Abstract
Osteoblastomas and aneurysmal bone cysts (ABC) are rare benign bone tumors that make up about 1%–2% of primary bone malignancies, typically occurring in young patients with a median age of 20 years, most commonly effecting the axial skeleton. ABCs may develop independently as primary lesions, or secondary to other bony lesions including osteoblastomas, chondroblastomas, and giant cell tumors. Treatment of unresectable or extensive osteoblastomas can be challenging. In 2013, the Food and Drug Administration (FDA) approved denosumab for the treatment of giant cell tumors of the bone due to its efficacy in these morbid bony lesions. Various case reports have shown that osteoblastomas can respond to denosumab. Furthermore, numerous ABC case reports have described the efficacy of denosumab in these situations. We herein describe a unique case of a young patient with an aggressive osteoblastoma and secondary ABCs who was successfully treated with denosumab.
Keywords: Osteoblastoma, aneurysmal bone cyst, bone sarcoma, osteosarcoma, denosumab, aggressive bone tumor, benign bone tumor
Introduction
Osteoblastoma are difficult to differentiate from other bone tumors including osteomas, ABCs, and osteosarcomas. The pathologic evaluation and definitive osteoblastoma diagnosis can be quite challenging as it shares common tumor morphology and genetic alterations with other bone lesions. The effective systemic treatments remain controversy.
Case
Our patient is a 20-year-old female who initially presented to an outside hospital in July 2017 with a 1-month history of progressive low back pain. Initial magnetic resonance (MR) showed a destructive, osteolytic, expansile lesion measuring 4.8 × 3.8 cm centered in the left aspect of the sacrum at the level of S2–S3 with areas of anterior cortical destruction extending into the left sacral neural foramina from S2 to S4. There was also a heterogeneously enhancing soft tissue component that was measured at 6.4 × 3.7 cm that was along the left piriformis muscle. A CT-guided core needle biopsy performed in August 2017 yielded an initial diagnosis of a high-grade osteosarcoma. She was subsequently treated as an osteosarcoma patient with MAP chemotherapy. She completed a total of 12 weeks of chemotherapy, with cumulative doses of cisplatin 360 mg/m2, doxorubicin 225 mg/m2, and methotrexate 24 g/m2. Surgical intervention was determined to result in substantial morbidity, and therefore, the patient underwent proton beam radiation in three fractions for a total dose of 54 Gy. However, due to the lack of increased bone mineralization in response to chemotherapy, the question of osteoblastoma rather than osteosarcoma was raised.
After reviewing the initial pathology, a second pathologist described the tissue specimen as a cellular tumor composed of proliferating osteoblasts with atypia and frequent mitotic figures associated with trabecule of woven bone. Neoplastic cells demonstrated mild cytologic atypia and scattered mitoses of normal configuration. In different areas, the tumors had undergone secondary hemorrhagic and cystic changes. Although difficult to definitively differentiate between the two, an overall impression of osteoblastoma was favored. As a result, a second biopsy was later obtained in December 2017. It in fact demonstrated no neoplastic cells, but instead a cystic wall with bland-appearing spindle cell proliferation, hemosiderin deposition, lamellar and woven bone with osteoclast-type giant cells along with a foci of fibrous tissue with plasmacytoid and epithelioid osteoblasts, with surrounding osteoid, all of which was consistent with an ABC. The specimen was negative for reportable single nucleotide variants, as well as negative for EWSR1/FUS/SS18/STAT6 rearrangements. After having three separate pathology consultations, the final diagnosis of an osteoblastoma with secondary ABC was made.
Based on the revised diagnosis of osteoblastoma, the decision was made to change her therapy to denosumab as surgery was still deemed to be too morbid. In May 2018, she was started on denosumab 120 mg every week for 4 weeks, and then monthly. At the time of writing this report, she has received 24 doses. Surveillance imaging throughout her treatment showed a decrease in the number and size of the cystic components, and an overall plain radiographic appearance of mineralization along with decreased FDG avidity on PET scan, suggestive of a treatment response (Figures 1 and 2). After nearly 24 months of treatment with denosumab, her latest MRI has continued to show stable to slightly decreased size of the lesion and loss of fluid level in the lesion (Figure 3). Additionally, her pain control has drastically improved and she was able to completely titrate off all pain medications within 3 months of starting denosumab.
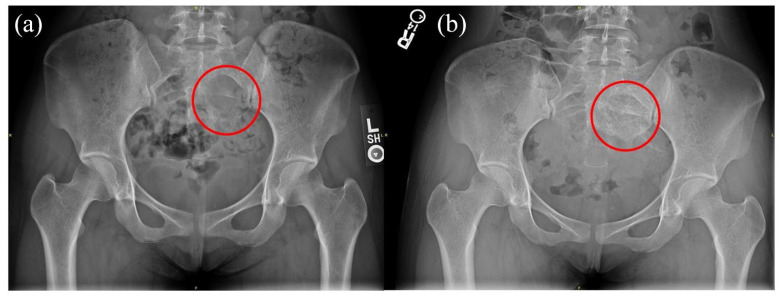
Figure 1.
