Abstract
Background
Chimeric antigen receptor-modified T (CAR T) cells are profoundly changing the standard of care in B-cell malignancies. This new therapeutic class induces a significant number of acute neurotoxicity, but data regarding mid- and long-term neurological safety are scarce. We evaluated mid-term neurological safety, with special emphasis on cognitive functions, in a series of adults treated with CAR T cells.
Methods
Patients treated in a single center with CD19-targeted CAR T cells for a relapsing B-cell lymphoma were prospectively followed up by neurologists. Before CAR T-cell infusion, all patients underwent neurological examinations with neuropsychological testing and filled out questionnaires assessing anxiety, depression, and cognitive complaints. Patients surviving without tumor progression were re-evaluated similarly, 6-12 months later.
Results
In this prospective cohort of 56 consecutive adult patients treated with CAR T cells, 27 were eligible for mid-term evaluation (median time 7.6 months). Twelve patients developed an acute and reversible neurotoxicity with median duration time of 5.5 days. In all patients, neurological examination on mid-term evaluation was similar to baseline. In self-assessment questionnaires, 63% of patients reported clinically meaningful anxiety, depression, or cognitive difficulties at baseline, a number reduced to 44% at the time of mid-term evaluation. On cognitive assessments, no significant deterioration was found when compared to baseline, in any cognitive functions assessed (verbal and visual memory, executive functions, language, and praxis), even in patients who developed acute neurotoxicity.
Conclusion
In this cohort of patients treated with CD19-targeted CAR T cells, we found no evidence for neurological or cognitive toxicity, 6-12 months after treatment.
Keywords: axicabtagene ciloleucel, B-cell lymphoma, brain, CAR T cells, immune effector cell-associated neurotoxicity syndrome, lymphoma, nervous system, neurotoxicity, tisagenlecleucel, toxicity
Key Points.
This study provides prospective data on mid-term neurotoxicity after CAR T-cell therapy.
No evidence for neurological deficits or cognitive disorders was found after 6-12 months.
Importance of the Study.
Chimeric antigen receptor-modified T (CAR T)-cell therapy is a highly promising treatment for hematological malignancies but is frequently associated with cytokine release syndrome and neurotoxicity. Neurotoxicity usually occurs within the first 2 weeks after CAR T-cell infusion and regresses rapidly with a median duration of 6 days. Data regarding long-term neurological safety of this new treatment modality are scarce. This study provides for the first time prospective data on mid-term neurotoxicity after CAR T-cell therapy in a homogeneous cohort of patients, evaluated both before and between 6 and 12 months after CAR T cells. Extensive neurological and cognitive examinations founded no evidence for mid-term neurological deficits or cognitive disorders, even in patients who developed an acute neurotoxicity after CAR T cells.
The recent clinical success of anti-CD19 chimeric antigen receptor-modified T-cell (CAR T) therapy represents a breakthrough in treatment modalities of B-cell hematological malignancies.1,2 However, CAR T-cell infusions have been associated with significant side effects, such as cytokine release syndrome (CRS) and acute neurotoxicity.3,4
Neurological symptoms are observed in approximately half of the patients and usually occur within the first 2 weeks after CAR T-cell infusion.3–9 This neurotoxicity typically includes cognitive impairments, movement disorders, and sometimes seizures.3–9 Neurological symptoms regress rapidly with a median duration of 6 days, although severe forms might lead to death in rare cases. In one series, severe neurotoxicity was associated with significantly shorter survival,9 but neurotoxicity was the cause of death in only one patient, suggesting that other factors such as high-risk disease may have contributed to the poor outcome in this series.
In contrast with the short-term evolution of acute neurotoxicity, data regarding the long-term neurological safety of this new treatment modality are scarce. This point is significant as these treatments have a high rate of durable remission, including in patients who developed neurotoxicity.10 In a study in which patients filled out self-report questionnaires, 47.5% of them complained of anxiety, depression, or cognitive disorders.11 Unfortunately, the baseline evaluation in this series is extracted from the medical records, while post-CAR T data are collected from questionnaires, thus impeding direct comparison between both endpoints. However, this series stresses the importance of studying long-term neurocognitive disorders that can remain underdiagnosed, yet impacting quality of life, in patients treated with CAR T cells. Such neurocognitive disorders have been described after chemotherapy or after allogeneic stem cell transplant, involving various neuropsychological domains, including executive functions, verbal working memory, visuospatial functions, processing speed and attention,11–15 and daily social and professional life.16
The objective of this study is to evaluate neurological safety between 6 and 12 months after infusions of CAR T cells, with special emphasis on cognitive functions, in a cohort of patients surviving without tumor progression for more than 6 months.
