Abstract
The immunoglobulin μ heavy-chain gene enhancer contains closely juxtaposed binding sites for ETS and leucine zipper-containing basic helix-loop-helix (bHLH-zip) proteins. To understand the μ enhancer function, we have investigated transcription activation by the combination of ETS and bHLH-zip proteins. The bHLH-zip protein TFE3, but not USF, cooperated with the ETS domain proteins PU.1 and Ets-1 to activate a tripartite domain of this enhancer. Deletion mutants were used to identify the domains of the proteins involved. Both TFE3 and USF enhanced Ets-1 DNA binding in vitro by relieving the influence of an autoinhibitory domain in Ets-1 by direct protein-protein associations. Several regions of Ets-1 were found to be necessary, whereas the bHLH-zip domain was sufficient for this effect. Our studies define novel interactions between ETS and bHLH-zip proteins that may regulate combinatorial transcription activation by these protein families.
The immunoglobulin (Ig) μ heavy-chain gene enhancer activates B-cell-specific gene transcription and DNA recombination (1, 6). A domain of the enhancer, which contains binding sites for three DNA binding proteins, is sufficient for transcriptional activity in B cells (17). The μA element binds Ets-1 and several other members of the ETS domain gene family; the μB element binds PU.1, a B-cell- and macrophage-specific transcription factor; and the third element, μE3, binds several members of the leucine zipper-containing basic helix-loop-helix (bHLH-zip) family of transcription factors. Of the three elements, μA and μB binding proteins have restricted tissue distributions, whereas μE3 binding proteins are present in all cell types examined.
The B-cell-specific function of the enhancer reflects two forms of combinatorial specificity. First, none of the three DNA binding proteins described above are restricted in expression only to those cell types where the Ig heavy-chain gene is expressed. Based on the expression patterns of the two ETS domain genes involved, we proposed that the cell specificity of the enhancer is determined by the overlap in tissue distribution of the two factors; that is, the enhancer is activated in those cell types where both PU.1 and Ets-1 are simultaneously present (17). Second, the transcriptional activity itself depends upon the appropriate juxtaposition of multiple elements of the enhancer. For example, multimerized versions of individual elements (such as μA, μB, or μE3) do not activate transcription in B cells, even though all the relevant binding proteins are expressed in these cells. Direct evidence of the requirement for appropriate juxtaposition between elements came from studies in which we changed the spacing or relative orientation of the μA, μB, or μE3 sites. Such altered enhancers were largely inactive in B-cell transfection assays (18).
Consistent with the proposed requirement for both μA and μB binding proteins for enhancer activity, coexpression of PU.1 and Ets-1 transactivated the tripartite (μA-μE3-μB) enhancer in nonlymphoid cells (17). In this context the μE3 site of the enhancer was necessary for transcriptional activation (21). Because only ETS domain genes were being transfected along with the reporter plasmid, we inferred that endogenous μE3 binding proteins were recruited to the enhancer in the presence of the transfected ETS protein. These results provided a plausible explanation for the observation that sites such as μE3, which bind ubiquitously expressed proteins, were occupied in vivo only in B cells (8). We suggested that tissue-restricted proteins may increase access of the ubiquitous factors to cell-specific enhancers.
In this paper we further explore transcription activation by the combination of ETS and bHLH-zip proteins. We found that TFE3 (4, 22), but not USF (11), activated the minimal μ enhancer in nonlymphoid cells in combination with either Ets-1 or PU.1. The domains of PU.1 or Ets-1 proteins necessary to synergize with TFE3 were identified and found to correspond closely with those previously shown to be important for PU.1 plus Ets-1 synergy (9). These results suggest that the present transfection studies with TFE3 reflect the situation in which an endogenous μE3 binding protein is used to activate the enhancer in the presence of cotransfected PU.1 plus Ets-1. In vitro experiments showed that TFE3 and USF enhanced Ets-1 DNA binding, and the bHLH-zip domain was sufficient for this purpose. Ets-1 deletion mutants were used in DNA binding and association assays to identify regions of Ets-1 that were important for the interaction with the bHLH-zip domain. The direct association between Ets-1 and TFE3 provides a mechanism by which the effects of an intramolecular inhibitory domain in Ets-1 (12, 15, 19, 26) may be relieved by an adjacent protein and a model for the selective recruitment of bHLH-zip proteins to the μ enhancer in B cells.
MATERIALS AND METHODS
Plasmids.
μ70-dependent reporter plasmids (wild type and μE3−) have been previously described (17). PU.1 and Ets-1 expression vectors were described in the work of Nelsen et al. (17).
Mammalian expression vectors.
Vectors for PU.1 and Ets-1 deletion mutants were described in the work of Erman and Sen (9). pEVRF-TFE3 was constructed by cloning a BamHI-KpnI fragment containing the mTFE3 cDNA from Gex.TFE3 (provided by Kathryn Calame, Columbia University, New York, N.Y.) into pEVRF-0 cut with the same enzymes. pEVRF.TFE3.S contains a portion of the TFE3 cDNA lacking the N-terminal transactivation domain; it was constructed by cloning a BglII (blunt)-XbaI fragment from pEVRF.TFE3 into pEVRF2 cut with SmaI and XbaI.
pEVRF.TFE3Δ contains only the bHLH-zip domain of mTFE3; it was constructed by cloning a BamHI-NcoI (blunt) fragment from pEVRF.TFE3.S into pEVRF3 cut with BamHI and SmaI.
TFE3(Uzip).
The bHLH-zip domain of USF was amplified by PCR with the oligonucleotides 5′-CCTTGGATCCCGAAGTCAGAAGCTCCC-3′ and 5′-CCGCTCATGAGCTCGAAGCAGCAG-3′, and the product was digested with BamHI and BspHI. pEVRF.TFE3 was digested with BamHI and XbaI and ligated with the NcoI-XbaI fragment of TFE3 and the PCR product described above. The resulting plasmid contains the bHLH-zip domain of USF and the 3′ end of TFE3. A BamHI-BglI fragment of TFE3 was cloned into this plasmid at the unique BamHI site. The correct orientation of the BamHI-BglII insert was identified by restriction digestion with BamHI and XbaI. 5′ and 3′ primers flanking the bHLH-zip domain were used to confirm the sequence across the junctions of the hybrid protein.
Bacterial expression plasmids. (i) His.Ets-1Δ167, -Δ231, and -Δ286.
BamHI fragments from pEVRF derivatives (9) were cloned into the BamHI site of pET28.
(ii) HA.Ets-1.
