The first infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in December 2019. The ensuing and ongoing pandemic continues to present significant public health challenges with important cardiovascular (CV) consequences. Despite the rapid development of effective vaccine programs, coronavirus disease 2019 (COVID-19) cases persist with newly evolving longer-term effects. Patients living with heart failure (HF) serve as a particularly vulnerable population wherein worse clinical outcomes ensue in the setting of acute COVID-19. Questions pertaining to mechanisms of disease activity, the unique vulnerability attributable to preexisting CV disease, and the natural history of postacute sequela are the subject of avid ongoing research. Changes in the patterns of care for patients with chronic diseases have prompted dramatic shifts in care delivery, with an increased reliance on remote monitoring systems and virtual visits. HF care has been uniquely impacted in this regard.
The present article, compiled by a multidisciplinary group of investigators, serves to outline the natural history of COVID-19 and its effects, pertinent discoveries, implications for clinical care, and remaining gaps in knowledge at the nexus of HF and COVID-19 in adults. As an important disclaimer, new information on COVID-19 emerges steadily. The references and concepts in this article reflect the state of knowledge at the time of review and writing, but are subject to change, predicated on anticipated future discovery. This scientific statement is intended, therefore, to serve as a document whereby progress may be assessed subsequently.
Pathophysiology
Viral Infection and CV Disease
Early during the pandemic, it became apparent that infection with SARS-CoV-2 resulted in systemic manifestations beyond respiratory compromise, with significant yet heterogenous presentations observed involving the CV system.1 , 2 These ranged from asymptomatic biomarker elevations to HF and cardiogenic shock requiring hemodynamic support.3, 4, 5, 6 Case reports of COVID-19 myocarditis garnered significant attention, yet confirmation of causation has proven elusive.
Myocardial Injury and Myocarditis: Defining the Problem
Before delineating the mechanisms by which viral infections may lead to cardiac pathologies, it is first critical to review commonly used terms of myocardial injury or cardiac injury. Myocardial injury can be defined most universally by increases in serum troponin concentrations,7 but has also been reported in the context of findings on advanced imaging. Main findings include abnormalities on cardiac magnetic resonance imaging (cMR) in T1 and T2 mapping, and late gadolinium enhancement, among others.8 The diagnosis of myocarditis, however, rests on the recognition of a clinical syndrome with abnormalities noted across multiple parameters.9 , 10 Although a pathological assessment of cardiac tissue by endomyocardial biopsy has been emphasized, the sensitivity of findings may vary. Further, it is increasingly recognized that cardiac injury from SARS-CoV-2 may not require myocyte death or an inflammatory cell infiltration.11 , 12 Thus, the term myocarditis has been reported in many settings based on varying criteria, including elevation in troponin concentrations, clinical signs of congestion, decrements in ejection fraction, and/or abnormal cMR findings, among others, in the absence of other explanatory causes.
Viral Infection and Myocardial Injury
To elucidate the role of viral infection in causing myocardial injury and potentially myocarditis, proposed mechanisms range from direct cellular invasion to active induction of detrimental immune responses (inflammatory or autoimmune). Endemic viruses such as coxsackie A and B, echoviruses, parvovirus B19, and viruses from the Herpesviridae family (such as human herpesvirus 6, Epstein–Barr virus, and cytomegalovirus) display primary CV tropism or lymphotropism and persistence in cardiac tissue. HIV and influenza A and B virus infections can also result in myocardial injury and myocarditis by enhanced immune system activation.12, 13, 14, 15 Epidemic H1N1 influenza strains such as the causative agent in the 1918 pandemic or the 2009 H1N1pdm09 virus have been correlated with myocarditis, with histologic studies in the latter displaying degenerated myocytes, infiltration of lymphocytes, and interstitial edema, but less often viral infiltration in myocytes itself.16 Seasonal coronaviruses have not been associated previously with cardiac abnormalities. In contrast, myocarditis and cardiomyopathy have now been reported with all 3 epidemic-prone beta-coronaviruses: Middle East respiratory syndrome virus and severe acute respiratory syndrome associated coronaviruses (SARS-CoV and SARS-CoV-2).17, 18, 19, 20 Infiltration of macrophages has been reported,21 but autopsy studies and some endomyocardial biopsy reports in cases of severe or fatal SARS-CoV-2 infections have not commonly demonstrated classic lymphocytic myocarditis based on Dallas or European Society of Cardiology working group criteria.9 , 10 , 22 , 23
Compared with SARS-CoV, SARS-CoV-2 targets angiotensin-converting enzyme 2 (ACE-2) receptors with greater affinity and across a broader range of organ systems, allowing its spike protein to gain cell entry mediated by host serine proteases TMPRSS2, cathepsin B, and cathepsin L.24, 25, 26 ACE-2 is expressed on a large variety of cardiac cells, including cardiomyocytes, pericytes, fibroblasts, and endothelial cells, as well as infected leukocytes and macrophages discovered in the myocardium.27 The latter suggests localization of virus to the heart at least during transient viremia.28 The proposed mechanisms of cardiac injury in patients with COVID-19 include direct infection with fusion of myocytes and apoptosis of cardiac and vascular endothelial cells, damage via proinflammatory dysregulated cytokine storm in response to the infection, and a propensity toward the development of microembolic and thrombotic involvement in vasculature.12 , 20 , 29 , 30 Rare cases of acute myocardial infarction with a high thrombus burden were reported in patients with COVID-19 and may have also contributed to ventricular dysfunction and, in some cases, cardiogenic shock.31, 32, 33 Additionally, owing to reports of autoimmune and autoinflammatory conditions such as multisystem inflammatory syndrome in children and multisystem inflammatory syndrome in adults, the role of infection leading to an immune response against self-epitopes has also been invoked.34, 35, 36, 37 Secondarily, respiratory dysfunction and hypoxemia, as well as dysregulation of the renin–angiotensin–aldosterone system, likely also contribute to cardiac findings in patients with COVID-19.12 Thromboembolic complications, such as deep vein thrombosis and pulmonary emboli, in addition to increases in pulmonary pressures from COVID-19–induced parenchymal lung disease leading to right HF have also been described.38 , 39
Although the precise pathophysiologic pathways may be multifactorial and incompletely understood, myocardial injury is more commonly encountered among patients with preexisting CV disease and is associated with worse clinical outcomes, including admission to the intensive care unit (ICU), ventricular dysfunction, arrhythmias, and death in patients with COVID-19. The degree of myocardial injury and myocardial stretch, as evidenced by cardiac troponin and natriuretic peptide elevations, have further shown to be strong predictors of adverse outcomes.4 , 40, 41, 42
COVID-19 in Patients With a History of HF
Early in the pandemic, advanced age and cardiometabolic comorbidities including diabetes, obesity, and hypertension were observed to be commonly associated with more severe forms of COVID-19.43 , 44 Mechanistic understanding of SARS-CoV-2 viral entry via the ACE-2 receptor led to concerns that patients with preexisting dysregulation of this neurohormonal axis, including patients with HF, may be particularly susceptible to severe COVID-19 and its related complications.45, 46, 47 This concern for increased susceptibility was reinforced by historical presentations of cardiac findings in other viral respiratory infections, such as influenza. For example, in a retrospective analysis of more than 8 million individuals with HF from the National Inpatient Sample, those diagnosed with influenza during hospitalization had higher rates of in-hospital mortality, acute respiratory failure, and acute renal failure, even after propensity matching.48 Together, these data identified patients with HF as possibly more vulnerable to serious adverse events associated with COVID-19.
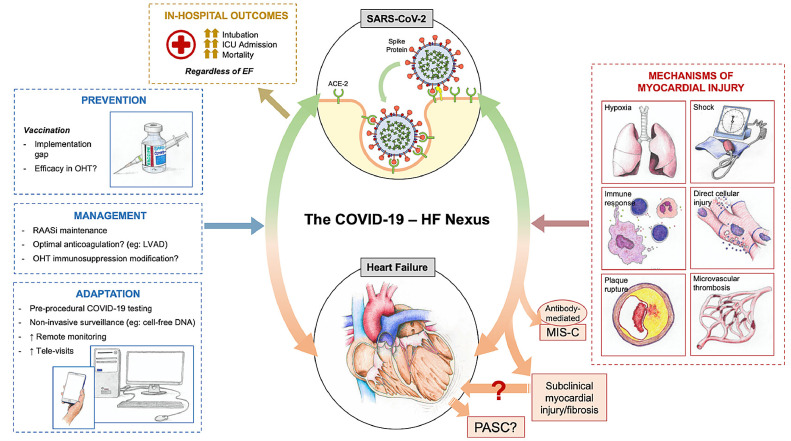
The incremental risk of poor in-hospital outcomes in patients with COVID and HF history has been demonstrated in 2 large retrospective studies. One analysis of 6439 patients admitted with COVID-19 across a large health system in New York City from February to June 2020 included 422 patients (6.6%) with HF. The study found that a history of HF was associated with a prolonged length of stay, increased need for ICU-level care, and greater rates of mechanical ventilation. The overall mortality among the cohort was 25.8%, although those with preexisting HF had significantly higher mortality as compared with those without (40.0% vs 24.9%; hazard ratio 1.88, 95% confidence interval 1.27–2.78).49 Importantly, the effect of a prior history of HF on worsening outcomes was observed across the spectrum of left ventricular ejection fraction and RAAS inhibitor use. Similar findings were reported using in a large, all-payer database inclusive of more than 1000 health care entities and health systems that included 132,312 patients with HF hospitalized from April to June 2020.50 Those with a history of HF and hospitalization with COVID-19 had significantly greater in-hospital resource use, including higher rates of ICU admission, mechanical ventilation, and renal replacement therapy as compared with those hospitalized with COVID-19 without HF. Among patients hospitalized with COVID-19, 24.2% of those with a history of HF died compared with 14.2% without a history of HF. In addition to increased mortality, a history of HF also was associated with greater morbidity in those hospitalized with COVID-19, with 41.0% of survivors requiring postacute care services as compared with 18.6% among those hospitalized with COVID-19 without a history of HF.50 Overall, these data suggest that patients with a history of HF (regardless of ejection fraction) represent a vulnerable group with greater predilection for COVID-19–related morbidity and mortality (Central Figure).
