Abstract
Malaria, a parasitic disease caused by protozoa belonging to the genus Plasmodium, continues to represent a formidable public health challenge. Despite being a preventable disease, cases reported among travelers have continued to increase in recent decades. Protection of travelers against malaria, a potentially life-threatening disease, is of paramount importance, and it is therefore necessary for healthcare professionals to be up to date with the most recent recommendations. The present review provides an update of the existent measures for malaria prevention among travelers.
Keywords: atovaquone/proguanil, chemoprophylaxis, Plasmodium falciparum, Plasmodium knowlesi, Plasmodium vivax, tafenoquine
Introduction
Malaria is a parasitic disease caused by protozoa belonging to the genus Plasmodium. There are four species that exclusively affect humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae. Plasmodium species that commonly infect non-human primates can also be responsible for a high proportion of human cases in certain parts of the world as is the case with Plasmodium knowlesi in southeast Asia and Plasmodium simium in Brazil.1–4 All species of malaria are transmitted by the bite of an infective female Anopheles mosquito. Malaria can be also transmitted through blood transfusion, needle sharing, laboratory accidents, organ transplantation, and congenitally from mother to fetus.5,6
Malaria continues to represent a formidable public health challenge. According to the most recent World Malaria Report published in 2020, there were an estimated 229 million cases of malaria worldwide in 2019 with 94% reported from the African region and mostly affecting children younger than 5 years of age. There were an estimated 409,000 deaths globally, with 95% occurring in sub-Saharan Africa.7 Although there has been progress in reducing the global prevalence and mortality attributable to malaria, the number of cases reported among travelers in the United States (US) has continued to have a stepwise increase of approximately 29.4 cases per year since 1972.6 A total of 2161 confirmed malaria cases were reported by the Centers for Disease Control and Prevention (CDC) in 2017, a 4% relative increase in confirmed cases compared with 2016 and the highest in 45 years. Most cases originated from West Africa (66.9%) and P. falciparum accounted for the majority of infections (70.5%). Most of the cases of malaria affected travelers who were visiting friends and relatives (VFR traveler) and only about a quarter of US residents with malaria reported taking any form of chemoprophylaxis. There were 27 pregnant women affected, of which 22 were hospitalized. Ten of the pregnant women were US residents, and none took prophylaxis to prevent malaria.6
Search strategy and selection criteria
We searched PubMed and Google Scholar for articles published up to June 30, 2021 with emphasis in the last 2 decades, using the terms ‘malaria,’ in combination with ‘traveler,’ ‘protection,’ ‘prophylaxis,’ ‘prevention.’ We reviewed these articles, and relevant articles in the references of these articles. Only articles published in English were included.
Clinical presentation
The severity of clinical manifestations due to malaria is primarily determined by previous exposures to Plasmodium spp. and the resulting immune status (i.e. premunition). The degree of parasitemia also plays a significant role in the pathogenetic mechanisms of the infection in the microvasculature. The ability of P. falciparum and, to a lesser degree, P. knowlesi to infect most stages of the lifespan of red cells correlates with higher levels of parasitemia and worse outcomes. Reports of life-threatening malaria caused by P. vivax are increasing in certain areas of the world, with thrombocytopenia being a potential marker of severity that requires further validation.8,9 Most travelers are considered non-immune to malaria and symptomatic disease is therefore seen across all age groups. The incubation period is of approximately 2 weeks for malaria caused by P. falciparum, P. knowlesi, and P. vivax. For P. ovale and P. malariae, the incubation period is of about 2–3 weeks and 18–35 days, respectively.10–13 Temperate climate P. vivax (P. vivax var hibernans) was of great importance until the middle of last century and was characterized by long incubation periods of up to 8–10 months. Temperate climate P. vivax malaria is still endemic in the Korean peninsula.14–16
The incubation period can also be altered (i.e. prolonged) by agents used for chemoprophylaxis and by antibiotics that are not commonly used to prevent or treat malaria such as rifampin, azithromycin, and ciprofloxacin.17–19 Malaria can present as early as 1 week after the initial exposure and as late as several years after leaving a malaria zone irrespective of chemoprophylaxis use. This is especially true with P. vivax or P. ovale when no liver stage schizonticide is taken as part of prophylaxis.13,18,19
The presentation of malaria can be vague and nonspecific. Fever, malaise, headache, chills, and sweats are common but gastrointestinal and respiratory complaints may predominate. Fever in a returning traveler should be considered a medical emergency and expedited evaluation for life-threatening infections is mandatory. Failure to consider the diagnosis of malaria is not infrequent and the diagnosis can be difficult, as fever is not always present during the initial evaluation. If the first blood films are negative, both thick and thin blood films or rapid diagnostics should be repeated twice 8–24 h apart.13,19
There are no pathognomonic physical exam findings for malaria but several variables could be used to predict a higher likelihood of the disease. Splenomegaly, fever, a white blood cell count <10,000 cells/l, platelet count <150,000/μl, hemoglobin <12 g/dl, eosinophils <5%, and hyperbilirubinemia have been associated with parasitemia.20,21 Malaria is a notifiable disease in every state of the United States.
Educating the traveler
The risk of acquiring malaria is determined by a variety of factors inherent to the traveler, itinerary, and the geographic area being visited. The risk assessment should be therefore individualized and ideally occur at least 4–6 weeks before departure.
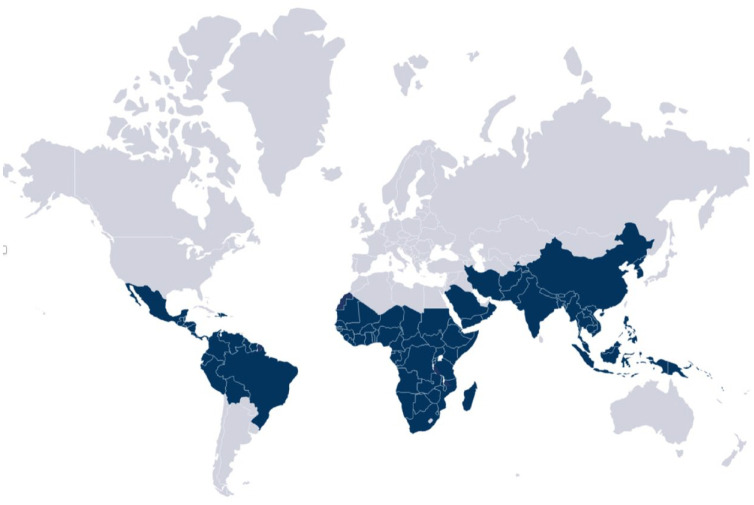
Malaria is endemic in 90 countries and territories (Figure 1) and its transmission is usually continuous throughout the year in tropical regions and seasonal in temperate zones of the world. The intensity and extent of transmission within a country varies and may be focal.7,22 There are several resources with country-specific data regarding malaria transmission to help clinicians with decision making. It is important to note that despite the great value offered by these resources, reliable data on area-specific risks within a predetermined region/country is difficult to predict23 and that several professional organizations publish recommendations that differ, sometimes to a great extent. In general, studies have shown that the highest risk of malaria transmission occurs in sub-Saharan Africa and Papua New Guinea.6,24–27 Chemoprophylaxis should be therefore prescribed routinely to travelers visiting these regions, Pakistan and India, regardless of whether they will be visiting an urban or rural setting. It is important to note that most, but not all, urban and tourist destinations in southeast Asia, Central and South America do not have sufficient risk of malaria transmission to warrant routine prophylaxis.27,28
Figure 1.
Malaria-endemic countries.
