Abstract
Fisp12 was first identified as a secreted protein encoded by a growth factor-inducible immediate-early gene in mouse fibroblasts, whereas its human ortholog, CTGF (connective tissue growth factor), was identified as a mitogenic activity in conditioned media of human umbilical vein endothelial cells. Fisp12/CTGF is a member of a family of secreted proteins that includes CYR61, Nov, Elm-1, Cop-1/WISP-2, and WISP-3. Fisp12/CTGF has been shown to promote cell adhesion and mitogenesis in both fibroblasts and endothelial cells and to stimulate cell migration in fibroblasts. These findings, together with the localization of Fisp12/CTGF in angiogenic tissues, as well as in atherosclerotic plaques, suggest a possible role for Fisp12/CTGF in the regulation of vessel growth during development, wound healing, and vascular disease. In this study, we show that purified Fisp12 (mCTGF) protein promotes the adhesion of microvascular endothelial cells through the integrin receptor αvβ3. Furthermore, Fisp12 stimulates the migration of microvascular endothelial cells in culture, also through an integrin-αvβ3-dependent mechanism. In addition, the presence of Fisp12 promotes endothelial cell survival when cells are plated on laminin and deprived of growth factors, a condition that otherwise induces apoptosis. In vivo, Fisp12 induces neovascularization in rat corneal micropocket implants. These results demonstrate that Fisp12 is a novel angiogenic inducer and suggest a direct role for Fisp12 in the adhesion, migration, and survival of endothelial cells during blood vessel growth. Taken together with the recent finding that the related protein CYR61 also induces angiogenesis, we suggest that Fisp12/mCTGF and CYR61 comprise prototypes of a new family of angiogenic regulators that function, at least in part, through integrin-αvβ3-dependent pathways.
Vessel formation and the consequent blood supply are fundamental to a wide spectrum of physiological and pathological processes. During embryonic development, blood vessels form by two distinct processes: vasculogenesis, in which vessels develop from progenitor stem cells that differentiate into endothelial cells, and angiogenesis, whereby new capillaries sprout from existing vessels (14, 45). In the adult, vessel formation is primarily restricted to angiogenesis during wound healing and in the female reproductive cycle (ovulation, implantation, pregnancy, and lactation). Abnormal angiogenesis underlies a variety of diseases, such as diabetic retinopathy, arthritis, psoriasis, atherosclerosis, and cancer (13). A complex process, angiogenesis requires first the degradation of the basal membrane surrounding the parental vasculature, migration of endothelial cells toward the angiogenic source, proliferation of these normally quiescent endothelial cells to form the new vessels and, finally, the alignment and organization of the growing endothelial cells to form circulating tubes that bring the blood supply to the target tissue (9, 14, 19, 45). While how these various steps are coordinated in vivo is not clearly understood, recent studies have led to the appreciation that angiogenesis is regulated by a delicate balance between multiple angiogenic inducers and inhibitors (5, 19).
Fisp12 (fibroblast-inducible secreted protein) was originally identified in mouse fibroblasts as a 38-kDa secreted protein encoded by a growth factor-inducible immediate-early gene (48). Its human ortholog is CTGF (connective-tissue growth factor), identified as a mitogenic activity in conditioned media of human umbilical vein endothelial cells (6). Both the mouse fisp12 and the human CTGF are immediate-early genes inducible by serum growth factors and encode secreted, cysteine-rich heparin-binding proteins (6, 8, 48). Upon secretion, Fisp12 exists in equilibrium between a soluble form in the culture medium and an extracellular matrix-associated form (31). Purified Fisp12 protein promotes cell adhesion and enhances growth-factor-induced mitogenesis in both fibroblasts and endothelial cells (31), whereas hCTGF stimulates DNA synthesis in fibroblasts and enhances the expression of type I collagen, fibronectin, and integrin α5 subunit (15). Fisp12/mCTGF level is induced in the granulation tissues during dermal wound healing (24, 33), and CTGF has been localized in a number of fibrotic disorders (22, 23, 25). These findings, together with the strong induction of CTGF by transforming growth factor (TGF)-β, has led to the proposal that CTGF works as a downstream mediator of TGF-β in tissue repair (17, 18).
Another growth-factor-inducible immediate-early gene, CYR61, encodes a protein that is structurally related to Fisp12/CTGF with ∼50% amino acid sequence homology and is coinduced with fisp12 by serum growth factors (38, 48). A remarkable diversity of activities has been detected for CYR61: (i) it promotes cell adhesion, migration, and proliferation of both endothelial cells and fibroblasts (1, 31, 32); (ii) it stimulates chondrogenic differentiation of limb bud mesenchyme (59); and (iii) it induces angiogenesis and promotes tumor growth in vivo (1). These diverse activities were put in a mechanistic context in a recent study in which CYR61 was shown to be a ligand of the integrin αvβ3 (30). Integrins are heterodimeric cell surface receptors capable of mediating a diverse array of cellular processes, including cell adhesion, migration, proliferation, differentiation, and survival (49, 51). Moreover, integrin αvβ3 is known to be involved in angiogenesis, tumor progression, and metastasis (56, 57). Thus, the interaction of CYR61 with integrin αvβ3 can explain many of its activities. Indeed, both CYR61-dependent endothelial cell adhesion and migration have been shown to be mediated through integrin αvβ3 (1, 30).
The structural and expression similarities of Fisp12/mCTGF and CYR61 suggest that they may regulate the same or overlapping cellular processes (31), prompting us to investigate the possible role of Fisp12/mCTGF in angiogenesis. Furthermore, CTGF is highly expressed in endothelial cells at the luminal site of advanced atherosclerotic lesions and in the newly formed vasa vasorum inside the plaques, suggesting a possible role for CTGF in angiogenesis in atherosclerotic plaques (39, 40). In this study, we demonstrate that purified Fisp12 protein mediates microvascular endothelial cell adhesion and stimulates cell migration in the same cell type. Both activities are mediated through the integrin αvβ3. Furthermore, Fisp12 promotes endothelial cell survival under a condition that otherwise induces apoptosis. In vivo, Fisp12 induces neovascularization in a rat corneal micropocket assay. Together, these results identify Fisp12 as a novel angiogenic inducer and suggest that Fisp12/mCTGF and CYR61 constitute prototypes of a new family of angiogenic regulators that function, at least in part, through integrin-mediated pathways.
MATERIALS AND METHODS
Proteins, antibodies, and peptides.
