Abstract
Histiocytoses are heterogeneous hematopoietic diseases characterized by the accumulation of CD68(+) cells with various admixed inflammatory infiltrates. The identification of the pivotal role of the mitogen-activated protein kinase (MAPK) pathway has opened new avenues of research and therapeutic approaches. We review the neurologic manifestations of 3 histiocytic disorders with frequent involvement of the brain and spine: Langerhans cell histiocytosis (LCH), Erdheim-Chester disease (ECD), and Rosai-Dorfman-Destombes disease (RDD). Central nervous system (CNS) manifestations occur in 10%-25% of LCH cases, with both tumorous or neurodegenerative forms. These subtypes differ by clinical and radiological presentation, pathogenesis, and prognosis. Tumorous or degenerative neurologic involvement occurs in 30%-40% of ECD patients and affects the hypothalamic-pituitary axis, meninges, and brain parenchyma. RDD lesions are typically tumorous with meningeal or parenchymal masses with strong contrast enhancement. Unlike LCH and ECD, neurodegenerative lesions or syndromes have not been described with RDD. Familiarity with principles of evaluation and treatment both shared among and distinct to each of these 3 diseases is critical for effective management. Refractory or disabling neurohistiocytic involvement should prompt the consideration for use of targeted kinase inhibitor therapies.
Keywords: central nervous system, Erdheim-Chester disease, Langerhans cell histiocytosis, MAPK pathway, Rosai-Dorfman-Destombes disease
Histiocytoses are rare diseases in adults and children, characterized by the accumulation of cells belonging to the mononuclear phagocyte system in various tissues and organs.1,2 The common histopathological features of histiocytoses are the presence of CD68(+) cells, accompanied by various degrees of tissue infiltration by inflammatory cells and/or fibrosis. In 2016, the classification of histiocytoses by the Histiocyte Society was revised based on histology, clinical and imaging phenotypes, and molecular alterations, broadly defining 5 groups of histiocytic disorders.3 More than 100 different types of histiocytoses have been described within these groups, with a wide range of organ manifestations and clinical phenotypes. When present, histiocytic infiltration of the nervous system and adjacent structures is an important cause of clinical symptomatology, functional impairments, and potential morbidity and mortality. Here we review the neurologic manifestations of 3 histiocytic diseases with frequent and heterogeneous involvement of the brain and spine: Langerhans cell histiocytosis (LCH), Erdheim-Chester disease (ECD), and Rosai-Dorfman-Destombes disease (RDD). We present the recent advances that molecular and genomic investigations have brought to the pathogenesis and therapy of the histiocytoses, and we review the management strategies of these 3 entities.
Molecular Pathogenesis of Histiocytoses
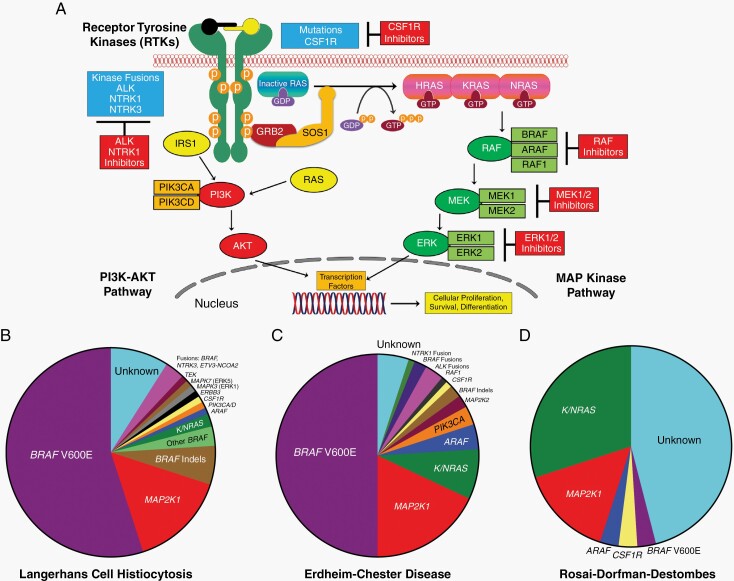
Research conducted in the last decade has offered key new insights into the pathogenesis of the histiocytoses and shifted the understanding of these diseases from autoimmune to clonal hematopoietic disorders. The first discovery came from an analysis of 61 LCH samples, which revealed the presence of the BRAFV600E mutation, an oncogenic driver in several human cancers, in 57% of them.4 Moreover, the detection of BRAFV600E mutations in CD34+ bone marrow cells of some LCH patients provided evidence that LCH is likely derived from hematopoietic progenitor cells. Several subsequent studies confirmed the presence of BRAFV600E in 50%-60% of LCH samples.5 Another critical step in understanding of histiocytoses pathogenesis came from the evidence that although the BRAFV600E mutation was found in almost 60% of LCH patients, 100% had activation of the mitogen-activated protein kinase (MAPK) pathway,4 with the description of other BRAF activating mutations or gene rearrangements, or other kinase mutations involving the MAPK pathway.6,7 Mutations in MAP2K1, which activate MEK1’s kinase activity, are the next most prevalent mutations in LCH after those of BRAF and occur in about 25% of cases.8–10 Other mutations in ARAF and MAP3K1 were also been reported.11 The presence of BRAF mutations was then investigated in ECD, and found in up to 65% of ECD cases, exclusive with other less frequent mutations or rearrangements of genes of the MAPK pathway (MAP2K1, NRAS, KRAS, and ARAF) also detected in ECD.11,12 ECD and LCH were subsequently classified together as clonal L-group histiocytoses by virtue of their shared dependence upon MAPK pathway mutations.3 The etiology of RDD, by contrast, designated as R-group in the above classification, is currently not as clearly defined. Unlike the L-group histiocytoses, recurrent kinase mutations have not been found to be invariably present in RDD. However, recent studies have identified BRAF, NRAS, KRAS, MAP2K1, or ARAF mutations in a subset of patients with RDD.11,13–15 This may reflect the notion that RDD is a heterogeneous entity with both immune and neoplastic forms. For ECD, LCH, and RDD, the identification of the pivotal role of the MAPK pathway has opened new avenues of research and therapeutic approaches (see Figure 1).
Fig. 1.
Overview of MAPK (mitogen-activated protein kinase) and PI3K-AKT signaling and the diverse kinase alterations discovered in select histiocytic neoplasms. (A) Diagram of the MAPK and PI3K-AKT signaling pathways with a description of the activation of the RAS proteins (HRAS, KRAS, and NRAS) with annotation of the signaling proteins affected by genetic alterations in the histiocytic neoplasms. (B) Pie chart illustrating a composite of the known kinase alterations in Langerhans cell histiocytosis. (C) Pie chart showing a composite of the known kinase alterations in Erdheim-Chester disease. (D) Pie chart demonstrating the published kinase alterations in Rosai-Dorfman-Destombes disease.
Langerhans Cell Histiocytosis
Overview
LCH is characterized by organ infiltration by histiocytes that share characteristics with the epidermal dendritic Langerhans cells but is derived from misguided differentiation of myeloid dendritic cell precursors.16 The diagnosis of LCH is based on appropriate clinical and radiological findings and a pathological demonstration of tissue infiltration by CD1a+/CD207+ histiocytes (Table 1). Biopsy is necessary in all virtual cases for confirming the diagnosis and molecular analysis. The disease predominantly affects young children, with a peak age of 1-3 years, and with male predilection. The clinical course varies from a self-limiting disease to a rapidly progressive one, leading to death.1 LCH is classified by a number of organ systems involved (single vs multisystem) and a number of disease sites (single vs multiple). “Risk-organ” involvement in LCH refers to involvement of the bone marrow, spleen, or liver and is associated with worse prognosis. The concept of “risk-organ”-related prognostication is considered relevant to the pediatric LCH context, not the adult setting in which this has not been examined. Clinical presentation of LCH includes variable constitutional symptoms (fever, weight loss), and local signs and symptoms depending on the involved organs, including bone pain, mucosal or cutaneous lesions, lymphadenopathy, or cytopenias. Estimated frequencies of site involvement in LCH vary by report. In adults, infiltration of bone is most frequent (60%-80% of cases), then lungs (15%-50% associated with smoking in adults), skin (15%-40%), liver or spleen (15%), and lymph nodes (5%-10%).2,17 In children, in a compiled analysis of 1741 patients,18 bone is also most frequently involved (77%), then skin (39%), lymph nodes (19%), liver (16%), spleen (13%), oral mucosa (13%), and lung (10%).
