Abstract
Teratocarcinosarcoma is a rare and aggressive tumor usually affecting the sinonasal tract. It arises primarily from the nasal cavity, paranasal sinuses with some reported cases arising from the nasopharynx and oral cavity and commonly referred to as Sinonasal Teratocarcinosarcoma (SNTC). We present the first case of teratocarcinosarcoma as a primary thyroid cancer in a 17-year-old male patient who presented with a rapidly growing anterior neck mass with no symptoms. Physical examination revealed circa 4 cm × 5 cm slightly right sided, non-tender, firm anterior neck swelling. A thyroid ultrasound revealed an enlarged thyroid gland with multiple thyroid nodes. Magnetic Resonance Imaging (MRI) of the head and neck showed no sinonasal tract tumor. Thyroidectomy and surgical resection of the tumor was performed. Histological examination revealed teratocarcinosarcoma of the thyroid gland, an analog to SNTC with no primary sinonasal tissue involvement. This implies that, teratocarcinosarcoma can occur in primary tissues other than sinonasal origin contrary to conventional knowledge.
Keywords: Teratocarcinosarcoma, thyroid, cancer, sinonasal, Germany
Introduction
Teratocarcinosarcoma is a rare and very aggressive tumor usually affecting the sinonasal tract. The tumor was previously called malignant teratoma, teratocarcinoma, blastoma, carcinosarcoma, teratoid carcinosarcoma mixed mesodermal tumor, and malignant mixed tumor. Heffner and Hyams1 named the tumor “teratocarcinosarcoma” based on the clinicopathological findings of a study of 20 cases of sinonasal tract neoplasm. The tumor is less than 1% of all cancers and about 3% of all malignant tumors of the head and neck. It arises commonly from the nasal cavity, paranasal sinuses with some reported cases arising from the nasopharynx and oral cavity.2–5 Majority of the cases reported exhibit male predominance with most of them found in the adult population of above 35 years.6,7 Histologically, teratocarcinosarcomas show heterogeneous morphology with variable proportions of benign and malignant epithelial, mesenchymal, and neuroepithelial elements.8,9 It is an aggressive tumor with mean survival reported to be 1.7 years with 60% mortality rate within 3 years as reported by Heffner and Hyams.1
The tumor is usually located in the sinonasal tract and rarely in the neck area. The usual complaints associated with it when located in the sinonasal tract are epistaxis, nasal obstruction, headache, and visual disturbance.5,9 Surgical resection followed by radiotherapy and a histology-specific multidrug chemotherapy has given good prognosis in some cases.10
Until date, there has not been any reported case of teratocarcinosarcoma arising primarily from the thyroid gland. We present the first case of teratocarcinosarcoma of the thyroid.
Case report
A 17-year-old male patient presented at our facility with a rapidly growing anterior neck mass of about 4 weeks duration. There was no history of fever, weight loss, night sweat, or thyroid hormone related symptoms. The family history was unremarkable for malignancy or tuberculosis.
Physical examination revealed circa 4 cm × 5 cm slightly right sided, nontender, soft to firm consistency, movable, roughly demarcated anterior neck swelling. The swelling moved slightly upon swallowing but not with protrusion of the tongue. There were no associated regional lymphadenopathy and the overlying skin was unremarkable.
The laboratory findings were as follows: Haemoglobin 16.5 g/dl, Leucocytes 8275/ul, Thrombocytes 27,300/ul, Calcium 4.98 mval/l, TSH 2.89 uU/ml, FT3 3.39 pg/ml, FT4 1.24 ng/dl, INR-1.030, Creatinine 1.02 mg/dl. Tumor markers: Alpha-fetoprotein (AFP) 1.74 ng/ml, Human chorionic gonadotropin (hCG) < 1.20 U/l, Calcitonin 2.36 pg/ml, Lactate dehydrogenase (LDH)309U/l (normal range < 248), Thyroglobulin (Tg) 29.8 ng/ml, Anti-thyroid peroxidase (anti-TPO)21.9 IU/ml (normal range 1–16).
A thyroid ultrasound revealed an enlarged thyroid gland with right lobe volume of 62 ml and left lobe volume of about 14 ml. A big lesion of about 40 ml was located around the caudal part of the right lobe. It also showed multiple thyroid nodes. Thyroid scintigraphy confirmed a multinodular goiter with “cold” nodes.
Magnetic resonance imaging (MRI) of the neck revealed diffused contrast enhanced right sided neck mass of about 8.2 cm × 3.4 cm × 3.6 cm with extension to the base of the tongue. Around the sinus sphenoidalis and sinus mastoidis was found little fluid without any evidence of tumor, infiltration or lymphadenopathy. (Figure 1).
Figure 1.