Ets-1 cDNA was excised from pEVRF-Ets-1 by using BamHI and SalI and cloned into pET28HA cut with the same enzymes.
(iii) Hemagglutinin (HA)-tagged Ets-1.
C-terminal deletions were generated by PCR with a 5′ primer, EVR1, and various 3′ primers as indicated below, with pEVRF-Ets-1 as the template. After amplification, the fragments were digested with BamHI and HindIII and cloned into pET28HA cut with the same enzymes. The primers EVR1 (5′-GGGGGATCTTGGTGGCGTG-3′), Ets(1–318) (5′-CCCGGGAAGCTTTCACTTGTCCTTGTTGAGGTC-3′), and Ets(1–227) (5′-AGTTAAAAGCTTTCACTTGATGGCAAAGTAGTC-3′) were used.
(iv) HA.Ets(168–318).
HisEtsΔ167 was used as the template in PCR with a 5′ primer corresponding to the T7 promoter in the vector and the 3′ primer [Ets(1–318)]. The amplified fragment was digested with BamHI and HindIII and cloned into pET28HA cut with the same enzyme.
pET28HA was generated by cloning a double-stranded oligonucleotide encoding the HA epitope tag into the BamHI site of pET28.
Transfections.
COS cell transfections have been previously described (17). NIH 3T3 transfections were done by using the same procedure. Reporter plasmids and transactivators were used in amounts of 2 μg each, and total DNA was kept at a constant amount of 6 μg with pEVRF expression vectors without inserts. Chloramphenicol acetyltransferase (CAT) enzyme assays (see Fig. 1 and 2) were done with [14C]chloramphenicol as a substrate and by using thin-layer chromatography to separate acetylated derivatives. Other CAT assays (see Fig. 3 and 4) were done by enzyme-linked immunosorbent assay (ELISA) (9).
FIG. 1.
Transcriptional synergy between ETS protein and bHLH-zip proteins in COS cells. μ70 enhancer-containing CAT reporter plasmids were transfected into COS nonlymphoid cells together with expression vectors for ETS proteins PU.1 and Ets-1 and bHLH-zip protein TFE3 or USF. The amount of total DNA was held constant by including pEVRF expression vector with no cDNA insert. The first bar shows the basal activity of the reporter in the absence of cotransfected transactivators, and the last bar shows the activity of the reporter with PU.1 plus Ets-1 as a positive control. Most transfections contained the wild-type μ70 reporter plasmid; bars marked μE3− contained the μ70 enhancer mutated at the μE3 site. (A) Effects of PU.1 and bHLH-zip proteins. (B) Effects of Ets-1 and bHLH-zip proteins. The y axis shows CAT enzyme activity assayed by thin-layer chromatography, normalized to levels seen in the absence of cotransfected transactivators. Results shown are obtained from three experiments carried out in duplicate. Error bars indicate standard deviations.
FIG. 2.
Transcriptional synergy between ETS protein and bHLH-zip proteins in NIH 3T3 cells. Cotransfection assays were carried out as described in the legend to Fig. 1 with μ70 enhancer-containing reporter plasmid and expression vectors for PU.1 (A), Ets-1 (B), TFE3, and USF. μ70 activity in the presence of cotransfected PU.1 and Ets-1 serves as the positive control. Results are obtained from three experiments carried out in duplicate. Error bars indicate standard deviations.
FIG. 3.
Domains of ETS proteins required to activate transcription with TFE3. N-terminal deletion mutants of PU.1 (A) or Ets-1 (B) were coexpressed in COS cells with TFE3 to activate transcription from a μ70 enhancer-dependent reporter plasmid. The Δ nomenclature signifies the absence of residues 1 to the indicated number in a particular deletion mutant. The Ets-1 mutant represented as ETS (B) contains only the ETS domain of Ets-1 (9) and is missing both N- and C-terminal residues. All deletion mutants have been previously described and have been shown to be expressed at comparable levels in transient transfection assays (9). CAT enzyme activity expressed from the reporter plasmid was assayed by ELISA and is shown on the y axis, normalized to the expression level in the absence of cotransfected transactivations (first bar). Results are averaged from three sets of transfections carried out in duplicate. Error bars indicate standard deviations.
FIG. 4.
Domains of TFE3 required to activate transcription with Ets-1. μ70 enhancer-containing reporter plasmids were cotransfected with expression vectors for Ets-1, TFE3, or TFE3 derivatives. CAT activity was assayed in whole-cell extracts by ELISA and is shown normalized to the activity of the reporter in the presence of full-length Ets-1 plus TFE3. TFE3S denotes a TFE3 derivative that lacks an N-terminal transactivation domain, and TFE3Δ denotes a protein containing only the bHLH-zip domain of TFE3. Results shown are averaged from two experiments done in duplicate. Error bars indicate standard deviations.
DNA binding assays.
Typical binding reactions used 100 to 200 ng of glutathione S-transferase (GST)-bHLH-zip protein and 400 to 600 ng of His.Ets-1 or its derivatives, together with 100 ng of poly(dI-dC) · (dI-dC), 75 mM NaCl, and 10% glycerol. The order of protein addition did not affect the observed outcomes. Nondenaturing polyacrylamide gel electrophoresis (PAGE) was carried out in 1× Tris-borate-EDTA with 4% gels. DNA probes were isolated as PstI-BamHI fragments from wild-type and mutated enhancers as indicated and end labeled with polynucleotide kinase and [γ-32P]ATP. Gels were visualized by autoradiography after being dried onto 3MM paper.
GST pull-down assays.
Pull-down assay conditions were derived from those described by Giese et al. (10). Briefly, 10 μl of glutathione beads per sample was washed twice with a 10× volume of TTBS (20 mM Tris [pH 7.6], 0.14 M NaCl, 0.1% Tween 20) containing 0.1% bovine serum albumin. GST fusion protein (4 to 8 μg) or equimolar amounts of GST protein were incubated with beads in a 300-μl volume for 1 h at 4°C. Protein-adsorbed beads were collected by centrifugation and resuspended in 300 μl of TTBS containing 0.2% bovine serum albumin and 50 μg of ethidium bromide per ml. Ets-1 derivatives (500 ng of full-length protein and equimolar amounts of deletion mutants) were incubated with the GST protein-bound beads for 2 h at 4°C. Beads were collected by centrifugation (1,000 rpm for 5 min in a microcentrifuge) and washed three times with 1 ml of TTBS. Adsorbed proteins were eluted by boiling the beads in sodium dodecyl sulfate (SDS)-PAGE sample buffer for 3 min and fractionated through SDS-containing 12% polyacrylamide gels. Ets-1 proteins were detected by immunoblotting with anti-Ets-1 antisera (1:500; Santa Cruz Immunochemical) or anti-HA antisera (1:200 dilution of 0.2 mg of stock per ml) (kindly provided by Michael Rosbash, Brandeis University, Waltham, Mass.).