Table 1.
Diagnostic Features, Clinical and Radiological Presentations, Molecular Biology, and Central Nervous System Manifestations in Histiocytoses
| Langerhans Cell Histiocytosis | Erdheim-Chester Disease | Rosai-Dorfman-Destombes Disease | |
|---|---|---|---|
| Distinctive clinical manifestations | • Constitutional symptoms (fatigue, night sweats), bone pain, skin lesions, anemia, and lymphadenopathy. • Cough, dyspnea, and apical-predominant nodular and/or cystic lung disease with interstitial changes. • Lytic lesions in the calvarium, base of skull, and axial skeleton. • Variable disease course (from slowly progressive to acute or subacute presentations). |
• Osteosclerosis in the legs (96% of cases). May be asymptomatic and only detected by radiotracer uptake in the distal ends of the femurs and the proximal and distal tibia. • Dense infiltration of perinephric fat, described as a “hairy kidney” on computed tomography, is a highly prevalent (68% of cases) finding. • Other organs involvements vary depending on BRAF status.19 Right atrium pseudo-tumor and cardiac involvements are more prevalent in BRAF-mutated patients. • The disease course is usually slow (over several years), but some symptoms and signs may be not clinically detected. |
• Bilateral, massive, and painless cervical lymphadenopathy with or without intermittent fevers, night sweats, and weight loss. • Extranodal involvement (skin, head, and neck, CNS, soft tissues, kidneys, ophthalmic manifestations) is present with or without lymphadenopathy. • Association with autoimmunity (eg, cytopenia, lupus). • The disease course is usually slow, over several months or years. |
| Distinctive pathological features | • Histopathological analysis demonstrates inflammatory lesions containing abundant CD68(+), CD163(+), CD1a+ Langerin+ S100+ histiocytes. | • Tissues are infiltrated by foamy CD68(+), CD163(+), Factor XIIIa(+), CD1a(−), and Langerin(−) histiocytes with fibrosis. • Touton giant cells are often present. • Positivity for S100 and emperipolesis have been rarely observed (2). |
• Typical findings include large pale histiocytes with cytoplasmic and nuclear S100 and fascin positivity, CD68 positivity, and variable CD163 and CD14 positivity. The cells are CD1a−/CD207− in contrast to LCH. • Emperipolesis is frequently present but may be variable, especially in extranodal sites. |
| Molecular features | • BRAFV600E mutation is present in 50% of cases. And can be detected in lesional tissue or cell-free DNA extracted from plasma. • BRAFV600 wild-type cases are characterized by activating mutations in the MAPK pathway. |
BRAFV600E mutation is present in 50% of cases. The presence of BRAF mutation is useful to confirm ECD in ambiguous cases. BRAFV600E mutations can be detected in lesional tissue cell-free DNA extracted from plasma. Ultrasensitive techniques are often needed for BRAFV600E determination because of low VAF in tissues.20 BRAFV600 wild-type cases are characterized by activating mutations in the MAPK pathway as well as ALK, NTRK, and others. |
Typically BRAFV600 wild type although few cases of BRAFV600E reported. NRAS, KRAS, MAP2K1, and ARAF mutations may be found in a subset of cases.15 |
Abbreviations: CNS, central nervous system; ECD, Erdheim-Chester disease; LCH, Langerhans cell histiocytosis; RDD, Rosai-Dorfman-Destombes disease.
Tumorous LCH of the Nervous System
Estimates of neurologic involvement of LCH, and other histiocytoses as well, vary in relation to their inclusion of non-parenchymal sites (eg, dura, base of skull) as “neurologic” (Table 2). True infiltration of the brain parenchyma is rare, occurring in ~5% of both adults and children, but considering all sites involving neurologic structures, these manifestations occur in 10%-25% of LCH cases.2,17,18 Neurologic involvement of LCH can be considered distinctly in its (1) tumorous or (2) neurodegenerative forms.21,22 These subtypes differ by clinical and radiological presentation, pathogenesis, and prognosis. Tumorous manifestations are characterized by one or more infiltrative lesions in cranial or spinal structures. The hypothalamic-pituitary-adrenal (HPA) axis is among the most commonly infiltrated site, occurring in up to 20% of patients. HPA infiltration presents clinically as diabetes insipidus (DI), and in some cases deficiency of anterior pituitary hormones; in adults, DI can precede an LCH diagnosis by months or even many years.23 Other sites of neurologic disease include the pachymeninges, choroid plexus, pineal gland, and brain parenchyma (Figure 2). Parenchymal lesions are typically located in the posterior fossa including the brainstem and cerebellar peduncles. Clinical manifestations of central nervous system (CNS) tumorous LCH reflect the site of the lesion but include headache, focal pain or swelling for calvarial disease, motor or sensory deficits for spinal lesions, or ataxia, dysarthria, and bulbar deficits for posterior fossa LCH. Special note should be made of LCH infiltration of the mastoid sinus which leads to otalgia, otorrhea, swelling, and fullness, and can masquerade as chronic/recurrent otitis media.24,25 Upon MRI evaluation, intracranial LCH lesions are expansile, T1-hypointense, T2-hyperintense, and strongly enhancing after gadolinium.26
Table 2.
Sites and Clinical Features of Neurologic Histiocytosis
| Site of Tumorous Disease | LCH | ECD | RDD | Sign and Symptoms |
|---|---|---|---|---|
| Osseous structures and sinuses | +++ | ++ | +++ | |
| Calvarium | +++ | ++ | +++ | • Focal pain • Swelling |
| Facial sinuses | + | ++ | +++ | • Chronic congestion • Airway obstruction • Facial fullness |
| Cavernous sinuses | − | ++ | + | • Facial pain or numbness |
| Maxilla, mandible | ++ | + | + | • Pain • Dental decay • Multiple tooth extractions |
| Mastoid | +++ | + | + | • Otalgia • Hearing loss • Pain and fullness |
| Meningeal structures | + | ++ | ++ | |
| Dura | + | ++ | ++ | • Headache • Dementia (rare, large bulky lesions) |
| Leptomeninges/CSF | + | + | + | • Cranial nerve deficits (eg, blindness, deafness) • Elevated intracranial pressure (ie, headache, nausea) • Radiculopathy |
| Hypothalamic-pituitary-adrenal axis | +++ | ++ | + | • Diabetes insipidus • Visual impairment (ie, via chiasmatic extension of hypothalamic lesions) • Hypogonadotropic hypogonadism |
| Brain/spine parenchyma | + | +++ | + | |
| Cerebral hemispheres | + | + | + | • Focal deficits • Cognitive impairment |
| Basal ganglia and thalamus | + | + | + | • Movement disorders, sensory disturbance (very rare) |
| Cerebellum | + | +++ | + | • Dysarthria • Truncal ataxia • Mood instability |
| Brainstem | + | ++ | + | • Diplopia • Limb weakness • Spasticity • Bulbar affect |
| Spinal cord | + | + | + | • Myelopathy • Spasticity |
| Neurodegeneration | + | + | NA | • Ataxia • Dysarthria • Cognitive impairment • Spasticity • Mood dysregulation |
Abbreviations: ECD, Erdheim-Chester disease; LCH, Langerhans cell histiocytosis; NA, not applicable; RDD, Rosai-Dorfman-Destombes disease.
+, Rare but described site of neurologic involvement; ++, common but not frequent site of neurologic involvement; +++, most frequent site(s) of neurologic involvement.
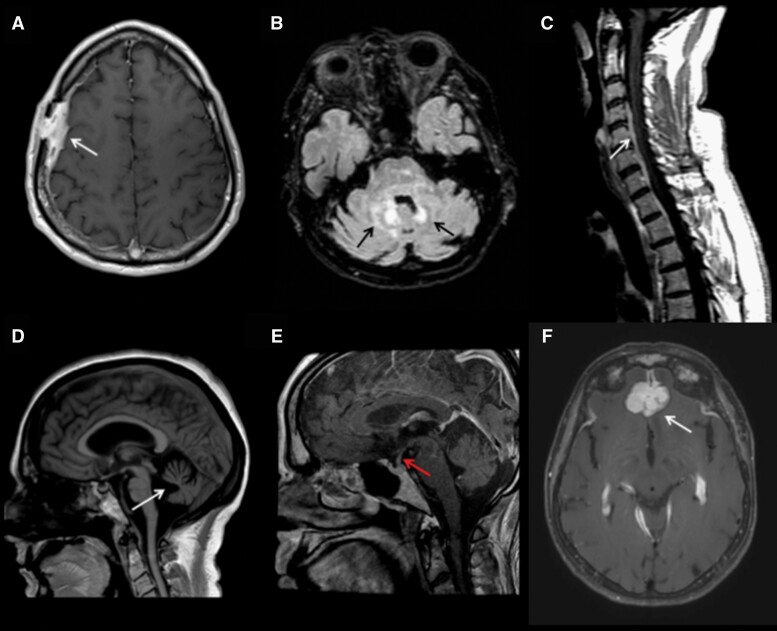
Fig. 2.
