG, from hyperpolarized [U-13C, U-2H]-glucose. In order to further confirm these results, we quantified 6-PG and lactate by spectrophotometric assays in extracts from cell suspensions that were rapidly frozen at the end of our hyperpolarized 13C-MRS experiments. We detected 6-PG but not lactate in NHATERT+ extracts and lactate but not 6-PG in NHAALT extracts (Supplementary Figure S3E). NHATERT− extracts showed significantly reduced 6-PG relative to NHATERT+, but lactate could not be detected. Silencing G6PDH, but not PGK1, in NHATERT+ cells reduced 6-PG production (Supplementary Figure S3F). We did not observe lactate in extracts from NHATERT+ G6PDH− or PGK− cells (Supplementary Figure S3G). These results suggest that, within the temporal window of the hyperpolarized 13C-MRS experiment (~5 minutes), hyperpolarized [U-13C, U-2H]-glucose metabolism to 6-PG distinguishes TERT-expressing cells from cells lacking TERT.
We confirmed the clinical relevance of these results in the patient-derived BT54 and SF10417 models. TERT silencing abrogated 6-PG production from hyperpolarized [U-13C, U-2H]-glucose in both models (Figure 2F, G and Supplementary Figure S3H, I). To assess whether hyperpolarized [U-13C, U-2H]-glucose provides a readout of response to TERT inhibition, we examined SF10417 cells treated with the telomerase inhibitor 6-thio-2′-deoxyguanosine.37 Induction of DNA damage (Supplementary Figure S3J) was associated with significantly reduced 6-PG production from hyperpolarized [U-13C, U-2H]-glucose in 6-thio-2′-deoxyguanosine-treated SF10417 cells (Figure 2H). Collectively, these results suggest that hyperpolarized [U-13C, U-2H]-glucose flux to 6-PG reports on TERT expression and response to telomerase inhibition in LGOG cells.
Hyperpolarized [U-13C, U-2H]-Glucose Metabolism to 6-PG Noninvasively Monitors TERT Expression in LGOGs In Vivo
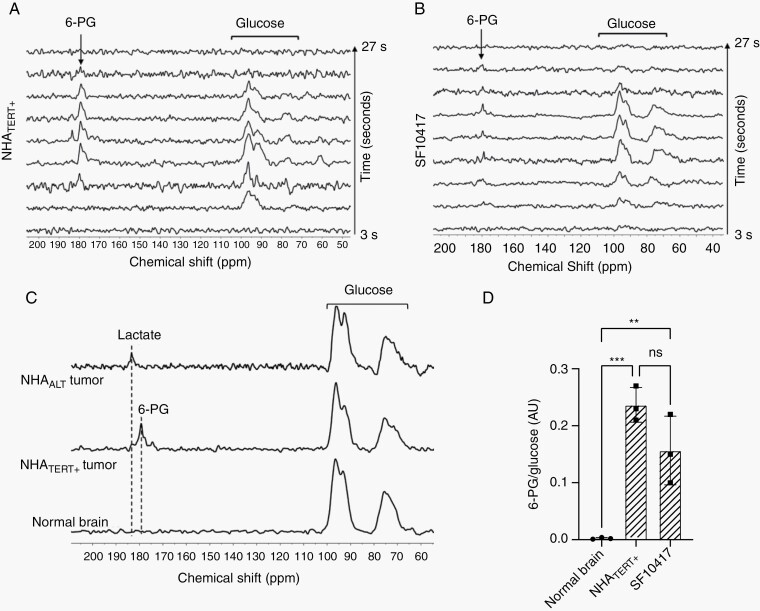
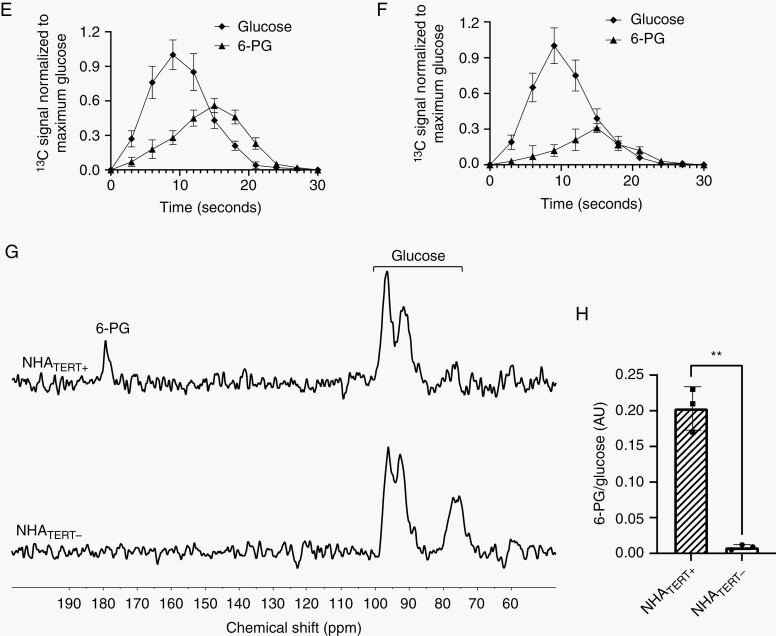
Next, we examined the feasibility of imaging TERT in vivo using hyperpolarized [U-13C, U-2H]-glucose. Dynamic 13C spectra were acquired from a 12 mm slab of the brain following intravenous injection of hyperpolarized [U-13C, U-2H]-glucose into rats bearing orthotopic NHATERT+, SF10417, or NHAALT tumors (tumor volume ~50 mm3). Consistent with our cell studies, we observed buildup of [1-13C]-6-PG in NHATERT+ and SF10417 tumor-bearing animals (Figure 3A, B), while NHAALT tumor-bearing rats produced [1-13C]-lactate (Supplementary Figure S4A). Tumor-free controls produced neither 6-PG nor lactate (Supplementary Figure S4B). These differences are highlighted in the summed 13C spectra shown in Figure 3C. The 6-PG/glucose ratio