Recombinant Fisp12 protein was purified from serum-free conditioned media of Sf9 insect cells infected with a baculovirus directing the expression of fisp12 essentially as described earlier (31). To eliminate the possibility of serum protein contamination, the Sf9 cells were washed three times after infection with baculovirus, and Fisp12 protein was purified from serum-free conditioned medium of infected Sf9 cells by using S-Sepharose column chromatography. Anti-Fisp12 antiserum was prepared by using a bacterial fusion protein containing amino acids 165 to 200 of Fisp12 linked to glutathione S-transferase (GST) as antigen. The antiserum was affinity purified by serial chromatography through a GST-Sepharose column and then a GST-Fisp12-Sepharose column (31). The resulting antibodies are highly specific for Fisp12 protein and do not cross-react with the structurally related family member Cyr61 purified in a similar manner (31). These anti-Fisp12 antibodies, produced against a Fisp12 fragment synthesized in bacteria, were used to block the activities of Fisp12 protein purified from serum-free conditioned media of baculovirus-infected insect cells.
Recombinant human basic fibroblast growth factor (bFGF) was obtained from Gibco-BRL. Vascular endothelial growth factor (VEGF) was obtained from R & D Systems. Human fibronectin and human vitronectin were obtained from Collaborative Research, Waltham, Mass. The monoclonal anti-αvβ3 antibody LM609 and anti-α5β1 antibody JBS5 were obtained from Chemicon. Synthetic peptides GRGDSP and GRGESP were purchased from Gibco-BRL.
Cell culture and cell adhesion assay.
Primary human dermal microvascular endothelial cells (HMVECs) isolated from a single neonatal donor were obtained from a commercial source (Clonetics) and grown in endothelial cell basal medium (EBM; Clonetics). Cell adhesion assays for HMVECs was performed under serum-free conditions essentially as described previously (30). Briefly, test proteins were diluted to the desired concentrations in phosphate-buffered saline (PBS), applied to 96-well microtiter plates (50 μl per well), and incubated at 4°C for 16 h. Unsaturated protein binding capacity was blocked with 1% bovine serum albumin (BSA) at room temperature for 1 h. HMVECs were washed twice with PBS and 1 mM EDTA and harvested by incubation in the same buffer for ∼10 min at room temperature. Cells were then washed with serum-free basal culture medium (EBM) and resuspended at 2.5 × 105 cells/ml in EBM containing 1% BSA and 10 mM HEPES (pH 7.2). Where indicated, EDTA or peptides were mixed with cells prior to plating, and antibodies were incubated with cells for 1 h at room temperature before plating. To each well, 50 μl of cell suspension was plated and, after incubation at 37°C for 30 min, wells were washed three times with PBS. Adherent cells were fixed with 10% formalin and stained with methylene blue, and adhesion was quantified by dye extraction and measurement of the absorbance at 620 nm as described earlier (41).
Cell migration assay.
The effects of Fisp12, bFGF, and VEGF on cell migration were assessed in 48-well modified Boyden chambers (Neuro Probe, Cabin John, Md.) as described earlier (43). HMVECs were serum starved in EBM for 24 h, resuspended at 7 × 105 cells/ml in EBM–0.1% BSA, and plated on the gelatinized surface of polycarbonate membrane (5-μm pore size; Costar, Cambridge, Mass.). Cells were allowed to attach for 2 h, and the chambers were inverted thereafter (cells are now in the lower chamber); test substances were then added to the upper chamber unless otherwise indicated. The membrane was removed after 4 h of incubation and stained with Diff-Quik (Baxter Diagnostics, Chicago, Ill.). Cells that migrated through the membrane to the upper chamber were counted under the microscope. Each experimental sample was counted in quadruplicate. To determine basal migration, the plating medium (EBM plus 0.1% BSA) plus the corresponding amount of Fisp12 buffer (50 mM morpholine ethane sulfonic acid, pH 6.0; 0.6 M NaCl; 2 mM EDTA; 0.5 mM phenyl methyl sulfonyl fluoride) was used as a test substance. Where indicated, bFGF was added at 10 ng/ml, and VEGF was added at 1 ng/ml in the same medium with Fisp12 buffer. In antibody blocking experiment, anti-Fisp12 antibody was incubated with Fisp12 protein at room temperature for 1 h before addition to the assay. In experiments to block integrin αvβ3, HMVECs were incubated with 50 μg of LM609 per ml for 1 h at room temperature with constant shaking before being plated on gelatinized membrane. The toxicity of various test substances was assessed by treating cells with each test substance in 24-well plates and measuring the trypan blue exclusion after a 16-h incubation; in no case was toxicity observed.
Rat corneal micropocket angiogenesis assay.
Neovascularization in vivo was examined by implantation of test substances formulated in Hydron pellets into rat corneas (43). Male Sprague-Dawley rats were anesthetized by intravenous injection of sodium pentobarbital, and ∼5 μl of Hydron pellets (Interferon Sciences, Inc., New Brunswick, N.J.) containing the test substance were implanted into micropockets made in the normal avascular corneal stroma 1 to 1.5 mm from the corneal limbus. In the blocking experiment, Fisp12 was preincubated with anti-Fisp12 antibodies for 1 h at room temperature before incorporation into Hydron pellets. Animals were perfused with colloidal carbon with heparin (100-U bolus) 7 days thereafter, and corneal neovascularization was examined.
Measurements of apoptosis and mitogenesis.
To measure apoptosis, HMVECs were serum starved for 24 h, resuspended in EBM plus 0.1% BSA, and plated on laminin-coated wells of Lab-Tek II chamber slide system (Nalge Nunc International). The cells were allowed to attach to the slides for 1.5 h and Fisp12 protein was then added and incubation was continued for an additional 20 h. After 20 h the medium was removed, and the cells were fixed in 4% paraformaldehyde solution (pH 7.4) for 30 min at room temperature and then processed for in situ detection of apoptosis by using the in situ cell death detection kit POD (Boehringer Mannheim). Cells were then lightly counterstained with hematoxylin, and the apoptotic nuclei were counted.
To measure proliferation, cells were grown in the same condition as that described above except that 10 μM bromodeoxyuridine (BrdUrd) was included in the medium for 24 h in the presence or absence of Fisp12 protein. Cells that incorporated the label were detected by using the BrdUrd staining kit (Calbiochem).
RESULTS
Fisp12 mediates endothelial cell adhesion through integrin αvβ3.