RESULTS
TFE3, not USF, activates transcription in combination with ETS proteins.
A tripartite domain of the μ enhancer, which contains the μA, μE3, and μB elements, can be activated in nonlymphoid cells by coexpressing the ETS domain proteins Ets-1 and PU.1. Mutations in any one of the three elements present in the enhancer abolish transactivation. Because only the μA and μB binding proteins are provided exogenously in this assay, we have proposed that an endogenous μE3 binding protein is recruited to the enhancer for transcriptional activity. To distinguish which μE3 binding protein cooperates with ETS proteins to activate the μ enhancer, we performed additional transfection studies with genes encoding different μE3 binding proteins.
Transfection of bHLH-zip genes with both PU.1 and Ets-1 did not increase transcriptional activity of the μ70 enhancer significantly over that seen with the ETS genes alone (data not shown). We surmise that in the presence of both ETS genes, the levels of endogenous μE3 binding proteins were sufficient for maximum transactivation. However, the transcriptional synergy between ETS and bHLH-zip genes was easily evident when individual ETS genes were used. The expression of PU.1 plus TFE3 in COS cells, but not that of either gene alone, efficiently activated the μ70 enhancer (Fig. 1A, first four bars). In contrast, the bHLH-zip protein USF was a much poorer transcriptional activator than TFE3 (Fig. 1A, bars 7 and 8). Synergistic activation by PU.1 and TFE3 required an intact μE3 element (Fig. 1A, bars 5 and 6), and was comparable to that observed with the coexpression of PU.1 and Ets-1 (Fig. 1A, bar 9). A similar pattern of activation was observed with Ets-1 and the two bHLH-zip proteins in COS cells (Fig. 1B), as well as in a second nonlymphoid cell line (Fig. 2). We inferred that TFE3, but not USF, cooperatively activated this μ enhancer domain together with ETS genes.
Domains of ETS proteins required for transcriptional activity.
If the mechanism of transcription activation in the ETS-TFE3 transfections in nonlymphoid cells reflects the situation in which an endogenous μE3 binding protein is used at the μE3 element, it is likely that the same domains of ETS proteins as those previously identified would be required for the synergy of PU.1 and Ets-1 (9). To strengthen the comparison between ETS-TFE3- and PU.1-Ets-1-mediated activations of the μ70 enhancer, we assayed deletion mutations of the ETS proteins together with TFE3.
Deletion mutants of PU.1 were coexpressed with full-length TFE3 and assayed for the activation of the μ70 reporter plasmid in COS cells (Fig. 3A). The removal of almost two-thirds of the N-terminal residues of PU.1 did not significantly affect its ability to transactivate the μ70 enhancer with TFE3. The last deletion (Δ162) retains only the DNA binding ETS domain of PU.1 and 14 carboxy-terminal amino acids and is an efficient transcription activator. The expression of various PU.1 derivatives in COS cells with these vectors has been previously shown to yield comparable levels of proteins (9). These results demonstrated that very similar domains of PU.1 cooperated with either Ets-1 or TFE3 to activate the μ70 enhancer and suggested that in both transfections we were assaying a common mechanism of transcription activation.
Unlike the observations with PU.1, the DNA binding domain of Ets-1 was not sufficient to cooperate with TFE3 in transcription activation (Fig. 3B, last bar); although the deletion of the first 167 amino acids of Ets-1 did not affect cooperation with TFE3, the removal of an additional 64 amino acids abolished the transactivation potential of this protein. Again the pattern of domain usage was very similar to that previously shown for Ets-1 cooperation with PU.1 (9). We conclude that the transactivation domain of Ets-1 was required to synergistically activate transcription with TFE3, whereas only the DNA binding domain of PU.1 was sufficient for this purpose.
Domains of bHLH-zip proteins required for transcriptional activity.
The transcription activation properties of USF and TFE3 are different. For example, USF does not activate transcription from distal enhancer elements, whereas TFE3 does (2). The results presented above indicated that USF did synergize with Ets-1 (or PU.1) to activate transcription. Because USF and TFE3 bind equally well to the μE3 element and enhance Ets-1 binding to the proximal μA site (see below), we assayed deletion mutants of TFE3 to identify the domain required for transcription synergy with Ets-1.
TFE3 has two activation domains (3), located on either side of the DNA binding domain. A deletion mutant of TFE3 lacking the N-terminal transactivation domain (TFE3S) synergized less well with Ets-1 to activate the μ70 enhancer (Fig. 4, first two bars). A further deletion of the carboxy-terminal transactivation domain (resulting in a protein containing only the bHLH DNA binding domain of TFE3 [TFE3Δ]) did not enhance transcription together with Ets-1 (Fig. 4, bar 3). Electrophoretical mobility shift assay (EMSA) analysis of extracts from transfected cells showed that TFE3Δ was expressed at high levels (data not shown). We conclude that both transactivation domains contribute to synergy with Ets-1.
The lack of transcription activation by USF is likely to be due to inappropriate transactivation domains that cannot synergize with Ets-1, at least in the context of the placement μA and μE3 sites in the μ enhancer. However, it was possible that the DNA binding domain of USF also contributed to the lack of transcription activation. To examine this question we created a hybrid protein containing the bHLH-zip domain of USF and the activation domains of TFE3 [TFE3(Uzip)] (Fig. 5A). The hybrid protein was expressed in transient transfection assays, alone or together with Ets-1 as shown by EMSA (Fig. 5B). TFE3(Uzip) synergized efficiently with cotransfected Ets-1 to activate the μ70 enhancer (Fig. 5C, bar 8). The level of reporter activity with the hybrid protein was comparable to that seen with TFE3 (Fig. 5B, bar 4); as expected, USF did not synergize with Ets-1 (Fig. 5C, bar 6). Furthermore, TFE3(Uzip)-dependent transcription required an intact μE3 sequence (Fig. 5C, bar 10). We conclude that the DNA binding domain of TFE3 is not essential to observe transcriptional synergy.
FIG. 5.