Central Figure.
The nexus of heart failure and coronavirus disease 2019 (COVID-19). ACE-2, angiotensin-converting enzyme 2; EF, ejection fraction; HF, heart failure; ICU, intensive care unit; LVAD, left ventricular assist device; MUS-C, multisystem inflammatory disorder in children; PASC, postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection; RAASi, renin–angiotensin–aldosterone system inhibitor.
Recognition of Acute HF in Patients With COVID-19
Challenges in the recognition of HF may be encountered owing to overlapping symptoms with respiratory compromise typically associated with COVID-19, including shortness of breath and pulmonary infiltrates on imaging. For example, both HF and pneumonia in the setting of COVID-19 can present with ground-glass opacities and thickened interlobular septae. The assessment of congestion, including signs and symptoms, as well as objective evidence by an elevated natriuretic peptides should occur routinely to help distinguish possible presenting features of HF; however, natriuretic peptides may also be high in the setting of pulmonary embolism or acute respiratory distress syndrome.51 In cases of suspected HF, pleural effusions, cardiomegaly, and inferior vena cava diameter enlargement may be more apparent and readily resolved with diuretic therapy.
Despite these diagnostic limitations, the assessment of congestion is relevant not only for those patients with a history of HF, but also for identifying new or incident HF. The point prevalence of new HF diagnoses in the setting of COVID-19 has not been well-reported; however, observational studies suggest SARS-CoV-2–related incident HF is likely infrequent. In an adjunct study of 6439 patients hospitalized with COVID-19 in New York City, only 37 (0.6%) were discharged with a new diagnosis of HF.49 , 52 Of these, 13 presented with shock (cardiogenic [n = 4], septic [n = 6], and mixed [n = 3]) and 5 patients presented with acute coronary syndrome. Notably, only 8 patients had neither CV disease nor any CV risk factors, whereas 14 had a history of overt CV disease and the other 15 had 1 or more risk factors that could have predisposed to the development of HF. The 8 individuals with new HF in the absence of CV risk factors tended to be younger, with lower body mass indices and fewer comorbidities than other patients with a new HF diagnosis.
Evidence of Myocardial Injury in the Subacute Setting
The mechanisms of viral injury leading to myocardial edema or fibrosis have been described to contribute to the high rates of left ventricular diastolic and systolic dysfunction in patients without epicardial or microvascular occlusions.53 In the subacute setting, cMR has revealed a high frequency of cardiac involvement in various forms, including changes in systolic function, increased myocardial native T1 representing a potential capillary leak, fibrosis in addition to raised myocardial native T2 typically indicative of myocardial edema, myocardial late gadolinium enhancement indicating fibrosis, or pericardial enhancement.19 For example, in a recent case series of 148 patients with positive troponin and severe COVID-19 in hospitals in London, 54% of hospitalized patients had magnetic resonance imaging abnormalities at a mean of 68 days after discharge, with 32% inflammatory and 28% ischemic patterns.8 A majority of patients (89%) had normal left ventricular function (ejection fraction 67% ± 11%). The rate of cMR abnormalities in young, previously healthy athletes who survived COVID-19 infection seems to be much lower, between 0.6% and 3.0%.54 Increased recognition of cardiac involvement as evidenced by advanced imaging techniques points to direct and indirect effects of SARS-CoV-2 infection on the CV system that may reflect new HF or potential for the development of HF over time.55
Management of HF During the COVID-19 Pandemic
Medical Management
Despite the concerns regarding a potentially increased propensity for more severe disease by way of enhanced viral entry via upregulated ACE2 receptors in patients on RAAS pathway inhibitors discussed elsewhere in this article,47 accumulating retrospective and prospective data as well as a joint statement by the American College of Cardiology/American Heart Association/Heart Failure Society of America affirm that RAAS pathway inhibitors should generally not be discontinued in patients with HF who are at risk for or hospitalized with COVID-19.56, 57, 58, 59 In fact, the discontinuation of these medications in patients not only with HF but also hypertension and coronary artery disease has not been shown to improve outcomes and likely deprives patients of evidence-based therapy.57
Statins have been studied as treatment in COVID-19. Multiple observational studies have shown improved outcomes among patients on chronic statin therapy who are hospitalized for COVID-19, potentially owing to their pleiotropic and anti-inflammatory effects,60, 61, 62 and randomized clinical trials are ongoing or will soon be reported. Patients with an alternative indication for statin therapy should remain on therapy, because there is no evidence that the halting of statins is beneficial in patients with COVID-19. In light of the known prothrombotic state associated with COVID-19 infection, various anticoagulation strategies are under investigation. Although retrospective data suggested benefits for intermediate- or full-dose anticoagulation,63 prospective studies have shown different findings based on disease severity.64, 65, 66 At a minimum, all hospitalized patients with HF with COVID-19 infection should receive prophylactic doses of anticoagulation, and some may benefit from therapeutic anticoagulation.67 Those patients with an alternative indication for therapeutic anticoagulation should continue this therapy provided there are no contraindications. Randomized control trial data have also informed the usefulness of corticosteroids in improving 28-day mortality among patients hospitalized with COVID-19.68 In an open-label trial of dexamethasone (intravenous or oral), 28% of patients had some form of heart disease. Patients with HF should be offered steroids among those requiring oxygen or invasive mechanical ventilation. Similarly, antiviral agents, monoclonal antibodies, and/or other immunomodulating therapies (ie, tocilizumab) should be offered to patients with HF in the appropriate clinical settings. Special attention may need to be paid to effective circulating volume in the setting of such therapies, particularly in patients with HF on chronic diuretic therapy.
| Therapy | Key Pharmacologic Considerations Among Patients With HF and COVID-19 |
|---|---|
| RAAS inhibition (ACEi, ARB, ARNI) | RAAS inhibition and guideline-directed medical therapy should not be disrupted in the acute setting of COVID-19 or thereafter |
| Statin | Currently, insufficient evidence to support routine statin use without other indications for statin therapy. However, patients on statin therapy should not have care disrupted |
| Anticoagulation | Evidence to guide recommendations as to optimal anticoagulation regimens for patients with HF has been recently completed with further data are forthcoming; recommendations may be different based on the severity of disease (moderate vs critically ill) per ACTIV-4 findings |
| Steroids and immunomodulating therapies (intravenous immunoglobulin) or monoclonal antibodies | May require careful monitoring of volume status and additional decongestive therapy as indicated |
| Antiviral agents | No evidence to support differential approach to use among patients with HF |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; COVID-19, coronavirus disease 2019; HF, heart failure; RAAS, renin–angiotensin–aldosterone system
Temporary Mechanical Circulatory Support
Data regarding the use of temporary mechanical circulatory support for patients with COVID-19 and cardiogenic shock are primarily limited to case reports and small case series.69, 70, 71 Cardiogenic shock in patients with COVID-19 should thus be managed similarly to those without COVID-19, with appropriate pharmacologic therapies selected based on clinical presentation. If mechanical circulatory support is indicated, venoarterial extracorporeal membrane oxygenation (ECMO) is often preferred for hemodynamic support as well as oxygenation, because most patients have significant coexisting hypoxemia. In acute respiratory distress syndrome with profound hypoxemia, differential oxygenation gradients may exist between blood traveling through the native circulation (arising from cardiac ejection) and the ECMO pump. In such scenarios, conversion to a venoarterial–venous ECMO cannulation strategy can be considered to deliver fully oxygenated blood through the pulmonary circulation while preventing compromise to cardiac recovery. Although data remain limited, the Extracorporeal Life Support Organization put forth specific guidelines regarding ECMO use in patients with COVID-19.72 In 1 report of 22 patients with COVID-19 on ECMO, 21 patients had respiratory failure and 7 had cardiac failure requiring arterial support.73 Ultimately, 12 patients (54.5%) survived hospitalization. The Extracorporeal Life Support Organization suggests that prepandemic criteria for the selection of ECMO candidates should be used; however, these criteria may not be universally applicable if resources are constrained.72
Impact of the Pandemic on Evaluation for Advanced HF Therapies
The pandemic has also impacted care for patients with HF without COVID-19 owing to significant changes in health care delivery.67 The early phases necessitated a reallocation of various hospital resources, increasing use of telemedicine, and placing limitations on elective procedures and testing. Thus, less urgent evaluations for left ventricular assist devices (LVAD) and heart transplantation faced significant delays.74 For example, cardiopulmonary exercise testing was often deferred because this test is an aerosolizing procedure and therefore requires special precautions. Similarly, placement of implantable cardioverter-defibrillators, stress testing, right heart catheterization, and other nonemergent diagnostic and therapeutic procedures were postponed early on and subsequently depending on COVID-19 case volume.74 Care adaptations have since largely allowed for the resumption of these services in many hospitals, but still require negative preprocedural COVID testing.
Left Ventricular Assist Devices
As the pandemic continues with varying densities of infection, decisions regarding the new implantation of durable LVADs were and continue to be highly dependent on various factors, including local policies, rate of SARS-CoV-2 infection in the surrounding area, and the availability of relevant resources, including ICU capacity. In settings of high rates of SARS-CoV-2 infection placing strain on hospital resources, LVAD implantation should be limited to the Interagency Registry for Mechanically Assisted Circulatory Support status 1–3 patients in whom implantation has unequivocal benefit among appropriately selected patients.75 Consideration should be given to the feasibility of outpatient LVAD follow-up care in the early postoperative period to minimize the risk of exposure to SARS-CoV-2. The social evaluation is particularly relevant and should include an assessment of home conditions that may place patients at increased risk for acquiring COVID-19 in the vulnerable postoperative phase.