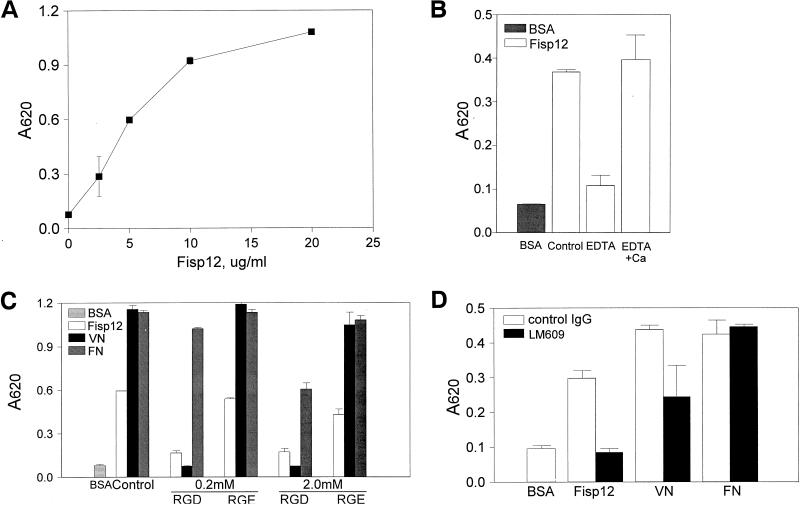
Previous studies showed that Fisp12 is secreted and is associated with the extracellular matrix, and immobilized Fisp12 protein serves as an adhesion substrate for human umbilical vein endothelial cells in a serum-containing medium (31). In this study, we have employed HMVECs as a culture system to examine cellular processes relevant to angiogenesis. To exclude the possibility that other serum proteins might participate in the Fisp12-mediated cell adhesion event, we examined adhesion under serum-free conditions with Fisp12 protein purified from serum-free conditioned medium of baculovirus-infected insect cells (see Materials and Methods). Under these conditions, immobilized Fisp12 mediated HMVEC adhesion in a dose-dependent manner (Fig. 1A). Cell adhesion to Fisp12 was blocked by affinity-purified anti-Fisp12 antibodies, thus confirming that the cell adhesion activity is attributable to Fisp12 (data not shown). Adhesion was also completely blocked by the presence of EDTA but was restored when Ca2+ was placed in the medium in addition to the EDTA (Fig. 1B). These results suggest that Fisp12 mediates cell adhesion through a divalent cation-dependent cell surface receptor.
FIG. 1.
Fisp12 mediates HMVEC adhesion through integrin αvβ3. HMVECs were washed and harvested in PBS with 1 mM EDTA, resuspended in serum-free medium, and plated on microtiter wells coated with indicated substrates. After incubation at 37°C for 30 min, adherent cells were fixed and stained with methylene blue, followed by quantitation by measuring the absorbance at 620 nm. Data shown are the means of duplicate determinations, and similar results were obtained in at least three separate experiments. Error bars represent the standard deviation(s) (SD). (A) Dose dependence of adhesion to Fisp12. HMVECs were plated onto microtiter wells coated with the indicated concentrations of purified Fisp12. (B) Divalent cation dependence. HMVEC adhesion to either BSA- or Fisp12-coated plates was determined; EDTA (10 mM) or Ca2+ (20 mM) was added where indicated. (C) Inhibition by RGD peptides. HMVECs were incubated with either buffer (control) or 0.2 or 2.0 mM GRGDSP or GRGESP peptides, as indicated, prior to addition to the microtiter wells coated with Fisp12 (5 μg/ml), 2 μg of fibronectin per ml (FN), or 0.1 μg of vitronectin per ml (VN). (D) HMVEC adhesion to Fisp12 was dependent on integrin αvβ3. Microtiter wells were coated with BSA, Fisp12 (2.5 μg/ml), fibronectin (2 μg/ml), or vitronectin (0.1 μg/ml). HMVECs were incubated with either normal mouse immunoglobulin G or the anti-αvβ3 antibody LM609 (50 μg/ml) prior to plating, and adhesion was measured at 30 min thereafter.
We have shown previously that CYR61, a protein structurally related to Fisp12, is a ligand of the integrin αvβ3 (30). By analogy to CYR61, we hypothesized that Fisp12 may also bind to an integrin receptor. We thus tested the effects of peptides containing the sequence RGD, which constitutes a recognition sequence by more than half of all known integrins, including αvβ3 (47). HMVEC adhesion to either Fisp12 or vitronectin was completely abolished by 0.2 mM RGD-containing peptide, whereas an RGE-containing peptide had little effect (Fig. 1C). HMVEC adhesion to fibronectin, which binds to integrin α5β1, was not affected until the RGD-peptide concentration reached 2 mM. The sensitivity of Fisp12-dependent cell adhesion to inhibition by an RGD-containing peptide indicated that this process may function through an integrin, and the relatively low inhibitory concentration (0.2 mM for Fisp12 and vitronectin) suggested that the integrin involved is likely αvβ3. Consistent with this notion, HMVEC adhesion to Fisp12 was completely abolished by LM609, a monoclonal antibody that is highly specific for the αvβ3 heterodimer (Fig. 1D). In contrast, LM609 had no effect on HMVEC adhesion to fibronectin, which binds integrin α5β1. Conversely, JBS5, a monoclonal antibody against α5β1, blocked adhesion to fibronectin but not to Fisp12 or vitronectin (data not shown). LM609 inhibited adhesion to vitronectin by about 50%, mostly likely because vitronectin can bind to both integrins αvβ3 and αvβ5 (12). Together, these results show that HMVEC adhesion to Fisp12 is mediated through the integrin receptor αvβ3.
Fisp12 stimulates HMVEC migration through an αvβ3-dependent pathway.
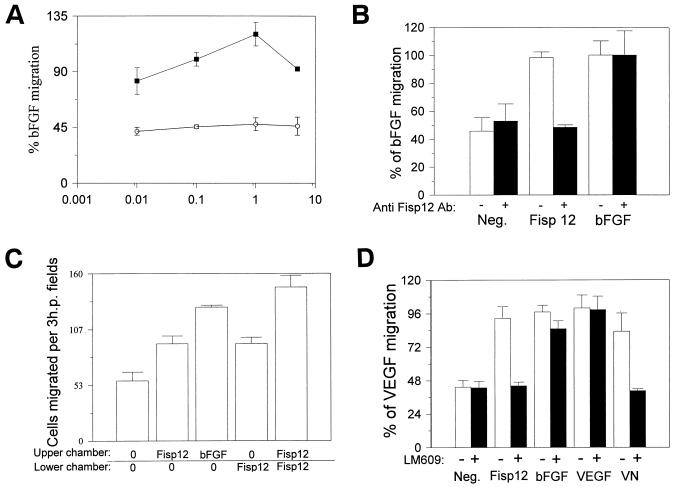
The integrin αvβ3 is known to be involved in a wide range of cellular functions, including cell migration. We thus investigated the possibility that Fisp12 may stimulate vascular endothelial cell migration. As shown in Fig. 2A, Fisp12-stimulated migration of HMVECs was dose dependent and detectable at 0.01 μg/ml, reaching a maximal level at 1 μg of Fisp12 per ml. A higher concentration of Fisp12 was less efficacious in enhancing HMVEC migration, resulting in a bell-shaped dose-response curve that is observed for many chemotactic factors. Fisp12-stimulated cell migration was completely blocked by affinity-purified anti-Fisp12 antibodies, indicating that this activity is an intrinsic property of Fisp12 (Fig. 2B).