Patients and Methods
Patients
Adult patients treated in Hôpital Saint-Louis (Paris) outside clinical trials with anti-CD19 CAR T-cell therapy (tisagenlecleucel or axicabtagene ciloleucel) for a relapsing lymphoma between October 2018 and August 2019 were included in this prospective cohort. Patients received lymphodepleting chemotherapy with cyclophosphamide and fludarabine before CAR T-cell infusion. This observational study was approved by the local institutional review board for ethics and clinical research (CLEA-2019-74) and conducted in compliance with STROBE Statement,17 and all patients signed an informed consent.
Neurological Follow-up
During the period reported here (October 2018 and August 2019), all lymphoma patients treated with CAR T cells underwent full neurological examinations and neuropsychological testing at baseline, within 5 days before CAR T-cell infusion. If acute neurotoxicity occurred, patients were monitored by a neurologist on a daily basis and treated according to the standard of care of our institution, with steroids and anticonvulsants when required. Neurotoxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAEv_5).18 Between 6 and 12 months after the infusion of CAR T cells, neurological examination and neuropsychological assessment were administered to patients still alive. To avoid any bias, patients who relapsed after CAR T cells and received another oncological treatment were not included in the analysis.
On the same day as neuropsychological testing, patients completed a set of self-administered questionnaires from the Hospital Anxiety and Depression Scale (HADS)19 and the Prospective and Retrospective Memory Questionnaire (QMRP)20 to collect data on anxiety, mood states, and cognitive complaints (see Supplementary Table 1). Anxiety was defined as “HADS anxiety” ≥ 8/21; depression as “HADS depression” ≥ 8/21; and memory complaints as “QMRP Prospective Memory” ≤ 21/40 or “QMRP Retrospective Memory” ≤ 19.4/40.
Cognitive performance was assessed by a trained neuropsychologist using a comprehensive battery of standardized neuropsychological tests. The overall cognitive level was assessed using the Mini-Mental State Examination (MMSE) and five cognitive domains were specifically examined: episodic memory, short-term memory, executive functions, language, and praxis. The neuropsychological tests used in this study have demonstrated reliability, validity, and availability of published cutoff and norms in the French population (see Supplementary Table 2). Some tests are frequently encountered in the international literature (MMSE, Rey-Osterrieth Complex Figure, Boston Diagnosis Aphasia Examination, Stroop Test, Trail Making Test-A and B, Digit Span forward and backward), while others are specific to French-speaking patients (French version of the Free and Cued Selective Reminding Test, Mahieux gestural praxis battery, naming task of 80 images). The choice of tests followed the recommendations of the International Cognition and Cancer Task Force (ICCTF)12 and of the Reflecting Group on Cognitive Evaluations in Oncology (GREC-Onco).21 The order of administration of tests and questionnaires was the same for all patients. To limit practice effects, alternate versions were used for the memory tests.
Statistical Analysis
Two kinds of analysis were conducted. First, we compared subjective (self-administered questionnaires) and objective (cognitive tests) measures before treatment and at mid-term evaluation, with the aim of highlighting a potential effect of treatment with CAR T cells on cognition.
For this statistical analysis, continuous variables are presented as median (range: minimum to maximum) in nonparametrical variables and as mean (standard deviation) in normally distributed variables. Nominal variables are presented as percentage. For continuous variables, analyses were carried out using either a t test or a Wilcoxon test according to the acceptance of the normality assumption (Shapiro-Wilk normality test, P = .10). Every test was done in the paired condition in order to reduce inter-individual variance. For the nominal variables, analyses were carried out with a nonparametric test (chi-square tests for within groups according to comparisons). P values were based on 2-sided tests, at a significance level of 0.05.
A second analysis was conducted in order to determine whether the existence of acute neurotoxicity after injection could influence the patients’ mid-term cognitive outcome. For this, a nonparametric test (Mann-Whitney test) was used since the assumptions of the parametric tests (normality and homogeneity) were not met.