Special considerations are required for patients on LVAD support who contract COVID-19.76 This population is at risk for severe COVID-19 infection owing to advanced age (in many circumstances), an increased number of comorbid conditions, and potentially compromised cellular immunity leading to a functionally immunosuppressed status.77 Cases have been reported of COVID-19 complicated by cytokine release syndrome leading to acute respiratory distress syndrome and multiorgan failure.78 Additionally, COVID-19 infection is associated with a proinflammatory and prothrombotic milieu that could pose additional problems in LVAD-supported patients who are already at increased risk for stroke and thrombosis. In cases of COVID-19 pneumonia-associated right ventricular dysfunction, adjustments may need to be made to LVAD speed considering inotropic support for the right ventricle. Prone positioning may present unique challenges in patients with LVADs owing to fear of driveline displacement or worsening of right ventricular hemodynamics,79 but is reported to have been conducted safely in highly monitored settings.75
Cardiac Transplantation
Heart transplant waitlist activity and volume were also impacted during the peak of the COVID-19 pandemic in the United States. Particularly during the early months, there was a significant increase in inactivated transplant candidates, with fewer new candidates added to the waitlist.80 Donor recovery also decreased owing to concerns regarding potential for COVID-19 positivity, initial lack of access to COVID-19 testing, and limitations in organ procurement organizations operation in the setting of COVID-19–associated policies around limited hospital access and travel. The number of heart transplants performed concomitantly decreased even in regions with a lower prevalence of COVID-19 owing to the effects of organ sharing.80 At many centers, only patients requiring hospital admission who qualified as United Network for Organ Sharing tiers 1–3 remained active on the transplant list wherein the risk of mortality owing to HF was deemed to outweigh the risk of COVID-19 exposure and need for resource conservation.81
The pandemic catalyzed many centers to switch to noninvasive surveillance strategies for ambulatory transplant recipients to detect rejection. The use of gene expression profiling and measurements of donor-derived cell-free DNA minimized exposure to health care personnel,82 , 83 mainly because endomyocardial biopsies were performed more selectively. The downstream clinical implications of this modified workflow on rejection rates and graft function and the associated survival outcomes in heart transplant recipients are of importance and undergoing further study.
The impact of COVID-19 infection among heart transplant recipients has been published in select reports. Among these, 2 New York Hospitals reported outcomes of 28 and 22 patients, and a group from Italy reported on 47 heart transplant recipients diagnosed with COVID-19. All 3 groups reported a case fatality of 25%–30%, highlighting the need for extra caution as to the avoidance of exposure to COVID-19 in heart transplant recipients, as well as the need to triage to higher levels of care if COVID-19 is contracted for such immunocompromised patients.84, 85, 86 A recent study of 99 patients with heart transplants and COVID-19 found a death rate of 15%; 64% required hospital admission87. Concerning immunosuppression, decreasing the dosage of calcineurin inhibitors and decreasing or temporarily discontinuing antimetabolites in the setting of COVID-19 infection may be considered on an individual basis. Yet, data as to optimal approaches are lacking.88 Additionally, drug interactions with COVID-19 therapeutics should be reviewed. Current vaccines against SARS-CoV-2 seem to be less effective in immunosuppressed patients,89 , 90 stressing the importance of continued infection control measures for heart transplant recipients, especially as the social measures imposed to combat SARS-CoV-2 transmissions are relaxed. It is incumbent on transplant programs to disseminate this information to coordinators and patients, and efforts to ensure vaccination among transplant recipients and those on the waitlist should be prioritized. Optimal vaccination strategies, including whether additional doses are required to confer immunity for solid organ transplant recipients, have been the subject of active investigation,91, 92, 93 and the US Food and Drug Administration recently authorized additional vaccine doses for immunocompromised individuals, including those with a solid organ transplant.94
COVID-19 Testing and Assessment Before Advanced Therapies Evaluation and Surgery
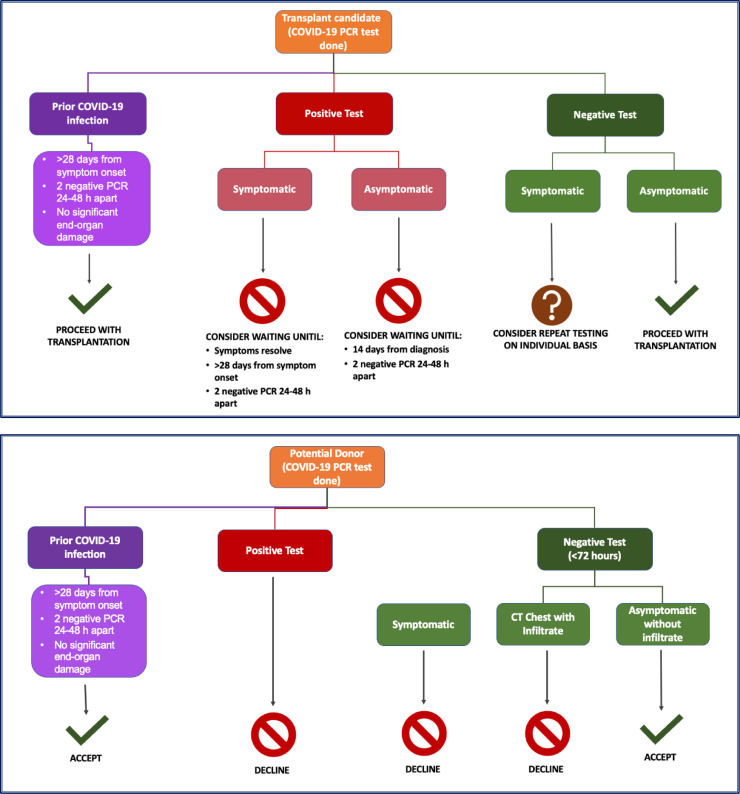
Before heart transplant surgery, donor and recipient testing for COVID-19 is performed routinely. According to a guidance document from the International Society for Heart and Lung Transplantation, donor testing should be performed within 72 hours of organ donation using polymerase chain reaction (PCR)-based testing by nasopharyngeal/oropharyngeal swab, sputum/tracheal aspiration, or bronchoalveolar lavage.75 There have been reports of donor-to-recipient SARS-CoV-2 transmission during lung transplantation despite negative donor upper respiratory tract testing,95 although it is unclear if this applies to other forms of organ donation (including heart transplant donation) or if lower respiratory tract testing (eg, bronchoalveolar lavage) would be a more optimal testing strategy. Potential algorithmic considerations regarding candidate and donor testing are presented in Fig. 1 .75
Fig. 1.
Algorithmic considerations for heart transplant candidate and donor severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing. COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction.
Hospitalized patients actively listed for heart transplantation should be cohorted in a COVID-19–free hospital location when possible. Ideally, nurses and additional staff caring for patients with COVID-19 should not be concomitantly assigned to patients awaiting transplant.81 PCR-based testing for COVID-19 should generally be performed for asymptomatic candidates within 5 days of surgery if a surgical date is known or if any new symptoms or exposures develop.
Heart transplantation and nonurgent surgical, electrophysiology, and catheter procedures for HF should likely be deferred if a candidate tests positive by PCR.75 , 96 Patients should only be reactivated or scheduled for procedures when clinical symptoms have resolved, more than 28 days have passed since the onset of symptoms, and/or 2 successive PCR-based tests at least 24–48 hours apart returned a negative result. Additionally, patients should not have sustained COVID-19–related end-organ damage. Enhanced imaging with high-resolution chest computed tomography scans may provide incremental risk assessment in select asymptomatic cases wherein a COVID-19 PCR test is positive. For asymptomatic patients, 14 days after diagnosis and 2 successive negative tests may serve as reasonable criteria for reactivation. For candidates at high risk for HF-related mortality without heart transplantation, decisions need to be individualized, and such timelines need to be considered in the clinical context of a risk–benefit assessment.75 There is limited evidence to guide similar recommendations for LVAD surgery; however, similar approaches could be considered. Of note, life-saving surgery (ie, pump thrombosis requiring exchange) should not be denied based on a positive PCR test alone—such patients should be offered surgery if clinically indicated, provided they do not have contraindications, with appropriate precautions in place.
Challenges and Innovations in HF Care Delivery During the Pandemic
Emergency department visits and hospitalizations for non–COVID-19 life-threatening diagnoses significantly decreased in the first one-half of 2020 compared with historical norms,97 , 98 including fewer admissions for acute coronary syndrome and HF.99 , 100 An excess in cardiac arrest and sudden death in the community during the peak of the pandemic was attributed to avoidance and fear of undue exposure.101 , 102 These realized secondary effects of the pandemic necessitated expeditious innovations in care delivery platforms specific to patients with HF.74
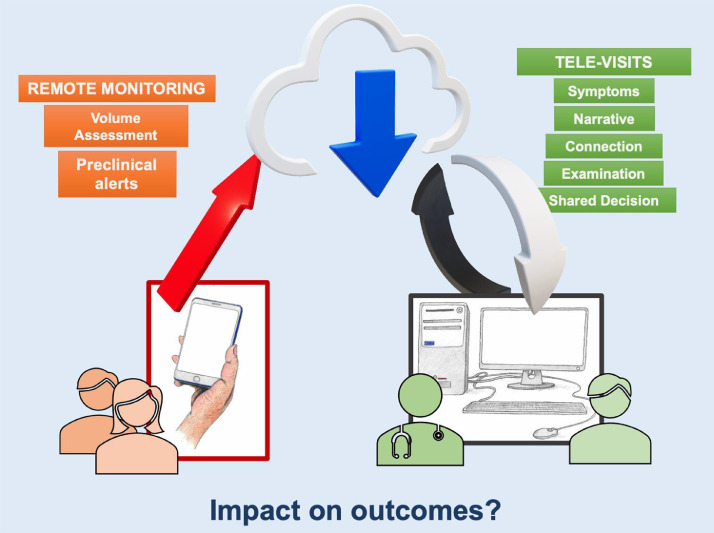
Technology was leveraged to facilitate virtual clinical assessments and data collection (Fig. 2 ).103 Regulatory reform by the Centers for Medicare and Medicaid Services with expanded reimbursement of telemedicine encounters may have aided greater adoption of these modalities. All aspects of care, including care for patients with LVAD or transplant recipients, were transformed to limit patient contact. As mentioned, gene expression profile and cell-free DNA testing partially substituted endomyocardial biopsies, including in centers that had not readily previously adopted noninvasive surveillance testing for transplant patients. Home anticoagulation monitoring and blood draws minimized in-person visits.104 Although virtual visits have been associated with better adherence to clinic follow-up (likely related to the relative ease of access and decreased burden of transportation),105 video examination alone (without supplemental remote monitoring and laboratory data) may be particularly challenging in the evaluation of high-risk patients who need careful and safe adjustment of guideline-directed medical therapies.106 , 107 Although assessing jugular venous distension and lower extremity edema by video examination is possible, supplemental data to inform intravascular volume status are frequently necessary for optimal decision-making.67 , 108 The impact of televisits in place of in-person visits on outcomes is unknown and requires investigation.