FIG. 2.
Fisp12 stimulates HMVEC migration through an αvβ3-dependent pathway. The migration of HMVECs was measured in a modified Boyden chamber assay. The cells placed in a lower chamber that migrated into the upper chamber were counted in three high-power fields for each condition ± the SD after a 4-h incubation at 37°C. As chemoattractants, bFGF (10 ng/ml), VEGF (1 ng/ml), vitronectin (5 μg/ml), and Fisp12 (1 μg/ml unless otherwise indicated) was placed in either the top or the bottom chamber, or both, as indicated. (A) Fisp12-stimulated cell migration is dose dependent. Cells that migrated into the upper well where the indicated amount of purified Fisp12 or the corresponding amount of the Fisp12 storage buffer was placed, were counted. The results are expressed as the percentage of bFGF-induced migration ± the SD. (B) Specific inhibition of Fisp12-induced cell migration by anti-Fisp12 antibodies. HMVEC migration was measured as described above by using either Fisp12 or bFGF as the chemoattractant. Where indicated, these proteins were preincubated with anti-Fisp12 antibodies (30 μg/ml) before addition to the upper wells. Neg., background migration in the absence of chemoattractant. (C) Fisp12 induces chemokinesis. The migration of HMVECs was measured in a checkerboard-type analysis. Fisp12 or bFGF were added to the upper chamber, the lower chamber, neither chamber, or both chambers as indicated. (D) Specific inhibition of Fisp12-induced HMVEC migration by anti-αvβ3 antibody. HMVEC migration was monitored by using Fisp12, vitronectin, bFGF, or VEGF as the chemoattractants. Where indicated cells were preincubated with 50 μg of LM609 per ml for 1 h before addition to the lower chamber. The results are expressed as the percentage of cells that migrated to VEGF. Neg., background migration in the absence of chemoattractant.
Stimulation of cell migration may be due to either a chemotactic (directed cell migration) or a chemokinetic (random cell movement) response. To investigate whether Fisp12 is chemotactic or chemokinetic for HMVECs, we carried out a checkerboard-type analysis in a modified Boyden chamber (Fig. 2C). Fisp12 was placed in the upper chamber (no cells), in the lower chamber (with cells), or in both. Cell migration from the lower chamber to the upper chamber was measured. HMVEC migration was stimulated to a similar extent when Fisp12 was placed in either the upper or the lower chamber, and the greatest stimulation was observed when Fisp12 was present in both chambers. Thus, even when Fisp12 was provided in the same chamber as the cells, it stimulated the migration of these cells to the other chamber where there was no Fisp12 protein. These results indicated that Fisp12 stimulates chemokinesis to enhance cell migration. However, when Fisp12 was present in both the top and the bottom chambers, more cell migration was observed than when Fisp12 was present in either the top or the bottom chambers alone. Therefore, it is possible that Fisp12 may be both chemotactic and chemokinetic, stimulating cell migration through both directed and nondirected cell movement.
Since Fisp12 mediates HMVEC adhesion through the integrin αvβ3, we tested whether Fisp12-stimulated cell migration also works through this integrin. As shown in Fig. 2D, Fisp12-induced cell migration was completely abolished when HMVECs were preincubated with LM609, as was vitronectin-stimulated cell migration. By contrast, neither VEGF-stimulated nor basal-cell migration was affected by LM609, whereas bFGF-stimulated cell migration was only minimally affected. These results show that Fisp12 stimulates HMVEC migration through an αvβ3-dependent mechanism.
Fisp12 promotes endothelial cell survival.
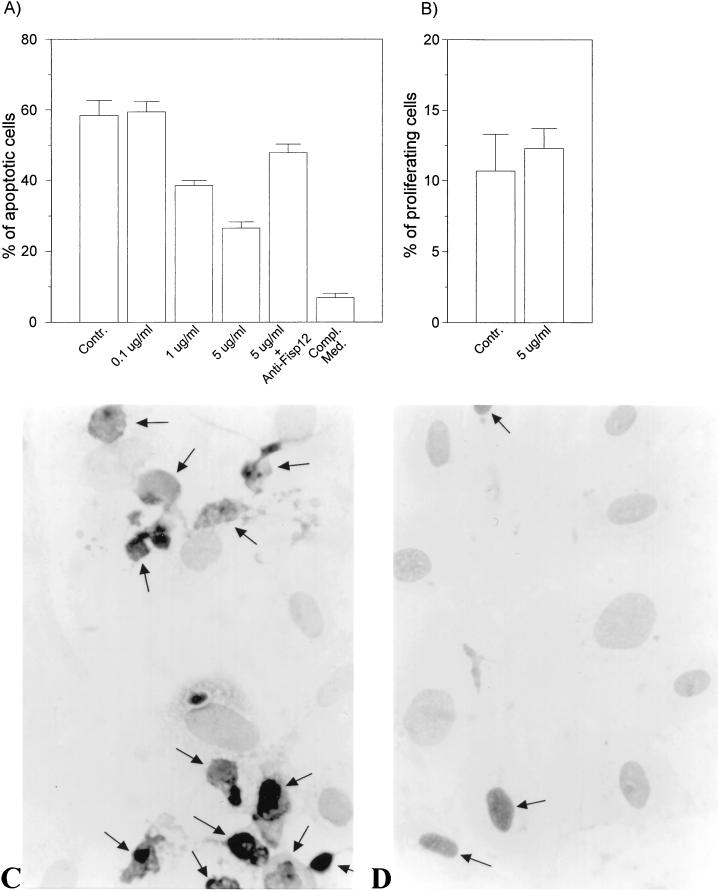
During neovascularization, endothelial cells from the parent vessels migrate toward the angiogenic target. Integrin antagonists induce apoptosis in angiogenic vascular endothelial cells, indicating that integrin-mediated signaling may be crucial for endothelial cell survival during angiogenesis (7, 56). Previous studies have shown that adhesion of endothelial cells to fibronectin or vitronectin, but not to laminin, sustains cell survival in a defined medium (58). These results suggested that ligation of the fibronectin or vitronectin integrin receptors, but not the laminin receptors, confer protection from apoptosis. To evaluate the potential role of Fisp12 in endothelial cell survival, we tested whether soluble Fisp12 can protect HMVECs from apoptosis when plated on laminin. HMVECs were deprived of growth factors for 18 h and plated on glass slides coated with laminin. Cells were then incubated with or without Fisp12 protein in the cell medium, and the number of apoptotic cells was determined by using an in situ terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (Fig. 3C and D). Under these conditions, Fisp12 promoted HMVEC survival in a dose-dependent manner (Fig. 3). Whereas no protection from cell death was observed when Fisp12 was present at 0.1 μg/ml, protection was evident when Fisp12 was present at 1.0 μg/ml and reached an even higher level when it was present at 5 μg/ml. Preincubation of Fisp12 with affinity-purified anti-Fisp12 antibodies inhibited the ability of Fisp12 to protect HMVECs from apoptosis (Fig. 3A). Likewise, incubation of these cells in the presence of complete medium with serum fully protected the cells from apoptosis. These results show that Fisp12 can promote endothelial cell survival.