For both analyses, given the large number of cognitive comparison (18 comparisons), the significance level was lowered to 0.003 (Bonferroni correction). Statistical analyses were performed using jamovi software (Version 1.2).
Results
Patients’ Characteristics
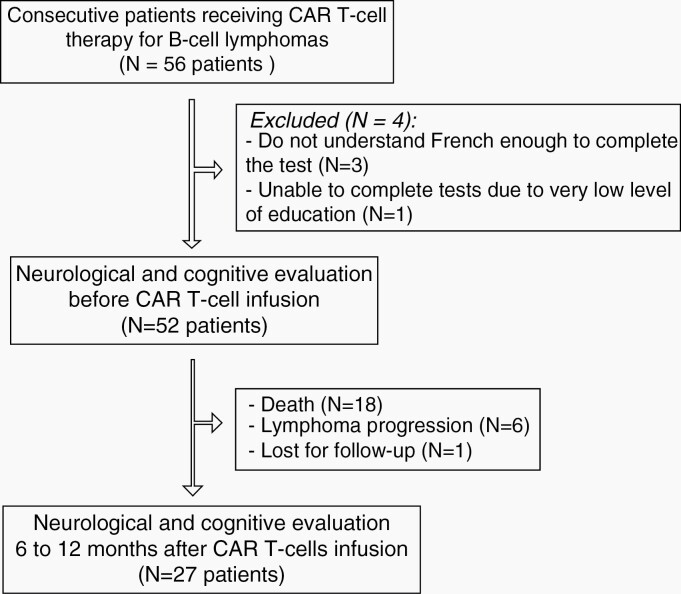
Fifty-six consecutive adult patients receiving anti-CD19 CAR T cells treatment for relapsed/refractory diffuse large B-cell lymphomas were screened. Four of these patients were not included in this study because they were not able to understand French enough to complete tests and questionnaires (n = 3) or had a very low level of education (n = 1), leaving 52 patients for a prospective follow-up. All these patients filled out self-questionnaires assessing anxiety, depression, and cognitive complaints and were examined by both a neurologist and a neuropsychologist to assess neurocognitive functions at baseline.
During follow-up, 18 of these 52 patients died of tumor progression (no neurological-related death), at a median of 2.3 months (0.3-12.6). Six other patients underwent treatment for progression, and one was lost to follow-up, leaving 27 disease-free patients for mid-term neurological evaluation (mean age ± SD: 58 ± 14 years old). Ten patients were treated with tisagenlecleucel and 17 with axicabtagene ciloleucel.
Six to 12 months after CAR T-cell infusion (median 7.6 months; range 6.1-12 months; Supplementary Table 4), all these 27 patients, among whom 12 had developed an acute neurotoxicity, filled out again the self-questionnaires and were re-examined by a neurologist and a neuropsychologist (see Figure 1, flowchart).
Fig. 1.
Flowchart of the patients analyzed in the study.
Neurological Assessment
Within the 27 patients, baseline neurological examination was considered normal in 12 patients (44%). Eleven patients (41%) had a peripheral sensory neuropathy probably due to previous chemotherapies, and four patients (15%) complained of radiculopathy due to local compression (mainly sciatica). Cerebral MRIs were done in all patients and were considered normal for age except in two patients; one showing punctiform Flair hyper-signals and one with multiple micro-bleeds.
Twelve patients developed an acute and regressive neurotoxicity (grade 1-2 in eight patients; grade 3-4 in four patients). The baseline characteristics of the 12 patients developing neurotoxicity did not differ from others in terms of mean age, level of education, and gender (see Supplementary Table 3). The median day of onset of neurological symptoms was day 7 (range 0-18). Neurological symptoms included cognitive disorders in most patients (n = 8). Four remaining patients without cognitive disorders had isolated cerebellar syndrome (n = 1), dyskinesia (n = 1), headaches (n = 1), and paraesthesia (n = 1). Detailed neurological symptoms are described in Table 1. All patients recovered from neurotoxicity within 6 weeks (six patients received steroids), with a median duration time for recovery of 5.5 days (1-42).
Table 1.