Fig. 2.
Virtual care adaptations during the coronavirus disease 2019 pandemic.
The global pandemic has accelerated remote monitoring technology innovation.109 Despite equivocal results of previous efforts,110 more recently, implantable remote pulmonary artery pressure monitoring in patients with HF and moderate symptoms has been shown to decrease HF-related hospitalizations111 and 30-day readmissions,112 and to maintain euvolemia during guideline-directed medical therapy optimization,113 including in patients with a preserved ejection fraction.114 Enhanced use of implantable hemodynamic monitoring during the pandemic to provide intravascular volume data and better guide HF treatment decisions has been reported.115 , 116 Certain implantable cardiac defibrillator systems include diagnostic and monitoring solutions to predict HF events.117 Particular algorithms can, for example, integrate a multivariate index of heart sounds, respiration rate, thoracic impedance, and physical activity to attempt to predict the degree of congestion, with early evidence of association with fewer HF hospitalizations.118 However, the usefulness of this technology in differentiating HF from COVID-19–related lung injury has yielded mixed results.119 , 120
The postpandemic state of remote monitoring and telemedicine for patients with HF is likely to include all forms of care, such as virtual, in-person, and remote assessments. Wearable devices may see accelerated adoption,121 as smartwatch heart rate and rhythm monitoring may effectively trigger the need to seek medical attention.122 Single-lead electrocardiographs and multivariate monitoring have also been used with promising results, leveraging machine learning algorithms to detect subclinical myocardial dysfunction.123 Economic support for the integration of such innovative platforms, along with appropriately designed workflows and processes to promote patient-activated therapy124 are required to advance telemedicine beyond the pandemic.
Racial, Ethnic, and Socioeconomic Disparities
The COVID pandemic has illuminated the impact of the vast inequities within the health system in the United States. Those with less access to health care are more likely to get COVID, have greater morbidity and mortality from COVID, and are least likely to get vaccinated.125 , 126 , 127 In a nearly 8000-patient analysis of the retrospective observational COVID-19 registry organized through the American Heart Association, Black (26%) and Hispanic (33%) patients together comprised more than one-half of hospitalized patients and were noted to be younger and more likely to be uninsured.128 Despite being younger, Black patients had a higher prevalence of preexisting conditions such as diabetes, hypertension, and obesity. Also, they were at increased risk of adverse outcomes such as mechanical ventilation and renal replacement therapy. Although there was no difference in major adverse cardiac events including death across race and ethnicity, 53% of all death was observed in Black and Hispanic patients representing a greater burden of morbidity and mortality in these otherwise under-represented minority groups. These observations are unfortunately rooted in a long-standing history of health care disparities wherein lack of access to healthy food, low health care literacy, and an inability to practice social distancing owing to housing density, among other factors, are disproportionately encountered among Black and Hispanic patients.129 Further compounding egregious observations of poor outcomes among these groups in the acute setting of COVID-19 is a greater hesitancy among these same populations to undergo vaccination owing to fear and mistrust of health care systems at large.126 Efforts to mitigate risk and improve rates of vaccination among under-represented populations require special attention.
The Postacute Sequelae of COVID-19 Syndrome
Persistent Symptoms and Evidence of Cardiac Injury
Although acute cases continue to arise in many countries around the globe, postacute infection symptoms indicative of nonlinear recovery have also been reported. After hospital discharge, patients (including those with no prior history of HF) may experience prevalent fatigue, muscle weakness, mild cognitive dysfunction (eg, fogginess), and sleep difficulties for up to 6 months.130 In fact, early studies have outlined not only the persistence of symptoms, but also the appearance of new symptoms, as well as detection of clinical signs suggesting that patients across the entire spectrum of acute infection can continue on to have postacute sequelae of SARS-CoV-2 infection, also referred to as “long COVID.” Regardless of hospitalization, impaired physical functioning and decreased exercise capacity can persist for weeks to months afterward and can negatively impact psychological well-being.131 Although there are no reports to date to suggest a predisposition for postacute sequelae of SARS-CoV-2 infection among patients with HF, understanding its underlying pathophysiology has implications for individual recovery, health systems preparedness, and the identification of tailored therapeutic interventions. Cardiac rehabilitation programs may be of particular benefit,132 tailored to patients with a history of HF, but also to younger patients with de novo cardiac sequelae. A recent study of patients with confirmed COVID-19 who had no cardiac complaints 2 weeks after diagnosis showed that recovered patients were more likely than healthy or risk-matched controls to have elevated cardiac injury biomarkers and abnormalities on cMR.19 Post-COVID myopericarditis has also been reported 6–8 weeks after diagnosis; currently limited to rare cases, myocarditis and pericarditis have also been reported after vaccination, particularly in younger adults.133, 134, 135 Despite worrisome early cohort studies with wide-ranging patient-reported symptoms and anecdotal clinical experience with COVID-19 survivors, postacute sequelae of SARS-CoV-2 infection as a syndrome remains ill-defined.136 A better understanding of pathobiological underpinnings of prolonged symptoms and associated ramifications are the subject of avid investigation, and longitudinal studies are needed with appropriate controls to help identify virus-specific vs critical illness-related cardiopulmonary limitations among patients with persistent symptoms.
Vaccination Against COVID-19
Within the first few months of the COVID-19 pandemic and publication of the genetic sequence of SARS-CoV-2, an effort of unprecedented speed commenced to develop and evaluate vaccine candidates. Vaccine manufacturers engineered strategies to introduce viral antigens or gene sequences to elicit immune responses to the virus spike protein, leading to decreased viral entry into host cells and thereby attenuated infection.137 The first SARS-CoV-2 vaccines to receive Emergency Use Authorization by the US Food and Drug Administration were messenger RNA and viral vector vaccines, and both were shown to significantly decrease symptomatic COVID-19. The viral vector platform involves replication of a deficient adenovirus that is engineered to deliver the SARS-CoV-2 genetic material to immune cells to express and present antigenic proteins to lymphocytes. Both vaccines rely on the production of antibodies to confer protection. Although studies in solid organ transplant recipients have found that most participants did not mount a significant antibody response after an initial vaccine dose and variable responses after second dose,89 , 138, 139, 140 vaccination remains safe90 and is strongly encouraged by multiple transplant societies.141, 142, 143 As mentioned, booster doses are now recommended for solid organ recipients and other immunosuppressed patients.
Geographic variability in the infrastructure required to vaccinate as many individuals as possible within a short time frame and vaccine hesitancy have affected overall vaccine uptake around the globe. Patients with preexisting CV disease, including HF, often were prioritized in earlier vaccination waves given the well-defined link between preexisting CV disease and more severe COVID-19–related adverse outcomes.144 Experience with the influenza vaccine has demonstrated that, despite strong support from professional cardiology organizations and the Centers for Disease Control and Prevention for annual vaccination, vaccine uptake in patients with HF is disappointingly low, coupled with consistent rates of vaccine refusal.145 , 146 Factors associated with COVID-19 vaccine hesitancy mirror what has been previously shown with other vaccines, including uncertainty about vaccine efficacy, an underappreciation of the necessity of vaccination, and general mistrust of vaccines. Given the poor outcomes demonstrated in observational studies among patients with HF who acquire COVID-19, educational and community efforts are needed to increase vaccination rates in this particularly vulnerable population.
Implementation trials in adjacent vaccination domains have indicated that behavioral economic principles, including intention prompts and active choice interventions, increased flu vaccination uptake across large populations.147 , 148 Similar trials of COVID-19 vaccination-related messaging are underway (NCT 04660703).
| Strategies shown to combat vaccine hesitancy and expand implementation |
|---|
| Clinician to patient education |
| Clinician audit and feedback |
| Patient outreach |
| Messaging that “a vaccine is reserved for you” |
| Point-of-care reminders |
| Active-choice and intention prompts |
Impact of the Pandemic on Ongoing Clinical Research
The conduct and interpretation of CV clinical trials have been severely affected by the COVID-19 pandemic.149 Recruitment in trials has been challenging because many recruiting sites were either completely prohibited from recruiting new patients or these activities had been severely curtailed.150 , 151 Ongoing trials have had challenges with study assessments and ascertainment of end points. Although many trials have converted some in-person visits to virtual visits successfully, this approach has not been practical when face-to-face procedures were required, such as for a 6-minute walk test, echocardiogram, or venipuncture for blood specimens. Outcomes trials have similarly been affected owing to increasing difficulty in ascertaining outcomes and have been impacted by the decrease in event rates for hospitalizations and urgent outpatient visits for CV events seen during the pandemic.99 These factors influence trials regardless of whether individual patients contract COVID-19.