differentiated NHATERT+ or SF10417 tumors from healthy brain (Figure 3D) while the lactate/glucose ratio differentiated NHAALT tumors from healthy brain (Supplementary Figure S4C). The temporal maximum (9 seconds post-injection; Figure 3E, F and Supplementary Figure S4D, E) and the SNR (Supplementary Figure S4F) of hyperpolarized [U-13C, U-2H]-glucose were similar in NHATERT+, SF10417, or NHAALT tumor-bearing animals and tumor-free controls, indicating that the observed differences in metabolism did not result from differences in glucose delivery. The maxima for 6-PG (15 seconds post-injection; Figure 3E, F) in TERT-expressing tumors and for lactate (21 seconds post-injection; Supplementary Figure S4D) in NHAALT tumors were delayed relative to glucose. Taken together with the lack of 6-PG or lactate in tumor-free rats, these results suggest that 6-PG or lactate production in tumor-bearing animals originates from metabolism within the tumor.
Fig. 3.
Hyperpolarized [U-13C, U-2H]-glucose metabolism can be noninvasively monitored in vivo. Representative 13C spectral array of hyperpolarized [U-13C, U-2H]-glucose metabolism in a rat bearing an orthotopic NHATERT+ (A) or SF10417 (B) tumor. (C) Representative summed 13C spectra of hyperpolarized [U-13C, U-2H]-glucose metabolism in rats bearing orthotopic NHATERT+ or NHAALT tumors or tumor-free rats. (D) 6-PG/glucose ratio from slab studies in tumor-free normal brain, NHATERT+ or SF10417 tumors. Dynamics of hyperpolarized [U-13C, U-2H]-glucose metabolism in rats bearing orthotopic NHATERT+ (E) or SF10417 (F) tumors. Representative summed 13C spectra (G) and quantification of 6-PG production from hyperpolarized [U-13C, U-2H]-glucose (H) in rats bearing orthotopic NHATERT+ tumors before (NHATERT+) or after doxycycline-mediated TERT silencing (NHATERT−). Abbreviation: TERT, telomerase reverse transcriptase.
To assess the ability of hyperpolarized [U-13C, U-2H]-glucose to report on response to TERT inhibition in vivo, we silenced TERT expression in a doxycycline-inducible manner in rats bearing orthotopic NHATERT+ tumors once tumors reached a volume of ~50 mm.3 TERT expression and telomerase activity were significantly reduced following doxycycline treatment without alterations in tumor volume (Supplementary Figure S5A–C), consistent with previous studies pointing to a lag period before cell death occurs due to TERT knockdown.38 Importantly, doxycycline-induced TERT silencing abrogated 6-PG production in vivo (Figure 3G, H). There was no difference in the SNR of hyperpolarized glucose (Supplementary Figure S5D), indicating that loss of 6-PG production did not result from differences in glucose delivery.