Certain populations are at higher risk of acquiring the disease or suffering from its complications. Immigrants who have settled in developed countries and return to their home countries as VFR travelers are highly vulnerable.6,23,26 Some of the factors that affect a VFR’s risk of illness include beliefs that they are immune to diseases that they might have acquired during childhood (e.g. malaria), access and trust of the healthcare (e.g. asylum seekers and new immigrants), lack of awareness of the risks associated with travel, cost, and language barriers among others.29,30 Pregnant women are at high risk of developing potentially fatal complications related to malaria.31 Women who are pregnant or who are likely to become pregnant should be advised against travel to malaria-endemic zones, as there is no chemoprophylactic regimen that is completely effective (see section 8.2 for more details).
There are many other aspects that need to be considered when determining the risk of acquisition of malaria. These include the length and season of the trip, rural versus urban setting, altitude of the destination, accommodation characteristics, outdoor exposure during night-time hours in locations with considerable exposure to mosquitoes, and adherence to mosquito avoidance precautions and chemoprophylaxis.23 Travel health practitioners need to inform travelers of the life-threatening nature of malaria infection and the importance of prevention.
Personal protection measures
Anopheles spp. feed mainly between dawn and dusk, and measures to decrease exposure during this time is of paramount importance. Contact with mosquitoes can be reduced by the use of screened accommodations and mosquito bed nets (preferably insecticide impregnated), appropriate application of repellents, and wearing clothes that cover most of the body.23,32–34
A wide array of popular devices designed to repel mosquitoes are available on the market. A few examples include coils, candles, and heat-generating devices. The effectiveness of these devices is not supported by robust studies and should therefore be used with the addition of proven measures.34
In general, topical repellents can be divided into synthetic chemicals and plant-derived essential oils.35,36 The best-known chemical insect repellents are N, N-diethyl-m-toluamide, also known as N, N-diethyl-3-methylbenzamide (DEET), and picaridin. Oil of lemon eucalyptus (OLE) or PMD (chemical name: para-mentahne-3,8 diol), IR3535 [chemical name: 3-(N-butyl-N-acetyl)-aminopropionic acid, ethyl ester], and 2-undecanone are considered either derivatives or synthetic products of natural materials.33,34
The efficacy and duration of protection are subject to factors such as ambient temperature and humidity, degree of perspiration and level of activity, exposure to water and other variables. The degree of protection to a determined species of mosquito or tick varies according to the active ingredient and the duration of protection is proportional to the concentration of the product.33,34 For DEET, picaridin, and IR3535, a concentration of at least 20% and for PMD 30% is recommended. Most repellents can be used on children aged >2 months with the exception of OLE, which should not be used on children aged <3 years. No additional precautions for using registered repellents on children or pregnant or lactating women are otherwise needed.33,34 It is also important to remember that DEET can have an unpleasant odor for some and can dissolve plastic. In addition, the sunscreen and repellent should be used as separate products and the sunscreen should be applied first.
The Environmental Protection Agency reviews and approves repellents based on efficacy and human safety. A repellency awareness graphic is available on the labels of insect repellents. More information is available on the following website: www.epa.gov/insect-repellents/repellency-awareness-graphic.
Chemoprophylactic agents
Please refer to Table 1 for doses and schemes used for the different drugs.
Table 1.
Chemoprophylactic agents in malaria.
| Drug | Adult dose | Pediatric dose | Special considerations |
|---|---|---|---|
| Atovaquone/proguanil | The adult tablet contains 250 mg of atovaquone and 100 mg of proguanil 1 adult tablet orally daily Begin 1–2 days prior to travel Take daily at the same timeContinue 7 days after leaving endemic area |
The pediatric tablet contains 62.5 mg of atovaquone and 25 mg of proguanil 5–8 kg: ½ pediatric tablet daily 8–10 kg: ¾ pediatric tablet daily 10–20 kg: 1 pediatric tablet daily 20–30 kg: 2 pediatric tablets daily 30–40 kg: 3 pediatric tablets daily 40 kg: 1 adult tablet daily Begin 1–2 days prior to travel Take daily at the same time Continue 7 days after leaving endemic area |
Contraindicated in severe renal impairment (CrCl <30 ml/min) Should be taken with food Not recommended in pregnant woman, when breastfeeding, and in children <5 kg |
| Doxycycline | 100 mg orally daily Begin 1–2 days prior to travel Take daily at the same time Continue 4 weeks after leaving endemic area |
⩾8 years of age: 2.2 mg/kg up to adult dose of 100 mg/day Begin 1–2 days prior to travel Take daily at the same time Continue 4 weeks after leaving endemic area |
Contraindicated in pregnant women and children <8 years of age |
| Mefloquine | 228 mg base (250 mg salt) orally once weekly Begin ⩾2 weeks before travel Take weekly on the same day of the week Continue 4 weeks after leaving endemic area |
⩽9 kg: 4.6 mg/kg base (5 mg/kg salt) orally once weekly >9–19 kg: ¼ tablet once weekly >19–30 kg: ½ tablet once weekly >30–45 kg: ¾ tablet once weekly >45 kg: 1 tablet once weekly Begin ⩾2 weeks before travel Take weekly on the same day of the week Continue 4 weeks after leaving endemic area |
Contraindicated in people with allergy to mefloquine or related compounds (quinine, quinidine) Contraindicated in people with active depression, psychosis, generalized anxiety disorder, and schizophrenia, seizures, and cardiac conduction abnormalities |
| Chloroquine phosphate | 300 mg base (500 mg salt) orally once weekly Begin 1–2 weeks before travel Take weekly on the same day of the week Continue 4 weeks after leaving endemic area |
5 mg/kg base (8.3 mg/kg salt) orally once weekly, up to a maximum dose of 300 mg base Begin 1–2 weeks before travel Take weekly on the same day of the week Continue 4 weeks after leaving endemic area |
May exacerbate psoriasis |
| Hydroxychloroquine sulfate | 310 mg base (400 mg salt) orally once weekly Begin 1–2 weeks before travel Take weekly on the same day of the week Continue 4 weeks after leaving endemic area |
5 mg/kg base (6.5 mg/kg salt) orally once weekly, up to a maximum dose of 310 mg base Begin 1–2 weeks before travel Take weekly on the same day of the week Continue 4 weeks after leaving endemic area |
|
| Primaquine | 30 mg base (52.6 mg salt) orally daily Begin 1–2 weeks before travel Take daily at the same timeContinue 7 days after leaving endemic area |
0.5 mg/kg base (0.8 mg/kg salt), up to a maximum dose of 30 mg base Begin 1–2 weeks before travelTake daily at the same timeContinue 7 days after leaving endemic area |
Contraindicated in people with G6PD deficiency Contraindicated during pregnancy and lactation, unless the infant being breastfed has a documented normal G6PD |
| Tafenoquine (Arakoda®) | 100 mg tablets 200 mg daily for 3 days prior to travel 200 mg weekly during travel, starting 1 week after last pre–travel dose) Take on the same day of the week 200 mg once 1 week after travel |
Not indicated | Only indicated in people ⩾18 years of age Contraindicated in people with G6PD deficiency Contraindicated during pregnancy and lactation, unless the infant being breastfed has a documented normal G6PD Contraindicated in people with a history of or current psychotic disorder |
CrCl, creatinine clearance; G6PD, glucose-6-phosphate dehydrogenase.