In clinical trials, several analytic approaches (such as sensitivity analyses) have been proposed to address some of the issues related to the pandemic, ranging from censoring at the start of the pandemic to excluding events thought to be COVID-19 related.152 For example, in the study of intravenous ferric carboxymaltose in patients with HF with iron deficiency, the investigators performed a prespecified pre–COVID-19 sensitivity analysis, censoring patients in each country at the date when its first COVID-19 patient was reported.153 Additionally, the broad-ranging impact of the pandemic and associated financial challenges led to the premature termination of some HF trials, including the Effect of Sotagliflozin on CV Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial.154 Because of the influence of the pandemic on patient quality of life, the interpretation of patient-related outcomes will prove especially challenging. Academic groups, industry, and regulatory authorities have issued several recommendations to address issues related to the conduct and interpretation of clinical trials during the pandemic.155, 156, 157 Overall, the challenges faced during the conduct and operation of these trials during the pandemic may inform alternative strategies for patient consent, participation, and engagement, which may ultimately make trial design more resilient.
The Clinician Voice
The pandemic has resulted in significant emotional and psychological tolls for clinicians at all levels of training. Studies have shown higher rates of depression, anxiety, and insomnia among physicians during the pandemic.158 , 159 Sources of stress include limited access to personal protective equipment, concerns about personal safety and that of loved ones related to COVID-19 exposure, and financial challenges associated with hiring freezes.160 Owing to variable in-person closures of schools and daycare programs, clinicians have been challenged to find alternate sources of childcare. Social distancing has compounded these stresses, leading to isolation. Moving forward, it is imperative for organizations and institutions to provide adequate support systems for access to mental health, emergency childcare, and mentorship. During peak infection rates, trainees may have been affected particularly, given their responding clinician role in academic medical centers. In addition, deferral of HF-specific testing, including cardiopulmonary testing, right heart catheterizations, and endomyocardial biopsies,67 , 161 may have led to decreased procedural exposure and training in competencies. HF trainees may have been particularly impacted by fewer HF admissions and restrictions placed on donor procurement and operating room personnel. Nursing programs were temporarily suspended (because students were unable to maintain adequate clinical practice hours), extending the time needed for licensure and certification. The practical realities and constraints of educating during the pandemic have led to an increase in innovative educational strategies and virtual platforms for education and data gathering from diverse knowledge dissemination platforms.162 , 163
The Patient Voice
From a patient perspective, the journey through COVID-19 is frequently paved with uncertainty and anxiety, compounded by a barrage of information appearing almost daily across national and international media and news platforms. Marked heterogeneity in disease presentation and course, variability in response to hospital-initiated therapies, and an inability to predict and ensure a full recovery, even with mild disease, all contribute to stress when suspecting or receiving a COVID-19 diagnosis.
In patients with preexisting HF, symptoms of acute HF decompensation and pulmonary manifestations of COVID may overlap, or mild chronic HF may become unstable. Additional challenges are imposed by potential medication changes, altered fluid balance, and other potential hazards, for example, deconditioning. For patients with mild HF who are recuperating from COVID-19 at home, understanding the cause of new or worsening cardiac-related symptoms, particularly chest pain or palpitations, can be stressful. Although chest pain is a relatively common symptom of COVID-19 and is frequently nonspecific, it may also represent a more serious CV event, including myocarditis, coronary ischemia, or pulmonary embolism. Patients need to be empowered to seek care if chest pain is persistent, recurrent, occurs with exertion, or is associated with other known markers of COVID-19 instability.131 , 164
During peaks of the pandemic, family, caregiver, and supporter presence was minimized, requiring novel adaptations in care (Table 1 ). Early engagement with palliative care and supportive cardiology services have been shown to provide essential care in many disease contexts, but may provide particular benefit in COVID-19. Engaging in early goals of care conversations, particularly given the challenges with in-person communication and uncertainty surrounding illness trajectory, is essential. Other limitations imposed during hospitalization, including restriction of patients’ mobilization outside of their room and bundling of care to purposely limit the number of personnel interactions, affect patients’ mental and emotional health. Quarantining and social distancing forced hospital personnel to develop new ways of communicating and supporting patients from afar that may have advantages even after COVID-19.165 , 166 Health care systems should consider the need for psychological and occupational health support for patients, particularly those with protracted illness courses.167
Table 1.
Solutions That Support Families and Caregivers and Supporters From Afar When Hospitalization Quarantining Rules Are in Place
| Communication Methodology | Support Benefits |
Web-based video-enabled teleconferencing with camera (ie, smartphone apps such as FaceTime, Zoom, Skype)
|
Families, caregivers, and supporters and Patients • • May enhance psychosocial and psychological well-being • Ability to visualize each other may decrease anxiety and stress • Allows for confirmation that patients’ conditions match communication received from clinical personnel |
| Families, caregivers, and supporters and hospital professionals • Enhanced level of psychosocial support to meet patient needs • Can use for shared decision-making or family conferences and discussions that require consensus or consent for clinical trials • Can be used to prepare families for next steps: improving health (discharge readiness) or deteriorating health (physical environment changes, surgery, palliative care) • Decreases misinformation and unreliable data being shared • May reinforce verbal communication and create normalization of current patient status |
|
Voice-only teleconferencing
|
This format may be perceived as a lower quality communication that can affect communication satisfaction between parties and overall distress related to visitation restrictions Families, caregivers, and supporters and hospital professionals • May enhance access to health care professionals, especially if inquiring about remaining informed of the plan of care or current patients’ condition |
Personal signs, photos, quotes, etc., taped to hospital room doors or walls
|
Patients and hospital professionals • Reminds patients that there is a purpose to the work they are living through during hospitalization—a life after discharge • Allows clinicians to “do something” to support families and patients in a personal way • Provides clinicians with a view of patients as people with lives outside of the hospital environment • May decrease psychological burden of health care professionals who are struggling with difficult emotions and regret |
Short (<2 minutes) taped recordings
|
• Families, caregivers, and supporters and Patients • Reminder that families, although not present, are thinking of the patient—can be replayed as often as desired to decrease stress and promote joy |
Early engagement with hospital-based support services
|
• Early engagement of palliative care services to support patients and facilitate communication • Early discussion with patients and families on engagement of chaplain or spiritual services |
Knowledge Gaps and Future Directions
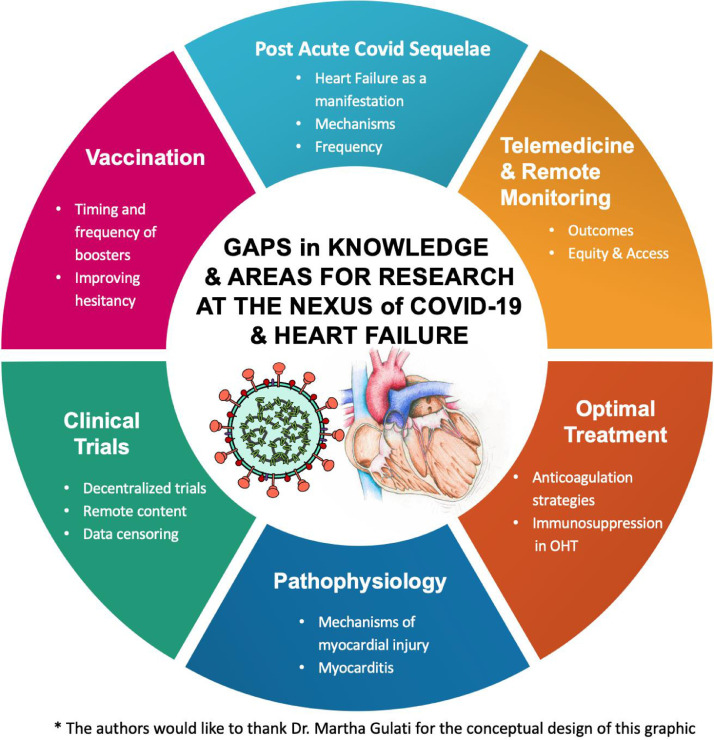
Tremendous progress in the battle against COVID-19 has been made in a short period, including the rapid development, validation, and distribution of vaccines. Nonetheless, several critical knowledge gaps and challenges remain168 (Fig. 3 ). Further investments are needed to establish a deeper understanding of the short-and long-term sequela of COVID-19 infection and the pandemic in general across all stakeholders.
Fig. 3.
Knowledge and gaps and areas of ongoing study. OHT, orthotopic heart transplantation.
Pathophysiology
First, important questions remain regarding the pathobiology of disease, principally regarding the effects of COVID on the heart. Although it is evident that patients with CV disease and HF in particular are at greater risk for more significant morbidity and mortality owing to COVID, it is uncertain why. Additional questions remain, especially regarding the degree and nature of myocardial injury, including COVID myocarditis. As with other causes of viral myocarditis, it is uncertain whether direct myocardial injury occurs from viral infection or whether CV sequelae are secondary to an unchecked immune response. In vitro and in vivo model systems have been developed to help elucidate mechanisms of COVID myocarditis; ideally, this ongoing work will have implications beyond COVID-19.
Treatment of COVID-19
One of the most pressing concerns remains the lack of therapeutics to treat COVID-19 effectively and reliably. Although several therapies are available, they are variably effective, particularly for severe infections. The results of ongoing studies will determine the efficacy of both novel and existing therapeutic agents. Of particular interest to the CV community is the effectiveness of specific anticoagulants in the prevention and treatment of COVID-19–associated thromboembolic complications. Whether or not tailored approaches are needed for patients with HF, LVADs, or transplantation remains to be understood. Notably, health care system investment in mitigating the disparities of contraction and outcomes with COVID-19 among Black and Hispanic communities should be a public health priority.