FIG. 3.
Fisp12 protects HMVECs from apoptosis. HMVECs were starved for 24 h prior to the addition to slides coated with 10 μg of laminin (Collaborative biosciences) per ml overnight at 4°C. Cells were then incubated in EBM for 20 h with or without the addition of Fisp12 protein, and apoptosis was monitored by using the TUNEL assay; the number of apoptotic cells was then counted. (A) Dose dependence of Fisp12-promoted cell survival. Values for HMVECs incubated in the absence of Fisp12 (control) or in the presence of indicated concentrations of Fisp12 are shown. Where indicated, Fisp12 was preincubated with anti-Fisp12 antibody prior to addition to the medium. Complete medium was added as a positive control. The percentages of apoptotic cells ± the SD from at least 500 cells counted are shown, and each experiment was done in triplicate. (B) Cell proliferation assay. HMVECs treated as described in panel A were labeled with BrdUrd for 24 h, and the percentages of cells incorporating label in the absence or presence of Fisp12 are shown. (C and D) Photomicrographs of HMVECs incubated in the absence or presence of 5 μg of Fisp12 per ml, respectively.
To address whether the increased number of surviving cells in the presence of Fisp12 might be due to cell proliferation rather than the survival of existing cells, we measured HMVEC proliferation in the presence or absence of Fisp12 via BrdUrd incorporation (Fig. 3B). Under the same conditions as for the TUNEL assay, the number of cells labeled with BrdUrd was not significantly different either in the presence (12.3 ± 1.4%) or absence (10.7 ± 2.6%) of Fisp12. Taken together, these results show that Fisp12 can promote the survival of endothelial cells under conditions that induce apoptosis.
Fisp12 induces neovascularization in vivo.
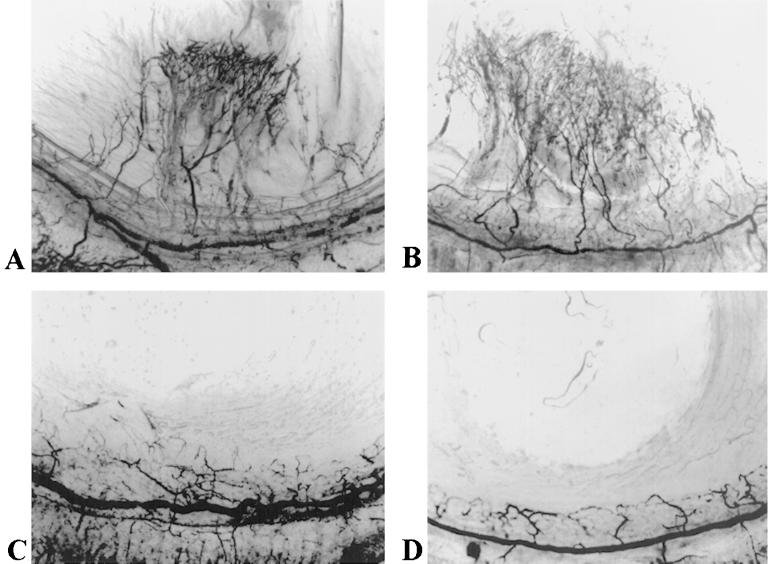
As discussed above, Fisp12 promotes vascular endothelial cell adhesion, migration, survival, and growth-factor-induced DNA synthesis, processes that are integral to vessel formation (Fig. 1 to 3) (6, 31). To assess the hypothesis that Fisp12 may regulate angiogenesis in vivo, we tested its ability to induce neovascularization by using a rat corneal micropocket assay. Hydron pellets formulated with purified Fisp12 induced neovascularization when implanted into rat corneas, whereas the vehicle control did not (Fig. 4; Table 1). Similar neovascularization was observed when corneas were implanted with Hydron pellets containing bFGF, a potent angiogenic factor. Preincubation of Fisp12 protein with specific neutralizing anti-Fisp12 antibodies before incorporation in Hydron pellets abolished the angiogenic activity of Fisp12 completely. These results show that Fisp12 can function as an angiogenic inducer in vivo.
FIG. 4.
Fisp12 induces neovascularization in rat corneas. Hydron pellets containing test substances were formulated and implanted into corneas of rats as described in Materials and Methods and Table 1. The formation of new blood vessels was visualized by perfusion with colloidal carbon 7 days after implantation. Hydron pellets contained in Fisp12 (A), bFGF (B), Fisp12 storage buffer (C), and Fisp12 protein preincubated with anti-Fisp12 antibodies (D) are shown.
TABLE 1.
Corneal vascularizationa
| Test substance | No. of corneas
|
|
|---|---|---|
| Vascularized | Unvascularized | |
| Fisp12 | 7 | 1 |
| bFGF | 4 | 0 |
| Fisp12 buffer | 0 | 4 |
| Fisp12 plus anti-Fisp12 | 0 | 4 |
Hydron pellets were formulated with purified Fisp12 (100 ng), Fisp12 storage buffer, bFGF (3 ng), or Fisp12 preincubated with anti-Fisp12 antibodies (2.3 μg) and implanted into rat corneas. Corneal vascularization was scored 7 days after implantation.
DISCUSSION
Appropriate adhesion of endothelial cells to the extracellular matrix is important for cell proliferation, migration, and survival. Endothelial cells must make proper contacts to each other and to the extracellular matrix (ECM) in order to construct and extend new vessel sprouts during angiogenesis (3, 54). It is clear that adhesion receptors are also versatile signaling receptors capable of transducing complex environmental cues, thus regulating myriad cellular processes (10, 16, 51). Antagonists of adhesion receptors can disrupt angiogenesis (7, 56), underscoring the crucial role of cell adhesion mechanisms in vessel development. We have identified here the immediate-early gene product Fisp12/mCTGF as a novel angiogenic inducer. The connection between endothelial cell adhesion and angiogenesis is further strengthened by the finding that Fisp12/mCTGF is an ECM-associated signaling molecule that mediates endothelial cell adhesion and migration through the integrin αvβ3.