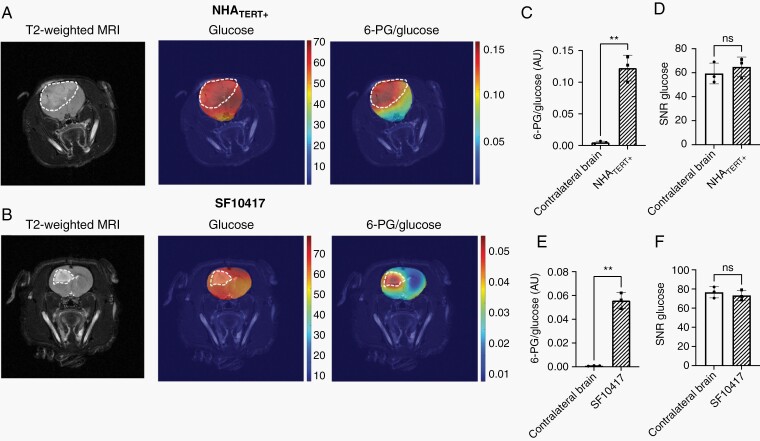
Finally, to assess the spatial distribution of hyperpolarized [U-13C, U-2H]-glucose metabolism, we performed 2D EPSI on rats bearing orthotopic NHATERT+ or SF10417 tumors. Metabolic heatmaps generated by overlaying 13C data acquired at 15 seconds post-injection of hyperpolarized [U-13C, U-2H]-glucose (corresponding to the time-point of maximum 6-PG in slab studies; see Figure 3E, F) on the anatomical MRI indicated localization of 6-PG to the tumor while glucose was distributed throughout the brain (Figure 4A, B). Importantly, consistent with the slab studies, the 6-PG/glucose ratio differentiated tumor from contralateral normal brain while there was no difference in the SNR of hyperpolarized [U-13C, U-2H]-glucose (Figure 4C–F). Collectively, these results suggest that 6-PG production from hyperpolarized [U-13C, U-2H]-glucose is a metabolic imaging biomarker of TERT expression in vivo, including in clinically relevant patient-derived LGOG models.
Fig. 4.
Hyperpolarized [U-13C, U-2H]-glucose noninvasively monitors tumor burden in LGOGs in vivo. (A) Left panel: Representative T2-weighted MRI from an NHATERT+ tumor-bearing rat with the tumor contoured in white. Middle panel: Metabolic heatmap of hyperpolarized [U-13C, U-2H]-glucose distribution overlaid over the corresponding MRI; Right panel: Metabolic heatmap of [1-13C]-6-PG distribution. (B) Left panel: Representative T2-weighted MRI from a rat bearing an orthotopic SF10417 tumor. The tumor is outlined in white. Middle panel: Metabolic heatmap of hyperpolarized [U-13C, U-2H]-glucose distribution overlaid over the corresponding MRI; Right panel: Metabolic heatmap of hyperpolarized 6-PG production. 6-PG/glucose ratio (C) and the SNR of hyperpolarized [U-13C, U-2H]-glucose (D) in rats bearing orthotopic NHATERT+ tumors. 6-PG/glucose ratio (E) and the SNR of hyperpolarized [U-13C, U-2H]-glucose (F) in SF10417 tumor-bearing rats. Abbreviations: LGOG, low-grade oligodendrogliomas; SNR, signal-to-noise ratio.
TERT Acts via SIRT2 to Modulate GLUT1 and G6PDH Expression in LGOGs
To identify the molecular mechanisms by which TERT modulates PPP flux in LGOGs, we interrogated enzymes and transporters involved in glucose metabolism upstream of 6-PG (see schematic in Figure 1D). In line with PPP flux (see Figure 1B), G6PDH expression (Supplementary Figure S6A) and activity (Figure 5A) were downregulated by doxycycline-induced TERT silencing in the NHATERT+ model. Expression of the glucose transporter GLUT1 (Figure 5B and Supplementary Figure S6B) and [2-13C]-glucose uptake (Supplementary Figure S6C) were also reduced in NHATERT− cells. There was no alteration in hexokinase activity (Supplementary Figure S6D). TERT silencing also downregulated GLUT1 and G6PDH in the patient-derived SF10417 model (Supplementary Figure S6E, F). Importantly, GLUT1 and G6PDH were downregulated in ex vivo tumor tissues resected from rats bearing NHATERT− tumors relative to NHATERT+ (Supplementary Figure S6G, H).