Vaccination
Specific questions persist regarding patients with advanced HF and, in particular, those who have undergone cardiac transplantation. Despite initial optimism, it seems that solid organ transplant recipients have worse outcomes with COVID-19 than expected. Recent data also suggest that these patients have a lower response to immunization. If this is the case, it is clear that mitigation strategies need to be developed, and the US Food and Drug Administration now recommends booster shots for immunocompromised patients, including those with solid organ transplantation. Whether immunosuppression should be altered for these patients at the time of vaccine administration is uncertain. Furthermore, the timing and frequency of booster vaccination for nontransplanted patients with HF remain unclear. Isolated cases of myocarditis and HF have been reported in the setting of vaccination.169 , 170 Understanding the underlying mechanisms, as well as patients more vulnerable to such reactions, will be of importance.
Postacute Covid Sequalae Syndrome
One of the most vexing challenges posed by COVID-19 revolves around the various postacute sequelae of SARS-CoV-2 infection syndromes associated with previous infection. These syndromes are increasingly recognized as a significant cause of morbidity among COVID-19 survivors and present with a myriad of symptoms, including a high prevalence of dysautonomia. Further study is required to elucidate the mechanisms of disease and accordingly develop effective therapeutic strategies. In particular, evidence of cardiac injury may predispose to the development of HF over time. How these patients should be followed longitudinally remains to be defined.
Impact of Remote Monitoring and Telemedicine on Outcomes
The COVID-19 pandemic will have a lasting impact, leading to fundamental changes in care delivery for patients living with HF. Structural changes were applied rapidly to allow telemedicine and remote monitoring accompanied by technological advances to support these changes. The impact of these changes on quality and outcomes remains uncertain, but data continue to accrue.
Psychosocial Impacts of the Pandemic
The COVID-19 pandemic may also result in a lasting psychological impact on recovered patients, families, and clinicians. In addition to longer-term physical sequela of COVID-19 infection, patients with protracted illness courses may experience significant debility and post-traumatic stress disorder after recovery. 171 Optimal methods for screening for mental health conditions including anxiety, depression, and post-traumatic stress disorder among survivors and their families remain an important area of further study. Finally, the pandemic placed monumental burdens on the health care workforce, leading to reports of clinician burnout which may persist even after the pandemic abates.172 , 173 Identifying novel strategies to screen for and mitigate mental health disturbances among health care professionals remain critical.
| Priorities for Future Research at the Intersection of COVID-19 and HF |
|---|
| Pathobiological understanding of mechanisms of cardiac injury |
| Optimal anticoagulation strategies in patients with HF and severe COVID-19 |
| Vaccine response and durable immunity in patients with advanced HF and cardiac transplantation |
| Large, registry-based data capture to understand incidence, clinical features, and sequela of PASC among patients with HF |
| Innovative implementation approaches to promote current and ongoing vaccination efforts, and HF medication optimization |
| Effective data capture on long-term effects of COVID-19 on patients with HF |
| Care interventions focused on mental, emotional, and spiritual health of patients, families, and clinicians afflicted by the COVID-19 pandemic |
COVID-19, coronavirus disease 2019; HF, heart failure; PASC, postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection.
Conclusions
The present scientific statement summarizes clinical and research discovery at the nexus of HF and COVID-19 to date, at the time of review and writing. Patients with HF are uniquely susceptible to adverse outcomes in the setting of COVID-19 infection, highlighting the importance of vaccination in this population. Incident HF is rare in the setting of acute COVID-19, although sustained markers of myocardial injury in the postacute setting lend questions as to whether HF may be a future consequence. Early phases of the pandemic prompted substantial shifts in care delivery, resulting in enhanced use of noninvasive and remote monitoring technologies and increases in telemedicine. However, the impact of these shifts will require exploration. Pathophysiologic underpinnings of COVID-19–related myocardial injury and postacute sequelae are the focus of ongoing investigation. Concepts presented herein are subject to change as new information and discovery emerge pertaining to COVID-19 and the umbrella of HF populations, including mechanical circulatory support and heart transplantation.
Disclosures
Dr Bhatt reports consulting fees from Sanofi Pasteur, Verve Therapeutics, and Clarivate and is supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training grant T32HL007604. Dr Adler has received consulting fees from Medtronic and Abbott. Dr Bhadelia has received research grants from NIH Fogarty Center, has received speaking honoraria from Agilent Technologies and Health Industry Distributors Association. Dr Sauer has received research grant funding and consulting or speaking honoraria from Abbott, Boston Scientific, Medtronic, Edwards, PreCARDIA, and Bioventrix. Dr Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, Respicardia, Sanofi Pasteur, Theracos, and has consulted for Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GSK, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, and Tremeau. Dr Vardeny reported research support from the National Institutes of Health and consulting with Sanofi-Pasteur Inc. Dr Lala reports speaker honoraria from Zoll Medical and Abbott. She serves on the DSMB for Suquana Medical and on an advisory board for Bioventrix. All other authors report no disclosures relevant to this article.
Footnotes
HFSA Scientific Statement Writing Committee:
COVID-19 Scientific Statement Co-Chairs: Ankeet S. Bhatt, MD, MBA and Anuradha Lala, MD
COVID-19 Scientific Statement Writing Committee Members: Eric D. Adler, MD; Nancy M. Albert, PhD, RN, FHFSA; Anelechi Anyanwu, MD; Nahid Bhadelia, MD, MALD; Leslie T. Cooper, MD; Ashish Correa, MD; Ersilia M. DeFilippis, MD; Emer Joyce, MD, PhD; Andrew J. Sauer, MD; Scott D. Solomon, MD; Orly Vardeny, PharmD; Clyde Yancy, MD, MSc
References
- 1.Giustino G, Pinney SP, Lala A, et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J Am Coll Cardiol. 2020;76:2011–2023. doi: 10.1016/j.jacc.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendren NS, Grodin JL, Drazner MH. Unique patterns of cardiovascular involvement in coronavirus disease-2019. J Card Fail. 2020;26:466–469. doi: 10.1016/j.cardfail.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chau VQ, Giustino G, Mahmood K, et al. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID-19. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007485. [DOI] [PubMed] [Google Scholar]
- 4.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudriot B, Mansour A, Thibault V, et al. Successful heart transplantation for COVID-19-associated post-infectious fulminant myocarditis. ESC Heart Fail. 2021;8:2625–2630. doi: 10.1002/ehf2.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng MP, Cau A, Lee TC, et al. Acute cardiac injury in coronavirus disease 2019 and other viral infections-a systematic review and meta-analysis. Crit Care Med. 2021;49:1558–1566. doi: 10.1097/CCM.0000000000005026. [DOI] [PubMed] [Google Scholar]
- 7.Januzzi JL, McCarthy CP. Trivializing an elevated troponin: adding insult to injury? J Am Coll Cardiol. 2019;73:10–12. doi: 10.1016/j.jacc.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 10.Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a. [DOI] [PubMed] [Google Scholar]
- 11.Bearse M, Hung YP, Krauson AJ, et al. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod Pathol. 2021;34:1345–1357. doi: 10.1038/s41379-021-00790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschöpe C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kühl U, Pauschinger M, Noutsias M, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 14.Ntusi NAB. HIV and myocarditis. Curr Opin HIV AIDS. 2017;12:561–565. doi: 10.1097/COH.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 15.Anderson MR, Geleris J, Anderson DR, et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection : a retrospective cohort study. Ann Intern Med. 2020;173:782–790. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ukimura A, Satomi H, Ooi Y, Kanzaki Y. Myocarditis associated with influenza A H1N1pdm2009. Influenza Res Treat. 2012;2012 doi: 10.1155/2012/351979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindner D, Fitzek A, Bräuninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76:1318–1324. doi: 10.1016/j.jacc.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topol EJ. COVID-19 can affect the heart. Science. 2020;370:408–409. doi: 10.1126/science.abe2813. [DOI] [PubMed] [Google Scholar]
- 21.Basso C, Leone O, Rizzo S, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami R, Sakamoto A, Kawai K, et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matheson NJ, Lehner PJ. How does SARS-CoV-2 cause COVID-19? Science. 2020;369:510–511. doi: 10.1126/science.abc6156. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atri D, Siddiqi HK, Lang JP, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicin L, Abplanalp WT, Mellentin H, et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020;41:1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma A, Garcia G, Wang Y, et al. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Bermejo JA, Kang S, Rockwood SJ, et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci Transl Med. 2021;13:eabf7872. doi: 10.1126/scitranslmed.abf7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choudry FA, Hamshere SM, Rathod KS, et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76:1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia S, Dehghani P, Grines C, et al. Initial findings from the North American COVID-19 Myocardial Infarction Registry. J Am Coll Cardiol. 2021;77:1994–2003. doi: 10.1016/j.jacc.2021.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Icenogle T. COVID-19: infection or Autoimmunity . Front Immunol. 2020;11:2055. doi: 10.3389/fimmu.2020.02055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker RC. COVID-19-associated vasculitis and vasculopathy. J Thromb Thrombolysis. 2020;50:499–511. doi: 10.1007/s11239-020-02230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 38.Sakr Y, Giovini M, Leone M, et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann Intensive Care. 2020;10:124. doi: 10.1186/s13613-020-00741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milligan GP, Alam A, Guerrero-Miranda C. Recognizing right ventricular dysfunction in coronavirus disease-2019-related respiratory illness. J Card Fail. 2020;26:476. doi: 10.1016/j.cardfail.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham JW, Vaduganathan M, Claggett BL, et al. Effects of sacubitril/valsartan on n-terminal pro-B-type natriuretic peptide in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8:372–381. doi: 10.1016/j.jchf.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Smilowitz NR, Jethani N, Chen J, et al. Myocardial injury in adults hospitalized with COVID-19. Circulation. 2020;142:2393–2395. doi: 10.1161/CIRCULATIONAHA.120.050434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J. 2020;41:1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panhwar MS, Kalra A, Gupta T, et al. Effect of influenza on outcomes in patients with heart failure. JACC Heart Fail. 2019;7:112–117. doi: 10.1016/j.jchf.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez-Garcia J, Lee S, Gupta A, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhatt AS, Jering KS, Vaduganathan M, et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9:65–73. doi: 10.1016/j.jchf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27:727–743. [Google Scholar]