Magnetic resonance imaging (MRI) of histiocytoses of the nervous system. (A) Axial T1 post-gadolinium MRI demonstrates calvarial-dural Langerhans cell histiocytosis (LCH). (B) Axial T2 FLAIR (fluid-attenuated inversion recovery) MRI demonstrates Erdheim-Chester disease (ECD) of the brainstem and cerebellum. (C) Sagittal T1 post-gadolinium mixed histiocytosis (ECD with Rosai-Dorfman-Destombes disease [RDD]) of the spine. (D) Sagittal T1 MRI demonstrates profound cerebellar atrophy in neurodegenerative LCH. (E) Sagittal T1 post-gadolinium MRI with enhancement and thickening of the infundibulum in a patient with LCH. (F) Axial T1 post-gadolinium MRI with RDD involving dura and bifrontal lobes.
Treatment of LCH with neurologic involvement depends on the extent and severity of the disease (Figure 3, Table 3). Solitary lesions of the calvarium or dura can be treated with surgical resection or low-dose radiotherapy and these interventions can frequently be curative. Multifocal disease or LCH involving the brain parenchyma is treated systemically with a variety of conventional (chemotherapeutic and/or immunosuppressive) agents. Current guidelines for conventional therapies,27–29 mainly based on the results of 4 prospective trials and a large cohort study,30–34 can be summarized as follows. The most frequently used first-line chemotherapy regimen for LCH in children is a combination of vinblastine with prednisolone, with treatment for up to 12 months.32 In a nationwide retrospective study of 20 LCH patients (median age 11.5; range 1-50) with CNS manifestations, vinblastine with (n = 9) or without (n = 11) steroids was efficacious and well tolerated for treating CNS LCH.35 In this study, 15 patients achieved an objective response, including 4 out of 6 who did not receive steroids (complete response in 5 cases and partial response in 10 cases), while 4 had stable disease and 1 patient progressed. Nonetheless, there is a lack of consensus about the role of vinblastine in adults with LCH, particularly in the setting of neurologic disease. Purine-analog chemotherapies cytarabine and cladribine given as monotherapies are effective for neurologic LCH; some specialists use these agents as first-line therapy while others reserve them for relapsed or refractory disease.27,36,37 The implications of BRAF and other MAPK pathway mutational status upon LCH treatment remain largely unclear. BRAF or MEK inhibitors have demonstrated efficacy in prospective trials of histiocytosis patients, although with a small proportion of LCH participants.38,39 In our view, this reflects the modest proportion of LCH patients who do not respond to conventional treatments, however, this specific population is in dire need of effective treatments. Therefore, the dramatic and well-established efficacy of targeted therapies in ECD leads us to recommend that LCH patients with refractory or clinically severe neurologic disease be treated with BRAF or MEK inhibitors to salvage neurologic function.
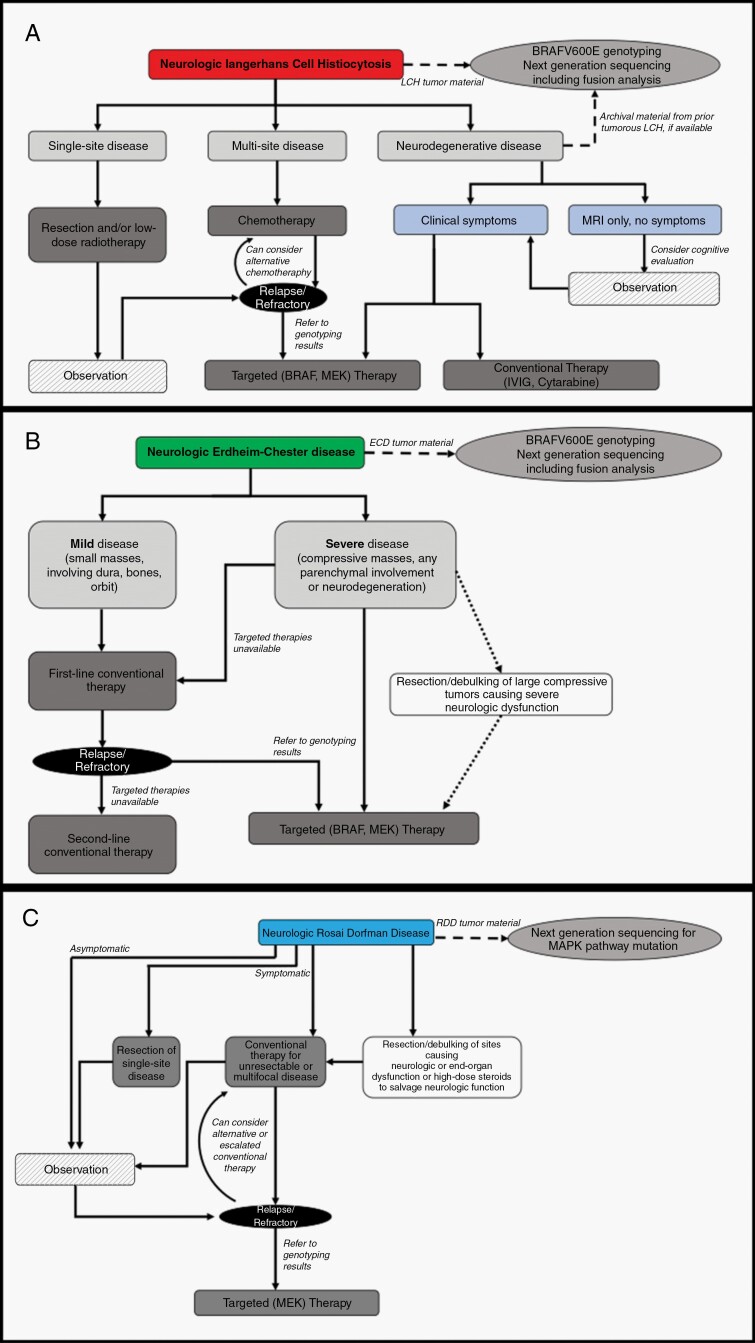
Fig. 3.
Management of Langerhans cell histiocytosis (A), Erdheim-Chester disease (B), and Rosai-Dorfman-Destombes disease (C) with nervous system involvement.
Table 3.
Systemic Treatments for Neurologic Histiocytosis
| Treatment | Regimen | Neurodegenative LCH | Tumorous LCH | ECD | RDD | Comment |
|---|---|---|---|---|---|---|
| Corticosteroid monotherapy | Prednisone 1 mg/kg day (or equivalent) until optimal response followed by 2-3 months taper | NR | NR | NR | +/− | Variable and non-lasting responses in RDD |
| Chemotherapy | ||||||
| Vinblastine/Prednisone | Vinblastine 6 mg/m2 (10 mg maximum) IV weekly × 6, followed by maintenance phase dosing every 3 weeks × 6-12 months; Prednisone 40 mg/m2 PO daily × 4 weeks, then taper, followed by 40 mg/m2 PO maintenance phase dosing days 1-5 every 3 weeks × 6-12 months | NR | + | NR | NR | Reasonable first-line treatment in LCH |
| Cytarabine | 100-150 mg/m2 IV days 1-5 (6-12 cycles; 28 days/cycle) | +/− | + | AD | AD | Reasonable first-line treatment in LCH, or if refractory to vinblastine Previously given for ND-LCH, however, has been replaced with BRAF/MEK inhibitors |
| Cladribine | 0.14 mg/kg IV days 1-5 or 5 mg/m2 IV days 1-5 (total of 6 cycles; 28 days/cycle) | NR | + | +/− | +/− | Reasonable first-line treatment in LCH or if refractory to vinblastine; modest evidence in ECD; anecdotal in RDD |
| Melphalan (intra-arterial) | 0.4 mg/kg | AD | AD | AD | +/− | Small series in 3 patients, 2/3 RDD |
| IVIG | 0.4 g/kg/day × 5 days | +/− | NR | NR | NR | Previously given for ND-LCH, however, has been replaced with BRAF/MEK inhibitors |
| Interferon-α | Pegylated 135 µg SC/week (standard dose) or 180 µg SC/week (high dose) or standard 3 mIU SC TIW (standard dose) or 6-9 mIU SC TIW (high dose) | NR | NR | + | NR | Reasonable treatment for clinically mild ECD, without parenchymal brain involvement |
| Anakinra | 100 mcg SC daily | NR | NR | +/− | NR | 2 cases of neurologic response in ECD |
| Targeted therapy | ||||||
| Vemurafenib | 480-960 mg twice daily | + | + | + | NR | Recommended for refractory or clinically severe symptomatic BRAFV600E-mutated diseasea |
| Dabrafenib | 75-150 mg twice daily | + | + | |||
| Cobimetinib | 20-60 mg daily for 21 of the 28-day cycle | + | + | + | +/− | Recommended for refractory or clinically severe BRAFV600 wild type or BRAFV600E-undefined disease |
| Trametinib | 1-2 mg daily |
Abbreviations: AD, absent data; ECD, Erdheim-Chester disease; LCH, Langerhans cell histiocytosis; NR, not recommended; RDD, Rosai-Dorfman-Destombes disease; SC, subcutaneous; TIW, three times per week.