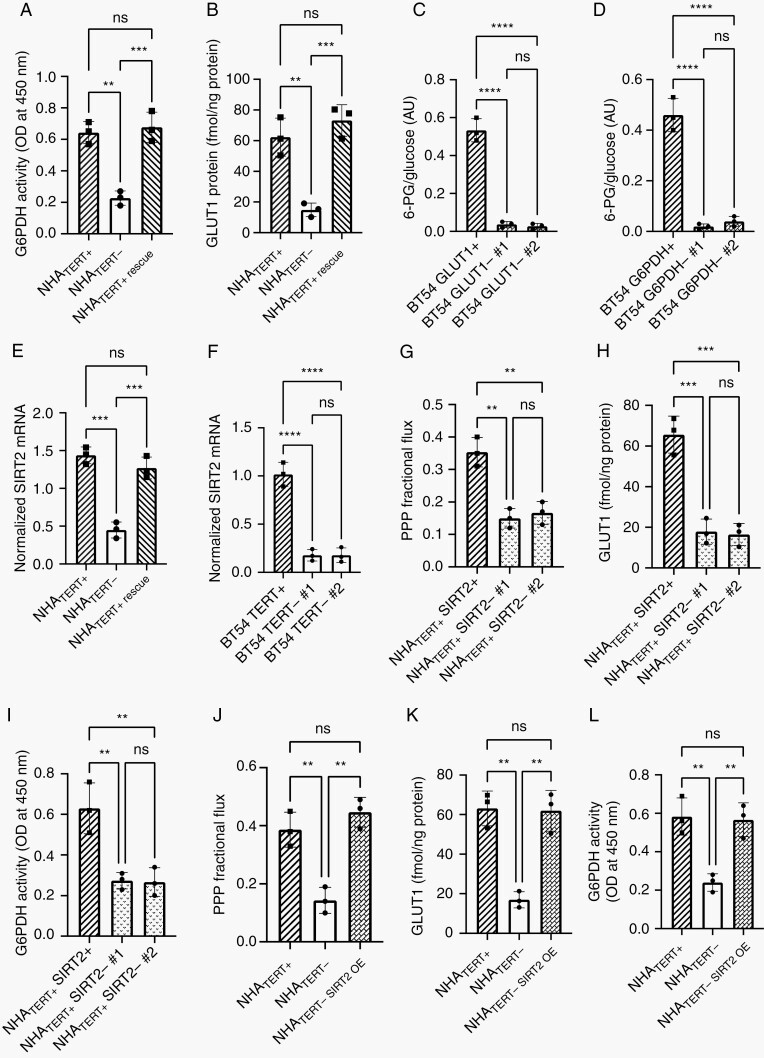
Fig. 5.
TERT mechanistically modulates glucose metabolism in LGOGs. G6PDH activity (A) and GLUT1 protein (B) in NHATERT+, NHATERT−, and NHATERT+ rescue cells. Effect of GLUT1 (C) or G6PDH (D) silencing on 6-PG production from hyperpolarized glucose in BT54 neurospheres. (E) SIRT2 expression in NHATERT+, NHATERT−, and NHATERT+ rescue cells. (F) Effect of TERT silencing on SIRT2 expression in BT54 neurospheres. Effect of SIRT2 silencing on PPP flux (G), GLUT1 protein (H), and G6PDH activity (I) in NHATERT+ cells. Effect of SIRT2 overexpression on PPP flux (J), GLUT1 protein (K), and G6PDH activity (L) in NHATERT− cells (NHATERT-SIRT2 OE). Abbreviations: LGOG, low-grade oligodendrogliomas; PPP, pentose phosphate pathway; TERT, telomerase reverse transcriptase.
To assess the role of GLUT1 and G6PDH in TERT-linked upregulation of PPP flux, we examined the effect of silencing GLUT1 or G6PDH in BT54 neurospheres. GLUT1 silencing (Supplementary Figure S7A) significantly reduced [2-13C]-glucose uptake (Supplementary Figure S7B). Although PPP fractional flux was unaltered (both PPP-derived [4-13C]-glutamate and glycolysis-derived [5-13C]-glutamate were reduced; Supplementary Figure S7C–E), 6-PG production from hyperpolarized [U-13C, U-2H]-glucose was significantly reduced in BT54 GLUT1− neurospheres (Figure 5C). These results suggest that TERT-mediated increase in GLUT1 leads to higher glucose uptake, which, in turn, leads to higher availability of glucose for PPP flux. Silencing G6PDH in BT54 neurospheres (Supplementary Figure S7F) also reduced 6-PG production from hyperpolarized [U-13C, U-2H]-glucose (Figure 5D), in line with results from the NHATERT+ model (Figure 2D).
The sirtuin SIRT2 has been shown to activate G6PDH and enhance NADPH production in mammalian cells.39,40 SIRT2 expression was significantly reduced by doxycycline-mediated TERT silencing in the NHATERT+ model (Figure 5E). TERT silencing also downregulated SIRT2 expression in BT54 neurospheres (Figure 5F). Importantly, silencing SIRT2 in NHATERT+ cells (Supplementary Figure S8A) downregulated PPP flux, GLUT1, and G6PDH (Figure 5G–I). Conversely, SIRT2 overexpression in NHATERT− cells (Supplementary Figure S8B) rescued the reduction in PPP flux, GLUT1, and G6PDH caused by TERT silencing (Figure 5J–L). Collectively, these results mechanistically link TERT to SIRT2, which, in turn, upregulates GLUT1 and G6PDH, resulting in higher glucose uptake and flux via the PPP.