Atovaquone/proguanil
Atovaquone inhibits the parasitic electron transport. Proguanil is metabolized through CYP2C19 to cycloguanil, which acts a parasitic dihydrofolate reductase inhibitor.37 The drug’s synergistic effect is caused by proguanil’s ability to increase atovaquone activity to collapse the mitochondrial membrane potential.38 Atovaquone is poorly absorbed, with bioavailability reaching 23% when taken with food. It is extensively protein bound (>99%) and is primarily (94%) hepatically eliminated with limited metabolism. It has a half-life of 55.9 h with multiple doses.37 Proguanil is extensively absorbed regardless of food with a bioavailability of 60–75%. Approximately 40–60% of proguanil is excreted renally with a half-life of 12–21 h.38
Studies have shown a 96–100% protection efficacy against P. falciparum. The medication is well tolerated with the most common side effects reported being abdominal pain, nausea, vomiting, and headache. Abbreviated regimens have been examined in an attempt to improve compliance. The doses used for the treatment of malaria (four tablets daily for 3 consecutive days) provides protection against malaria for >4 weeks. A study done in Australia examined the efficacy of this regimen in adults traveling to malaria-endemic areas with low-to-medium risk for up to 4 weeks. Most participants complied with the regimen, although 43.3% reported side effects. No traveler developed malaria, although the study was not designed and did not have the statistical power to determine effectiveness.39 An observational study designed to detect prophylactic failure with a twice-weekly regimen of atovaquone/proguanil among long-term expatriates (⩾6 months) in West Africa found no cases of malaria. In comparison, the malaria rates were 11.7/1000 person-months in the group taking no prophylaxis, and 2.06/1000 person-months in the group taking weekly mefloquine.40 Other investigators have examined and concluded that discontinuation of the medication 1 day after return as opposed to the 7 days did not alter efficacy.41 Despite these promising results, a modified regimen of atovaquone/proguanil needs to be supported by more robust data from clinical trials with a larger sample size and higher-risk destinations.42
Doxycycline
Doxycycline inhibits protein synthesis by binding to the 30S ribosomal subunit. Doxycycline may also inhibit dihydroorotate dehydrogenase, a mitochondrial enzyme involved in pyrimidine synthesis.43 Doxycycline is almost completely absorbed in the duodenum with a bioavailability of 95%. Food decreases absorption by 20%. The volume of distribution is 0.7 l/kg with protein binding of 82–93%. It achieves high concentrations in the liver and it is excreted unchanged with 35–60% in the urine and the rest in feces.44
Doxycycline has an efficacy between 92% and 96% for P. falciparum and 98% for primary P. vivax infection. The most common side effects are photosensitivity, nausea, vomiting, and an increased frequency of vaginal yeast infections.45 The medication should be taken with at least 8 oz of water while in an upright position and not just before going to bed, to avoid esophagitis. Doxycycline monohydrate or enteric-coated doxycycline hyclate are better tolerated than generic doxycycline hyclate. It is also common practice to prescribe 150 mg of fluconazole for women that intend to use the medication as a standby treatment for vaginal candidiasis. There are insufficient data to make recommendations regarding the interchangeability between doxycycline and minocycline for this indication. Minocycline should be stopped 1–2 days before travel for those taking it chronically and be replaced with doxycycline. The minocycline can be resumed once the course of doxycycline has been completed.45
Mefloquine
The exact mechanism of action is unknown but protein synthesis inhibition has been hypothesized.46 Mefloquine is well absorbed with a bioavailability of 87–89%. It has high protein binding of 98%, with a mean volume of distribution of 22 l.47 Mefloquine is metabolized by CYP3A4 into carboxymefloquine, an inactive metabolite.48 Its terminal half-life is 14–21 days with primarily bile and feces elimination.47
Controversy has surrounded the use of this agent due to its safety profile.49,50 Mefloquine has been associated with a lengthy list of side effects including gastrointestinal discomfort, headache, and dizziness. More severe neuropsychiatric side effects such as visual disturbances, severe depression leading to suicide, sensory and motor neuropathies, memory deficits, hallucinations, aggression, seizures, psychosis, irreversible vertigo, and encephalopathy have been reported. The US Food and Drug Administration (FDA) issued a black-box warning in 2013 about the risk of neuropsychiatric side effects. In the US, any traveler receiving a prescription for mefloquine must also receive a copy of the FDA medication guide, which can be found at https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019591s023lbl.pdf.51 The European Medicines Agency updated the product information and mandated that all European Union members ensure that healthcare professionals are aware and communicate to their patients the risk neuropsychiatric and other adverse events. In addition, it was stipulated that only travelers without a contraindication to the medication receive a prescription and that the traveler carry an alert card at all times.52
The risk of developing severe or disabling neuropsychiatric side effects ranges from 1/607 to 1/20,000 compared with a rate for chloroquine of 1/1181 to 1/13,600.50 Mefloquine is contraindicated in the setting of allergy to the medication or related compounds (e.g. quinine) or any excipient. Active or recent history of depression, generalized anxiety disorder, psychosis, schizophrenia, convulsions, cardiac conduction abnormalities and treatment with halofantrine or ketoconazole are also contraindications.52
Mefloquine is an ideal chemoprophylactic agent for long-term travelers, children, and pregnant women, but due to the potential toxicities, it should be reserved when other agents are contraindicated and for areas with high malaria risk such as sub-Saharan African and parts of Oceania.
Chloroquine and hydroxychloroquine
Chloroquine and hydroxychloroquine’s exact mechanism of action is unknown. They are thought to concentrate in lysosomes and interfere with parasitic processing of hemoglobin.53 Chloroquine has a bioavailability of 89%. Hydroxychloroquine has a bioavailability of 74%. The pharmacokinetics of these drugs are complex, with a large volume of distribution (greater than 50,000 l has been reported), three-compartment pharmacokinetics, and reported half-lives of over 100 days. They both concentrate in the liver. Approximately 23–38% is excreted unchanged in the urine, 17–18% is excreted as metabolites in the urine, with the remainder being excreted in feces or stored long term in lean tissues.54
The medication is well tolerated with the most common reported side effects being gastrointestinal disturbances, dizziness, blurred vision, insomnia, and pruritus. The medication can exacerbate psoriasis, and although rare in the doses used for prophylaxis, retinopathy can occur. The medication is safe in pregnancy.51
Primaquine
The exact mechanism of action of primaquine is unknown. Possible mechanisms are impeding mitochondrial metabolism or oxidative stress. It is rapidly absorbed with a plasma peak in 1–3 h and a volume of distribution of 3 l/kg. It accumulates in the brain, liver, skeletal muscle, lungs, and heart. Primaquine is metabolized into carboxyprimaquine and other metabolites, by oxidases and dehydrogenases. Less than 5% is eliminated unchanged in the urine.55
Primaquine phosphate has two roles in prevention. It can be used as a causal prophylactic agent against all Plasmodium spp. and for presumptive anti-relapse therapy (PART), also known as terminal prophylaxis, for P. vivax and P. ovale. The dosing should overlap with the blood schizonticide and therefore when chloroquine, doxycycline, or mefloquine are used for primary prophylaxis, primaquine is usually taken during the last 2 weeks of postexposure prophylaxis. When atovaquone–proguanil is used for prophylaxis, primaquine may be taken during the final 7 days of atovaquone–proguanil, and then for an additional 7 days. Primaquine should be given concurrently with the primary prophylaxis medication. However, if that is not feasible, the primaquine course in the form of terminal prophylaxis should still be administered after the primary prophylaxis medication has been completed.56 Terminal prophylaxis with primaquine (or tafenoquine, see below) is particularly important for long-term travelers returning from highly endemic areas of P. vivax transmission in the Pacific Islands (i.e. Papua New Guinea, Vanuatu, and Solomon Islands) or among those returning from countries that constitute the Horn of Africa.