Neurological Symptomatology in the 12 Patients Who Developed an Acute Neurotoxicity
| Period | CTC Grade | Cognitive Disorders | Other Symptoms | |
|---|---|---|---|---|
| 1 | Days 1-8 | 1 | None | Dyskinesia of the upper limbs |
| 2 | Days 2-9 | 1 | Dyscalculia | - |
| 3 | Days 10-19 | 1 | None | Truncal and kinetic cerebellar syndrome |
| 4 | Days 8-15 | 1 | None | Dysesthesia |
| 5 | Days 15-20 | 2 | Lexical dysgraphia, executive disorders | Truncal and kinetic cerebellar syndrome |
| 6 | Days 5-10 | 2 | Drowsiness, dysgraphia, executive disorders | Truncal and kinetic cerebellar syndrome |
| 7 | Days 5-7 | 2 | None | Headaches |
| 8 | Days 18-23 | 2 | Executive disorders | Myoclonus |
| 9 | Days 9-42 | 3 | Drowsiness, aphasia with comprehension disorders, ideatory apraxia | Truncal and kinetic cerebellar syndrome |
| 10 | Days 6-8 | 3 | Perplexity, simple order comprehension disorder, anomic aphasia, complete ideatory apraxia | - |
| 11 | Days 8-26 | 3 | Drowsiness, aphasia, agraphia, executive disorders | Truncal and kinetic cerebellar syndrome, myoclonus |
| 12 | Days 6-12 | 4 | Drowsiness, major agraphia, acalculia, dysarthria |
In all patients, neurological examination on mid-term evaluation (over 6 months) was similar to baseline. Namely, we found no evidence for tremor, seizures, sensorimotor, or cerebellar deficits in both groups, including in the 12 patients who developed an acute neurotoxicity.
Self-Questionnaires
At baseline and at time of mid-term follow-up, all the 27 patients were asked to fill out the HADS and QMRP questionnaires19,20 assessing anxiety, depression, and memory complaints (Table 2; Supplementary Table 4). All questionnaires were retrieved. The most frequent complaints were anxiety, reported by 48% of patients at baseline, with a significant decrease at the time of follow-up (30% of patients). Only two patients reported anxiety at mid-term evaluation but not at baseline. Memory complaints, reported by eight patients (30%) at baseline (prospective memory in seven and retrospective memory in five), persisted in only three patients (11%) after 6 months.
Table 2.
Percentage of Patients With Self-Assessment of Anxiety, Depression, and Cognitive Complaints Before (Baseline) and 6-12 Months (Mid-Term) After Post-CAR T Therapy (Patients Can Present Several Signs)
| Baseline Evaluation (%) | Mid-Term Evaluation (%) | P | |
|---|---|---|---|
| HADS—anxiety | 48 | 30 | .09 |
| HADS—depression | 11 | 7 | .56 |
| QMRP prospective memory | 26 | 11 | .04 |
| QMRP retrospective memory | 19 | 7.4 | .18 |
Abbreviations: CAR T, chimeric antigen receptor-modified T cells; HADS, Hospital Anxiety and Depression Scale; QMRP, Prospective and Retrospective Memory Questionnaire.
Cognitive Assessment
Extensive and validated cognitive assessments for verbal and visual memory, executive functions, language, and praxis were administrated to all patients (not lost for follow-up) both before and 6-12 months after CAR T-cell infusions. At baseline, all patients’ performance was within normal limits.
At the time of mid-term evaluation, no significant difference was found when compared to baseline, in all cognitive functions assessed, neither when each patient assessment was compared to its own baseline assessment (Supplementary Table 5), nor when the cohort was taken as a whole (Table 3). Performances were even improved in three tests assessing visuo-constructive functions (copy of the Rey-Osterrieth Complex Figure), visuospatial memory (recall of the Rey-Osterrieth Complex Figure), and working memory (digit span).
Table 3.