TERT Expression Is Associated With Metabolic Reprogramming in LGOG Patient Biopsies
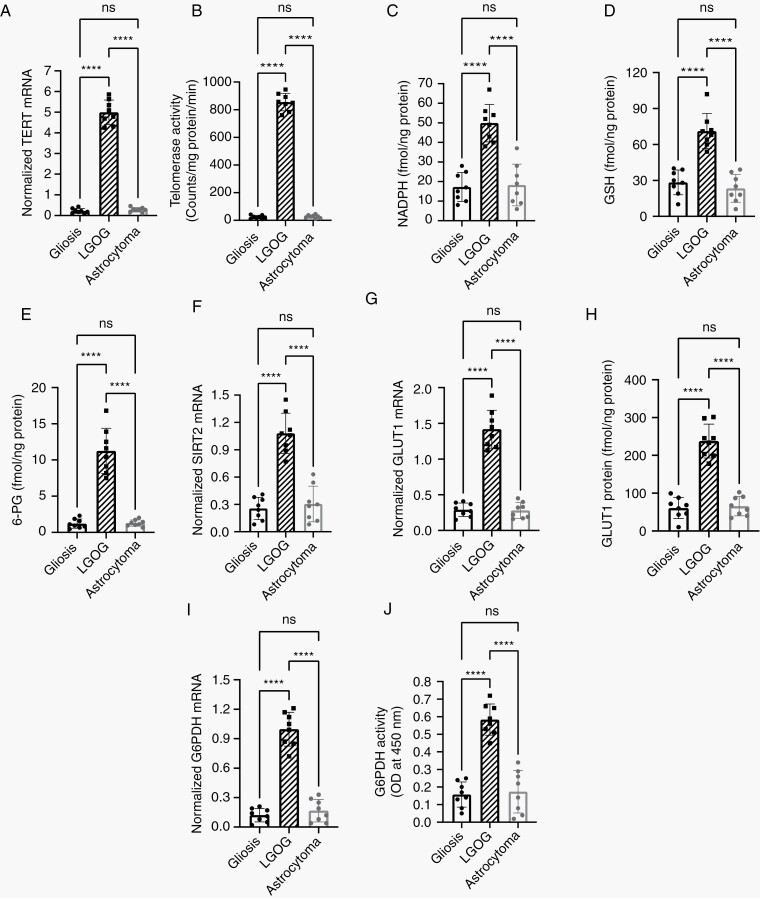
To evaluate the clinical relevance of our metabolic biomarkers, we examined biopsies from LGOG patients and compared them to non-neoplastic gliosis biopsies and biopsies from low-grade astrocytoma tumors that do not express TERT.9,10 In line with the results from preclinical models, TERT expression and telomerase activity in LGOG biopsies (Figure 6A, B) were associated with elevated NADPH, GSH, 6-PG, SIRT2, GLUT1, and G6PDH relative to gliosis and astrocytoma (Figure 6C–J). These results link TERT to glucose metabolism in patient samples and point to the potential clinical utility of hyperpolarized glucose for assessing TERT status.
Fig. 6.
TERT expression is linked to glucose metabolism in LGOG biopsies. TERT mRNA (A), telomerase activity (B), NADPH (C), GSH (D), 6-PG (E), SIRT2 mRNA (F), GLUT1 mRNA (G), GLUT1 protein (H), G6PDH mRNA (I), and G6PDH activity (J) in LGOG, gliosis, and astrocytoma biopsies. Abbreviations: GSH, glutathione; LGOG, low-grade oligodendrogliomas; TERT, telomerase reverse transcriptase.
Discussion
TERT is indispensable for tumor proliferation, including in LGOGs.1,2 Using NHAs that have been engineered to silence TERT in a doxycycline-inducible manner as well as patient-derived LGOG models, we show that TERT upregulates glucose flux via the PPP and elevates antioxidant capacity. Importantly, we show that hyperpolarized [U-13C, U-2H]-glucose flux to 6-PG via the PPP can be used for noninvasive imaging of TERT expression in preclinical LGOG models in vivo.
Although we cannot rule out the possibility that glucose metabolism is modulated by other molecular pathways, our results point to a mechanistic association between TERT and the PPP in LGOGs. TERT silencing downregulates PPP flux, NADPH, and GSH in the NHATERT+ and patient-derived LGOG models. Mechanistically, TERT acts by increasing GLUT1-mediated glucose uptake and upregulating G6PDH, the rate-limiting enzyme for 6-PG and NADPH synthesis. Importantly, the ability of SIRT2 overexpression to restore PPP flux, GLUT1, and G6PDH in NHATERT− cells and, conversely, the reduction in PPP flux, GLUT1, and G6PDH in NHATERT+ cells in which SIRT2 is silenced, identify SIRT2 as a novel mediator of TERT-induced alterations in glucose metabolism.