+, Recommended; +/−, recommended in the context of modest evidence.
aRosai-Dorfman disease is nearly invariably BRAFV600 wild type. Rare exceptions would be appropriate for BRAF inhibitor therapy.
Neurodegenerative LCH
Neurodegenerative LCH (ND-LCH) is a highly rare but devastating form of the disease, occurring in fewer than 5% of patients.21 ND-LCH refers to a syndrome of progressive multi-domain neurologic deterioration that often arises many years after tumorous LCH is treated and presumed to be cured. Some patients have both tumorous and ND-LCH. Clinically, ND-LCH in children and adolescents manifests most commonly as a progressive cerebellar syndrome, accompanied by cognitive dysfunction and behavioral disturbances.40 Adult ND-LCH patients suffer predominantly from cerebellar deficits with variable cognitive impairment. Radiologic abnormalities can be observed throughout the brain parenchyma but are most prevalent in the posterior fossa. ND-LCH lesions are characterized by non-expansile, T2, and fluid-attenuated inversion recovery (FLAIR) intense lesions in the cerebellar peduncles, medial cerebellar structures (peri-dentate region), basal ganglia, and/or brainstem. It should be noted that MRI changes can be seen in patients without clinical symptomatology, although many such patients will develop symptoms eventually.41 In a study of 13 ND-LCH patients,42 posterior fossa was involved in 12 patients (92%), showing a symmetrical T2 hyperintensity of the cerebellar white matter areas (n = 7), a circumscribed T1 hyperintensity of the dentate nuclei (n = 5), definite hyperintense T2 areas in the adjacent pontine tegmentum white matter (n = 9), and/or hyperintensity of the pontine pyramidal tracts (n = 4). Cerebellar atrophy was noted in 8 cases, and diffuse atrophy in 3 cases.
Cerebrospinal fluid (CSF) profile is typically unremarkable in LCH without pleocytosis or elevated protein. Osteopontin was the only consistently elevated CSF protein in patients with ND-LCH compared with patients with other brain pathologies, among 121 unique proteins associated with inflammation and/or neurodegeneration that were assessed in CSF samples from 40 patients.43 The BRAFV600E mutation can be detected in cell-free DNA in the CSF in 10% of patients with ND-LCH, at a lower frequency than in blood.43
Given the clinical morbidity of ND-LCH, significant efforts have been made to better understand its epidemiology, pathogenesis, and risk factors. In a national prospective registry of pediatric LCH patients, 36/1897 (1.9%) were ultimately diagnosed with ND-LCH.44 The 10-year cumulative incidence was 4.1%. ND-LCH typically affected (in 69% of cases) patients previously treated for a multisystem LCH without risk-organ involvement. This study also showed that pituitary gland, skin, base skull or orbit tumoral lesions, and BRAFV600E mutation, were frequently associated with neurodegenerative phenotype.
The etiology and pathogenesis of ND-LCH are still undefined, and both immunologic and neoplastic mechanisms have been postulated. Brain biopsies of patients with ND-LCH are rare, although in 1 neuropathologic series, infiltrating T cells without characteristic CD1a+/CD207+ LCH lesional cells were observed, and histopathology was noted to be reminiscent of immune encephalitis.22 This finding has led to an enduring notion of paraneoplasia driving ND-LCH, although this has never been demonstrated directly. More recently, several avenues of research have indicated that mutational events likely underlie ND-LCH. In the postmortem analysis of 1 patient who died from ND-LCH, BRAFV600E+ cells were identified in brainstem (13% of cells), including the pons (8%) and cerebellum (5%), with aggregates of perivascular mutated cells in areas of active demyelination. These areas enriched for BRAFV600E+ cells corresponded to characteristic areas of T2 hyperintensity illustrated in a brain MRI from the same patient. Additionally, BRAFV600E+ cells with monocyte phenotype (CD14+, CD33+, CD163+, P2RY12−) were found in perivascular area of 3 brain specimens of ND-LCH patients.43 In the same study, patients with ND-LCH were found to have BRAFV600E+ circulating peripheral blood mononuclear cells. These data suggest the possibility that ND-LCH lesions arise from CNS-infiltrating BRAFV600E-mutated hematopoietic (myeloid/monocytic) cells. However, a different line of research suggests that ND-LCH is driven not by mutated hematopoietic cells, but rather by somatic mutations occurring during organogenesis in yolk-sac erythro-myeloid progenitors, leading to mutated tissue-derived macrophages (ie, microglia in the CNS). In a mouse model, mosaic expression of BRAFV600E in yolk-sac erythro-myeloid precursors resulted in clonal expansion of tissue-resident macrophages and a severe late-onset neurodegenerative disorder.45 Neurobehavioural signs, astrogliosis, deposition of amyloid precursor protein, synaptic loss, and neuronal death were driven by extracellular signal-regulated kinase (ERK)-activated microglia. In these mice, neurodegeneration was ameliorated by BRAF inhibitor administration.45 Further research is necessary to provide clarity about these disparate conceptualizations of the pathogenesis of ND-LCH.
Treatment of ND-LCH is challenging, and conventional therapies have yielded modest results. Disease stabilization has been observed in patients treated with all-trans retinoic acid in 1 report,46 treated with intravenous immune globulin (IVIG) alone,47 or with IVIG and chemotherapy.48 Clinical and radiologic improvement was reported in a small series of patients receiving cytarabine-based chemotherapy regimens.49 Recently, 3 of the 4 patients with ND-LCH treated with BRAF inhibitor following worsening despite conventional chemotherapy experienced significant clinical and radiologic improvement.43 This has led to increased enthusiasm about targeted therapies for ND-LCH, and further studies and clinical experience are still needed. Our view is that early implementation of BRAF and MEK inhibitors, for BRAFV600E-mutated and BRAFV600 wild type or undefined respectively, will gain traction in the coming years.
Erdheim-Chester Disease
Overview
First described in 1930,50 ECD is a rare, multisystemic non-LCH, characterized by an infiltration of various organs by xanthomatous histiocytes, Touton giant cells, lymphocytes, and scattered plasma cells with surrounding fibrosis. The histiocytes are positive by immunohistochemistry for CD68, and CD163, negative for CD1a and Langerin, and have a variable expression of S-100 protein. Clinical and radiological presentations of ECD differ strongly from those of LCH.51 ECD mainly affects adults around 50-60 years of age, with a slight male predominance (2-3/1), although rare pediatric cases have been reported. ECD diagnosis relies on consistent clinic-radiological features, the presence of long-bone involvement, compatible histopathology, and exclusion of differential diagnoses (Table 1).51,52
ECD clinical phenotypes are widely heterogeneous, ranging from indolent and minimally symptomatic to progressive, disabling, and life-threatening forms. Disease infiltration of the long bones of the legs is an iconic feature of ECD, occurring in up to 96% of cases, and is characterized by symmetric osteosclerosis of the diaphyseal and metaphyseal regions of the femora and tibia.51 Other manifestations suggestive of ECD are cardiovascular infiltration, occurring in 50% of patients, seen around the aorta or large vessels, pericardium, right coronary artery, and interatrial wall. Also, retroperitoneal fibrosis visualized as contrast-enhancing perinephric sheathing (“hairy kidneys”) seen on abdominal CT is a common finding. ECD-like xanthogranulomatous histiocytosis that is limited to the nervous system, ie, neurologic juvenile or adult xanthogranuloma (in children and adults, respectively) is an ultra-rare form of non-LCH with similar neurologic manifestations to ECD.