- 52.Alvarez-Garcia J, Jaladanki S, Rivas-Lasarte M, et al. New heart failure diagnoses among patients hospitalized for COVID-19. J Am Coll Cardiol. 2021;77:2260–2262. doi: 10.1016/j.jacc.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freaney PM, Shah SJ, Khan SS. COVID-19 and heart failure with preserved ejection fraction. JAMA. 2020;324:1499–1500. doi: 10.1001/jama.2020.17445. [DOI] [PubMed] [Google Scholar]
- 54.Martinez MW, Tucker AM, Bloom OJ, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6:745–752. doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yancy CW, Fonarow GC. Coronavirus disease 2019 (COVID-19) and the heart-is heart failure the next chapter? JAMA Cardiol. 2020;5:1216–1217. doi: 10.1001/jamacardio.2020.3575. [DOI] [PubMed] [Google Scholar]
- 56.Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopes RD, Macedo AVS, de Barros E, Silva PGM, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokoyama Y, Aikawa T, Takagi H, Briasoulis A, Kuno T. Association of renin-angiotensin-aldosterone system inhibitors with mortality and testing positive of COVID-19: meta-analysis. J Med Virol. 2021;93:2084–2089. doi: 10.1002/jmv.26588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biswas M, Kali MSK. Association of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers with risk of mortality, severity or SARS-CoV-2 test positivity in COVID-19 patients: meta-analysis. Sci Rep. 2021;11:5012. doi: 10.1038/s41598-021-84678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Permana H, Huang I, Purwiga A, et al. In-hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis. Pharmacol Rep. 2021;73:769–780. doi: 10.1007/s43440-021-00233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta A, Madhavan MV, Poterucha TJ, et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun. 2021;12:1325. doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torres-Peña JD, Pérez-Belmonte LM, Fuentes-Jiménez F, et al. Prior treatment with statins is associated with improved outcomes of patients with COVID-19: data from the SEMI-COVID-19 Registry. Drugs. 2021;81:685–695. doi: 10.1007/s40265-021-01498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.INSPIRATION Investigators. Sadeghipour P, Talasaz AH, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeFilippis EM, Reza N, Donald E, Givertz MM, Lindenfeld J, Jessup M. Considerations for heart failure care during the COVID-19 pandemic. JACC Heart Fail. 2020;8:681–691. doi: 10.1016/j.jchf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papageorgiou J-M, Almroth H, Törnudd M, van der Wal H, Varelogianni G, Lawesson SS. Fulminant myocarditis in a COVID-19 positive patient treated with mechanical circulatory support - a case report. Eur Heart J Case Rep. 2021;5:ytaa523. doi: 10.1093/ehjcr/ytaa523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ranard LS, Fried JA, Abdalla M, et al. Approach to acute cardiovascular complications in COVID-19 infection. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badulak J, Antonini MV, Stead CM, et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67:485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agerstrand C, Dubois R, Takeda K, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019: crisis standards of care. ASAIO J. 2021;67:245–249. doi: 10.1097/MAT.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 74.Reza N, DeFilippis EM, Jessup M. Secondary impact of the COVID-19 pandemic on patients with heart failure. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007219. [DOI] [PubMed] [Google Scholar]
- 75.Benden CM, Budev M, Hopkins P, Courtwright A, et al. Guidance from the International Society of Heart and Lung Transplantation regarding the SARS CoV-2 pandemic. J Heart Lung Transplant. 2021;40:169–171. [Google Scholar]
- 76.Birati EY, Najjar SS, Tedford RJ, et al. Characteristics and outcomes of COVID-19 in patients on left ventricular assist device support. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.120.007957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ankersmit HJ, Tugulea S, Spanier T, et al. Activation-induced T-cell death and immune dysfunction after implantation of left-ventricular assist device. Lancet. 1999;354:550–555. doi: 10.1016/s0140-6736(98)10359-8. [DOI] [PubMed] [Google Scholar]
- 78.Chau VQ, Oliveros E, Mahmood K, et al. The imperfect cytokine storm: severe COVID-19 with ARDS in a patient on durable LVAD support. JACC Case Rep. 2020;2:1315–1320. doi: 10.1016/j.jaccas.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sobol I, Yuzefpolskaya M, Roth Z, et al. Characteristics and outcomes of patients with a left ventricular assist device with coronavirus disease-19. J Card Fail. 2020;26:895–897. doi: 10.1016/j.cardfail.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeFilippis EM, Sinnenberg L, Reza N, et al. Trends in US heart transplant waitlist activity and volume during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Cardiol. 2020;5:1048–1052. doi: 10.1001/jamacardio.2020.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeFilippis EM, Farr MA, Givertz MM. Challenges in heart transplantation in the era of COVID-19. Circulation. 2020;141:2048–2051. doi: 10.1161/CIRCULATIONAHA.120.047096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khush KK, Patel J, Pinney S, et al. Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: a prospective multicenter study. Am J Transplant. 2019;19:2889–2899. doi: 10.1111/ajt.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sperry BW, Khumri TM, Kao AC. Donor-derived cell-free DNA in a heart transplant patient with COVID-19. Clin Transplant. 2020;34:e14070. doi: 10.1111/ctr.14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020;5:1165–1169. doi: 10.1001/jamacardio.2020.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singhvi A, Barghash M, Lala A, et al. Challenges in heart transplantation during COVID-19: a single-center experience. J Heart Lung Transplant. 2020;39:894–903. doi: 10.1016/j.healun.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bottio T, Bottio L, Fiocco A, et al. COVID-19 in Heart transplant recipients: a multicenter analysis of the Northern Italian outbreak. JACC Heart Fail. 2021;9:52–61. doi: 10.1016/j.jchf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Genuardi MV, Moss N, Najjar SS, et al. Coronavirus disease 2019 in heart transplant recipients: Risk factors, immunosuppression, and outcomes. J Heart Lung Transplant. 2021;4:926–935. doi: 10.1016/j.healun.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105:e56–e57. doi: 10.1097/TP.0000000000003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385 doi: 10.1056/NEJMc2108861. 662–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;15:L21–0282. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hall VG, Ferreira VH, Ku T, et al. Randomized Trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;11 doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised [cited 2021 Aug 16]. 2021
- 95.Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021;21:2885–2889. doi: 10.1111/ajt.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar D, Manuel O, Natori Y, et al. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773–1779. doi: 10.1111/ajt.15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pines JM, Zocchi MS, Black BS, et al. The effect of the COVID-19 pandemic on emergency department visits for serious cardiovascular conditions. Am J Emerg Med. 2021;47:42–51. doi: 10.1016/j.ajem.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baum A, Schwartz MD. Admissions to Veterans Affairs Hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324:96–99. doi: 10.1001/jama.2020.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76:280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solomon MD, McNulty EJ, Rana JS, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 101.Holland M, Burke J, Hulac S, et al. Excess cardiac arrest in the community during the COVID-19 pandemic. JACC Cardiovasc Interv. 2020;13:1968–1969. doi: 10.1016/j.jcin.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baldi E, Sechi GM, Mare C, et al. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med. 2020;383:496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abraham WT, Fiuzat M, Psotka MA, O'Connor CM. Heart failure collaboratory statement on remote monitoring and social distancing in the landscape of COVID-19. JACC Heart Fail. 2020;8:692–694. doi: 10.1016/j.jchf.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yehya A, Shah KS, Mitter SS, et al. Challenges and the innovations in the care of advanced heart failure patients during COVID-19. Heart Fail Rev. 2021;12:1–4. doi: 10.1007/s10741-021-10074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gorodeski EZ, Moennich LA, Riaz H, Jehi L, Young JB, Tang WHW. Virtual versus in-person visits and appointment no-show rates in heart failure care transitions. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007119. [DOI] [PubMed] [Google Scholar]
- 106.Puwanant S, Sinphurmsukskul S, Krailak L, et al. The impact of the coronavirus disease and Tele-Heart Failure Clinic on cardiovascular mortality and heart failure hospitalization in ambulatory patients with heart failure. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thibodeau JT, Gorodeski EZ. Telehealth for uptitration of guideline-directed medical therapy in heart failure. Circulation. 2020;142:1507–1509. doi: 10.1161/CIRCULATIONAHA.120.050582. [DOI] [PubMed] [Google Scholar]
- 108.Gorodeski EZ, Goyal P, Cox ZL, et al. Virtual visits for care of patients with heart failure in the era of COVID-19: a statement from the Heart Failure Society of America. J Card Fail. 2020;26:448–456. doi: 10.1016/j.cardfail.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gensini GF, Alderighi C, Rasoini R, Mazzanti M, Casolo G. Value of telemonitoring and telemedicine in heart failure management. Card Fail Rev. 2017;3:116–121. doi: 10.15420/cfr.2017:6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition – Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176:310–318. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047–1057. doi: 10.1016/S0140-6736(18)31880-4. [DOI] [PubMed] [Google Scholar]
- 112.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 113.Nassif ME, Qintar M, Windsor SL, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation. 2021;143:1673–1686. doi: 10.1161/CIRCULATIONAHA.120.052503. [DOI] [PubMed] [Google Scholar]