Neuropsychological Tests in Our Series, at Baseline and 6-12 Months (Mid-Term Evaluation) After CAR T-Cell Infusion
| Name of Neuropsychological Tests | Baseline Evaluation Median (Min-Max) and Mean (SD) | Mid-Term Evaluation Median (Min-Max) and Mean (SD) | P |
|---|---|---|---|
| Mini-Mental State Examination (Score/30) | 29 (25-30) | 30 (23-30) | 08 |
| Episodic memory | |||
| French Free and Cued Selective Reminding Test (learning score %) | 50% (−14% to 83%) | 57% (−14% to 100%) | .20 |
| French Free and Cued Selective Reminding Test (cueing score %) | 94% (54% to 100%) | 92% (64% to 100%) | .45 |
| French Free and Cued Selective Reminding TestDelayed Free Cued consolidation (score—% of forgetfulness) | 6.7% (−21% to 75%) | 7.1% (−44% to 63%) | .60 |
| Visuospatial Memory (Recall of the Rey-Osterrieth Complex Figure—score/36) | 21 (5-33) | 26 (18-36) | <.001 |
| Short-term Memory | |||
| Digit Span forward and backward (score/19) | 9 (4-15) | 10 (6-17) | .002 |
| Executive functions | |||
| Trail Making Test part A (seconds) | 33 (18-80) | 34 (16-80) | .16 |
| Trail Making Test part B (seconds) | 84 (42-291) | 75 (31-318) | .31 |
| Trail Making Test part B (time) − Trail Making Test part A (seconds) | 51 (16-253) | 41 (13-275) | .19 |
| Stroop test: time of interference part − time of denomination part (seconds)a | 58.5 (32-190) | 56 (23-152) | .008 |
| Language | |||
| Oral naming task of 80 images (score/80) | 79 (66-80) | 79 (71-80) | 1 |
| Semantic verbal fluency (number of items in 2 minutes) | 31 (17-56) | 30 (21-60) | .58 |
| Dictation of the Boston diagnostic aphasia examination (score/3) | 3 (1-3) | 3 (0-3) | .60 |
| Repetition (words/sentences) of the Boston diagnostic aphasia examination (score/26) | 26 (25-26) | 26 (25-26) | 1 |
| Praxis | |||
| Copy of the Rey-Osterrieth Complex Figure—score/36 (constructive praxis) | 34 (27-36) | 36 (30-36) | <.001 |
| Symbolic gestures of the Mahieux gestural praxis battery—score/5 (gestural praxis) | 5 (4-5) | 5 (5-5) | 1 |
| Pantomimes of the Mahieux gestural praxis battery—score/10 (gestural praxis) | 10 (10-10) | 10 (7-10) | .37 |
| Imitation of abstract gestures of the Mahieux gestural praxis battery—score/8 (gestural praxis) | 8 (7-8) | 8 (7-8) | .23 |
Abbreviation: CAR T, chimeric antigen receptor-modified T cells.
an = 26 patients as one refused to perform the Stroop test. The significance level was lowered to 0.003 (Bonferroni correction).
In the three patients complaining of memory disorders on self-questionnaires after 6 months, cognitive tests were strictly similar to baseline. No evidence for mid-term cognitive impairment was seen in the 12 patients who did develop an acute neurotoxicity, including the four patients who developed grade 3-4 neurotoxicity with severe acute cognitive disorders (Supplementary Tables 5 and 6).
Discussion
We report here a cohort of patients treated in a single center with anti-CD19 CAR T cells for relapsed/refractory B-cell lymphoma and prospectively followed up by neurologists to monitor potential mid-term (6-12 months) neurological and/or cognitive disorders.
The incidence of acute neurological disorders attributed to CAR T cells was 40%, occurring with a median delay after CAR T-cell infusion of 7 days. All patients recovered within 2 months and none of them developed another acute neurological deficit afterwards. Both this incidence and the evolution are in line with the literature.3–9 In a previous study, no clear difference in terms of frequency of neurotoxicity between the type of CAR T cells (tisagenlecleucel or axicabtagene ciloleucel) was found.9 There are some reports of patients with remittent neurological deficits after neurotoxicity onset, but these neurological events resolved within 2 months after CAR T-cell infusions.4,7,9
When neurologically evaluated between 6 and 12 months after treatment with CAR T, none of the patients showed any evidence for mid-term neurological deficits. All patients showed similar neurological evaluations at baseline and follow-up, including those who had developed acute neurotoxicity. This observation contrasts with the series of Cordeiro et al. that reports late or long-lasting neurological events in 9/86 patients including vascular events, Alzheimer’s dementia, peripheral neuropathy, restless legs, migraine, seizures, and psychiatric events.22 However, baseline evaluation is lacking in this series making the relationship of these heterogeneous symptoms with CAR T cells unclear in this heavily pretreated population, at risk for chemotherapy-related cognitive impairment. Physiopathology of acute neurotoxicity remains unknown. Current hypotheses are alteration of the blood-brain barrier due to cytokine release and/or infiltration with lymphocytes and CAR T cells4,23,24 which might be related to CD19 expression in brain mural cells.25 Some series also mentioned cases of vascular events.8,22 Our study was not intended to clarify the physiopathology, but the return to baseline at 6 months in patients who developed an acute neurotoxicity without any recurrent episodes, fits better with a transient blood-brain barrier alteration than with autoimmune or vascular disorders.