Studies have used positron emission tomography (PET) or optical imaging for TERT detection.41,42 However, the radioactive nature of PET limits longitudinal imaging due to concerns of radiation exposure while the restricted depth penetration of optical imaging limits application to gliomas. Recent studies have also used MRI combined with radiomics or 1H-MRS to assess TERT status in LGOGs.43,44 Our study identifies a complementary hyperpolarized 13C-glucose-based method of imaging TERT status. 6-PG production from hyperpolarized [U-13C, U-2H]-glucose is consistently associated with TERT expression in LGOG cells and orthotopic tumors in vivo. In contrast, NHAALT cells and tumors that do not express TERT and, instead, use the ALT pathway for telomere maintenance,22,23 produce lactate from hyperpolarized [U-13C, U-2H]-glucose, further confirming the utility of 6-PG production as a biomarker of TERT in LGOGs. Importantly, our results are significant in light of the limited utility of 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) for glioma imaging, which stems from high glucose uptake in the normal brain.45 The ability of 6-PG production to demarcate tumor from contralateral brain in our EPSI studies indicates that monitoring hyperpolarized 13C-glucose metabolism to 6-PG, as opposed to monitoring glucose uptake by FDG-PET, can be useful for imaging glioma burden in vivo.
As an endogenous nutrient that readily crosses the blood-brain barrier46 and is non-toxic at the doses in our study, hyperpolarized [U-13C, U-2H]-glucose has potential for clinical translation. The main limitation is the short T1 (~14 seconds at 3T). However, 6-PG has a relatively longer T1 (~32 seconds at 3T),47 suggesting that clinically useful information can still be derived from the use of hyperpolarized [U-13C, U-2H]-glucose. A recent study48 also achieved ~70% polarization for hyperpolarized [U-13C, U-2H]-glucose (as opposed to ~24% in our study), a method that could significantly improve the SNR. The use of singly labeled [2-13C, U-2H]-glucose36 could also increase SNR since the resulting 6-PG peak would be a singlet.
Although some TERT inhibitors have encountered toxicity issues in clinical trials due to stem cell inhibition,3 next-generation TERT inhibitors such as 6-thio-2′-deoxyguanosine have been successful in preclinical studies and are now in clinical trials.49 Our studies show that 6-thio-2′-deoxyguanosine inhibits 6-PG production from hyperpolarized [U-13C, U-2H]-glucose in the patient-derived SF10417 model. Importantly, doxycycline-induced TERT silencing in vivo abrogates 6-PG production in the NHATERT+ model. These results point to the potential utility of hyperpolarized [U-13C, U-2H]-glucose in monitoring response to TERT inhibitors and, thereby, enabling their clinical translation.
Glioma patient management is heavily dependent on MRI. However, distinguishing tumor from lesions such as gliosis and edema can be difficult using MRI. Importantly, MRI-based treatment response assessment is complicated by the occurrence of pseudoprogression, ie, treatment-related effects such as necrosis and inflammation that mimic tumor recurrence.50 Our studies showing that TERT expression is associated with alterations in glucose metabolism in LGOG patient biopsies relative to gliosis point to the potential utility of metabolic imaging of TERT status for differentiating tumor from gliosis. Further studies in vivo are needed to fully determine the utility of hyperpolarized glucose in differentiating tumors from gliosis, pseudoprogression, or pseudoresponse.
In summary, our study mechanistically links TERT expression to elevated PPP flux and identifies hyperpolarized [U-13C, U-2H]-glucose as a potential metabolic imaging probe of TERT in LGOGs. By enabling noninvasive assessment of a hallmark of tumor immortality, our study has the potential to improve longitudinal tumor imaging and treatment response monitoring in LGOG patients.
Supplementary Material
Funding
This work was supported by: National Institutes of Health (R01CA239288 to P.V., S.M.R.; UCSF SPORE Career Enhancement Award P50CA97257 to P.V.; P01CA118816 to S.M.R., R01NS105087 to R.O.P., P41EB013598 Center grant), Department of Defense (W81XWH201055315 to P.V.), American Cancer Society (131715-RSG-18-005-01-CCE to P.E.Z.L.), NICO.
Conflict of interest statement. The authors have no conflicting interests to disclose.