- 114.Abraham WT. The role of implantable hemodynamic monitors to manage heart failure. Cardiol Clin. 2017;35:273–279. doi: 10.1016/j.ccl.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 115.Bayes-Genis A, Codina P, Abdul-Jawad Altisent O, et al. Advanced remote care for heart failure in times of COVID-19 using an implantable pulmonary artery pressure sensor: the new normal. Eur Heart J Suppl. 2020;22(Suppl Ptt):P29–P32. doi: 10.1093/eurheartj/suaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oliveros E, Mahmood K, Mitter S, Pinney SP, Lala A. Letter to the editor: pulmonary artery pressure monitoring during the COVID-19 pandemic in New York City. J Card Fail. 2020;26:900–901. doi: 10.1016/j.cardfail.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boehmer JP, Hariharan R, Devecchi FG, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the multisense study. JACC Heart Fail. 2017;5:216–225. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 118.Treskes RW, Beles M, Caputo M-L, et al. Clinical and economic impact of HeartLogicTM compared with standard care in heart failure patients. ESC Heart Fail. 2021;8:1541–1551. doi: 10.1002/ehf2.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mitter SS, Alvarez-Garcia J, Miller MA, Moss N, Lala A. Insights from HeartLogic multisensor monitoring during the COVID-19 pandemic in New York City. JACC Heart Fail. 2020;8:1053–1055. doi: 10.1016/j.jchf.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shumway JL, Stolen CM, Ahmed R, Plumer M, Capodilupo RC. Case reports of implantable cardiac device physiologic sensor changes in subjects with coronavirus disease-2019 infection. J Card Fail 27:373–8. [DOI] [PMC free article] [PubMed]
- 121.Bayoumy K, Gaber M, Elshafeey A, et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021 doi: 10.1038/s41569-021-00522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bumgarner JM, Lambert CT, Hussein AA, et al. Smartwatch algorithm for automated detection of atrial fibrillation. J Am Coll Cardiol. 2018;71:2381–2388. doi: 10.1016/j.jacc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 123.Mastoris I, Sauer AJ. Opening the “black box” of artificial intelligence for detecting heart failure. ASAIO J. 2021;67:322–323. doi: 10.1097/MAT.0000000000001401. [DOI] [PubMed] [Google Scholar]
- 124.Allen LA, Venechuk G, McIlvennan CK, et al. An electronically delivered patient-activation tool for intensification of medications for chronic heart failure with reduced ejection fraction: the EPIC-HF trial. Circulation. 2021;143:427–437. doi: 10.1161/CIRCULATIONAHA.120.051863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Risk for COVID-19 infection, hospitalization, and death by race/ethnicity | CDC. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html [cited 2021 Jun 11] 2021
- 126.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 127.CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic, [cited 2021 Jun 11]. 2021
- 128.Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 Cardiovascular Disease Registry. Circulation. 2020;143:2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Churchwell K, Elkind MSV, Benjamin RM, et al. Call to action: structural racism as a fundamental driver of health disparities: a Presidential Advisory from the American Heart Association. Circulation. 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 130.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 132.Barker-Davies RM, O'Sullivan O, Senaratne KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020;54:949–959. doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bajaj R, Sinclair HC, Patel K, et al. Delayed-onset myocarditis following COVID-19. Lancet Respir Med. 2021;9:e32–e34. doi: 10.1016/S2213-2600(21)00085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.CDC. Myocarditis and pericarditis following mRNA COVID-19 vaccination. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html [cited 2021 Jun 11]. 2021
- 135.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021 Jun 2 doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 138.Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021 Mar 23 doi: 10.1136/annrheumdis-2021-220289. annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peled Y, Ram E, Lavee J, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40:759–762. doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Havlin J, Svorcova M, Dvorackova DE, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40:754–758. doi: 10.1016/j.healun.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aslam S, Goldstein DR, Vos R, et al. COVID-19 vaccination in our transplant recipients: the time is now. J Heart Lung Transplant. 2021;40:169–171. doi: 10.1016/j.healun.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.American Society of Transplantation. Statement on COVID-19 vaccination in solid organ transplant recipients. https://www.myast.org/statement-covid-19-vaccination-solid-organ-transplant-recipients [cited 2021 May 31]. 2021
- 143.SARS-CoV-2 Vaccination in Heart and Lung Transplantation. Recommendations from the ISHLT COVID-19 Task Force March 15, 2021.
- 144.Driggin E, Maddox TM, Ferdinand KC, et al. ACC health policy statement on cardiovascular disease considerations for COVID-19 vaccine prioritization: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:1938–1948. doi: 10.1016/j.jacc.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhatt AS, Liang L, DeVore AD, et al. Vaccination trends in patients with heart failure: insights from Get With The Guidelines-Heart Failure. JACC Heart Fail. 2018;6:844–855. doi: 10.1016/j.jchf.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 146.Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine. 2020;38:6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yokum D, Lauffenburger JC, Ghazinouri R, Choudhry NK. Letters designed with behavioural science increase influenza vaccination in Medicare beneficiaries. Nat Hum Behav. 2018;2:743–749. doi: 10.1038/s41562-018-0432-2. [DOI] [PubMed] [Google Scholar]
- 148.Milkman KL, Patel MS, Gandhi L, et al. A megastudy of text-based nudges encouraging patients to get vaccinated at an upcoming doctor's appointment. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2101165118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Psotka MA, Abraham WT, Fiuzat M, et al. Conduct of clinical trials in the era of COVID-19: JACC scientific expert panel. J Am Coll Cardiol. 2020;76:2368–2378. doi: 10.1016/j.jacc.2020.09.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Selvaraj S, Greene SJ, Khatana SAM, Nathan AS, Solomon SD, Bhatt DL. The landscape of cardiovascular clinical trials in the United States initiated before and during COVID-19. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gaudino M, Arvind V, Hameed I, et al. Effects of the COVID-19 pandemic on active non-COVID clinical trials. J Am Coll Cardiol. 2020;76:1605–1606. doi: 10.1016/j.jacc.2020.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.United States Food and Drug Administration. Statistical Considerations for Clinical Trials During the COVID-19 Public Health Emergency Guidance for Industry. June 2020.
- 153.Ponikowski P, Kirwan B-A, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396:1895–1904. doi: 10.1016/S0140-6736(20)32339-4. [DOI] [PubMed] [Google Scholar]
- 154.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 155.Vaduganathan M, Butler J, Krumholz HM, Itchhaporia D, Stecker EC, Bhatt DL. Regulation of cardiovascular therapies during the COVID-19 public health emergency. J Am Coll Cardiol. 2020;76:2517–2521. doi: 10.1016/j.jacc.2020.09.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.United States Food and Drug Administration. Guidance on Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency. August 2021.
- 157.European Medicines Agency. Implications of coronavirus disease (COVID-19) on methodological aspects of ongoing clinical trials. https://www.ema.europa.eu/en/implications-coronavirus-disease-covid-19-methodological-aspects-ongoing-clinical-trials [cited 2021 May 28]. 2021
- 158.Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hadley MB, Lampert J, Zhang C. Cardiology Fellowship during the COVID-19 pandemic: lessons from New York City. J Am Coll Cardiol. 2020;76:878–882. doi: 10.1016/j.jacc.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rao P, Diamond J, Korjian S, et al. The impact of the COVID-19 pandemic on cardiovascular fellows-in-training: a national survey. J Am Coll Cardiol. 2020;76:871–875. doi: 10.1016/j.jacc.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.DeFilippis EM, Stefanescu Schmidt AC, Reza N. Adapting the educational environment for cardiovascular fellows-in-training during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2630–2634. doi: 10.1016/j.jacc.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Parwani P, Choi AD, Lopez-Mattei J, et al. Understanding social media: opportunities for cardiovascular medicine. J Am Coll Cardiol. 2019;73:1089–1093. doi: 10.1016/j.jacc.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 163.Almarzooq ZI, Lopes M, Kochar A. Virtual learning during the COVID-19 pandemic: a disruptive technology in graduate medical education. J Am Coll Cardiol. 2020;75:2635–2638. doi: 10.1016/j.jacc.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Joyce E. COVID-19, myocarditis, and the other side of the bed. Eur J Heart Fail. 2020;22:2187–2189. doi: 10.1002/ejhf.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hado E, Friss Feinberg L. Amid the COVID-19 pandemic, meaningful communication between family caregivers and residents of long-term care facilities is imperative. J Aging Soc Policy. 2020;32:410–415. doi: 10.1080/08959420.2020.1765684. [DOI] [PubMed] [Google Scholar]
- 166.Azoulay E, Cariou A, Bruneel F, et al. Symptoms of anxiety, depression, and peritraumatic dissociation in critical care clinicians managing patients with COVID-19. A cross-sectional study. Am J Respir Crit Care Med. 2020;202:1388–1398. doi: 10.1164/rccm.202006-2568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Facing up to long COVID. Lancet. 2020;396:1861. doi: 10.1016/S0140-6736(20)32662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Varshney AS, Wang DE, Bhatt AS, et al. Characteristics of clinical trials evaluating cardiovascular therapies for coronavirus disease 2019 registered on ClinicalTrials.gov: a cross sectional analysis. Am Heart J. 2021;232:105–115. doi: 10.1016/j.ahj.2020.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 170.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Janiri D, Carfì A, Kotzalidis GD, et al. Posttraumatic stress disorder in patients after severe COVID-19 infection. JAMA Psychiatry. 2021;78:567–569. doi: 10.1001/jamapsychiatry.2021.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shapiro J, McDonald TB. Supporting clinicians during Covid-19 and beyond - learning from past failures and envisioning new strategies. N Engl J Med. 2020;383:e142. doi: 10.1056/NEJMp2024834. [DOI] [PubMed] [Google Scholar]
- 173.Dzau VJ, Kirch D, Nasca T. Preventing a parallel pandemic - a national strategy to protect clinicians’ well-being. N Engl J Med. 2020;383:513–515. doi: 10.1056/NEJMp2011027. [DOI] [PubMed] [Google Scholar]