Patient-reported outcomes (PROs), measuring health status directly from a questionnaire filled out by patients, is an interesting tool for evaluation of treatment-related toxicities, although self-assessment might be subjected to bias.26–28 In our cohort, the rate of meaningful anxiety, depression, or cognitive difficulties reported by patients was higher at baseline than at the mid-term evaluation and this improvement was statistically significant for anxiety and prospective and retrospective memory complaint questionnaires. The main complaint being anxiety, this trend makes sense for patients achieving remission of their hematological malignancies. However, the level of anxiety remained high in our cohort (30%) when compared to the general French population,29 probably because hindsight after remission was still short (6-12 months). In a series of 40 long-term survivors after CAR T cells, assessed 1-5 years after remission, the mean self-reported neuropsychiatric status was not clinically meaningfully different from the general US population.11 In line with this latter series, our cohort was similar to the general French population in terms of depressive signs.29
We did not find evidence for mid-term cognitive deterioration after CAR T cells, neither when the cohort was taken as a whole, nor when individual patients were compared to their own baseline assessments. Better still, we saw a significant improvement in performance in three cognitive tests involving visuospatial memory and working memory. Several factors may account for this finding; the most likely being the reduction in anxiety which is well known to have deleterious impact on memory and working memory,30 or a practice effect leading to improvement in cognitive test procedures.31 Secondly, we analyzed the subset of patients who previously developed a short-term neurotoxicity, presuming that these patients could be more at risk for long-term deficits, especially if they had developed acute cognitive disorders. Yet, even in this subgroup, no mid-term cognitive disorders were found. Interestingly, the subset of patients complaining of memory disorders in self-questionnaires was not identified with extensive cognitive assessment, highlighting the limitations of PROs. In the Ruark series, 37.5% of patients reported at least one cognitive difficulty on long-term evaluation.11 This figure is much higher than the 11% of patients complaining of memory disorders in our series, but the discrepancy probably relies on the different methodology and tests used in the Ruark series (no baseline assessment, only one item assessing memory, longer time interval of the assessment). Evaluation at 2 years in our cohort is underway to assess whether cognition could worsen over time. We acknowledge that the small number of patients included is a limitation of the study. However, in such neurocognitive studies with a systematic evaluation at baseline, it is necessary to find a compromise between the number of participants and the extensiveness of neurocognitive evaluation, which is time-consuming (about 2 hours per patient), requires active participation of the patients, and is at high risk of loss to follow-up. Only patients who did respond to CAR T cells and did not require any other oncological interventions were analyzed in this study. This choice was made with the idea of minimizing bias, but makes us unable to comment on the cognitive outcomes in patients with progression, which might have been more at risk of neurotoxicity. Among our 12 patients who developed acute neurotoxicity, none of them had seizures. This is not surprising as seizures are not so frequently encountered in CAR T cells.9 However, this might also have introduced a bias in our analysis, as prolonged seizures and/or cerebral edema might represent a risk factor for poor cognitive outcomes. Further studies on cohorts of patients who did experience seizures will be needed to address this point.
In conclusion, although CD19-targeted CAR T-cell therapy induces a significant rate of acute neurotoxicity, we found no evidence in this cohort of patients for mid-term (more than 6 months) neurological or cognitive toxicity. This is an important point for extending the indications for this type of treatment and should be checked for other types of CAR T treatments.
Supplementary Material
Funding
This study was supported by ADNA and AP-HP.
Conflict of interest statement. C.B.: honoraria from Gilead; A.F.C: consultant for BMS and honoraria from Gilead, Janssen; C.T.: consultant/advisory board member for Amgen, Celgene, Jazz Pharmaceuticals, Kite, Novartis, Servier Roche—Financial support (institution): Roche, Hospira, Celgene, Novartis.