Authorship statement. P.V. and S.M.R. conceptualized the research; P.V. designed the experiments; P.V., G.B., C.T., and V.A. performed experiments; A.M.G. assisted with cell and in vivo studies; P.E.Z.L. contributed to the design of imaging studies; H.A.L., J.F.C., and R.O.P. provided cell lines; P.V. wrote the manuscript; G.B. commented on the manuscript; P.V., S.M.R., and R.O.P. secured funding.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 2. Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet. 2019;20(5):299–309. [DOI] [PubMed] [Google Scholar]
- 3. Bell RJ, Rube HT, Xavier-Magalhães A, et al. Understanding TERT promoter mutations: a common path to immortality. Mol Cancer Res. 2016;14(4):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akıncılar SC, Khattar E, Boon PL, Unal B, Fullwood MJ, Tergaonkar V. Long-range chromatin interactions drive mutant TERT promoter activation. Cancer Discov. 2016;6(11):1276–1291. [DOI] [PubMed] [Google Scholar]
- 5. Mancini A, Xavier-Magalhaes A, Woods WS, et al. Disruption of the beta1L Isoform of GABP reverses glioblastoma replicative immortality in a TERT promoter mutation-dependent manner. Cancer Cell. 2018;34(3):513–528.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korber V, Yang J, Barah P, et al. Evolutionary trajectories of IDHWT glioblastomas reveal a common path of early tumorigenesis instigated years ahead of initial diagnosis. Cancer Cell. 2019;35(4):692–704.e12. [DOI] [PubMed] [Google Scholar]
- 7. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 8. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dilley RL, Greenberg RA. ALTernative telomere maintenance and cancer. Trends Cancer. 2015;1(2):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferreira MSV, Sørensen MD, Pusch S, et al. Alternative lengthening of telomeres is the major telomere maintenance mechanism in astrocytoma with isocitrate dehydrogenase 1 mutation. J Neurooncol. 2020;147(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pekmezci M, Rice T, Molinaro AM, et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017;133(6):1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Tergaonkar V. Noncanonical functions of telomerase: implications in telomerase-targeted cancer therapies. Cancer Res. 2014;74(6):1639–1644. [DOI] [PubMed] [Google Scholar]
- 13. Bagheri S, Nosrati M, Li S, et al. Genes and pathways downstream of telomerase in melanoma metastasis. Proc Natl Acad Sci USA. 2006;103(30):11306–11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmad F, Dixit D, Sharma V, et al. Nrf2-driven TERT regulates pentose phosphate pathway in glioblastoma. Cell Death Dis. 2016;7:e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahmad F, Patrick S, Sheikh T, et al. Telomerase reverse transcriptase (TERT) - enhancer of zeste homolog 2 (EZH2) network regulates lipid metabolism and DNA damage responses in glioblastoma. J Neurochem. 2017;143(6):671–683. [DOI] [PubMed] [Google Scholar]
- 16. Ahmed S, Passos JF, Birket MJ, et al. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci. 2008;121(Pt 7):1046–1053. [DOI] [PubMed] [Google Scholar]
- 17. Indran IR, Hande MP, Pervaiz S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res. 2011;71(1):266–276. [DOI] [PubMed] [Google Scholar]
- 18. Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, et al. Hyperpolarized 13C MRI: path to clinical translation in oncology. Neoplasia. 2019;21(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viswanath P, Li Y, Ronen SM. C-13 Hyperpolarized MR spectroscopy for metabolic imaging of brain tumors. In: Pope WB, ed. Glioma Imaging: Physiologic, Metabolic, and Molecular Approaches. Cham: Springer International Publishing; 2020:191–209. [Google Scholar]
- 20. Park I, Larson PEZ, Gordon JW, et al. Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn Reson Med. 2018;80(3):864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohba S, Mukherjee J, Johannessen TC, et al. Mutant IDH1 expression drives TERT promoter reactivation as part of the cellular transformation process. Cancer Res. 2016;76(22):6680–6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukherjee J, Johannessen TC, Ohba S, et al. Mutant IDH1 cooperates with ATRX loss to drive the alternative lengthening of telomere phenotype in glioma. Cancer Res. 2018;78(11):2966–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Viswanath P, Batsios G, Mukherjee J, et al. Non-invasive assessment of telomere maintenance mechanisms in brain tumors. Nat Commun. 2021;12(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones LE, Hilz S, Grimmer MR, et al. Patient-derived cells from recurrent tumors that model the evolution of IDH-mutant glioma. Neurooncol Adv. 2020;2(1):vdaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelly JJ, Blough MD, Stechishin OD, et al. Oligodendroglioma cell lines containing t(1;19)(q10;p10). Neuro Oncol. 2010;12(7):745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Viswanath P, Radoul M, Izquierdo-Garcia JL, et al. Mutant IDH1 gliomas downregulate phosphocholine and phosphoethanolamine synthesis in a 2-hydroxyglutarate-dependent manner. Cancer Metab. 2018;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Viswanath P, Radoul M, Izquierdo-Garcia JL, et al. 2-Hydroxyglutarate-mediated autophagy of the endoplasmic reticulum leads to an unusual downregulation of phospholipid biosynthesis in mutant IDH1 gliomas. Cancer Res. 2018;78(9):2290–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodrigues TB, Serrao EM, Kennedy BW, Hu DE, Kettunen MI, Brindle KM. Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose. Nat Med. 2014;20(1):93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cawthorne C, Swindell R, Stratford IJ, Dive C, Welman A. Comparison of doxycycline delivery methods for Tet-inducible gene expression in a subcutaneous xenograft model. J Biomol Tech. 2007;18(2):120–123. [PMC free article] [PubMed] [Google Scholar]