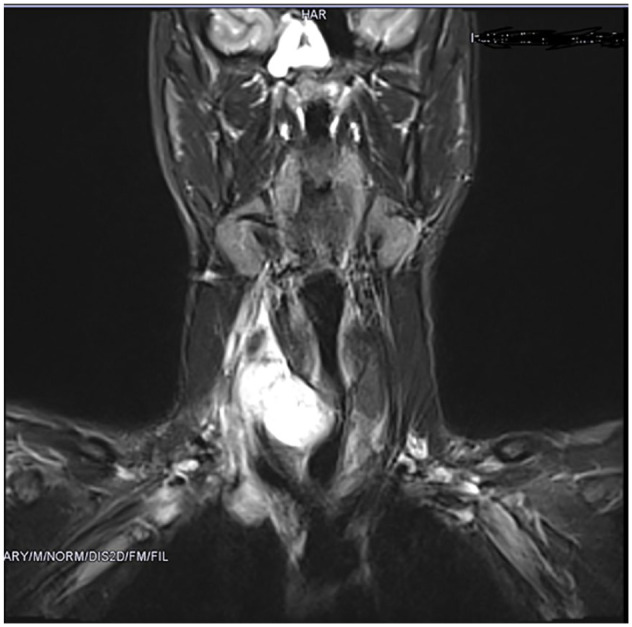
MRI of the neck revealed diffused contrast enhanced right sided neck mass with extension to the base of the tongue.
Exploration, thyroidectomy and surgical resection of the tumor was carried out on the 21.01.2019. We found an enlarged multinodular thyroid gland with diffuse tumor of the right lobe infiltrating the nearby tissues and pressing on the trachea.
Frozen section histological examination revealed a tumor most likely to be germ cell tumor and sensitive to Chemotherapy. Based on these intraoperative and the histological findings, we carried out near-total thyroidectomy aiming at tumor debulking and tracheal decompression without causing damage to the nearby organs.
The postoperative was uneventful, and the patient was discharged on the fifth postoperative day. The final histological examination revealed a teratocarcinosarcoma of the thyroid gland, an analog to the sinonasal teratocarcinosarcoma (Figures 2–4).
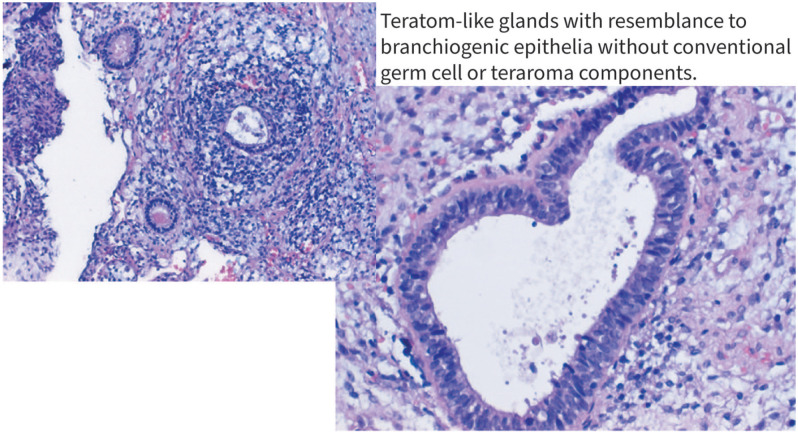
Figure 2.
Teratom-like glands with resemblance to branchiogenic epithelia without conventional germ cell or teratoma components.
Figure 3.
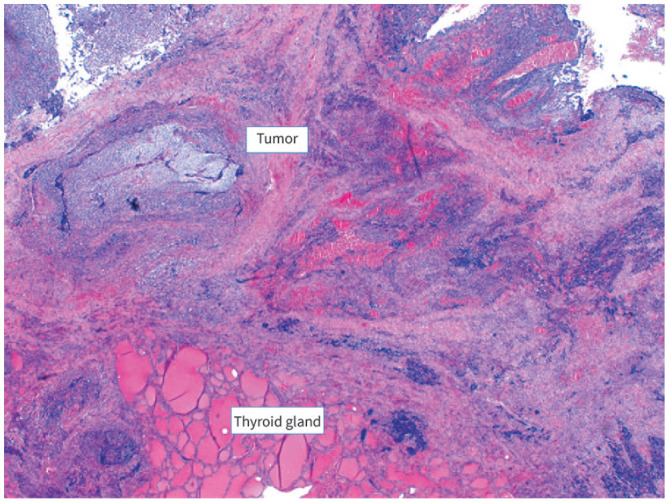
Thyroid gland with sarcomatous tumor cells.
Figure 4.