Plain X-ray: (a) pre-denosumab showed lytic lesion in left sacrum and (b) post-denosumab showed increased calcification in lesion.
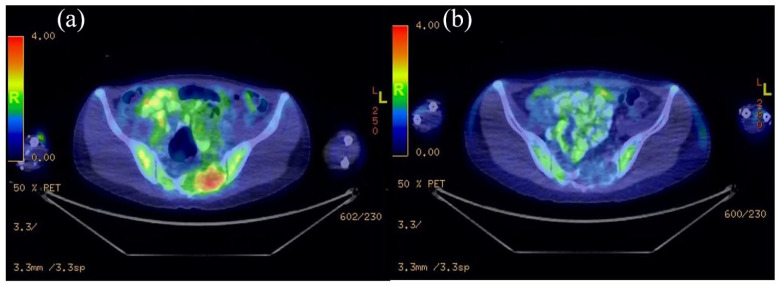
Figure 2.
(a) PET scan of pre-denosumab showed metabolically active lesion in sacrum and (b) post-denosumab showed no metabolic activity.
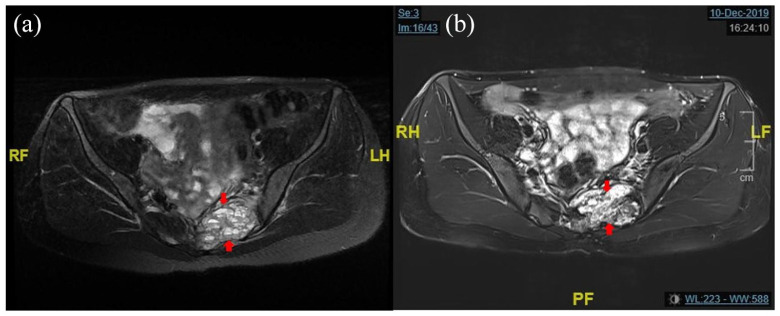
Figure 3.
(a) The fluid levels seen here are very similar to those seen in ABCs and (b) loss of fluid levels in lesion.
Discussion
The pathogenesis and genetic alterations of osteoblastomas remain unclear; however, chromosomal abnormalities were proposed to account for the proliferative potential of these lesions.6 Copy number losses involving chromosomes 22 and 14 have been reported to be associated with osteoblastomas.7 Additionally, a more recently study by Fittall et al.6 demonstrated that osteoblastomas had alterations in the AP-1 transcription factors FOS and FOSB, which were suggested to be diagnostic markers for osteoblastomas and osteoid osteomas as they are not seen in other bone tumors including osteosarcomas.
Osteoblastoma are difficult to differentiate from other bone tumors including osteomas, ABCs, and osteosarcomas. On plain radiographs, it is typically radiolucent with lytic features, a rim of reactive sclerosis or internal calcifications, and a component of well-demarcated central ossification. Secondary ABCs may also be present. They may also be surrounded by sclerotic bone and mimic osteoid osteomas.8 MRI findings include hypo to iso-intense appearance on T1-weighted images with low signal foci representing calcification, and hyper-intensity on T2-weighted caused by soft tissue edema.8
While most tumors grow locally, some are quite extensive and expand into the intracortical region, thus appearing as an ABC.8 Although uncommon, some aggressive osteoblastomas are destructive and can be mistaken for malignant tumors.8
The pathologic evaluation and definitive osteoblastoma diagnosis can be quite challenging as it shares common tumor morphology and genetic alterations with other bone lesions including osteoid osteoma, osteosarcoma, and osteoblast-like osteosarcoma. Histologically, it is composed of woven bone rimmed by plump osteoblasts and scattered osteoclasts that gradually merge with the adjacent normal bone.1,2,9 Cystic changes and secondary ABC formation can be present. The mitotic rate can be focally high, but without atypical forms. In rare cases, foci of benign appearing cartilage can also be found.1
In rare instances, stromal cells in osteoblastomas may show pronounced atypia, which can be a diagnostic pitfall. Bahk and Mirra9 reported the case series of pseudoanaplastic bone tumors, including two osteoblastomas that were initially judged to be osteosarcoma. The lack of mitotic activity, specifically of atypical mitoses, was helpful in the diagnosis of osteoblastoma.
The difficulty in accurately differentiating osteoblastomas from other bony lesions is also highlighted in another case report by Ruggieri et al.10 which describes an osteoblastoma patient who was initially diagnosed with telangiectatic osteosarcoma treated with chemotherapy. For our patient, distinguishing between osteoblastoma and osteosarcoma on a small biopsy was very challenging due to histologic overlap and a small sample of tissue, and lesion located in axial bone. This case was reassessed when systemic treatment did not induce mineralization, which called into question the diagnosis of osteosarcoma.