The efficacy for prophylaxis is considered to be over 85% against P. falciparum and P. vivax and around 95% when used for PART. The most common side effects are abdominal pain, nausea, and vomiting. Severe hemolysis in persons with glucose-6-phosphate-dehydrogenase (G6PD) deficiency and methemoglobinemia can occur. The medication is contraindicated in the setting of G6PD deficiency, nicotinamide dehydrogenase methemoglobin reductase deficiency, pregnancy, known hypersensitivity to primaquine, and illnesses that manifest with tendency for granulocytopenia. A G6PD testing should be performed before use of the medication.56
Tafenoquine
Tafenoquine’s exact mechanism of action is unknown. Mitochondrial dysfunction leads to Plasmodium spp. death. Absorption is slow and increased with a high-fat meal. It is extensively (>99.5%) protein bound with a volume of distribution of 1600 l. The terminal half-life is 15 days. It is thought to be excreted unchanged, but complete information on excretion is unknown.57
Tafenoquine succinate is formulated as either a 100 mg or 150 mg tablet. The medication has been approved by the FDA and in Australia for the primary prevention of malaria for persons aged ⩾18 years and for the radical cure of P. vivax in persons older than 16 years of age.57–59 It is important to note that the medication is approved in two separate formulations from two different manufacturers. The 60° Pharmaceuticals manufacture Arakoda® and Kodatef® for causal prophylaxis in the US and Australia, respectively. In a partnership with Medicines for Malaria Venture, GlaxoSmithKline (GSK) manufactures Krintafel® and Kozenis® for radical cure of P. vivax, also in the US and Australia, respectively. Tafenoquine is also licensed for PART. For P. ovale, tafenoquine can be used off label for radical cure.60–62
Tafenoquine has only been studied for radical cure of P. vivax malaria when used with chloroquine, and the medication should therefore be co-administered with chloroquine only. CDC continues to recommend the off-label use of tafenoquine for radical cure of P. ovale and like with P. vivax, it should be co-administered with chloroquine only.
Tafenoquine has a half-life of approximately 2 weeks allowing weekly administration for primary prophylaxis after a loading dose and one dose when used for radical cure. When used for primary prophylaxis, the medication is taken at a dose of 200 mg daily for 3 days prior to travel, weekly during travel, and then once after return. The last dose should be taken 7 days after the last maintenance dose while in the malaria-endemic area. For the radical cure of P. vivax, the dose is 300 mg once.57,58 The efficacy of the medication for causal prophylaxis varies between 86% and 100%. The malaria recurrence-free rate at 6 months ranges from 62% to 89%.58
The medication is contraindicated in G6PD-deficient individuals. Quantitative G6PD testing (rather than qualitative, which is usually appropriate for primaquine use) is required, which might logistically be difficult to accomplish for last-minute travelers.63,64 The medication is contraindicated in pregnancy and in breastfeeding women if the infant has G6PD deficiency or if the infant’s G6PD status is unknown. Its safety in children has not been established.58
Malaria standby emergency treatment (SBET)
The concept describes the self-administration of emergent malaria treatment brought from the country of origin for use when no medical attention is available or for use after the diagnosis has been established. The topic is controversial and the appropriate setting for its use varies according to different national guidelines.65 There is great variation in the proportion of travelers that appropriately use this strategy as response to the development of fever which is of primordial importance given the life-threatening nature of the disease.66
For travelers taking chemoprophylaxis and for whom SBET is prescribed, the drug that is being used for chemoprophylaxis should not be used for treatment. Once treatment is completed, chemoprophylaxis should be resumed. If atovaquone/proguanil is being used, it can be resumed immediately after treatment. If another agent is being used, it can be resumed 1 week after completion of treatment. Atovaquone/proguanil and artemether/lumefantrine, two regimens approved in the US, can be used for SBET. Artemether/lumefantrine can be used during pregnancy.67,68 The medications should be bought and taken from the country of origin, given the high rates of counterfeit in malaria-endemic countries.51 Mefloquine should be avoided for this indication given the potential toxicity. In addition, artemether/lumefantrine should not be used for SBET if mefloquine is being used for chemoprophylaxis.51 Other regimens such as the combination of doxycycline and quinine require multiple doses which are frequently associated with side effects.
SBET alone can be considered if traveling to low malaria transmission areas such as most of southeast Asia and South America. If possible, SBET should only be considered when traveling to remote areas where medical attention is hampered by a lack of medical services, or quality medications and access to a medical evaluation is not readily available within 24 h.65 It is important to emphasize to the traveler that the development of fever requires immediate evaluation regardless of SBET use.
Vaccine
Vaccines have not been approved for the prevention of malaria among travelers. The RTS,S/AS01 vaccine has been studied in two phase III trials in Africa with ages ranging from 5–17 months.69–71 The largest malaria vaccine trial done in Africa included 15,459 infants and young children and showed that the vaccine prevented 39% of cases of malaria and 29% of cases of severe malaria over a 4-year follow-up period. For those that received four doses, 1774 cases of malaria were prevented for every 1000 children vaccinated. Efficacy waned with time.69 A recommendation to include the vaccine in the national immunization programs has not been made, and a pilot study is currently underway in Ghana, Kenya, and Malawi.72 Although vaccines can have a great impact in achieving the goals of malaria eradication, further research is needed to be considered ideal for travelers.73
Choosing an antimalarial agent for chemoprophylaxis
The choice of a chemoprophylactic agent (Tables 2–4) requires consideration of several factors such as the travel destination, layovers, season, length of travel, traveler’s health, potential side effects and medication interactions, preference, and cost.
Table 2.
Cost of malaria chemoprophylaxis for adults for one week of travel, including required pre- and post-travel dosing. Prices obtained from GoodRx (https://www.goodrx.com/).
| Cost of malaria chemoprophylaxis for adults | |
|---|---|
| Atovaquone/proguanil | $24.05 |
| Doxycycline | $13.93 |
| Mefloquine | $20.84 |
| Chloroquine phosphate | $31.92 |
| Hydroxychloroquine sulfate | $6.93 |
| Primaquine | $33.59 |
| Tafenoquine (Arakoda® in the US) | $279.52* |
Arakoda pricing based on 16-dose pill pack and exceeds the number of tablets needed for 1 week of travel.
US, United States.
Table 3.
Advantages and disadvantages of malaria chemoprophylaxis.
| Drug | Advantages | Disadvantages |
|---|---|---|
| Atovaquone/proguanil | Effective in all areas Option for last-minute travelers Appropriate for travelers who prefer daily medication A suitable option for short trips Can be taken by children >5 kg Well tolerated |
Cannot be taken with renal dysfunction Cannot be taken by women who are pregnant, or breastfeeding children <5 kg Cost |
| Doxycycline | Effective in all areas Option for last-minute travelers Appropriate for travelers who prefer daily medication Available in most countries Can prevent other travel-related illnesses |
Cannot be taken by children <8 years of age Cannot be taken in pregnancy Can cause photosensitivity, vaginal yeast infections, and gastrointestinal upset Must be taken 4 weeks after travel completion |
| Mefloquine | Appropriate for travelers who prefer taking weekly medication Useful for long trips Can be used in pregnancy Can be taken by children |
Can only be used in areas with mefloquine sensitivity Not an attractive option for last-minute travelers Cannot be used in psychiatric conditions, seizure disorder, or cardiac abnormalities |
| Chloroquine phosphate | Appropriate for travelers who prefer taking weekly medications Useful for long trips Can be used in pregnancy Can be taken by children |
Can only be used in areas with chloroquine sensitivity Not an attractive option for last-minute travelers May exacerbate psoriasis |
| Hydroxychloroquine sulfate | Appropriate for travelers who prefer taking weekly medication Useful for long trips Can be used in pregnancy |
Can only be used in areas with chloroquine sensitivity Not an attractive option for last-minute travelers |
| Primaquine | Appropriate for travelers who prefer taking daily medications A suitable option for short trips Option for last minute travelers if G6PD testing is available |
Can be used in areas with principally Plasmodium vivax Cannot be used in pregnancy and lactation Cannot be used in travelers with G6PD deficiency Can causes gastrointestinal upset |
| Tafenoquine (Arakoda® in the US) | Effective in all areas Option for last minute travelers if G6PD testing is available Appropriate for travelers who prefer weekly medication A suitable option for short trips |
Cannot be used in children Contraindicated during pregnancy and lactation, unless the infant being breastfed has a documented normal G6PD Cannot be used in travelers with G6PD deficiency Cannot be used in psychiatric conditions Expensive |
G6PD, glucose-6-phosphate dehydrogenase; US, United States.
Table 4.