Neurologic ECD
Neurologic involvement occurs in 30%-40% of ECD patients, and typically affects the HPA axis, meninges, and brain parenchyma (Figure 2, Table 2).53 HPA axis involvement can lead to various neuro-endocrinopathies (including DI in 17%-47%), hypersomnia, and visual impairment if there is an extension to the optic chiasm. Pachymeningeal thickening can mimic a nodular meningioma or cause a diffuse plaque-like expansion of dural structures. These lesions may be asymptomatic or cause compression of cranial nerves, spinal cord, or brain parenchyma. Intraparenchymal ECD lesions have a proclivity for the posterior fossa like LCH but can involve the cerebral hemisphere as well. Additionally, retro-orbital lesions are seen in 20%-30% of cases, DI in 17%-47%, and both are associated with other neurologic manifestations.20,53,54 An additional feature suggestive of ECD is the sheathing of intracranial vessels that can lead to ischemic stroke or compression of adjacent structures.55 In a study of 40 patients, radiographic evidence of CNS involvement (ie, dural, brain, including Fazekas score >1, or spinal cord) occurred in 22 (55%). The MRI lesions were mainly seen in dura (6/41), brainstem (9/39), cerebellum (8/39), spinal cord (2/16), spinal epidural region (2/16), hypothalamic-pituitary axis (17/39), and orbits (13/42). T2 white matter abnormalities (Fazekas score ≥1) were present in 21/34 patients.56 The same characteristics were observed in another study of 33 patients, in which the hypothalamic-pituitary axis was involved in 16/33 (53%), with 6 cases of micronodular or nodular masses of the infundibular stalk, meninges in 5, bilateral symmetric T2 high signal intensity in the dentate nucleus areas in 3, and intracranial periarterial infiltration in 3 patients.19 Non-tumorous atrophic changes in the brainstem and cerebellum have been observed in ECD, although neurodegenerative phenomena have not been characterized as they have been in LCH.20 In a study of self-reported symptoms in 50 ECD patients, 26 (52%) reported memory impairment and 17 (34%) reported difficulty with concentration.57 In a volumetric neuroimaging study comparing 11 ECD patients without tumorous neurologic involvement to age-matched controls, patients had diffuse bihemispheric reduction in cortical thickness and subcortical gray matter volumes.58
Neurologic ECD Treatment
Neurologic involvement of ECD is associated with poor prognosis,20 but the prognosis is closely linked to CNS phenotype, with neurodegenerative forms having the worst prognosis. Treatments options (Table 3) in ECD include anti-neoplastic agents (interferon [IFN]-α and pegylated IFN-α [PEG-IFN-α], cladribine), anti-inflammatory agents (anakinra, sirolimus, infliximab, and tocilizumab), and, since the discovery of the major role of the MAPK pathway, therapies targeting BRAF or MEK (Figure 3). As a general rule, neurologic ECD requires more intensive therapy than non-neurologic forms of the disease in order to prevent morbidity and mortality. The first established ECD treatment was IFN-α,59 and this was associated with better overall survival compared with other therapies in a cohort study of 46 ECD patients.60 Responses in the nervous system to IFN-α have been mixed, and in 1 study prolonged treatment with higher doses was needed to achieve responses.61 Anakinra, an interleukin (IL)-1 receptor antagonist, has been studied in a small number of ECD patients with variable efficacy and only anecdotal neurological responses.62,63 Additional biological agents that have been investigated in ECD, including infliximab and tocilizumab, have not had efficacy in neurologic forms of the disease.64,65 Cladribine demonstrated an overall response rate of 53% of 21 patients in 1 retrospective analysis, including 5 patients with CNS involvement.66 Vemurafenib has achieved robust and durable responses with both systemic and neurologic ECD in 1 prospective trial and in numerous case reports and series,39,67–71 including patients with severe and life-threatening neurologic illness. This led to the US Food and Drug Administration’s approval of vemurafenib for treating BRAFV600E-mutated ECD.72 Similarly favorable responses have been seen with dabrafenib.73 However, chronic treatment may be needed as 1 prospective study demonstrated that ECD relapsed upon cessation of vemurafenib in 75% of cases.68 MEK inhibitors (cobimetinib and trametinib) have shown dramatic efficacy in BRAFV600 wild-type ECD, also in 1 prospective trial and several case series.39,74 Given the morbidity posed by neurologic ECD, BRAF, and/or MEK inhibitors should be considered first-line therapy in cases of tumorous parenchymal disease and in neurodegenerative forms of ECD. BRAFV600E-mutated patients can be treated with BRAF inhibitor monotherapy, or combined with MEK inhibitors as is done in other BRAFV600E-mutated cancers to reduce toxicities,68 although the benefit of combination therapy in ECD has not been clearly shown. BRAF wild-type patients with neurologic involvement, or even neurologic ECD patients with unknown mutational status, should be treated with MEK inhibitor monotherapy.39 In 1 retrospective series of 30 patients with neurologic ECD, targeted (BRAF, MEK, or ALK inhibiting) therapies led to partial or complete response by MRI in 24 (89%) of cases vs 20% for chemotherapy or immunosuppressive therapies. Mutations outside of the MAPK pathway, including alterations in NTRK, ALK, and CSF1R, have been recently documented,75 and these should be pursued if MEK inhibition is not efficacious and an alternative therapeutic target is identified.
Rosai-Dorfman-Destombes Disease
Overview
RDD is another rare non-LCH, first described by the French pathologist Destombes in 1965,76 then recognized as a distinct clinicopathological entity by Rosai and Dorfman in 1969.77 RDD mainly occurs in children and young adults but can affect all ages. It is characterized by the accumulation of large pale CD68+, S100+, and CD1a− histiocytes, with frequent histological findings of emperipolesis, referring to the trafficking of intact leukocytes through the cytoplasm of histiocytes, and varying proportions of IgG4/IgG plasma cells.14 RDD belongs to the R-group of the revised classification, and several subtypes have been described, including inherited forms, overlap with immunoglobulins (Ig)-G4 related disease, or association with autoimmune disorders.3 The predominant clinical presentation of RDD is a massive, bilateral, and painless cervical lymphadenopathy. However, extranodal localizations have been documented in 43% of patients,14 most frequently involving the skin, ear-nose-throat, CNS, soft tissue, and bones.78
Neurologic RDD
Neurologic RDD involvement has been described in both adults and children, with a mean age at presentation of 39 years and a male prevalence (male:female ratio = 1.8:1.0).79 Neurologic RDD can be isolated or associated with extra-neurological manifestations, and clinical presentation is typically indolent in its pace.80 Conversely to nodal forms of the disease, CNS manifestations of RDD mainly occur in older adults.79 In a review of 210 cases of the literature published until 2014, RDD was isolated to the CNS in 174 cases, while in 36 patients, it was part of a systemic disseminated disease.79 In the same study, 167 patients (80%) had intracranial lesions, 24 (11%) had spinal involvement, and 19 (9%) had both intracranial and spinal involvement. Tumorous lesions, predominantly of the pachymeninges, can lead to compression of neurologic structures, leading to various neurological signs. Intraparenchymal lesions are quite rare but can occur. Presenting symptoms include headaches, motor or sensory abnormalities, cranial nerve deficits, and less frequent seizures or gait difficulty. A large variety of presentations have been described: spinal cord compression, conus cauda syndrome, hydrocephalus, cognitive dysfunction, or seizures.79 Radiological presentation of CNS RDD includes nodular meningioma-like lesions, diffuse thickening of the dura, or masses in the parenchyma, orbits, or cavernous sinuses (Table 2). RDD lesions typically present as homogenous, T1 isointense lesions, T2 hypo or isointense, with contrast enhancement. Perilesional edema can also be seen. Unlike LCH and ECD, neurodegenerative lesions or syndromes have not been described with RDD.