Purified Fisp12/mCTGF is capable of a number of pro-angiogenic activities in culture. Previous studies have shown that Fisp12/mCTGF promotes endothelial cell proliferation by augmenting growth-factor-induced DNA synthesis (31). In this study, we show that Fisp12/mCTGF mediates HMVEC adhesion and migration (Fig. 1 and 2), both through an integrin-dependent mechanism. In addition, Fisp12/mCTGF promotes endothelial cell survival (Fig. 3). Thus, Fisp12/mCTGF in culture promotes endothelial cell proliferation, adhesion, migration, and survival, each component steps in the process of angiogenesis. Furthermore, Fisp12/mCTGF induces neovascularization in a corneal micropocket assay (Fig. 4), demonstrating that it functions as an angiogenic inducer in vivo. The activities of Fisp12 were blocked by specific anti-Fisp12 antibodies (Fig. 1, 2, and 4). These affinity-purified antibodies were derived from antiserum raised against a bacterially expressed Fisp12 polypeptide antigen, whereas the Fisp12 protein used for activity assays was purified from serum-free conditioned media of baculovirus-infected insect cells. Thus, inhibition of Fisp12 activities by the antibodies provided compelling evidence that these activities are attributed to the Fisp12 protein.
fisp12/mCTGF is a member of a growing gene family, named the CCN family (CTGF, CYR61, and Nov) (4), that includes (i) CYR61, whose mouse, human, and chicken forms are inducible by serum growth factors in a manner similar to that of Fisp12/CTGF (38, 48, 52); (ii) Nov, a gene aberrantly expressed in myeloblastosis-associated virus-induced nephroblastomas and in some Wilm’s tumors but downregulated by serum and mitogenic conditions (29, 50); (iii) Elm-1, a gene expressed in low-metastatic but not in high-metastatic clones of the K-1735 murine melanoma cell line (20); and (iv) WISP-3, a related gene identified through screening an expressed sequence tag database (42). Proteins encoded by these genes exhibit conservation of all 38 cysteines in their secreted portion, share at least 40 to 50% amino acid sequence homology with one another and are comprised of four conserved domains, each encoded by a separate exon (4, 18, 48). Another homologous gene, Cop-1/WISP-2 (42, 60), encodes a protein that lacks precisely the carboxyl-terminal domain but is otherwise equally conserved with the other members of the protein family.
Insights into the biological functions and mechanism of actions of this family of proteins are beginning to emerge based on the activity studies of CYR61 and Fisp12/CTGF. Both Fisp12/mCTGF and CYR61 have been shown to promote the adhesion, migration, and proliferation of both fibroblasts and endothelial cells (Fig. 1 and 2) (31, 32) and to induce neovascularization in vivo (Fig. 4) (1). CTGF also enhances the expression of type I collagen and fibronectin in fibroblasts (15). In this study, we showed that HMVEC adhesion to Fisp12/mCTGF was inhibited by EDTA, RGD-containing peptide, and the specific anti-αvβ3 monoclonal antibody LM609 (Fig. 1), indicating that HMVEC adhesion to Fisp12 is mediated through the integrin αvβ3. Furthermore, Fisp12 stimulates HMVEC migration, also through an integrin-αvβ3-dependent mechanism (Fig. 2). Likewise, CYR61-dependent HMVEC adhesion and migration were also mediated through the integrin αvβ3 (1, 30). These findings suggest that Fisp12/mCTGF and CYR61 may act, at least in part, through integrin αvβ3 to induce angiogenesis. In a recent study, we showed that CYR61 binds to the integrin αvβ3 directly as a ligand, with half-maximal binding to immobilized integrin αvβ3 occurring at 5 nM CYR61 in a solid-phase binding assay (30). The dependence of Fisp12-mediated endothelial cell adhesion and migration on the integrin αvβ3, as well as its structural homology to CYR61, are consistent with the notion that Fisp12/mCTGF may also function through direct binding to integrin αvβ3. Together, these data implicate the CCN family of proteins as novel angiogenic regulators that may function through integrin-αvβ3-dependent mechanisms (1, 30).
Despite the structural similarities among members of the CCN family, these proteins may have antithetical activities to one another in the same processes. Several examples illustrate the paradigm of homologous proteins playing opposing roles to regulate angiogenesis. Thus, proliferin induces angiogenesis, whereas the homologous proliferin-related protein inhibits angiogenesis (26). Likewise, members of the C-X-C chemokine family are either inducers or inhibitors of angiogenesis, the former group being distinguished by the presence of the ELR sequence motif (53). In addition, whereas angiopoietin-1 promotes vessel wall assembly, the homologous angiopoietin-2 disrupts this process (34, 55). Since CYR61 and fisp12/CTGF are induced by mitogens, whereas nov and cop1 are repressed by mitogens, proteins encoded by these genes may play opposing roles. While the finding that both CYR61 and Fisp12 are inducers of neovascularization implicates the CCN family of proteins in angiogenesis, whether other members of the family might act to promote or inhibit angiogenesis remains to be experimentally determined.
The survival of endothelial cells is known to be dependent on the ligation of integrins to appropriate ligands (2, 58). The monoclonal antibody against integrin αvβ3 (LM609) disrupts angiogenesis in chick chorioallantoic membrane and in quail embryos in a process that involves apoptosis of endothelial cells (7, 11, 56). Thus, proper ligation of integrin αvβ3 is critical for promoting the survival of angiogenic endothelial cells. Studies in cell culture systems have shown that serum-starved endothelial cells plated on laminin undergo apoptosis, whereas those plated on fibronectin or vitronectin are protected from apoptotic death (58). These results suggest that ligation of the αvβ3 or α5β1 integrins, but not of the α2β1 or α6β1 integrins, can confer protection against apoptosis in endothelial cells. Here we show that soluble Fisp12/mCTGF protects starved HMVECs plated on laminin from apoptosis (Fig. 3), suggesting that Fisp12/mCTGF can promote the survival of angiogenic endothelial cells. Although the mechanism of Fisp12/mCTGF action in this system is unclear, the simplest interpretation is that it acts through integrin αvβ3 to protect cells from apoptosis.
The finding that Fisp12/mCTGF is an angiogenic inducer helps to shed new light on its biological functions during growth and development. During mouse embryogenesis, Fisp12/mCTGF is localized in angiogenic cell types, including the trophoblastic giant cells of the placenta (31). These cells produce an arsenal of angiogenic factors, including VEGF, bFGF, proliferin, and CYR61 (26, 27, 37). Expression of Fisp12/mCTGF in these trophoblasts may help to promote the angiogenesis required for maternal vessel growth towards the developing embryo, thus providing nutrients for fetal development. Fisp12/mCTGF is also localized in chondrocytes of the hypertrophic cartilage (35), where invading endothelial sprouts deliver osteoblasts that will initiate the process of endochondral ossification. Angiogenesis induced by the hypertrophic cartilage is thus a necessary step towards the replacement of cartilage by bone. In addition to embryonic development, Fisp12/mCTGF is induced during cutaneous wound healing in the adult rodent (24, 33). In this instance the role of Fisp12 may include the induction of angiogenesis in the granulation tissue, in addition to serving as a chemotactic and mitogenic factor for fibroblasts to promote wound repair.