Drug interactions with malaria chemoprophylaxis agents.74
| Drug | Interactions |
|---|---|
| Atovaquone/proguanil | Anti-retrovirals (e.g. efavirenz, ritonavir, saquinavir, indinavir) |
| Rifamycins | |
| Tetracycline | |
| Metoclopramide | |
| Warfarin | |
| Doxycycline | Antacids |
| Bismuth subsalicylate | |
| Oral iron | |
| Rifampin | |
| Anti-epileptic medications (e.g. carbamazepine, phenytoin, phenobarbital) | |
| Warfarin | |
| Methotrexate | |
| Mefloquine | Medications that affect cardiac conduction (e.g. digoxin, B-blockers, amiodarone) |
| Anti-epileptic medications (e.g. carbamazepine, phenobarbital, levetiracetam) | |
| Rifampin | |
| Ketoconazole | |
| Warfarin | |
| Chloroquine phosphate | Antacids |
| Cyclosporine | |
| Tamoxifen | |
| Anti-retrovirals (e.g. ritonavir) | |
| Antipsychotics (cisapride, dronedarone) Antidepressants (e.g. citalopram, escitalopram, fluoxetine, tricyclics) |
|
| QT-prolonging medications | |
| Carbamazepine | |
| Praziquantel | |
| Thyroxine | |
| Hydroxychloroquine sulfate | Antipsychotics |
| Medications that affect cardiac conduction (e.g. digoxin, B-blockers, amiodarone) | |
| Fluoroquinolones | |
| Cyclosporine | |
| Tamoxifen | |
| Primaquine | Antipsychotics |
| Medications that affect cardiac conduction, e.g. (digoxin, B-blockers, amiodarone) | |
| Tafenoquine (Arakoda® in the US) | Cisplatin |
| Oxaliplatin | |
| Metformin |
B-blockers, beta blockers; US, United States.
P. falciparum has developed resistance to all antimalarials, and knowledge about its geographic distribution is important in decision making.51,75 Resistance to chloroquine was first observed in southeast Asia and South America in the 1950s and subsequently spread to most parts of the world excluding the Caribbean and Central America, west of the Panama Canal.76 Resistance is the result of a point mutation in the PfCRT protein that localizes to the digestive vacuole of the Plasmodium spp. This results in the inability of chloroquine to concentrate within the digestive vacuole and form complexes with toxic heme moieties that interfere with detoxification mechanisms.77–80
Resistance of P. vivax to chloroquine was documented in 1989 when Australians repatriated from Papua New Guinea failed routine treatment.81 Subsequent reports from Indonesia, Myanmar, and India corroborated findings.82 The cause of resistance in P. vivax remains elusive due to the nature of the parasite’s dormant phase in the liver, low parasitemia, and difficulty in distinguishing relapse, recrudescence, and reinfection.83
Special populations
Infants, children, and adolescents
Prevention recommendations for malaria in children are similar to that in adults and include assessment of risk based on travel itinerary, education of mosquito avoidance, determination of chemoprophylaxis, and educating parents to seek early medical care if fever develops during or soon after travel.84 All chemoprophylactic agents used in adults, with the exception of tafenoquine, are available for children. There are several observations to remember: atovaquone/proguanil should not be administered to children weighing less than 5 kg and doxycycline in those younger than 8 years of age. The product’s label should be carefully followed for pediatric dosing of malarial chemoprophylaxis. Due to difficulty in pediatric dosing, pulverizing tablets into specified dosing can be done, with the assistance of a pharmacist. Due to bitter taste, medications can be mixed into or crushed into food. Specific contraindication for use of repellents in children was described earlier in this document.
Pregnancy and breastfeeding
Pregnant women are particularly susceptible to malaria infection due to immunologic changes that occur during pregnancy. P. falciparum can concentrate in the placenta and lead to miscarriage, stillbirth, preterm birth, and low birth weight.85–87 Congenital infection with detectable parasitemia from 24 h to 7 days of life is another important complication.88–90
Pregnant women should be advised not to travel, if at all possible, as no chemoprophylactic regimen is 100% effective. For women that chose to travel to a malaria-endemic region, emphasis on mosquito avoidance measures and chemoprophylaxis should be provided. In the US, malaria chemoprophylaxis in pregnancy is limited to mefloquine and chloroquine.
In some countries, atovaquone/proguanil is used for either treatment or prophylaxis of malaria during pregnancy. Although the available safety data for its use in pregnancy are reassuring, well-established trials are needed before a definite recommendation can be made.91,92 Doxycycline is also used during the first trimester in several European countries if there are compelling reasons for chemoprophylaxis and if no alternative is available. It is important to remember that doxycycline needs to be administered for 4 weeks after return from a malaria-endemic region.93
In all women of childbearing age, plans for conception during travel should be discussed. All breastfeeding mothers should be counseled that maternal use of chemoprophylaxis does not provide protection to a breastfed infant, as only a limited amount of medication is secreted in the breastmilk. All chemoprophylactic agents can be administered to breastfeeding mothers except primaquine and tafenoquine, unless G6PD deficiency has been excluded in both the mother and newborn.
Immunosuppressed travelers
Immunosuppressed patients, including those with human immunodeficiency virus (HIV), are more likely to develop severe malaria.22 Immunosuppressed travelers should be provided chemoprophylaxis similarly to those who are immunocompetent, with additional consideration of the possibility of drug interactions.
Interactions with malaria prophylaxis are primarily seen with cancer-related therapies, anti-rejection medications used after transplantation, and medications used to treat HIV (Table 4). Drug interactions related to anti-retrovirals can be checked on the University of Liverpool HIV Drug Interactions website (https://www.hiv-druginteractions.org/). Typical cancer-directed therapies that can result in interactions include the use of tamoxifen with chloroquine that results in an increased risk of retinopathy, increased levels of platinum-based chemotherapies with tafenoquine, and increased levels of methotrexate when used with doxycycline. In those who have undergone transplantation, chloroquine can increase cyclosporine levels. In travelers with HIV, interactions occur primarily with protease-inhibitor-based regimens and with some non-nucleoside reverse-transcriptase inhibitors such as efavirenz, nevirapine, and etravirine.74,94
Long-term travelers
A long-term traveler is a person visiting a malaria-endemic region for longer than 6 months. Long-term travelers represent a high-risk group, as they tend to underuse personal protective equipment and adhere poorly to anti-malaria prophylaxis.32
Chloroquine has a good safety record for the treatment of rheumatologic conditions, suggesting it can be used for long-term malaria prophylaxis. For long-term use (>5 years) of chloroquine, a baseline eye examination with bi-annual follow up is recommended to screen for potential retinal toxicity.51 Long-term mefloquine use is shown to be safe. Tolerability has been variable and related to neuropsychiatric events that usually occur early in the course of prophylaxis.32,95,96 Atovaquone/proguanil has been used up to a duration of 34 weeks with good tolerability, with diarrhea being the primary side effect.97
The tolerability of doxycycline was evaluated in a study of 600 military personnel in Cambodia for 1 year and 900 men deployed to Somalia for 4 months. The medication was well tolerated, with gastrointestinal events and photosensitivity being the most common reported side effects.98 Another study with 228 US Peace Corps volunteers who took doxycycline prophylaxis for an average of 19 months showed a similar side-effect profile.99 Doxycycline is approved by the FDA for a duration of 4 months. The use of primaquine for up to 52 weeks is safe with mild non-clinically significant methemoglobinemia being the most common side effect.100
Relapse prevention
PART is an intervention used to eradicate the quiescent liver hypnozoites of P. vivax or P. ovale with medications such as primaquine and tafenoquine. Primaquine is FDA approved at a dose of 0.25 mg/kg (15 mg base) daily for 14 days.51 The World Health Organization guidelines recommend a higher dose of primaquine (0.5 mg/kg or 30 mg base daily for 14 days) for strains of P. vivax acquired in East Asia and Oceania,101 and CDC recommends the higher dose regardless of geographic site of acquisition.51 Tafenoquine should be administered as a single 300 mg dose and it should ideally overlap with blood-stage treatment or the last dose of prophylaxis. If this is not feasible, tafenoquine may be taken as soon as possible afterward.62
The use of PART is appropriate for travelers that have visited P. vivax-endemic areas, even if P. falciparum is present, and especially for prolonged stays.50 It is important to remember that if either primaquine or tafenoquine are used as prophylactic agents, PART is not needed.