Treatments and outcomes of CNS RDD are widely heterogeneous, and little published data exists to guide treatment. Therapeutic options include observation, surgery, radiotherapy, steroids, and various immunosuppressive or chemotherapeutic agents (Figure 3). Surgical resection can be curative for unifocal disease, and debulking surgery may be warranted in case of spinal cord compression or other lesions causing an immediate risk of neurologic deterioration.39 Long-term remissions with resection alone have been reported in the isolated intracranial disease. Steroids are usually helpful in reducing lesional size and improving symptoms, although responses have been variable and are not thought to be durable.81 Also, high doses are usually required for brain lesions. Variable responses with some success have been observed with cladribine,82 rituximab,83 lenalidomide, and dexamethasone.84 Little is known about the efficacy of targeted therapies in neurologic RDD, as has been observed in L-group histiocytoses. Few reports have shown the response of RDD lesions to MEK inhibitors, but no patient had CNS manifestations.11,39
Mixed Histiocytosis
Patients with overlapping forms of histiocytosis have been described in many instances, primarily mixed ECD and LCH,85–89 and this entity is characterized almost invariably by BRAFV600E mutations. Recently, mixed forms of ECD and RDD have been described,90 predominantly harboring mutations in MAP2K1. Neurologic presentations of these mixed histiocytoses have not been characterized; however, it is our experience of the overlap entities that their clinical phenotype, as well as responses to treatment, are more representative of ECD rather than the co-occurring disease. From the standpoint of treatment, it is suggested that therapy is formulated along the lines of treating ECD in these very rare cases.
Challenges and Future Directions for Neurohistiocytosis
Despite the insights and therapeutic advances gained with the identification of targetable MAPK pathway mutations in the histiocytoses, there continue to be challenges and knowledge gaps in the evaluation and management of patients with neurohistiocytic disease. First, achieving a diagnosis of neurologic ECD, LCH, or RDD can be a protracted process, punctuated by misdiagnoses. Pathology studies have demonstrated that biopsies of neurologic structures frequently do not demonstrate typical LCH, ECD, or RDD morphology, rather they can mimic non-neoplastic inflammatory22,54,91 or fibroinflammatory92,93 processes. Specifically, cases of isolated HPA LCH pose a vexing diagnostic challenge as lesions are radiologically minimal and difficult to sample surgically. Molecular advances have allowed for diagnosis of neurologic histiocytosis by way of identification of MAPK pathway mutations in cases with clinical suspicion for histiocytosis but nonspecific biopsy findings.94 Difficulty in diagnosing neurohistiocytosis underscores the importance of awareness among clinicians of these diseases, their clinical spectrum, and the role of detailed molecular analysis, even of equivocal biopsies. Another emerging challenge is suboptimal CNS penetration of existing kinase inhibitor therapies. BRAF and MEK inhibitors are both substrates of P-glycoproteins95 and their efflux by the blood-brain barrier leads to limited drug levels within the nervous system. There are limited reports of unfavorable CNS responses to BRAF inhibitor monotherapy in BRAFV600E-mutated ECD that are salvaged with combined BRAF/MEK inhibitor therapy.96 Our collective clinical experience reflects a related phenomenon of differential systemic vs neurologic responses to targeted therapies, with neurologic sites of disease responding to a lesser degree than systemic sites. In some cases, suboptimal response can be augmented by higher dosing or combined treatment, but a price is paid with toxicity and tolerability. Last, patients with isolated neurohistiocytosis also present an unmet need for effective and durable local therapies in order to spare systemic treatment. There is a recent report of intra-arterial chemotherapy as highly effective in 3 such cases, including HPA neurohistiocytosis,97 and further investigation of other creative local interventions is needed.
Conclusions
Neurologic manifestations of LCH, ECD, and RDD are fascinating and heterogeneous clinical entities with disabling forms in some patients. Treatment outcomes have historically been variable, and particularly dismal with severe and degenerative neurologic forms of the disease. The molecular advances in the understanding of histiocytic neoplasms, however, and the advent of targeted therapies have profoundly modified the management and outlook for these diseases. Awareness of the spectrum of these clinical entities and the diagnostic and therapeutic potential of molecular analysis is critical to improving identification and management of these patients.
Funding
This work was supported by the National Institutes of Health/National Cancer Institute Core Grant [P30 CA008748] and by the Frame Fund, Applebaum Foundation, and Joy Family West Foundation (E.L.D.).
Conflict of interest statement. Dr. O.A.-W. has served as a consultant for H3B Biomedicine, Foundation Medicine Inc., Merck, and Janssen and serves on the Scientific Advisory Board of Envisagenics Inc., unrelated to the current manuscript; O.A.-W. has received prior research funding from H3B Biomedicine unrelated to the current manuscript. Dr. E.L.D. discloses editorial support from Pfizer Inc., outside the submitted work. F.C.-A. and J.H. are investigators of an academic study about efficacy of the MEK inhibitor cobimetinib in histiocytoses. Other authors declare they have no conflicts of interest to report.
References
- 1.Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379(9):856–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haroche J, Cohen-Aubart F, Rollins BJ, et al. . Histiocytoses: emerging neoplasia behind inflammation. Lancet Oncol. 2017;18(2):e113–e125. [DOI] [PubMed] [Google Scholar]
- 3.Emile JF, Abla O, Fraitag S, et al. ; Histiocyte Society . Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badalian-Very G, Vergilio JA, Degar BA, et al. . Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116(11):1919–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berres ML, Lim KP, Peters T, et al. . BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups. J Exp Med. 2014;211(4):669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satoh T, Smith A, Sarde A, et al. . B-RAF mutant alleles associated with Langerhans cell histiocytosis, a granulomatous pediatric disease. PLoS One. 2012;7(4):e33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansal R, Quintanilla-Martinez L, Datta V, Lopategui J, Garshfield G, Nathwani BN. Identification of the V600D mutation in Exon 15 of the BRAF oncogene in congenital, benign Langerhans cell histiocytosis. Genes Chromosomes Cancer. 2013;52(1):99–106. [DOI] [PubMed] [Google Scholar]
- 8.Brown NA, Furtado LV, Betz BL, et al. . High prevalence of somatic MAP2K1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood. 2014;124(10):1655–1658. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborty R, Hampton OA, Shen X, et al. . Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood. 2014;124(19):3007–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson DS, van Halteren A, Quispel WT, et al. . MAP2K1 and MAP3K1 mutations in Langerhans cell histiocytosis. Genes Chromosomes Cancer. 2015;54(6):361–368. [DOI] [PubMed] [Google Scholar]
- 11.Diamond EL, Durham BH, Haroche J, et al. . Diverse and targetable kinase alterations drive histiocytic neoplasms. Cancer Discov. 2016;6(2):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haroche J, Charlotte F, Arnaud L, et al. . High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. [DOI] [PubMed] [Google Scholar]
- 13.Fatobene G, Haroche J, Hélias-Rodzwicz Z, et al. . BRAF V600E mutation detected in a case of Rosai-Dorfman disease. Haematologica. 2018;103(8):e377–e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abla O, Jacobsen E, Picarsic J, et al. . Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson TE, Wachsmann M, Oliver D, et al. . BRAF mutation leading to central nervous system Rosai-Dorfman disease. Ann Neurol. 2018;84(1):147–152. [DOI] [PubMed] [Google Scholar]