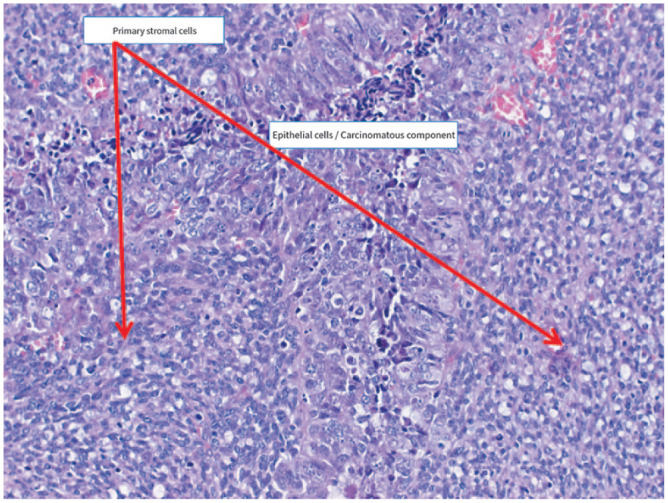
Primary stromal cells, epithelial cells with carcinomatous elements.
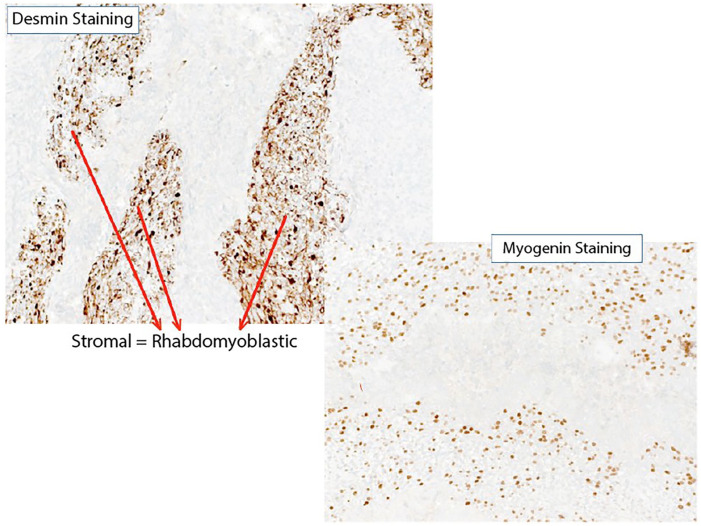
Immunnohistochemical staining with desmin and myogenin revealed rhabdomyoblastic differentiation of the stromal component (Figure 5). Subsequently, SALL4 staining was done to exclude immature teratoma with rhabdomyosarcomatous foci.
Figure 5.
Immunohistochemical staining with desmin and myogenin showing rhabdomyoblastic differentiation of the stromal component.
Staging Computed tomography (CT) of the thorax, Magnetic Resonance Imaging (MRI) of the abdomen, Head and Neck as well as Full Body Scintigraphy did not show any evidence of metastasis or primary tumor focus. The patient was given chemotherapy with Cisplatin, Etoposid, and Ifosfamid. The tumor was found to be regressive after the chemotherapy. A second staged surgical resection was carried out on the third of April 2019. This was followed with Radiochemotherapy. The Patient is currently doing well and is on surveillance.
Discussion
Teratocarcinosarcoma is a rare and very aggressive tumor usually affecting the sinonasal tract. Its occurrence is more likely to be explained by a divergent differentiation of multipontential adult somatic stem cell. The absence of germ cell components and chromosome 12 p makes a germ cell origin highly unlikely.11 We found a case in the thyroid as a primary thyroid tumor which is the first to be reported. In our patient, the SALL4 staining which is sensitive and specific for germ cell tumors did not confirm the presence of germ cell components. This supports the somatic stem cell divergent differentiation against the germ cell origin theory.
Our finding is also contrary to the conventional knowledge that Teratocarcinosarcoma arises primarily from the nasal cavity, paranasal sinuses, nasopharynx, and oral cavity and thus commonly referred to as Sinonasal Teratocarcinosarcoma.4,8,9
On the other hand, the major cancers of the thyroid gland are papillary carcinoma (75%–85%), follicular carcinoma (10%–20%), Hürthle cell carcinoma, medullary carcinoma (circa 8%), and anaplastic thyroid cancer (less than 5%).12 Other less common cancers include Thyroid lymphoma, squamous cell thyroid carcinoma, and sarcoma of the thyroid.12,13
The tumor makers associated with conventional thyroid gland cancers such as calcitonin and thyroglobulin were not detected in the case of our patient.
This rare case of Teratocarcinosarcoma of the thyroid is worth noting, so that clinicians will consider it as a differential diagnosis for thyroid tumors especially in a situation of ambiquity. This implies that, pathologists should now actively look for it in primary thyroid tumors.