There are several important caveats with the use primaquine. Adherence to a 14-day course is poor, and life-threatening hemolytic reactions may occur if G6PD deficiency is not recognized. To address the first limitation, two randomized controlled trials in G6PD-normal patients compared a shorter primaquine regimen consisting of 1 mg/kg day for 7 days with high-dose primaquine for 14 days. Both studies showed no difference in efficacy between the 7-day and 14-day course. However, there were more side effects reported in the 7-day arm in one of the studies.102,103
Blood donation
Transfusion-transmitted malaria was first described in the turn of last century and is an important form of transmission in malaria-endemic areas.104 A recent review concluded that the median worldwide prevalence of malaria parasitemia in healthy blood donors was 10.54% by microscopy, 5.36% by molecular techniques, and 0.38% by rapid diagnostic tests.105
Storage of blood under refrigeration is deleterious to Plasmodium spp. Refrigeration at 4°C decreases parasitemia rapidly, nonetheless Plasmodium falciparum has been shown to survive in stored whole blood or plasma at this temperature for approximately 18 days and can remain microscopically detectable for up to 28 days when frozen but with diminished infectivity.106
The incubation period of transfusion-transmitted malaria is longer than mosquito-transmitted malaria which could lead to a lack of suspicion and delay in the diagnosis of the disease; especially in non-endemic regions.104 A study that examined transfusion-transmitted malaria in the US between 1963 and 1999 found that the median incubation period was 10 days but ranged from 1 to 180 days.107
As of April of 2020, the FDA recommends that non-resident travelers of an endemic country or those who are residents of an endemic country but have lived in a non-endemic region for more than 3 consecutive years and are returning from malaria-endemic areas defer blood donation for 3 months after arrival as opposed to 1 year as was previously recommended. This group does not necessarily need to defer donation, as pathogen reduction techniques could be used and allow the collection of components from otherwise-eligible donors. For former residents of malaria-endemic regions that have lived in a non-endemic region for less than 3 consecutive years and for people that have been diagnosed with malaria, donation of blood products should be deferred for 3 years, and this recommendation remains unaltered in the most recent update.108
Conclusion
The prevention of malaria in travelers continues to be challenging. A multitude of factors determine the risk of malaria acquisition among travelers. The knowledge of such factors and the available preventive measures are of vital importance in being able to provide evidence-based recommendations to travelers. This knowledge should not be restricted to specialized travel medicine professionals as a significant percentage of travelers seek attention from general practitioners. It is therefore imperative for practitioners to be familiar with the most recent guidance and available resources (e.g. internet based, specialized travel clinics available in the community, etc.) to be able to provide safe, effective, and affordable care to the traveler.
Footnotes
Authors contributions: Conceptualization, retrieval of articles for review, critical revision of original draft and approval of final manuscript: Nelson I. Agudelo Higuita, Miranda McGhee, Bryan White, Carlos Franco-Paredes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Nelson Iván Agudelo Higuita  https://orcid.org/0000-0002-9363-6280
https://orcid.org/0000-0002-9363-6280
Carlos Franco-Paredes  https://orcid.org/0000-0001-8757-643X
https://orcid.org/0000-0001-8757-643X
Contributor Information
Nelson Iván Agudelo Higuita, Department of Medicine, Section of Infectious Diseases, University of Oklahoma Health Science Center, 800 Stanton L. Young Blvd., Suite 7300, Oklahoma City, OK 73104, USA.
Bryan Pinckney White, Infectious Diseases Clinical Pharmacist, Oklahoma University Medical Center, Oklahoma City, OK, USA.
Carlos Franco-Paredes, Department of Medicine, University of Colorado Denver School of Medicine, Aurora, CO, USA.
Miranda Ann McGhee, Department of Medicine, Section of Infectious Diseases, University of Oklahoma Health Science Center, 800 Stanton L. Young Blvd., Suite 7300, Oklahoma City, OK 73104, USA.
References
- 1.Brasil P, Zalis MG, De Pina-Costa A, et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Glob Health 2017; 5: e1038–e1046. [DOI] [PubMed] [Google Scholar]
- 2.Singh B, Kim Sung L, Matusop A, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004; 363: 1017–1024. [DOI] [PubMed] [Google Scholar]
- 3.Fornace KM, Brock PM, Abidin TR, et al. Environmental risk factors and exposure to the zoonotic malaria parasite Plasmodium knowlesi across northern Sabah, Malaysia: a population-based cross-sectional survey. Lancet Planet Health 2019; 3: e179–e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber BE, Rajahram GS, Grigg MJ, et al. World malaria report: time to acknowledge Plasmodium knowlesi malaria. Malar J 2017; 16: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruell H, Hamacher L, Jennissen V, et al. On taking a different route: an unlikely case of malaria by nosocomial transmission. Clin Infect Dis 2017; 65: 1404–1406. [DOI] [PubMed] [Google Scholar]
- 6.Mace KE, Lucchi NW, Tan KR.Malaria surveillance–United States, 2017. MMWR Surveill Summ 2021; 70: 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization, 2020. [Google Scholar]
- 8.Rahimi B, Thakkinstian A, White NJ, et al. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J 2014; 13: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naing C, Whittaker MA.Severe thrombocytopaenia in patients with vivax malaria compared to falciparum malaria: a systematic review and meta-analysis. Infect Dis Poverty 2018; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins WE, Jeffery GM.Plasmodium ovale: parasite and disease. Clin Microbiol Rev 2005; 18: 570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins WE, Jeffery GM.Plasmodium malariae: parasite and disease. Clin Microbiol Rev 2007; 20: 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashley EA, Pyae Phyo A, Woodrow CJ.Malaria. Lancet 2018; 391: 1608–1621. [DOI] [PubMed] [Google Scholar]
- 13.Kain KC, Keystone JS.Malaria in travelers. Epidemiology, disease, and prevention. Infect Dis Clin North Am 1998; 12: 267–284. [DOI] [PubMed] [Google Scholar]
- 14.Petersen E, Severini C, Picot S.Plasmodium vivax malaria: a re-emerging threat for temperate climate zones? Travel Med Infect Dis 2013; 11: 51–59. [DOI] [PubMed] [Google Scholar]
- 15.Bahk YY, Lee HW, Na BK, et al. Epidemiological characteristics of re-emerging vivax malaria in the Republic of Korea (1993-2017). Korean J Parasitol 2018; 56: 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White NJ.Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J 2011; 10: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahl EL, Rosenthal PJ.Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother 2007; 51: 3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz E, Parise M, Kozarsky P, et al. Delayed onset of malaria—implications for chemoprophylaxis in travelers. New Engl J Med 2003; 349: 1510–1516. [DOI] [PubMed] [Google Scholar]
- 19.Dorsey G, Gandhi M, Oyugi JH, et al. Difficulties in the prevention, diagnosis, and treatment of imported malaria. Arch Intern Med 2000; 160: 2505–2510. [DOI] [PubMed] [Google Scholar]
- 20.D’Acremont V, Landry P, Mueller I, et al. Clinical and laboratory predictors of imported malaria in an outpatient setting: an aid to medical decision making in returning travelers with fever. Am J Trop Med Hyg 2002; 66: 481–486. [DOI] [PubMed] [Google Scholar]