- 16.Collin M, Bigley V, McClain KL, Allen CE. Cell(s) of origin of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 2015;29(5):825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal G, Young JR, Koster MJ, et al. ; Mayo Clinic Histiocytosis Working Group . The Mayo Clinic Histiocytosis Working Group consensus statement for the diagnosis and evaluation of adult patients with histiocytic neoplasms: Erdheim-Chester disease, Langerhans Cell histiocytosis, and Rosai-Dorfman disease. Mayo Clin Proc. 2019;94(10):2054–2071. [DOI] [PubMed] [Google Scholar]
- 18.Grois N, Pötschger U, Prosch H, et al. ; DALHX- and LCH I and II Study Committee . Risk factors for diabetes insipidus in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2006;46(2):228–233. [DOI] [PubMed] [Google Scholar]
- 19.Drier A, Haroche J, Savatovsky J, et al. . Cerebral, facial, and orbital involvement in Erdheim-Chester disease: CT and MR imaging findings. Radiology. 2010;255(2):586–594. [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Aubart F, Emile JF, Carrat F, et al. . Phenotypes and survival in Erdheim-Chester disease: results from a 165-patient cohort. Am J Hematol. 2018;93(5):E114–E117. [DOI] [PubMed] [Google Scholar]
- 21.Grois N, Fahrner B, Arceci RJ, et al. . Central nervous system disease in Langerhans cell histiocytosis. J Pediatr. 2010;156(6):873–881.e1. [DOI] [PubMed] [Google Scholar]
- 22.Grois N, Prayer D, Prosch H, Lassmann H; CNS LCH Co-operative Group . Neuropathology of CNS disease in Langerhans cell histiocytosis. Brain. 2005;128(Pt 4):829–838. [DOI] [PubMed] [Google Scholar]
- 23.Sagna Y, Courtillot C, Drabo JY, et al. . Endocrine manifestations in a cohort of 63 adulthood and childhood onset patients with Langerhans cell histiocytosis. Eur J Endocrinol. 2019;181(3):275–285. [DOI] [PubMed] [Google Scholar]
- 24.Zheng H, Xia Z, Cao W, et al. . Pediatric Langerhans cell histiocytosis of the temporal bone: clinical and imaging studies of 27 cases. World J Surg Oncol. 2018;16(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Ambrosio N, Soohoo S, Warshall C, Johnson A, Karimi S. Craniofacial and intracranial manifestations of Langerhans cell histiocytosis: report of findings in 100 patients. AJR Am J Roentgenol. 2008;191(2):589–597. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Duverneuil N, Idbaih A, Hoang-Xuan K, et al. ; French Langerhans Cell Histiocytosis Study Group . MRI features of neurodegenerative Langerhans cell histiocytosis. Eur Radiol. 2006;16(9):2074–2082. [DOI] [PubMed] [Google Scholar]
- 27.Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donadieu J, Chalard F, Jeziorski E. Medical management of Langerhans cell histiocytosis from diagnosis to treatment. Expert Opin Pharmacother. 2012;13(9):1309–1322. [DOI] [PubMed] [Google Scholar]
- 29.Haupt R, Minkov M, Astigarraga I, et al. ; Euro Histio Network . Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60(2):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gadner H, Grois N, Arico M, et al. ; Histiocyte Society . A randomized trial of treatment for multisystem Langerhans’ cell histiocytosis. J Pediatr. 2001;138(5):728–734. [DOI] [PubMed] [Google Scholar]
- 31.Gadner H, Grois N, Pötschger U, et al. ; Histiocyte Society . Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood. 2008;111(5):2556–2562. [DOI] [PubMed] [Google Scholar]
- 32.Gadner H, Minkov M, Grois N, et al. ; Histiocyte Society . Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121(25):5006–5014. [DOI] [PubMed] [Google Scholar]
- 33.Morimoto A, Ikushima S, Kinugawa N, et al. ; Japan Langerhans Cell Histiocytosis Study Group . Improved outcome in the treatment of pediatric multifocal Langerhans cell histiocytosis: results from the Japan Langerhans Cell Histiocytosis Study Group-96 protocol study. Cancer. 2006;107(3):613–619. [DOI] [PubMed] [Google Scholar]
- 34.Rigaud C, Barkaoui MA, Thomas C, et al. . Langerhans cell histiocytosis: therapeutic strategy and outcome in a 30-year nationwide cohort of 1478 patients under 18 years of age. Br J Haematol. 2016;174(6):887–898. [DOI] [PubMed] [Google Scholar]
- 35.Ng Wing Tin S, Martin-Duverneuil N, Idbaih A, et al. ; French LCH Study Group . Efficacy of vinblastine in central nervous system Langerhans cell histiocytosis: a nationwide retrospective study. Orphanet J Rare Dis. 2011;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weitzman S, Braier J, Donadieu J, et al. . 2′-Chlorodeoxyadenosine (2-CdA) as salvage therapy for Langerhans cell histiocytosis (LCH). Results of the LCH-S-98 protocol of the Histiocyte Society. Pediatr Blood Cancer. 2009;53(7):1271–1276. [DOI] [PubMed] [Google Scholar]
- 37.Goyal G, Abeykoon JP, Hu M, et al. ; Mayo Clinic-University of Alabama at Birmingham Histiocytosis Working Group . Single-agent cladribine as an effective front-line therapy for adults with Langerhans cell histiocytosis. Am J Hematol. 2021;96(5):E146–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diamond EL, Subbiah V, Lockhart AC. Vemurafenib for BRAF V600-mutant Erdheim-Chester disease and Langerhans cell histiocytosis: analysis of data from the histology-independent phase 2, open-label VE-BASKET study. JAMA Oncol. 2019;4(3):384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diamond EL, Durham BH, Ulaner GA, et al. . Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567(7749):521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Guennec L, Decaix C, Donadieu J, et al. . The cognitive spectrum in neurodegenerative Langerhans cell histiocytosis. J Neurol. 2014;261(8):1537–1543. [DOI] [PubMed] [Google Scholar]
- 41.Yeh EA, Greenberg J, Abla O, et al. ; North American Consortium for Histiocytosis. Evaluation and treatment of Langerhans cell histiocytosis patients with central nervous system abnormalities: current views and new vistas. Pediatr Blood Cancer. 2018;65(1):1–10. [DOI] [PubMed] [Google Scholar]
- 42.Martin-Duverneuil N, Idbaih A, Hoang-Xuan K, et al. ; French Langerhans Cell Histiocytosis Study Group . MRI features of neurodegenerative Langerhans cell histiocytosis. Eur Radiol. 2006;16(9):2074–2082. [DOI] [PubMed] [Google Scholar]
- 43.McClain KL, Picarsic J, Chakraborty R, et al. . CNS Langerhans cell histiocytosis: common hematopoietic origin for LCH-associated neurodegeneration and mass lesions. Cancer. 2018;124(12):2607–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Héritier S, Barkaoui MA, Miron J, et al. . Incidence and risk factors for clinical neurodegenerative Langerhans cell histiocytosis: a longitudinal cohort study. Br J Haematol. 2018;183(4):608–617. [DOI] [PubMed] [Google Scholar]
- 45.Mass E, Jacome-Galarza CE, Blank T, et al. . A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease. Nature. 2017;549(7672):389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Idbaih A, Donadieu J, Barthez MA, et al. . Retinoic acid therapy in “degenerative-like” neuro-Langerhans cell histiocytosis: a prospective pilot study. Pediatr Blood Cancer. 2004;43(1):55–58. [DOI] [PubMed] [Google Scholar]
- 47.Imashuku S, Fujita N, Shioda Y, et al. ; Japan LCH Study Group (JLSG) . Follow-up of pediatric patients treated by IVIG for Langerhans cell histiocytosis (LCH)-related neurodegenerative CNS disease. Int J Hematol. 2015;101(2):191–197. [DOI] [PubMed] [Google Scholar]
- 48.Imashuku S, Okazaki N, Nakayama M, et al. ; Japan LCH Study Group . Treatment of neurodegenerative CNS disease in Langerhans cell histiocytosis with a combination of intravenous immunoglobulin and chemotherapy. Pediatr Blood Cancer. 2008;50(2):308–311. [DOI] [PubMed] [Google Scholar]
- 49.Allen CE, Flores R, Rauch R, et al. . Neurodegenerative central nervous system Langerhans cell histiocytosis and coincident hydrocephalus treated with vincristine/cytosine arabinoside. Pediatr Blood Cancer. 2010;54(3):416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chester W. Über Lipoidgranulomatose. Virchows Arch. 1930;279(2):561–602. [Google Scholar]
- 51.Goyal G, Heaney ML, Collin M, et al. . Erdheim-Chester disease: consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood. 2020;135(22):1929–1945. [DOI] [PubMed] [Google Scholar]