This may also imply that; the tumor may be found as a primary tumor in other tissues of the body as opposed to previous knowledge of it being exclusively a sinonasal tract tumor.
The histological finding of immature neuroectodermal element, epithelial carcinomatous component as well as sarcomatous cell areas as shown is the gold standard for its diagnosis. A differential diagnosis of immature malignant teratoma is highly unlikely based on the histological findings.
The tumor predominantly affects adults and males in comparison to female, with male to female ratio of about 4:1.6 Despite being male, our patient happened to be in the unusual age group of presentation contrary to most literature findings; with the age of presentation usually above 35 years.7
The initial diagnosis could be a challenge to the clinician, especially when it occurs in an atypical location and age group as it happened in the case of our patient.
Even though the tumor is aggressive with high mortality rate, surgical resection followed by radiotherapy and a histology-specific multidrug chemotherapy has given good prognosis in some cases. There are no laid down management guidelines for it, but studies have shown that a multimodality approach involving chemotherapy with or without radiotherapy results in good outcome.
This implies that early diagnosis and institution of treatment is paramount to achieving good prognosis. Excision and aggressive sampling of the tumor for histopathological examination is necessary to avoid erroneous diagnosis.
Because of the high rate of recurrence of the tumor, a regular follow-up is necessary for early detection.
Acknowledgments
The authors do not have any acknowledgements.
Footnotes
Authors’ contributions: AOA and KMN are equally responsible for conception, drafting and design of the case study. AOA, KMN, and AB are responsible for literature search and intellectual content. AOA, AB, and MB participated actively from the beginning with the patients as part of the surgical team. All authors read and approved the final manuscript.
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Malteser Waldkrankenhaus ST. Marien Erlangen. Approval number not applicable.
Informed Consent: Written informed consent was obtained. A copy is available upon request
ORCID iD: Akwasi Ofori Abayie  https://orcid.org/0000-0002-1594-5803
https://orcid.org/0000-0002-1594-5803
References
- 1.Heffner DK, Hyams VJ. Teratocarcinosarcoma (malignant teratoma?) of the nasal cavity and paranasal sinuses A clinicopathologic study of 20 cases. Cancer 1984; 53(10): 2140–2154. [DOI] [PubMed] [Google Scholar]
- 2.Endo H, Hirose T, Kuwamura KI, et al. Case report: Sinonasal teratocarcinosarcoma. Pathol Int 2001; 51(2): 107–112. [DOI] [PubMed] [Google Scholar]
- 3.Foong YC, Murdolo V, Naiman N, et al. Sinonasal teratocarcinosarcoma: a case report. J Med Case Rep 2017; 11(1): 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takasaki K, Sakihama N, Takahashi H. A case with sinonasal teratocarcinosarcoma in the nasal cavity and ethmoid sinus. Eur Arch Otorhinolaryngol 2006; 263(6): 586–591. [DOI] [PubMed] [Google Scholar]
- 5.Wei S, Carroll W, Lazenby A, et al. Sinonasal teratocarcinosarcoma: report of a case with review of literature and treatment outcome. Ann Diagn Pathol 2008; 12(6): 415–425. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC, 2005. [Google Scholar]
- 7.Smith SL, Hessel AC, Luna MA, et al. Sinonasal teratocarcinosarcoma of the head and neck: a report of 10 patients treated at a single institution and comparison with reported series. Arch Otolaryngol Head Neck Surg 2008; 134(6): 592–595. [DOI] [PubMed] [Google Scholar]
- 8.Prasad KC, Pai RR, Padmanabhan K, et al. Terato-carcinosarcoma of the nose, paranasal sinuses and nasopharynx. J Laryngol Otol 2003; 117(4): 321–324. [DOI] [PubMed] [Google Scholar]
- 9.Chao KK, Eng TY, Barnes J, et al. Sinonasal teratocarcinosarcoma. Am J Clin Oncol 2004; 27(1): 29–32. [DOI] [PubMed] [Google Scholar]
- 10.Nitsche M, Hermann RM, Christiansen H, et al. Rationale for individualized therapy in Sinonasal Teratocarcinosarcoma (SNTC): case report. Onkologie 2005; 28(12): 653–656. [DOI] [PubMed] [Google Scholar]
- 11.Thomas J, Adegboyega P, Iloabachie K, et al. Sinonasal teratocarcinosarcoma with yolk sac elements: a neoplasm of somatic or germ cell origin? Ann Diagn Pathol 2011; 15(2): 135–139. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Thyroid cancer, https://www.cancer.gov/types/thyroid (1980, accessed 19 May 2019).
- 13.NORD National Organization for Rare Disorders. Thyroid cancer, https://rarediseases.org/rare-diseases/thyroid-cancer/ (accessed 19 May 2019).