- 21.Taylor SM, Molyneux ME, Simel DL, et al. Does this patient have malaria? JAMA 2010; 304: 2048–2056. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. World malaria report 2018. Geneva: World Health Organization, 2018. [Google Scholar]
- 23.Freedman DO.Clinical practice. Malaria prevention in short-term travelers. New Engl J Med 2008; 359: 603–612. [DOI] [PubMed] [Google Scholar]
- 24.Leder K, Black J, O’Brien D, et al. Malaria in travelers: a review of the GeoSentinel surveillance network. Clin Infect Dis 2004; 39: 1104–1112. [DOI] [PubMed] [Google Scholar]
- 25.Harvey K, Esposito DH, Han P, et al. Surveillance for travel-related disease—GeoSentinel surveillance system, United States, 1997-2011. MMWR Surveill Summ 2013; 62: 1–23. [PubMed] [Google Scholar]
- 26.Mace KE, Arguin PM, Lucchi NW, et al. Malaria surveillance—United States, 2016. MMWR Surveill Summ 2019; 68: 1–35. [DOI] [PubMed] [Google Scholar]
- 27.Steinhardt LC, Magill AJ, Arguin PM.Review: malaria chemoprophylaxis for travelers to Latin America. Am J Trop Med Hyg 2011; 85: 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behrens RH, Carroll B, Hellgren U, et al. The incidence of malaria in travellers to South-east Asia: is local malaria transmission a useful risk indicator? Malar J 2010; 9: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angell SY, Cetron MS.Health disparities among travelers visiting friends and relatives abroad. Ann Intern Med 2005; 142: 67–72. [DOI] [PubMed] [Google Scholar]
- 30.Leder K, Tong S, Weld L, et al. Illness in travelers visiting friends and relatives: a review of the GeoSentinel surveillance network. Clin Infect Dis 2006; 43: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 31.Rogerson SJ, Desai M, Mayor A, et al. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 2018; 18: e107–e118. [DOI] [PubMed] [Google Scholar]
- 32.Chen LH, Wilson ME, Schlagenhauf P.Prevention of malaria in long-term travelers. JAMA 2006; 296: 2234–2244. [DOI] [PubMed] [Google Scholar]
- 33.Moore SJ, Mordue Luntz AJ, Logan JG.Insect bite prevention. Infect Dis Clin North Am 2012; 26: 655–673. [DOI] [PubMed] [Google Scholar]
- 34.Alpern JD, Dunlop SJ, Dolan BJ, et al. Personal protection measures against mosquitoes, ticks, and other arthropods. Med Clin North Am 2016; 100: 303–316. [DOI] [PubMed] [Google Scholar]
- 35.Fradin MS, Day JF.Comparative efficacy of insect repellents against mosquito bites. New Engl J Med 2002; 347: 13–18. [DOI] [PubMed] [Google Scholar]
- 36.Goodyer LI, Croft AM, Frances SP, et al. Expert review of the evidence base for arthropod bite avoidance. J Travel Med 2010; 17: 182–192. [DOI] [PubMed] [Google Scholar]
- 37.McKeage K, Scott L.Atovaquone/proguanil: a review of its use for the prophylaxis of Plasmodium falciparum malaria. Drugs 2003; 63: 597–623. [DOI] [PubMed] [Google Scholar]
- 38.Marra F, Salzman JR, Ensom MH.Atovaquone-proguanil for prophylaxis and treatment of malaria. Ann Pharmacother 2003; 37: 1266–1275. [DOI] [PubMed] [Google Scholar]
- 39.Lau CL, Ramsey L, Mills LC, et al. Drug-free holidays: compliance, tolerability, and acceptability of a 3-day atovaquone/proguanil schedule for pretravel malaria chemoprophylaxis in Australian travelers. Clin Infect Dis 2019; 69: 137–143. [DOI] [PubMed] [Google Scholar]
- 40.Lachish T, Bar-Meir M, Eisenberg N, et al. Effectiveness of twice a week prophylaxis with atovaquone-proguanil (Malarone®) in long-term travellers to west Africa. J Travel Med 2016; 23: taw064. [DOI] [PubMed] [Google Scholar]
- 41.Leshem E, Meltzer E, Stienlauf S, et al. Effectiveness of short prophylactic course of atovaquone-proguanil in travelers to sub-Saharan Africa. J Travel Med 2014; 21: 82–85. [DOI] [PubMed] [Google Scholar]
- 42.Savelkoel J, Binnendijk KH, Spijker R, et al. Abbreviated atovaquone-proguanil prophylaxis regimens in travellers after leaving malaria-endemic areas: a systematic review. Travel Med Infect Dis 2018; 21: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaillard T, Madamet M, Pradines B.Tetracyclines in malaria. Malar J 2015; 14: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agwuh KN, MacGowan A.Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 2006; 58: 256–265. [DOI] [PubMed] [Google Scholar]
- 45.Tan KR, Magill AJ, Parise ME, et al. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg 2011; 84: 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong W, Bai XC, Sleebs BE, et al. Mefloquine targets the Plasmodium falciparum 80S ribosome to inhibit protein synthesis. Nat Microbiol 2017; 2: 17031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer KJ, Holliday SM, Brogden RN.Mefloquine. A review of its antimalarial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1993; 45: 430–475. [DOI] [PubMed] [Google Scholar]
- 48.Piedade R, Traub S, Bitter A, et al. Carboxymefloquine, the major metabolite of the antimalarial drug mefloquine, induces drug-metabolizing enzyme and transporter expression by activation of pregnane X receptor. Antimicrob Agents Chemother 2015; 59: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlagenhauf P, Adamcova M, Regep L, et al. The position of mefloquine as a 21st century malaria chemoprophylaxis. Malar J 2010; 9: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen LH, Wilson ME, Schlagenhauf P.Controversies and misconceptions in malaria chemoprophylaxis for travelers. JAMA 2007; 297: 2251–2263. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. CDC yellow book 2018: health information for international travel. New York: Oxford University Press, 2017. [Google Scholar]
- 52.Schlagenhauf P, Hatz C, Behrens R, et al. Mefloquine at the crossroads? Implications for malaria chemoprophylaxis in Europe. Travel Med Infect Dis 2015; 13: 192–196. [DOI] [PubMed] [Google Scholar]
- 53.Foley M, Tilley L.Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther 1998; 79: 55–87. [DOI] [PubMed] [Google Scholar]
- 54.Browning DJ.Pharmacology of chloroquine and hydroxychloroquine. Hydroxychloroquine and chloroquine retinopathy. New York, NY: Springer New York, 2014. pp.35–63. [Google Scholar]
- 55.Vale N, Moreira R, Gomes P.Primaquine revisited six decades after its discovery. Eur J Med Chem 2009; 44: 937–953. [DOI] [PubMed] [Google Scholar]
- 56.Hill DR, Baird JK, Parise ME, et al. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg 2006; 75: 402–415. [PubMed] [Google Scholar]
- 57.Frampton JE.Tafenoquine: first global approval. Drugs 2018; 78: 1517–1523. [DOI] [PubMed] [Google Scholar]
- 58.Baird JK.Tafenoquine for travelers’ malaria: evidence, rationale and recommendations. J Travel Med 2018; 25: tay110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan KR, Hwang J.Tafenoquine receives regulatory approval in USA for prophylaxis of malaria and radical cure of Plasmodium vivax. J Travel Med 2018; 25: tay071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu CS, Freedman DO.Tafenoquine and G6PD: a primer for clinicians. J Travel Med 2019; 26: taz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Advice for travelers. Med Lett Drugs Ther 2019; 61: 153–160. [PubMed] [Google Scholar]
- 62.Haston JC, Hwang J, Tan KR.Guidance for using Tafenoquine for prevention and antirelapse therapy for malaria–United States, 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White NJ, Nosten FH.SERCAP: is the perfect the enemy of the good? Malar J 2021; 20: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chu CS, Hwang J.Tafenoquine: a toxicity overview. Expert Opin Drug Saf 2021; 20: 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Behrens R.Standby emergency treatment of malaria for travellers to low transmission destinations. Does it make sense or save lives? J Travel Med 2017; 24: tax034. [DOI] [PubMed] [Google Scholar]