- 30. Larson PE, Kerr AB, Chen AP, et al. Multiband excitation pulses for hyperpolarized 13C dynamic chemical-shift imaging. J Magn Reson. 2008;194(1):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brekke EM, Walls AB, Schousboe A, Waagepetersen HS, Sonnewald U. Quantitative importance of the pentose phosphate pathway determined by incorporation of 13C from [2-13C]- and [3-13C]glucose into TCA cycle intermediates and neurotransmitter amino acids in functionally intact neurons. J Cereb Blood Flow Metab. 2012;32(9):1788–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stincone A, Prigione A, Cramer T, et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2015;90(3):927–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christensen CE, Karlsson M, Winther JR, Jensen PR, Lerche MH. Non-invasive in-cell determination of free cytosolic [NAD+]/[NADH] ratios using hyperpolarized glucose show large variations in metabolic phenotypes. J Biol Chem. 2014;289(4):2344–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris T, Degani H, Frydman L. Hyperpolarized 13C NMR studies of glucose metabolism in living breast cancer cell cultures. NMR Biomed. 2013;26(12):1831–1843. [DOI] [PubMed] [Google Scholar]
- 35. Timm KN, Hartl J, Keller MA, et al. Hyperpolarized [U-2H, U-13C]Glucose reports on glycolytic and pentose phosphate pathway activity in EL4 tumors and glycolytic activity in yeast cells. Magn Reson Med. 2015;74(6):1543–1547. [DOI] [PubMed] [Google Scholar]
- 36. Mishkovsky M, Anderson B, Karlsson M, et al. Measuring glucose cerebral metabolism in the healthy mouse using hyperpolarized 13C magnetic resonance. Sci Rep. 2017;7(1):11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mender I, Gryaznov S, Dikmen ZG, Wright WE, Shay JW. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2′-deoxyguanosine. Cancer Discov. 2015;5(1):82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel PL, Suram A, Mirani N, Bischof O, Herbig U. Derepression of hTERT gene expression promotes escape from oncogene-induced cellular senescence. Proc Natl Acad Sci USA. 2016;113(34):E5024–E5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu SN, Wang TS, Li X, Wang YP. SIRT2 activates G6PD to enhance NADPH production and promote leukaemia cell proliferation. Sci Rep. 2016;6:32734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang YP, Zhou LS, Zhao YZ, et al. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 2014;33(12):1304–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jung KO, Youn H, Kim SH, Kim YH, Kang KW, Chung JK. A new fluorescence/PET probe for targeting intracellular human telomerase reverse transcriptase (hTERT) using Tat peptide-conjugated IgM. Biochem Biophys Res Commun. 2016;477(3):483–489. [DOI] [PubMed] [Google Scholar]
- 42. Liu M, Wang RF, Zhang CL, et al. Noninvasive imaging of human telomerase reverse transcriptase (hTERT) messenger RNA with 99mTc-radiolabeled antisense probes in malignant tumors. J Nucl Med. 2007;48(12):2028–2036. [DOI] [PubMed] [Google Scholar]
- 43. Park YW, Ahn SS, Park CJ, et al. Diffusion and perfusion MRI may predict EGFR amplification and the TERT promoter mutation status of IDH-wildtype lower-grade gliomas. Eur Radiol. 2020;30(12):6475–6484. [DOI] [PubMed] [Google Scholar]
- 44. Fukuma R, Yanagisawa T, Kinoshita M, et al. Prediction of IDH and TERT promoter mutations in low-grade glioma from magnetic resonance images using a convolutional neural network. Sci Rep. 2019;9(1):20311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. la Fougère C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13(8):806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hertz MM, Paulson OB. Glucose transfer across the blood–brain barrier. Adv Metab Disord. 1983;10:177–192. [DOI] [PubMed] [Google Scholar]
- 47. Moreno KX, Harrison CE, Merritt ME, Kovacs Z, Malloy CR, Sherry AD. Hyperpolarized δ‐[1‐ 13C]gluconolactone as a probe of the pentose phosphate pathway. NMR Biomed. 2017;30(6):e3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Capozzi A, Patel S, Wenckebach WT, Karlsson M, Lerche MH, Ardenkjær-Larsen JH. Gadolinium effect at high-magnetic-field DNP: 70% 13C polarization of [U-13C] glucose using trityl. J Phys Chem Lett. 2019;10(12):3420–3425. [DOI] [PubMed] [Google Scholar]
- 49. Sengupta S, Sobo M, Lee K, et al. Induced telomere damage to treat telomerase expressing therapy-resistant pediatric brain tumors. Mol Cancer Ther. 2018;17(7):1504–1514. [DOI] [PubMed] [Google Scholar]
- 50. Villanueva-Meyer JE, Mabray MC, Cha S. Current clinical brain tumor imaging. Neurosurgery. 2017;81(3):397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.