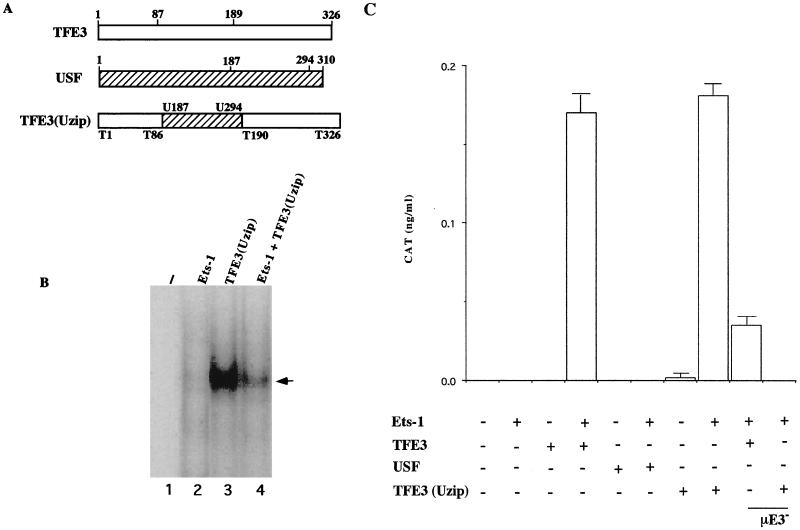
Analysis of a TFE3-USF fusion protein. (A) In TFE3(Uzip) the bHLH-zip domain of TFE3 was replaced by the corresponding domain of USF, as detailed in Materials and Methods. The first schematics show the structures of murine TFE3 and USF. The bHLH-zip domain of TFE3 is located between residues 87 and 189 and that of USF is located at the C terminus of the protein between residues 187 and 294. TFE3(Uzip) contains the first 86 amino acids of TFE3 (T1 to T86), followed by residues 187 to 294 from USF (U187 to U294) and residues 190 to 326 from TFE3 (T190 to T326). The structure of the hybrid protein was confirmed by DNA sequencing. (B) For functional studies TFE3(Uzip) was expressed in COS cells and expression levels were assayed by EMSA. The probe (lane 1) was a PstI-BamHI fragment of the μ enhancer-containing μA, μE3, and μB sequences. Extracts used were obtained from COS cells transfected with expression vectors for the proteins indicated. The arrow marks the position of a complex obtained only in TFE3(Uzip)-expressing cells. (C) COS cells were transfected with the μ70 reporter plasmids and expression vectors for various proteins. The amount of total DNA was kept constant by the inclusion of empty expression vectors. The wild-type μ70 reporter plasmid was used, except for the last two bars for which the reporter contained a μE3-mutated μ70 fragment. CAT enzyme levels (in nanograms per milliliter) are indicated on the y axis. The results shown were obtained by averaging two sets of experiments carried out in duplicate. Error bars indicate standard deviations.
bHLH-zip proteins enhance Ets-1 DNA binding.
Based on DNase I footprint and EMSA analysis, we have recently shown that TFE3 enhances Ets-1 DNA binding (7). Here we further explored the interaction between ETS and bHLH-zip proteins. Bacterially expressed TFE3 generated a broad nucleoprotein complex (Fig. 6A, lane 1), whereas Ets-1 did not yield a detectable complex because its DNA binding is weakened by two inhibitory domains that flank the DNA binding ETS domain (12, 15, 19, 26). We confirmed that the full-length Ets-1 used here, as well as deletion mutants of Ets-1 used below, was capable of DNA binding when assayed with a high-affinity binding site (Fig. 6E). As expected, Ets-1 and Ets-1-Δ167, which contain the N-terminal inhibitory domain, bound weakly compared to mutants in which this domain was missing (Fig. 6E, compare lanes 2 and 3 to lanes 4 and 5). As shown earlier (7), the coincubation of Ets-1 and TFE3 resulted in a nucleoprotein complex that migrated more slowly than the complex seen with TFE3 alone (Fig. 6A). The slower-migrating complex was not formed on a probe containing a mutated μA (Ets-1 binding) site (Fig. 6A, lanes 4 to 6), and no distinct complexes were observed on a probe mutated at the μE3 site (Fig. 6A, lanes 7 to 9). Formation of these DNA-protein complexes did not depend on the μB site which binds PU.1 (data not shown). Thus, both proteins (Ets-1 and TFE3) and both sites (μA and μE3) were required to generate the lower-mobility complex. Based on these observations we conclude that this complex contains Ets-1 and TFE3 bound simultaneously to DNA. Thus, TFE3 enhanced Ets-1 binding to DNA. When PU.1 and TFE3 were used in such assays, both proteins bound to DNA individually and together, with no indication of cooperative binding (data not shown).
FIG. 6.
bHLH-zip proteins enhance Ets-1 DNA binding. Ets-1 protein expressed in bacteria was used in EMSA in the presence of full-length TFE3 (A), full-length USF (B), a deletion mutant of TFE3, TFE3S (C), and a TFE3 derivative containing only the bHLH-zip domain (D). Binding reactions utilized three probes: a wild-type μ enhancer fragment containing the μA, μE3, and μB elements (WT) and the same fragment with either a mutated μA element (μA−) or a mutated μE3 element (μE3−). Each probe was used in sets of three binding assays that contained either protein alone or both proteins together. Full-length Ets-1 alone did not yield a detectable nucleoprotein complex under these conditions, whereas all bHLH-zip derivatives bound DNA well (lanes 1 in panels A to D). Arrows indicate the positions of supershifted complexes generated when both proteins were coincubated with the WT probe (lanes 3); μA and μE3− probes did not yield these supershifted complexes (lanes 6 and 9). bHLH-zip proteins were expressed as GST fusion proteins and purified from bacterial extracts by adsorption to glutathione-agarose resins. Ets-1 was expressed as a six-histidine-tagged protein and purified by adsorption to nickel agarose resin. (E) Histidine-tagged Ets-1 derivatives were assayed for DNA binding by using a high-affinity Ets-1 binding site. The Δ nomenclature indicates the residues that were deleted from the N terminus in the derivatives. Equal amounts of proteins purified by adsorption to nickel chelate columns were used.
To check whether other bHLH-zip proteins could also enhance Ets-1 DNA binding, we used GST-USF in similar assays. GST-USF alone yielded two distinct nucleoprotein complexes (Fig. 6B, lane 1); because protein is limiting under the conditions of our assay, it is likely that the faster complex represents a truncated version of USF. The coincubation of Ets-1 and USF led to a shift of both complexes seen with USF alone (Fig. 6B, lane 3). As seen with TFE3, the supershift of the USF complexes required an intact Ets-1 binding site (Fig. 6B, lanes 4 to 6), and no complexes were observed on a μE3 mutated probe (Fig. 6B, lanes 7 to 9). We conclude that a second μE3 binding bHLH-zip protein also enhanced Ets-1 DNA binding. However, increased Ets-1 binding is not sufficient for transcription activation, because USF and Ets-1 do not synergize in the cotransfection assays. This indicates that an appropriate transactivation domain on the bHLH-zip protein is required.