- 66.Tan R, Elmers J, Genton B.Malaria standby emergency treatment (SBET) for travellers visiting malaria endemic areas: a systematic review and meta-analysis. J Travel Med 2019; 26: taz027. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization Malaria Policy Advisory Committee and Secretariat. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of eighth biannual meeting (September 2015). Malar J 2016; 15: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ballard SB, Salinger A, Arguin PM, et al. Updated CDC recommendations for using Artemether-Lumefantrine for the treatment of uncomplicated malaria in pregnant women in the United States. MMWR Morb Mortal Wkly Rep 2018; 67: 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.RTS SClinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olotu A, Fegan G, Wambua J, et al. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. New Engl J Med 2013; 368: 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olotu A, Fegan G, Wambua J, et al. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. New Engl J Med 2016; 374: 2519–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adepoju P.RTS,S malaria vaccine pilots in three African countries. Lancet 2019; 393: 1685. [DOI] [PubMed] [Google Scholar]
- 73.Teneza-Mora N, Lumsden J, Villasante E.A malaria vaccine for travelers and military personnel: requirements and top candidates. Vaccine 2015; 33: 7551–7558. [DOI] [PubMed] [Google Scholar]
- 74.Lewis J, Gregorian T, Portillo I, et al. Drug interactions with antimalarial medications in older travelers: a clinical guide. J Travel Med 2020; 27. taz089. [DOI] [PubMed] [Google Scholar]
- 75.Ashley EA, Dhorda M, Fairhurst RM, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. New Engl J Med 2014; 371: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hyde JE.Drug-resistant malaria—an insight. FEBS J 2007; 274: 4688–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sidhu AB, Verdier-Pinard D, Fidock DA.Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science2002; 298: 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sullivan DJ, Jr, Matile H, Ridley RG, et al. A common mechanism for blockade of heme polymerization by antimalarial quinolines. J Biol Chem 1998; 273: 31103–31107. [DOI] [PubMed] [Google Scholar]
- 79.Pagola S, Stephens PW, Bohle DS, et al. The structure of malaria pigment beta-haematin. Nature 2000; 404: 307–310. [DOI] [PubMed] [Google Scholar]
- 80.Ginsburg H, Ward SA, Bray PG.An integrated model of chloroquine action. Parasitol Today 1999; 15: 357–360. [DOI] [PubMed] [Google Scholar]
- 81.Rieckmann KH, Davis DR, Hutton DC.Plasmodium vivax resistance to chloroquine? Lancet 1989; 2: 1183–1184. [DOI] [PubMed] [Google Scholar]
- 82.Baird JK.Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother 2004; 48: 4075–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noisang C, Meyer W, Sawangjaroen N, et al. Molecular detection of antimalarial drug resistance in Plasmodium vivax from returned travellers to NSW, Australia during 2008-2018. Pathogens 2020; 9: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shetty AK, Woods CR.Prevention of malaria in children. Pediatr Infect Dis J 2006; 25: 1173–1176. [DOI] [PubMed] [Google Scholar]
- 85.Desai M, Ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7: 93–104. [DOI] [PubMed] [Google Scholar]
- 86.Rogerson SJ, Hviid L, Duffy PE, et al. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis 2007; 7: 105–117. [DOI] [PubMed] [Google Scholar]
- 87.Rogerson SJ.Management of malaria in pregnancy. Indian J Med Res 2017; 146: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feresu SA, Harlow SD, Woelk GB.Risk factors for prematurity at Harare Maternity Hospital, Zimbabwe. Internet J Epidemiol 2004; 33: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 89.Guyatt HL, Snow RW.Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev 2004; 17: 760–769. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thapar RK, Saxena A, Devgan A.Congenital malaria. Med J Armed Forces India 2008; 64: 185–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andrejko KL, Mayer RC, Kovacs S, et al. The safety of atovaquone-proguanil for the prevention and treatment of malaria in pregnancy: a systematic review. Travel Med Infect Dis 2019; 27: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mayer RC, Tan KR, Gutman JR.Safety of atovaquone-proguanil during pregnancy. J Travel Med 2019; 26. tay138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McKinney KL, Wu HM, Tan KR, et al. Malaria in the pregnant traveler. Journal of Travel Medicine 2020; 27(4): taaa074. DOI: 10.1093/jtm/taaa074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castelli F, Patroni A.The human immunodeficiency virus-infected traveler. Clin Infect Dis 2000; 31: 1403–1408. [DOI] [PubMed] [Google Scholar]
- 95.Lobel HO, Miani M, Eng T, et al. Long-term malaria prophylaxis with weekly mefloquine. Lancet 1993; 341: 848–851. [DOI] [PubMed] [Google Scholar]
- 96.Rombo L, Angel VH, Friman G, et al. Comparative tolerability and kinetics during long-term intake of Lariam and Fansidar for malaria prophylaxis in nonimmune volunteers. Trop Med Parasitol 1993; 44: 254–256. [PubMed] [Google Scholar]
- 97.Overbosch D.Post-marketing surveillance: adverse events during long-term use of atovaquone/proguanil for travelers to malaria-endemic countries. J Travel Med 2003; 10(Suppl. 1): S16–S20; discussion S21–S23. [DOI] [PubMed] [Google Scholar]
- 98.Shanks GD, Roessler P, Edstein MD, et al. Doxycycline for malaria prophylaxis in Australian soldiers deployed to United Nations missions in Somalia and Cambodia. Mil Med 1995; 160: 443–445. [PubMed] [Google Scholar]
- 99.Korhonen C, Peterson K, Bruder C, et al. Self-reported adverse events associated with antimalarial chemoprophylaxis in Peace Corps volunteers. Am J Prev Med 2007; 33: 194–199. [DOI] [PubMed] [Google Scholar]
- 100.Baird JK, Lacy MD, Basri H, et al. Randomized, parallel placebo-controlled trial of primaquine for malaria prophylaxis in Papua, Indonesia. Clin Infect Dis 2001; 33: 1990–1997. [DOI] [PubMed] [Google Scholar]
- 101.Milligan R, Daher A, Graves PM.Primaquine at alternative dosing schedules for preventing relapse in people with Plasmodium vivax malaria. Cochrane Database Syst Rev 2019; 7: CD012656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taylor WRJ, Thriemer K, von Seidlein L, et al. Short-course primaquine for the radical cure of Plasmodium vivax malaria: a multicentre, randomised, placebo-controlled non-inferiority trial. Lancet 2019; 394: 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chu CS, Phyo AP, Turner C, et al. Chloroquine versus dihydroartemisinin-piperaquine with standard high-dose primaquine given either for 7 days or 14 days in Plasmodium vivax malaria. Clin Infect Dis 2019; 68: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verra F, Angheben A, Martello E, et al. A systematic review of transfusion-transmitted malaria in non-endemic areas. Malar J 2018; 17: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahmadpour E, Foroutan-Rad M, Majidiani H, et al. Transfusion-transmitted malaria: a systematic review and meta-analysis. Open Forum Infect Dis 2019; 6: ofz283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chattopadhyay R, Majam VF, Kumar S.Survival of Plasmodium falciparum in human blood during refrigeration. Transfusion 2011; 51: 630–635. [DOI] [PubMed] [Google Scholar]
- 107.Mungai M, Tegtmeier G, Chamberland M, et al. Transfusion-transmitted malaria in the United States from 1963 through 1999. New Engl J Med 2001; 344: 1973–1978. [DOI] [PubMed] [Google Scholar]
- 108.US Food and Drug Administration. Revised recommendations to reduce the risk of transfusion-transmitted malaria. Silver Spring, MD: US Food and Drug Administration, 2020. [Google Scholar]