A potentially important role for Fisp12/CTGF in diseases is also implicated by its angiogenic activity. For example, whereas human CTGF was undetectable in normal vessels, high levels of expression were found in endothelial cells at the luminal site of advanced atherosclerotic lesions and in the newly formed vasa vasorum inside the atherosclerotic plaques (39, 40). Thus, CTGF may act as an angiogenic factor in the diseased vessel, leading to advanced atherosclerotic lesions (36). The angiogenic activity of Fisp12/CTGF also portends a potential role in tumorigenesis. It is now established that solid tumors must acquire the ability to induce angiogenesis in order to secure the necessary nutrients for successful tumor growth and development (5, 13). Although the role of Fisp12/CTGF in tumorigenesis has not been fully investigated, CTGF expression has been correlated with endothelial cells of cutaneous vascular tumors such as pyogenic granuloma, angiopiloma, and angioleiomyoma (21). These findings suggest a potential contribution of the angiogenic activity of Fisp12/CTGF in these tumors.
CTGF expression is also significantly induced in a number of fibrotic diseases and in systemic sclerosis (scleroderma) (22), a chronic generalized autoimmune disorder characterized by cutaneous and visceral fibrosis. Affected tissues in these diseases are marked by proliferation of connective tissue and significant deposition of ECM and are correlated with TGF-β expression (22, 28). TGF-β, which stimulates fibrosis and angiogenesis in vivo (46), induces CTGF expression and may work through CTGF to execute some of its biological effects (17). The pathogenesis of sclerosis may reflect, in part, a dynamic imbalance between angiogenesis and fibrosis. A recent study showed that biopsies from patients with systemic sclerosis induce significant angiogenic response on chick chorioallantoic membrane, demonstrating the presence of angiogenic inducers in these tissues (44). The precise roles of Fisp12/CTGF in development, wound healing, vascular and fibrotic diseases, and cancer are likely to be influenced by the presence of other growth factors, cytokines, and angiogenic inducers and/or inhibitors. Future inquiries into the interplay between the angiogenic activity of Fisp12/CTGF in endothelial cells and its proliferative and matrix remodeling functions in fibroblasts may prove illuminating.
ACKNOWLEDGMENTS
We thank Maria L. Kireeva and George Yang for useful reagents. We are also grateful to Noël Bouck and Olga Volpert for many helpful suggestions and comments.
This work was supported by grants from the National Cancer Institute (CA46565 and CA80080).
REFERENCES
- 1.Babic A, Kireeva M L, Kolesnikova T V, Lau L F. CYR61, product of a growth factor-inducible immediate-early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates R C, Lincz L F, Burns G F. Involvement of integrins in cell survival. Cancer Metastasis Rev. 1995;14:191–203. doi: 10.1007/BF00690291. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff J. Cell adhesion and angiogenesis. J Clin Invest. 1997;99:373–376. doi: 10.1172/JCI119168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- 5.Bouck N, Stellmach V, Hsu S C. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 6.Bradham D M, Igarashi A, Potter R L, Grotendorst G R. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks P C, Montgomery A M P, Rosenfeld M, Reisfel R, Hu T, Klier G, Cheresh D. Integrin alpha V beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 8.Brunner A, Chinn J, Neubauer M, Purchio A F. Identification of a gene family regulated by transforming growth factor-beta. DNA Cell Biol. 1991;10:293–300. doi: 10.1089/dna.1991.10.293. [DOI] [PubMed] [Google Scholar]
- 9.Bussolino F, Mantovni A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997;22:251–256. doi: 10.1016/s0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- 10.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 11.Drake C J, Cheresh D A, Little C D. An antagonist of integrin αvβ3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci. 1995;108:2655–2661. doi: 10.1242/jcs.108.7.2655. [DOI] [PubMed] [Google Scholar]
- 12.Felding-Habermann B, Cheresh D A. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. Blood vessel formation: what is its molecular basis. Cell. 1997;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 15.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst G R. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 16.Giancotti F G. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 17.Grotendorst G R. Connective tissue growth factor: a mediator of TGF-β action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 18.Grotendorst G R, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- 19.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto Y, Shindo-Okada N, Tani M, Nagamachi Y, Takeuchi K, Shiroishi T, Toma H, Yokota J. expression of the Elm1 gene, a novel gene of the CCN (connective tissue growth factor, Cyr61/Cef10, and neuroblastoma overexpressed gene) family, suppresses in vivo tumor growth and metastasis of K-1735 murine melanoma cells. J Exp Med. 1998;187:289–296. doi: 10.1084/jem.187.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Igarashi A, Hayashi N, Nashiro K, Takehara K. Differential expression of connective tissue growth factor gene in cutaneous fibrohistiocyte and vascular tumors. J Cutaneous Pathol. 1998;25:143–148. doi: 10.1111/j.1600-0560.1998.tb01706.x. [DOI] [PubMed] [Google Scholar]
- 22.Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Fujimoto M, Grotendorst G R, Takehara K. Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders. J Invest Dermatol. 1996;106:729–733. doi: 10.1111/1523-1747.ep12345771. [DOI] [PubMed] [Google Scholar]
- 23.Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Grotendorst G R, Tekehara K. Significant correlation between connective tissue growth factor gene expression and skin sclerosis in tissue sections from patients with systemic sclerosis. J Invest Dermatol. 1995;105:280–284. doi: 10.1111/1523-1747.ep12318465. [DOI] [PubMed] [Google Scholar]
- 24.Igarashi A, Okochi H, Bradham D M, Grotendorst G R. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito Y, Aten J, Bende R J, Oemar B S, Rabelink T J, Weening J J, Goldschmeding R. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53:853–861. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- 26.Jackson D, Volpert O, Bouck N, Linzer D I H. Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science. 1994;266:1581–1584. doi: 10.1126/science.7527157. [DOI] [PubMed] [Google Scholar]