The region of greatest similarity between TFE3 and USF is in the DNA binding bHLH-zip domain; this is reflected in the indistinguishable DNA binding specificities of the two proteins (5). Other parts of TFE3 and USF are considerably less similar, which suggested that the common property of enhancing Ets-1 binding may be determined by the bHLH-zip domain. However, it was also possible that structural features of TFE3 and USF that were not immediately obvious from the primary sequence contributed to its effects on Ets-1. To distinguish between these possibilities, we used deletion mutants of TFE3 together with full-length Ets-1 in DNA cobinding assays in vitro. TFE3S, a truncated version of TFE3 lacking the N-terminal transactivation domain, formed a distinct nucleoprotein complex on a μ enhancer probe (Fig. 6C, lane 1). The coincubation of TFE3S and full-length Ets-1 generated a distinguishable complex (Fig. 6C, lane 3), compared to Ets-1 alone (Fig. 6C, lane 2). As before, the supershifted complex did not form on a μA− probe (Fig. 6C, lanes 4 to 6), and no discrete complexes were formed on a μE3− probe (Fig. 6C, lanes 7 to 9). These observations suggested that the N-terminal region of TFE3 plays a minor, if any, role in the stabilization of Ets-1 DNA binding.
The bHLH-zip domain of TFE3 (TFE3Δ) was expressed in bacteria as a GST fusion protein and used for further EMSA studies. GST-ΔTFE3 also enhanced Ets-1 DNA binding to a wild-type μ enhancer probe (Fig. 6D, lanes 1 to 3) but not to a probe mutated at the μA site (Fig. 6D, lanes 4 to 6). These results indicated that the bHLH-zip domain of TFE3 was sufficient to enhance DNA binding by Ets-1 and that other parts of TFE3 (and by extrapolation, USF) played a lesser role.
Two regions of Ets-1 interact with TFE3.
We next wished to identify the domains of Ets-1 that interacted with TFE3 or USF. Two N-terminal deletions of Ets-1 were used in DNA cobinding assays with TFE3 or USF. Both Ets-1Δ167 and Ets-1Δ231 did not bind well to DNA by themselves (Fig. 7A, lanes 2 and 5). However, both proteins yielded supershifted complexes when coincubated with TFE3 proteins (Fig. 7A, lanes 3 and 6), compared to the complex generated by TFE3 alone (Fig. 7A, lanes 1 and 4). Similar results were seen with TFE3Δ, a deletion mutant containing only the bHLH-zip domain of TFE3 (Fig. 7B). Studies using GST-USF in place of TFE3 gave identical results to those in Fig. 7A (data not shown). The ETS domain of Ets-1 was also used in similar assays. However, because this Ets-1 derivative binds DNA well, there was no obvious cooperative binding with TFE3 or its derivatives, although occupancy of both μA and μE3 sites was readily observed (data not shown). These observations suggested that the inhibition of Ets-1 DNA binding by intramolecular interactions was diminished in the presence of the bHLH-zip domains of TFE3-USF, thereby resulting in enhanced Ets-1 binding to the μA site.
FIG. 7.
DNA binding by Ets-1 deletion mutants with bHLH-zip proteins. Two deletion mutants of Ets-1, Δ167 and Δ231, were assayed for DNA binding on a wild-type μ enhancer probe together with full-length TFE3 (A) or the bHLH-zip domain of TFE3 (TFE3Δ) (B). Both Ets-1 deletions retain the domains that inhibit Ets-1 DNA binding and therefore bind poorly to DNA in the absence of additional proteins (panel A, lanes 2 and 5; panel B, lanes 2 and 4). Arrows on the left indicate the positions of a supershifted complex formed with Ets-1(Δ167), and those on the right indicate the position of a supershifted complex formed with Ets-1(Δ231). The triangles mark the position of the TFE3-DNA or TFE3Δ-DNA complexes in panels A and B, respectively.
The EMSA experiments above, however, did not reveal which parts of Ets-1 interacted with bHLH-zip proteins. We therefore examined the interaction of Ets-1 with TFE3 or USF in solution. Equal amounts of N-terminal Ets-1 truncations (Fig. 8E) were incubated with glutathione-Sepharose beads containing GST protein or GST-TFE3. Ets-1 derivatives retained on the beads were visualized by immunoblotting after the separation of proteins by SDS-PAGE. The greater retention of Ets-1 derivatives on GST-TFE3 than on GST alone was interpreted as indicating a specific interaction between Ets-1 and TFE3. GST-TFE3 retained full-length Ets-1 (labeled 1–440) and the first two deletion mutants but not the smallest Ets-1 derivative (Δ286) which bound to the GST-containing column as well (Fig. 8A). To examine C-terminal deletion mutants we tagged Ets-1 with a 15-amino-acid epitope from the influenza virus HA. Tagged full-length Ets-1 and two C-terminal deletions containing 318 and 227 amino acids of Ets-1 bound specifically to GST-TFE3 beads (Fig. 8B, lanes 1 to 6). However, an internal fragment of Ets-1, encompassing residues 168 to 318, bound to both GST and GST-TFE3 beads (Fig. 8B, lanes 7 and 8), indicating that this polypeptide did not interact specifically with TFE3. Because USF protein enhanced DNA binding by Ets-1 as efficiently as TFE3, we tested whether USF also interacted directly with Ets-1 in solution. The use of Ets-1 derivatives described above with GST-USF retained on glutathione-Sepharose showed the same binding pattern as that seen with GST-TFE3 (Fig. 8C and D). Again, we noted that Ets-1(Δ286) bound to both GST and GST-USF, which indicated nonspecific interactions. These observations were consistent with the results of DNA cobinding assays and indicated that both TFE3 and USF proteins interacted directly with Ets-1. It is likely that this interaction, at least in part, results in enhanced DNA binding by Ets-1 in the presence of these bHLH-zip proteins.
FIG. 8.