Systemic treatment options for unresectable or extensive lesions consist of bisphosphonates and RANK ligand inhibitors. As mentioned, osteoblastomas consists largely of osteoblasts. Immature osteoblasts expressed a higher level of RANKL compare to mature cells, leading to increased osteoclastic activity and bone resorption.11 Thus, by inhibiting the RANK-RANKL pathway, osteoclastic activity can be reduced, shifting bone turnover toward re-ossification.3,11
The combination of osteoblastoma with secondary ABCs reported about 14% in case series.12 The efficacy of denosumab has anecdotally been demonstrated in ABCs.5 The etiology of ABCs remain unknown; however, several hypothesis have been proposed. For instance, clonal t(16:17) translocation resulting in TRE17 overexpression may contribute in altering osteoclast/osteoblast homeostasis, culminating in bone loss/destruction in primary ABCs. Also, ABCs may develop as a reaction to bone neoplasm and/or previous trauma. The pathologic finding of ABCs commonly result in cells that express osteoclastic lineage markers. The expression of RANKL on multi-nucleated giant cells within ABC suggest that RANKL inhibition may reduce osteoclastic activity and as a result control disease progression.13 The patient in this report had improved pain symptoms, and achieved complete tumor ossification. Moreover, two additional cases reports describe how denosumab induced osteoblastoma regression, converting the destructive tumor into a solid ossified structure that resulted in significantly improved symptoms.4,14
Conclusion
Osteoblastomas with secondary ABCs have a heterogeneous presentation, and in many cases may be challenging to differentiate from other bone tumors. This is especially true for aggressive osteblastomas in young patients, which mimic malignant tumors—making it a very difficult diagnosis and a potential diagnostic pitfall. Finally, this case suggested that osteoblastomas with secondary ABCs can be effectively treated with denosumab.
Acknowledgments
Information Technology Center, University of California, Los Angeles.
Footnotes
Author contributions: KW and JC drafted the initial manuscript. ME, KM, and SN provided data. KW, JC, FH, and AS reviewed the final draft and helped in critical analysis.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Reporting of individual rare cases does not require Ethical Committee approval at our institute—University of California, Los Angeles.
Informed consent: Written and informed consent was taken from patient and family.
ORCID iD: Jomjit Chantharasamee  https://orcid.org/0000-0002-1237-331X
https://orcid.org/0000-0002-1237-331X
References
- 1.Lucas DR, Unni KK, McLeod RA, et al. Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol 1994; 25(2): 117–134. [DOI] [PubMed] [Google Scholar]
- 2.Vergel De, Dios AM, Bond JR, Shives TC, et al. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer 1992; 69(12): 2921–2931. [DOI] [PubMed] [Google Scholar]
- 3.Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 2010; 11(3): 275–280. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds JJ, Rothenfluh DA, Athanasou N, et al. Neoadjuvant denosumab for the treatment of a sacral osteoblastoma. Eur Spine J 2018; 27(Suppl. 3): 446–452. [DOI] [PubMed] [Google Scholar]
- 5.Kurucu N, Akyuz C, Ergen FB, et al. Denosumab treatment in aneurysmal bone cyst evaluationof nine cases. Pediatr Blood Cancer. 2018;65:e26926. 10.1002/pbc.26765 [DOI] [PubMed] [Google Scholar]
- 6.Fittall MW, Mifsud W, Pillay N, et al. Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat Commun 2018; 9(1): 2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nord KH, Nilsson J, Arbajian E, et al. Recurrent chromosome 22 deletions in osteoblastoma affect inhibitors of the Wnt/beta-catenin signaling pathway. PLoS One 2013; 8(11): e80725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenspan A.Benign bone-forming lesions: osteoma, osteoid osteoma, and osteoblastoma. Clinical, imaging, pathologic, and differential considerations. Skeletal Radiol 1993; 22(7): 485–500. [DOI] [PubMed] [Google Scholar]
- 9.Bahk W-J, Mirra JM.Pseudoanaplastic tumors of bone. Skeletal Radiol 2004; 33(11): 641–648. [DOI] [PubMed] [Google Scholar]
- 10.Ruggieri P, Huch K, Mavrogenis AF, et al. Osteoblastoma of the sacrum: report of 18 cases and analysis of the literature. Spine 2014; 39(2): E97–E103. [DOI] [PubMed] [Google Scholar]
- 11.Atkins GJ, Kostakis P, Pan B, et al. RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res 2003; 18(6): 1088–1098. [DOI] [PubMed] [Google Scholar]
- 12.Della Rocca C, Huvos AG. Osteoblastoma: varied histological presentations with a benign clinical course. An analysis of 55 cases. Am J Surg Pathol 1996; 20(7): 841–850. [DOI] [PubMed] [Google Scholar]
- 13.Pelle DW, Ringler JW, Peacock JD, et al. Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res 2014; 164(2): 139–148. [DOI] [PubMed] [Google Scholar]
- 14.Kooner P, Ferguson P.The use of denosumab in osteoblastoma of the metacarpal. J Hand Surg Am 2019; 44(11): 994.e1–994.e6. [DOI] [PubMed] [Google Scholar]