Authorship statement. Conception and design: C.B. and A.F.C. Acquisition of data (acquired and managed patients, provided facilities, etc.): D.M., C.B., L.S.-V., R.U., C.T., R.D.B., and A.F.C. Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis): C.M. and A.F.C. Writing, review, and/or revision of the manuscript: D.M., C.B., C.M., L.S.-V., R.U., S.C., C.T., R.D.B., and A.F.C. Study supervision: A.F.C.
References
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. . Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;377: 45–56. [DOI] [PubMed] [Google Scholar]
- 3.Topp MS, Gökbuget N, Stein AS, et al. . Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. [DOI] [PubMed] [Google Scholar]
- 4.Gust J, Hay KA, Hanafi LA, et al. . Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santomasso BD, Park JH, Salloum D, et al. . Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Santomasso BD, Locke FL, et al. . ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. [DOI] [PubMed] [Google Scholar]
- 7.Karschnia P, Jordan JT, Forst DA, et al. . Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–2221. [DOI] [PubMed] [Google Scholar]
- 8.Rubin DB, Danish HH, Ali AB, et al. . Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain. 2019;142(5):1334–1348. [DOI] [PubMed] [Google Scholar]
- 9.Belin C, Devic P, Ayrignac X, et al. . Description of neurotoxicity in a series of patients treated with CAR T-cell therapy. Sci Rep. 2020;10(1):18997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster SJ, Svoboda J, Chong EA, et al. . Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruark J, Mullane E, Cleary N, et al. . Patient-reported neuropsychiatric outcomes of long-term survivors after chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2020;26(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 13.Nelson WL, Suls J. New approaches to understand cognitive changes associated with chemotherapy for non-central nervous system tumors. J Pain Symptom Manage. 2013;46(5):707–721. [DOI] [PubMed] [Google Scholar]
- 14.Rusiewicz A, DuHamel KN, Burkhalter J, et al. . Psychological distress in long-term survivors of hematopoietic stem cell transplantation. Psychooncology. 2008;17(4):329–337. [DOI] [PubMed] [Google Scholar]
- 15.Bevans M, El-Jawahri A, Tierney DK, et al. . National Institutes of Health hematopoietic cell transplantation late effects initiative: the patient-centered outcomes working group report. Biol Blood Marrow Transplant. 2017;23(4):538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodin G, Yuen D, Mischitelle A, et al. . Traumatic stress in acute leukemia. Psychooncology. 2013;22(2):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 18.Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed April 24, 2020.
- 19.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 20.Guerdoux-Ninot E, Martin S, Jailliard A, Brouillet D, Trouillet R. Validity of the French Prospective and Retrospective Memory Questionnaire (PRMQ) in healthy controls and in patients with no cognitive impairment, mild cognitive impairment and Alzheimer disease. J Clin Exp Neuropsychol. 2019;41(9):888–904. [DOI] [PubMed] [Google Scholar]
- 21.Taillia H, Léger I, Moroni C, et al. . [The battery of the GREC-ONCO (Reflecting Group on Cognitive Evaluations in ONCOlogy)]. In Hugonot-Diener L, Thomas-Antérion C, Sellal F, eds. GREMOIRE 2. Tests and Scales of Neurological Diseases with Cognitive Symptomatology. Paris: De Boeck/Solal, 2015:33–37. [Google Scholar]
- 22.Cordeiro A, Bezerra ED, Hirayama AV, et al. . Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taraseviciute A, Tkachev V, Ponce R, et al. . Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 2018;8(6):750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019;111(7):646–654. [DOI] [PubMed] [Google Scholar]
- 25.Parker KR, Migliorini D, Perkey E, et al. . Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T Immunotherapies. Cell. 2020;183(1):126–142.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):319–325. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty R, Sidana S, Shah GL, et al. . Patient-reported outcomes with chimeric antigen receptor T cell therapy: challenges and opportunities. Biol Blood Marrow Transplant. 2019;25(5):e155–e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lépine JP, Gasquet I, Kovess V, et al. . [Prevalence and comorbidity of psychiatric disorders in the French general population]. Encephale. 2005;31(2):182–194. [DOI] [PubMed] [Google Scholar]
- 30.Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. . A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106:1–27. [DOI] [PubMed] [Google Scholar]
- 31.Wesnes K, Pincock C. Practice effects on cognitive tasks: a major problem? Lancet Neurol. 2002;1(8):473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.