In vitro association of Ets-1 and bHLH-zip proteins. Glutathione-Sepharose beads containing GST protein, GST-TFE3 (A and B), or GST-USF (C and D) were incubated with full-length Ets-1(1–440) or Ets-1 deletion mutants. After extensive washing, proteins retained on the beads were fractionated by SDS-PAGE, and Ets-1 derivatives were visualized by immunoblotting with anti-Ets-1 antiserum (A and C) or an anti-HA epitope monoclonal antibody (B and D). N-terminal deletion mutants of Ets-1 are shown in panels A and C (Δ167, Δ231, or Δ286). C-terminal truncations were tagged with an HA peptide tag at the N terminus and are numbered to indicate the amino acid residues that are retained in the deletion mutants. ∗1–440 is a full-length Ets-1 tagged with the HA epitope to serve as the positive control for the C-terminal deletion mutants. The specific interaction between Ets-1 derivatives and bHLH-zip proteins was judged by increased retention on GST-TFE3- or GST-USF-containing beads compared to GST alone. The approximately equal input of Ets-1 derivatives for the association assays was confirmed by Western blot analysis with 40% of the material incubated with GST or GST-bHLH-zip (Input). Association assays were analyzed on gels separate from the input quantitation and do not represent the proportion of input protein bound. The inputs for gels in panels B and D are shown above panel B. (E) Summary of in vitro association assays. Full-length Ets-1 is shown on line 1, with the known domains indicated by different shadings. N-terminal truncations are on lines 2 to 4. Full-length Ets-1 containing the HA epitope at the N terminus (line 5) and C-terminal truncations are shown (lines 6 to 8). Binding of these Ets-1 derivatives to full-length TFE3 or USF is summarized at the right with plus and minus signs.
Two conclusions may be drawn from the results of the in vitro association assays with Ets-1 deletions (Fig. 8E). First, the comparison of Fig. 8E, lines 3 and 4, shows that deleting most of the previously characterized N-terminal inhibitory domain reduced interaction with TFE3. However, this deleted region was not sufficient for the interaction with TFE3 (Fig. 8E, line 8). The comparison of Fig. 8E, lines 2 and 8, showed that the ETS domain of Ets-1 contributed to TFE3 binding, though it was clearly not sufficient (line 4). The simplest interpretation of these observations is that TFE3 and USF make weak contacts with both the N-terminal inhibitory domain and the ETS domain, which can only be detected in our assay when both domains are present together. We note, however, that we cannot rule out the possibility that TFE3-USF makes contact with a conformational determinant created only when both domains are together. Second, the comparison of Fig. 8E, lines 7 and 8, suggested that an N-terminal domain of Ets-1 can also bind to TFE3-USF. The information shown in Fig. 8E, line 8, suggested that a part of the transactivation domain plus the N-terminal inhibitory domain was not sufficient for this interaction. Overall, we conclude that TFE3 and USF make two distinct contacts with Ets-1; one of these involves the N-terminal inhibitory domain plus the ETS domain and may function to reduce the inhibition of DNA binding by Ets-1, and the second involves the transcription activation domain of Ets-1, which may promote transcriptional synergy (Fig. 3B) between Ets-1 and an appropriate bHLH-zip protein.
As described above, DNA binding assays suggested that cooperative DNA binding required only the bHLH-zip domain of TFE3. We therefore checked whether this domain was also sufficient for a solution association between the two proteins. Carboxy-terminal deletions of Ets-1 were used in pull-down assays with GST-ΔTFE3, a fusion protein containing only the bHLH-zip domain of TFE3. Full-length Ets-1, as well as both C-terminal truncations, associated with GST-ΔTFE3 (Fig. 9, lanes 1 to 6), whereas Ets-1(168–318) interacted with GST as well as GST-ΔTFE3 (Fig. 9, lanes 7 and 8). The bHLH-zip domain was therefore sufficient to mediate interactions with Ets-1.
FIG. 9.
bHLH-zip domain of TFE3 associates with Ets-1 derivatives in vitro. GST pull-down assays were carried out as described in the legend to Fig. 6 with Ets-1 C-terminal deletions and either GST (negative control; odd-numbered lanes) or GST-TFE3Δ, a fusion protein containing only the bHLH-zip domain of TFE3. After elution from beads, Ets-1 derivatives were visualized by immunoblotting with an antibody against the HA epitope. Ets-1 derivatives used for association assays were analyzed by Western blotting to confirm equal protein input (Input). Numbers to the left indicate molecular size markers.
DISCUSSION
Several analyses of the μE3 element of the Ig μ enhancer have provided considerable insights into the properties of bHLH-zip proteins such as TFE3 and USF (13, 16). However, most studies to date have examined the function of the isolated μE3 motif, although there is evidence that this element is not an efficient transcriptional activator by itself. Most importantly, multimerized μE3 elements do not reconstitute enhancer activity in either lymphoid or nonlymphoid cells, despite the ubiquitous expression of several μE3 binding proteins (4). Usually μE3 sites are combined with other sequence elements to produce regulatory sequences, as in the case of the μ enhancer in which the μE3 element functions together with ETS protein binding sites μA and μB. To approximate the combinatorial use of different factors, we examined the transcriptional synergy between bHLH-zip and ETS proteins. We show that TFE3, but not USF, activates the μ70 enhancer together with ETS proteins, though both proteins enhance Ets-1 DNA binding. We also investigated the mechanism of enhancement of Ets-1 DNA binding.
The close correlation between site requirement for μ70 activity in B cells and in nonlymphoid cells transfected with PU.1 and Ets-1 suggests that the transfection assay mimics important aspects of B-cell enhancer activity. Transactivation by PU.1 and Ets-1 requires the intervening μE3 element, indicating that endogenous μE3 binding proteins are recruited to the enhancer in the presence of two coexpressed ETS genes. Because either ETS gene alone does not activate the μ70 enhancer, it is likely that endogenous μE3 binding proteins cannot be brought to the enhancer with only one ETS protein. However, when TFE3 was coexpressed together with a single ETS gene, the enhancer was efficiently activated through two sites only. That is, raising the level of TFE3 in a cell reduced the requirement for a second ETS gene. Overexpression of TFE3 results in significant activation of μ enhancer fragments by this protein alone (4), indicating that the requirement for combinations of factors is further reduced under these conditions. Taken together, these observations indicate that TFE3 utilization at multicomponent regulatory sequences is enhanced by ETS proteins and provide evidence for the idea that combining regulatory elements allows the efficient use of transcription factors whose access to DNA is otherwise limited. The restricted use of TFE3 in the absence of other factors may be the reason that the μE3 site in the μ enhancer is occupied in vivo in B cells only (8). In these cells, interactions of μE3 binding proteins with ETS proteins may enable the formation of a stable complex that can be detected by in vivo footprinting.