- 52.Diamond EL, Dagna L, Hyman DM, et al. . Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Estrada-Veras JI, O’Brien KJ, Boyd LC, et al. . The clinical spectrum of Erdheim-Chester disease: an observational cohort study. Blood Adv. 2017;1(6):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozkaya N, Rosenblum MK, Durham BH, et al. . The histopathology of Erdheim-Chester disease: a comprehensive review of a molecularly characterized cohort. Mod Pathol. 2018;31(4):581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathis S, Godenèche G, Haroche J, et al. . Long-term outcome of basilar stenosis in Erdheim-Chester disease: a case report. Medicine (Baltimore). 2016;95(36):e4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parks NE, Goyal G, Go RS, Mandrekar J, Tobin WO. Neuroradiologic manifestations of Erdheim-Chester disease. Neurol Clin Pract. 2018;8(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diamond EL, Reiner AS, Buthorn JJ, et al. . A scale for patient-reported symptom assessment for patients with Erdheim-Chester disease. Blood Adv. 2019;3(7):934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diamond EL, Hatzoglou V, Patel S, et al. . Diffuse reduction of cerebral grey matter volumes in Erdheim-Chester disease. Orphanet J Rare Dis. 2016;11(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haroche J, Amoura Z, Trad SG, et al. . Variability in the efficacy of interferon-alpha in Erdheim-Chester disease by patient and site of involvement: results in eight patients. Arthritis Rheum. 2006;54(10):3330–3336. [DOI] [PubMed] [Google Scholar]
- 60.Arnaud L, Hervier B, Néel A, et al. . CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117(10):2778–2782. [DOI] [PubMed] [Google Scholar]
- 61.Hervier B, Arnaud L, Charlotte F, et al. . Treatment of Erdheim-Chester disease with long-term high-dose interferon-α. Semin Arthritis Rheum. 2012;41(6):907–913. [DOI] [PubMed] [Google Scholar]
- 62.Cohen-Aubart F, Maksud P, Saadoun D, et al. . Variability in the efficacy of the IL1 receptor antagonist anakinra for treating Erdheim-Chester disease. Blood. 2016;127(11):1509–1512. [DOI] [PubMed] [Google Scholar]
- 63.Diamond EL, Abdel-Wahab O, Durham BH, et al. . Anakinra as efficacious therapy for 2 cases of intracranial Erdheim-Chester disease. Blood. 2016;128(14):1896–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen-Aubart F, Maksud P, Emile JF, et al. . Efficacy of infliximab in the treatment of Erdheim-Chester disease. Ann Rheum Dis. 2018;77(9):1387–1390. [DOI] [PubMed] [Google Scholar]
- 65.Goyal G, Shah MV, Call TG, et al. . Efficacy of biological agents in the treatment of Erdheim-Chester disease. Br J Haematol. 2018;183(3):520–524. [DOI] [PubMed] [Google Scholar]
- 66.Goyal G, Shah MV, Call TG, Litzow MR, Hogan WJ, Go RS. Clinical and radiologic responses to cladribine for the treatment of Erdheim-Chester disease. JAMA Oncol. 2017;3(9):1253–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haroche J, Cohen-Aubart F, Emile JF, et al. . Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAFV600E-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411–418. [DOI] [PubMed] [Google Scholar]
- 68.Cohen Aubart F, Emile JF, Carrat F, et al. . Targeted therapies in 54 patients with Erdheim-Chester disease, including follow-up after interruption (the LOVE study). Blood. 2017;130(11):1377–1380. [DOI] [PubMed] [Google Scholar]
- 69.Hyman DM, Puzanov I, Subbiah V, et al. . Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Euskirchen P, Haroche J, Emile JF, Buchert R, Vandersee S, Meisel A. Complete remission of critical neurohistiocytosis by vemurafenib. Neurol Neuroimmunol Neuroinflamm. 2015;2(2):e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen-Aubart F, Emile JF, Maksud P, et al. . Marked efficacy of vemurafenib in suprasellar Erdheim-Chester disease. Neurology. 2014;83(14):1294–1296. [DOI] [PubMed] [Google Scholar]
- 72.The Lancet Haematology. Orphan drug approval for Erdheim-Chester disease. Lancet Haematol. 2017;4(12):e562. [DOI] [PubMed] [Google Scholar]
- 73.Bhatia A, Ulaner G, Rampal R, et al. . Single-agent dabrafenib for BRAFV600E-mutated histiocytosis. Haematologica. 2018;103(4):e177–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen Aubart F, Emile JF, Maksud P, et al. . Efficacy of the MEK inhibitor cobimetinib for wild-type BRAF Erdheim-Chester disease. Br J Haematol. 2018;180(1):150–153. [DOI] [PubMed] [Google Scholar]
- 75.Durham BH, Lopez Rodrigo E, Picarsic J, et al. . Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25(12):1839–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Destombes P. Adénites avec surcharge lipidique, de l’enfant ou de l’adulte jeune, observées aux Antilles et au Mali. Bull Soc Pathol Exot. 1965;58:1169–1175. [PubMed] [Google Scholar]
- 77.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol. 1969;87(1):63–70. [PubMed] [Google Scholar]
- 78.Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19–73. [PubMed] [Google Scholar]
- 79.Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. . Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haroche J, Abla O. Uncommon histiocytic disorders: Rosai-Dorfman, juvenile xanthogranuloma, and Erdheim-Chester disease. Hematology Am Soc Hematol Educ Program. 2015;2015:571–578. [DOI] [PubMed] [Google Scholar]
- 81.Goyal G, Ravindran A, Young JR, et al. . Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612–615. [DOI] [PubMed] [Google Scholar]
- 83.Pagel JM, Lionberger J, Gopal AK, Sabath DE, Loeb K. Therapeutic use of Rituximab for sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Am J Hematol. 2007;82(12):1121–1122. [DOI] [PubMed] [Google Scholar]
- 84.Al-Ghawas MS, Ng T, Chen LYC. Confirmed efficacy of lenalidomide and dexamethasone in unresectable cutaneous facial Rosai-Dorfman-Destombes disease. Mayo Clin Proc Innov Qual Outcomes. 2019;3(1):94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haroche J, Arnaud L, Cohen-Aubart F, et al. . Erdheim-Chester disease. Rheum Dis Clin North Am. 2013;39(2):299–311. [DOI] [PubMed] [Google Scholar]
- 86.Furmanczyk PS, Bruckner JD, Gillespy T 3rd, Rubin BP. An unusual case of Erdheim-Chester disease with features of Langerhans cell histiocytosis. Skeletal Radiol. 2007;36(9):885–889. [DOI] [PubMed] [Google Scholar]
- 87.Tsai JW, Tsou JH, Hung LY, Wu HB, Chang KC. Combined Erdheim-Chester disease and Langerhans cell histiocytosis of skin are both monoclonal: a rare case with human androgen-receptor gene analysis. J Am Acad Dermatol. 2010;63(2):284–291. [DOI] [PubMed] [Google Scholar]
- 88.Naruse H, Shoda H, Okamoto A, Oka T, Yamamoto K. A case of osteoarthropathy due to Erdheim-Chester disease with overlapping Langerhans’ cell infiltration. Intern Med. 2010;49(12):1225–1228. [DOI] [PubMed] [Google Scholar]
- 89.Caoduro C, Ungureanu CM, Rudenko B, et al. . 18F-fluoride PET/CT aspect of an unusual case of Erdheim-Chester disease with histologic features of Langerhans cell histiocytosis. Clin Nucl Med. 2013;38(7):541–542. [DOI] [PubMed] [Google Scholar]
- 90.Razanamahery J, Diamond EL, Cohen-Aubart F, et al. . Erdheim-Chester disease with concomitant Rosai-Dorfman like lesions: a distinct entity mainly driven by MAP2K1. Haematologica. 2020;105(1):e5–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhatia A, Hatzoglou V, Ulaner G, et al. . Neurologic and oncologic features of Erdheim-Chester disease: a 30-patient series. Neuro Oncol. 2020;22(7):979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165–170. [DOI] [PubMed] [Google Scholar]
- 93.Boissaud-Cooke MA, Bhatt K, Hilton DA, Muquit S. Isolated intracranial Rosai-Dorfman disease: case report and review of the literature. World Neurosurg. 2020;137:239–242. [DOI] [PubMed] [Google Scholar]
- 94.de la Fuente MI, Rosenblum MK, Diamond EL, Tabar VS, Omuro A. Erdheim-Chester disease among neuroinflammatory syndromes: the case for precision medicine. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakji-Dupré L, Le Rhun E, Templier C, Desmedt E, Blanchet B, Mortier L. Cerebrospinal fluid concentrations of vemurafenib in patients treated for brain metastatic BRAF-V600 mutated melanoma. Melanoma Res. 2015;25(4):302–305. [DOI] [PubMed] [Google Scholar]
- 96.Mazor RD, Weissman R, Luckman J, et al. . Dual BRAF/MEK blockade restores CNS responses in BRAF-mutant Erdheim-Chester disease patients following BRAF inhibitor monotherapy. Neurooncol Adv. 2020;2(1):vdaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Francis JH, Gobin yp, Nasany RA, et al. . Intra-arterial melphalan for neurologic non-langerhans-cell histiocytosis. Neurology. 2021. doi: 10.1212/WNL.0000000000012070. [DOI] [PMC free article] [PubMed] [Google Scholar]