- 27.Jackson M R, Carney E W, Lye S J, Ritchie J W K. Localization of two angiogeneic growth factors (PDECGF and VEGF) in human placentae throughout gestation. Placenta. 1994;15:341–353. doi: 10.1016/0143-4004(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez S A, Hitraya E, Varga J. Pathogenesis of scleroderma. Rheum Dis Clin N Am. 1996;22:647–674. doi: 10.1016/s0889-857x(05)70294-5. [DOI] [PubMed] [Google Scholar]
- 29.Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J, Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992;12:10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kireeva M L, Lam S C T, Lau L F. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin αvβ3. J Biol Chem. 1998;273:3090–3096. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- 31.Kireeva M L, Latinkić B V, Kolesnikova T V, Chen C-C, Yang G P, Abler A S, Lau L F. Cyr61 and Fisp12 are both signaling cell adhesion molecules: comparison of activities, metablism, and localization during development. Exp Cell Res. 1997;233:63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- 32.Kireeva M L, Mo F-E, Yang G P, Lau L F. Cyr61, product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16:1326–1334. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latinkić B V. Ph.D. thesis. Chicago, Ill: The University of Illinois at Chicago; 1994. [Google Scholar]
- 34.Maisonpierre P C, Suri C, Jones P F, Bartunkova S, Wiegand S J, Radziejewski C, Comption D, McClain J, Aldrich T H, Papadopoulos N, Daly T J, Davis S, Sato T N, Yancopoulos G D. Aniopoietin-2, a natural antagonist for Tis2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi T, Kimura Y, Tamura T, Ichikawa H, Yamaai Y-I, Sugimoto T, Takigawa Y M. Cloning of a mRNA preferentially expressed in chondrocytes by differential display-PCR from a human chondrocytic cell line that is identical with connective tissue growth factor (CTGF) mRNA. Biochem Biophys Res Commun. 1997;234:206–210. doi: 10.1006/bbrc.1997.6528. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien E R, Garvin M R, Dev R, Stewart D K, Hinohara T, Simpson J B, Schwartz S M. Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol. 1994;145:883–894. [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien T P, Lau L F. Expression of the growth factor-inducible immediate early gene cyr61 correlates with chondrogenesis during mouse embryonic development. Cell Growth Differ. 1992;3:645–654. [PubMed] [Google Scholar]
- 38.O’Brien T P, Yang G P, Sanders L, Lau L F. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990;10:3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oemar B S, Luscher T F. Connective tissue growth factor: friend or foe? Arterioscler Thromb Vasc Biol. 1997;17:1483–1489. doi: 10.1161/01.atv.17.8.1483. [DOI] [PubMed] [Google Scholar]
- 40.Oemar B S, Werner A, Garnier J-M, Do D-D, Godoy N, Nauck M, Marz W, Rupp J, Pech M, Luscher T F. Human connective tissue growth factor is expressed in advanced atheroslerotic lesions. Circulation. 1997;95:831–839. doi: 10.1161/01.cir.95.4.831. [DOI] [PubMed] [Google Scholar]
- 41.Oliver M H, Harrison N K, Bishop J E, Cole P J, Laurent G J. A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci. 1989;92:513–518. doi: 10.1242/jcs.92.3.513. [DOI] [PubMed] [Google Scholar]
- 42.Pennica D, Swanson T, Welsh J W, Roy M A, Lawrence D A, Lee J, Brush J, Taneyhill L A, Deuel B, Lew M, Watanabe C, Cohen R L, Melhem M F, Finley G, Quirke P, Goddard A D, Hillan K J, Gurney A L, Botstein D, Levine A J. WISP genes are members of the connective tissue growth factor family that are up-regulated in Wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polverini P J, Bouck N P, Rastinjad F. Assay and purification of naturally occurring inhibitor of angiogenesis. Methods Enzymol. 1991;198:440–441. doi: 10.1016/0076-6879(91)98044-7. [DOI] [PubMed] [Google Scholar]
- 44.Ribatti D, Cantatore F P, Vacca A, D’Amore M, Ria R, Roncali L, Pipitone V. Systemic sclerosis stimulates angiogenesis in the chick embryo chorioallantoic membrane. Clin Rheumatol. 1998;17:115–120. doi: 10.1007/BF01452256. [DOI] [PubMed] [Google Scholar]
- 45.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 46.Roberts A B, Sporn M B, Assoian R K, Smith J M, Roche N S, Wakefield L M, Heine U I, Liotta L A, Falanga V, Kehrl J H, Fauci A S. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 48.Ryseck R-P, Macdonald-Bravo H, Mattei M-G, Bravo R. Structure, mapping, and expression of fisp-12, a growth factor-inducible gene encoding a secreted cysteine-rich protein. Cell Growth Differ. 1991;2:225–233. [PubMed] [Google Scholar]
- 49.Sastry S K, Horwitz A F. Adhesion-growth factor interactions during differentiation: an integrated biological response. Dev Biol. 1996;180:455–467. doi: 10.1006/dbio.1996.0319. [DOI] [PubMed] [Google Scholar]
- 50.Scholz G, Martinerie C, Perbal B, Hanafusa H. Transcriptional down regulation of the nov proto-oncogene in fibroblasts transformed by p60v-src. Mol Cell Biol. 1996;16:481–486. doi: 10.1128/mcb.16.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz M A, Schaller M D, Ginsburg M H. Integrins: emergining paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 52.Simmons D L, Levy D B, Yannoni Y, Erikson R L. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci USA. 1989;86:1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strieter R M, Polverini P J, Kunkel S L, Arenber D A, Burdick M D, Kasper J, Dzuiba J, Van Dammes J, Walz A, Marriott D, Chan S-Y, Roczniak S, Shanafelt A B. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 54.Stromblad S, Cheresh D A. Integrins, angiogenesis and vascular cell survival. Chem Biol. 1996;3:881–885. doi: 10.1016/s1074-5521(96)90176-3. [DOI] [PubMed] [Google Scholar]
- 55.Suri C, Jones P F, Patan S, Bartunkova S, Maisonpierre P C, Davis S, Sato T N, Yancopoulos G D. Requisite role of angiopoietin-1, a ligand for the TIE-2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 56.Varner J A. The role of vascular cell integrins alpha v beta 3 and alpha v beta 5 in angiogenesis. In: Goldberg I D, Rosen E M, editors. Regulation of angiogenesis. Basel, Switzerland: Birkhauser Verlag; 1997. pp. 361–390. [DOI] [PubMed] [Google Scholar]
- 57.Varner J A, Cheresh D A. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 58.Wary K K, Mainiero F, Isakoff S J, Marcantonio E E, Giancotti F G. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 59.Wong M, Kireeva M L, Kolesnikova T V, Lau L F. Cyr61, product of a growth factor-inducible immediate-early gene, regulates chondrogenesis in mouse limb bud mesenchymal cells. Dev Biol. 1997;192:492–508. doi: 10.1006/dbio.1997.8766. [DOI] [PubMed] [Google Scholar]
- 60.Zhang R, Averboukh L, Zhu W, Zhang H, Jo H, Dempsey P J, Coffey R J, Pardee A B, Liang P. Identification of rCop-1, a new member of CCN growth factor family, as a negative regulator or cell transformation. Mol Cell Biol. 1998;18:6131–6141. doi: 10.1128/mcb.18.10.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]