Recently Sieweke et al. (23) showed that Ets-1 and USF synergistically activate a fragment of the human immunodeficiency virus type 1 distal enhancer that contains one E box and an ETS binding site. Though TFE3 and USF were not compared in that study, the results demonstrate that there are circumstances in which Ets-1 and USF can cooperatively activate transcription. The reason for the lack of transactivation of the μ enhancer by this combination is not yet clear; however, it could be a consequence of the differences in the sequences of the elements of the human immunodeficiency virus and μ enhancers or the difference in the distances between the elements in the two regulatory sequences.
In further exploring the basis for Ets-1-TFE3 synergy in vitro with EMSA and GST pull-down assays, we found that several regions of Ets-1 were important for the interaction with the bHLH-zip domain of TFE3. Although it was possible that cooperative transcriptional activation between the two factors was a manifestation of the cooperative binding, the interaction domain identified in this study suggested two categories of interactions—those that were pertinent to transcription enhancement and those that were pertinent to the enhancement of DNA binding. Each will be discussed independently. The carboxy-terminal deletion of Ets-1, Ets-1(*1–227), contains most of a previously identified transcription activation domain (and additional N-terminal residues); this domain interacted with TFE3 in vitro (Fig. 6), and the deletion of this domain abolished transcriptional synergy (Fig. 3), suggesting that this interaction may participate in transcriptional activation, for example, by appropriately juxtaposing transcription activation domains in TFE3 and Ets-1. However, a deletion mutant of Ets-1 that lacked this N-terminal interaction domain, Ets-1(Δ231), also associated directly with TFE3 and showed enhanced DNA binding in the presence of TFE3. Because this deletion mutant did not activate transcription, we conclude that enhancement of DNA binding can be separated from cooperative transcriptional activation by these two factors. This is also evident from the observation that USF enhanced Ets-1 DNA binding but was not an efficient transactivator in combination with Ets-1. We suggest that in the intact Ets-1 protein, enhanced DNA binding is mediated by TFE3 interacting with the carboxy-terminal portion of Ets-1 (containing the inhibitory domains and the DNA binding ETS domain) and transcriptional synergy is facilitated by interactions with its N-terminal domains.
The presence of two domains that inhibit DNA binding by Ets-1 makes it important to characterize the mechanisms by which this inhibition may be relieved, because efficient DNA binding is presumably a prerequisite for transcriptional activation. Only the Runt domain-containing transcription factor CBFα/PEBP2α (14, 25) has been previously shown to enhance Ets-1 DNA binding (10, 24, 27). This interaction was shown to be of importance in the regulation of T-cell receptor α and β gene enhancers. While there are some similarities, there are also significant differences in the nature of interactions between Ets-1 and either TFE3 (shown in this study) or core-binding factor (CBF) (10, 24, 27). Firstly, both TFE3 and CBF interact with Ets-1 via their respective DNA binding domains—the bHLH-zip domain of TFE3 as shown in this study and the Runt domain of CBF (10). Secondly, both TFE3 and CBF interact directly with N-terminal parts of Ets-1. However, in contrast with CBF, TFE3 also interacts with the C-terminal 210 amino acids of Ets-1 as assayed by both DNA binding and GST pull-down assays, whereas the Runt domain of CBF does not interact with an Ets-1 derivative similar to our Ets-1(Δ167). These observations suggest that the inhibition of Ets-1 DNA binding may be relieved in different ways by different factors.
The comparison of the EMSA and association data with the derivatives Ets-1(Δ231), Ets-1(Δ286), and Ets-1(*168–318) suggests a plausible mechanism for the enhancement of Ets-1 DNA binding by TFE3 (Fig. 10). Peterson et al. have proposed that an α-helical segment of the Ets-1 N-terminal inhibitory domain interacts with the DNA binding ETS domain to inhibit DNA binding (20). A protein such as TFE3 that enhances Ets-1 binding could in principle relieve inhibition by (i) interacting with the inhibitory domain so as to move it away from the DNA binding ETS domain, (ii) interacting with the ETS domain so that the inhibitory domain-ETS domain interaction is disrupted, or (iii) a combination of both of these effects. In the GST pull-down assays, we found that neither the N-terminal inhibitory domain alone [Ets-1(*168–318)] nor a fragment containing the ETS domain plus the C-terminal inhibitory domain alone [Ets-1(Δ286)] could interact with TFE3; however, Ets-1(Δ231) which contained both these portions was sufficient for this purpose. The simplest interpretation of this observation is that TFE3 simultaneously touches both the N-terminal inhibitory domain and the C-terminal part of Ets-1 to enhance DNA binding (see above). We note that Sieweke et al. (23) found that the ETS domain of Ets-1 was sufficient to interact with the bHLH-zip domain of USF. In our studies as well, we detected a weak interaction of USF with a C-terminal fragment of Ets-1 (Fig. 8E). However, this was not detected with TFE3, suggesting that additional interactions were required to stabilize Ets-1-bHLH-zip protein association. The observation that an N-terminal domain of Ets-1 [Ets-1(*1–227)] associates with both TFE3 and USF supports the idea of more than one interacting domain being involved. We propose that TFE3 protein wedges between the inhibitory and DNA binding domains to disrupt the autoinhibitory interactions (Fig. 10). However, we recognize that a model in which TFE3 binds a conformation of Ets-1 that is dependent on both the inhibitory and ETS domains cannot be ruled out at present. Overall, our studies define novel interactions between ETS proteins and bHLH-zip proteins that may be important for the combinatorial transcription activation by these families of proteins.
FIG. 10.
Schematic summary of the enhancement of Ets-1 DNA binding by TFE3. Inhibition of Ets-1 DNA binding by a helix (Inh.) as proposed by Peterson et al. (20) is shown in the center. Model 1 indicates a pathway in which the interaction of a second protein (shaded box) with the inhibitory domain allows the DNA binding domain to be revealed. In model 2, the second protein interacts with the DNA binding domain, resulting in the displacement of the inhibitory helix. However, GST pull-down assays show that TFE3-Ets-1 interaction requires both the inhibitory and DNA binding domains, suggesting that TFE3 makes contact with both domains to accentuate Ets-1 binding, as shown in model 3.
ACKNOWLEDGMENTS
Expression vectors for bHLH-zip proteins were kindly provided by Kathryn Calame (Columbia University, New York, N.Y.). We thank B. Nikolajczyk and W. Dang for comments on the manuscript and Elaine Ames for its preparation.
This work was supported by an NIH grant (GM 38925) to R.S.
REFERENCES
- 1.Alessandrini A, Desiderio S V. Coordination of immunoglobulin DJH transcription and D-to-JH rearrangement by promoter-enhancer approximation. Mol Cell Biol. 1991;11:2096–2107. doi: 10.1128/mcb.11.4.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artandi S E, Cooper C, Shrivastava A, Calame K. The basic helix-loop-helix-zipper domain of TFE3 mediates enhancer-promoter interaction. Mol Cell Biol. 1994;14:7704–7716. doi: 10.1128/mcb.14.12.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artandi S E, Merrell K, Avitahl N, Wong K, Calame K. TFE3 contains two activation domains, one acidic and the other proline-rich, that synergistically activate transcription. Nucleic Acids Res. 1995;23:3865–3871. doi: 10.1093/nar/23.19.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckmann H, Su L-K, Kadesch T. TFE3: A helix-loop-helix protein that activates transcription through the immunoglobulin enhancer μE3 motif. Genes Dev. 1990;4:167–179. doi: 10.1101/gad.4.2.167. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell T K, Huang J, Ma A, Kretzner L, Alt F W, Eisenman R N, Weintraub H. Binding of Myc proteins to canonical and noncanonical DNA sequences. Mol Cell Biol. 1993;13:5216–5224. doi: 10.1128/mcb.13.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackwell T K, Moore M W, Yancopoulos G D, Suh H, Lutzker S, Selsing E, Alt F W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986;324:585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- 7.Dang W, Sun X-H, Sen R. ETS-mediated cooperation between basic helix-loop-helix motifs of the immunoglobulin μ heavy-chain gene enhancer. Mol Cell Biol. 1998;18:1477–1488. doi: 10.1128/mcb.18.3.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ephrussi A, Church G M, Tonegawa S, Gilbert W. B lineage-specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985;227:134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- 9.Erman B, Sen R. Context dependent transactivation domains activate the immunoglobulin μ heavy chain gene enhancer. EMBO J. 1996;15:4665–4675. [PMC free article] [PubMed] [Google Scholar]
- 10.Giese K, Kingsley C, Kirshner J R, Grosschedl R. Assembly and function of a TCRα enhancer complex is dependent on LEF-1 induced DNA bending and multiple protein-protein interactions. Genes Dev. 1995;9:995–1008. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]
- 11.Gregor P D, Sawadogo M, Roeder R G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990;4:1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- 12.Hagman J, Grosschedl R. An inhibitory carboxyl-terminal domain in Ets-1 and Ets-2 mediates differential binding of ETS family factors to promoter sequences of the mb-1 gene. Proc Natl Acad Sci USA. 1992;89:8889–8893. doi: 10.1073/pnas.89.19.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadesch T. Helix-loop-helix proteins in the regulation of immunoglobulin gene transcription. Immunol Today. 1992;13:31–36. doi: 10.1016/0167-5699(92)90201-h. [DOI] [PubMed] [Google Scholar]
- 14.Kamachi Y, Ogawa E, Asano M, Ishida S, Murakami Y, Satake M, Ito Y, Shigesada K. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol. 1990;64:4808–4819. doi: 10.1128/jvi.64.10.4808-4819.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim F, Kraut N, Frampton J, Graf T. DNA binding by c-Ets-1, but not v-Ets, is repressed by an intramolecular mechanism. EMBO J. 1992;11:643–652. doi: 10.1002/j.1460-2075.1992.tb05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merrell K, Wells S, Henderson A, Gorman J, Alt F, Stall A, Calame K. The absence of the transcription activator TFE3 impairs activation of B cells in vivo. Mol Cell Biol. 1997;17:3335–3344. doi: 10.1128/mcb.17.6.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelsen B, Tian G, Erman B, Gregoire J, Maki R, Graves B, Sen R. Regulation of lymphoid-specific immunoglobulin μ heavy chain gene enhancer by ETS-domain proteins. Science. 1993;261:82–86. doi: 10.1126/science.8316859. [DOI] [PubMed] [Google Scholar]
- 18.Nikolajczyk B, Nelsen B, Sen R. Precise alignment of sites required for μ enhancer activation in B cells. Mol Cell Biol. 1996;16:4544–4554. doi: 10.1128/mcb.16.8.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nye J A, Petersen J, Gunther C V, Jonsen M D, Graves B J. Interaction of murine Ets-1 with GGA-binding sites establishes the ETS domain as a new DNA-binding motif. Genes Dev. 1992;6:975–990. doi: 10.1101/gad.6.6.975. [DOI] [PubMed] [Google Scholar]
- 20.Peterson J M, Skalicky J J, Donaldson L W, Mcintosh L P, Alber T, Graves B. Modulation of transcription factor Ets-1 DNA binding: DNA-induced unfolding of an α-helix. Science. 1995;269:1866–1869. doi: 10.1126/science.7569926. [DOI] [PubMed] [Google Scholar]
- 21.Rao E, Dang W, Tian G, Sen R. A three protein-DNA complex on a B cell-specific domain of the immunoglobulin μ heavy chain gene enhancer. J Biol Chem. 1997;272:6722–6732. doi: 10.1074/jbc.272.10.6722. [DOI] [PubMed] [Google Scholar]
- 22.Roman C, Matera A G, Cooper C, Artandi S, Blain S, Ward D C, Calame K. mTFE3, an X-linked transcriptional activator containing basic helix-loop-helix and zipper domains, utilizes the zipper to stabilize both DNA binding and multimerization. Mol Cell Biol. 1992;12:817–827. doi: 10.1128/mcb.12.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sieweke M H, Tekotte H, Jarosch U, Graf T. Cooperative interaction of Ets-1 with USF-1 required for HIV-1 enhancer activity in T cells. EMBO J. 1998;17:1728–1739. doi: 10.1093/emboj/17.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun W, Graves B J, Speck N A. Transactivation of the Moloney murine leukemia virus and T-cell receptor β-chain enhancers by cbf and ets requires intact binding sites for both proteins. J Virol. 1995;69:4941–4949. doi: 10.1128/jvi.69.8.4941-4949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Wang Q, Crute B E, Melnikova I N, Keller S R, Speck N A. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol. 1993;13:3324–3339. doi: 10.1128/mcb.13.6.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasylyk C, Kerckaert J-P, Wasylyk B. A novel modulator domain of Ets transcription factors. Genes Dev. 1992;6:965–974. doi: 10.1101/gad.6.6.965. [DOI] [PubMed] [Google Scholar]
- 27.Wotoon D, Ghysdael J, Wang S, Speck N A, Owen M J. Cooperative binding of Ets-1 and core binding factor to DNA. Mol Cell Biol. 1994;14:840–850. doi: 10.1128/mcb.14.1.840. [DOI] [PMC free article] [PubMed] [Google Scholar]