Abstract
Background
Focal chondral defects of the knee are common. Several surgical techniques have been proposed for the management of chondral defects: microfractures (MFX), osteochondral autograft transplantation (OAT), autologous matrix-induced chondrogenesis (AMIC) and autologous chondrocyte implantation (ACI)—first generation (pACI), second generation (cACI) and third generation (mACI). A Bayesian network meta-analysis was conducted to compare these surgical strategies for chondral defects in knee at midterm follow-up.
Methods
This Bayesian network meta-analysis was conducted according to the PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions. PubMed, Google Scholar, Embase and Scopus databases were accessed in July 2021. All the prospective comparative clinical trials investigating two or more surgical interventions for chondral defects of the knee were accessed. The network meta-analyses were performed through a Bayesian hierarchical random-effects model analysis. The log odds ratio (LOR) effect measures were used for dichotomic variables, while the standardized mean difference (SMD) for the continuous variables.
Results
Data from 2220 procedures (36 articles) were retrieved. The median follow-up was 36 (24 to 60) months. The ANOVA test found good baseline comparability between symptoms duration, age, sex and body mass index. AMIC resulted in higher Lysholm score (SMD 3.97) and Tegner score (SMD 2.10). AMIC demonstrated the lowest rate of failures (LOR −0.22) and the lowest rate of revisions (LOR 0.89). As expected, MFX reported the lower rate of hypertrophy (LOR −0.17) followed by AMIC (LOR 0.21). No statistically significant inconsistency was found in the comparisons.
Conclusion
AMIC procedure for focal chondral defects of the knee performed better overall at approximately 3 years’ follow-up.
Keywords: Knee, Chondral defects, Autologous chondrocyte implantation, Osteochondral autograft transplantation, Autologous matrix-induced chondrogenesis
Introduction
Focal chondral defects of the knee are common [1]. Avascularity and hypocellularity, along with minimal metabolic activity of cartilage, lead to a limited self-repair capability [2–4]. Chondral defects represent one of the major challenges for orthopaedic surgeons [5]. If left untreated, they negatively impact patient quality of life, reducing their sporting activities and resulting in premature osteoarthritis [6–8]. Knee chondral defects are 20% more common in athletes [9], increasing up to 50% in those who underwent ACL reconstructive surgery [10, 11]. Symptomatic knee chondral defects often require surgery. Microfractures (MFX) represent the traditional approach to these lesions [12]. During osteochondral autograft transplantation (OAT), single or multiple autologous osteochondral grafts are harvested from a donor site and transplanted into the chondral defect [13]. Another surgical technique, namely autologous chondrocyte implantation (ACI), has been in use since 1994 [14]. At ACI, a sample of hyaline cartilage is harvested from a non-weightbearing zone of the distal femur and the chondrocytes are expanded in vitro. In the first generation (periosteal ACI or pACI), expanded chondrocytes are injected into the defect beneath an autologous periosteal membrane [15]. In the second generation (collagenic ACI or cACI), the periosteal membrane is replaced by a collagenic membrane [16]. In the third generation (matrix-induced ACI or mACI), harvested chondrocytes are directly cultivated over a membrane that will then be used to cover the defect [17]. Recently, autologous matrix-induced chondrogenesis (AMIC) has been proposed to manage chondral defect [18, 19]. In AMIC, following MFX of the chondral defect, a membrane is used to cover the lesion in a single step surgery [8, 20]. AMIC exploits the regenerative potential of bone-marrow derived cells. Given the complexity of these injuries, and the number of surgical techniques for knee chondral defects, a Bayesian network meta-analysis was conducted to compare these strategies for the surgical management of focal chondral defects of the knee at midterm follow-up. The purpose of the present study compared efficacy of these strategies in terms of clinical scores and complications.
Methods
Search strategy
This Bayesian network meta-analysis was conducted according to the PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions [21]. The PICOT framework was preliminary pointed out:
P (Problem): knee chondral defect
I (Intervention): surgical management
C (Comparison): pACI, cACI, mACI, AMIC, OAT, MFX
O (Outcomes): clinical scores and complications
T (Timing): ≥ 12 months follow-up
Data source and extraction
Two authors (**;**) independently conducted the literature search. PubMed, Google Scholar, Embase and Scopus databases were accessed in July 2021. The following keywords were used in the database search bar using the Boolean operators AND/OR: chondral, cartilage, articular, damage, defect, injury, chondropathy, knee, pain, periosteum, membrane, matrix-induced, autologous, chondrocyte, autograft, transplantation, implantation, mACI, pACI, cACI, AMIC, OAT, cylinder, osteochondral, transplantation, autologous matrix-induced chondrogenesis, microfractures, mosaicplasty, management, surgery, outcomes, revision, failures, hypertrophy. No time constrains were set for the search. The same authors screened separately the resulting articles for inclusion. The full-text of the articles of interest was accessed. A cross reference of the bibliography of the full-text articles was conducted. Disagreements were solved by a third author (**).
Eligibility criteria
All the clinical trials that compare two or more surgical interventions for knee chondral defects were accessed. Given the authors’ language abilities, articles in English, German, Italian, French and Spanish were eligible. Only prospective studies levels I to II of evidence, according to Oxford Centre of Evidence-Based Medicine [22], were considered. Only studies focusing on AMIC, OAT, MFX and ACI were considered in the present investigation. Only studies that clearly stated the surgical procedures were included. Studies involving patients with end-stage joint osteoarthritis were not eligible, nor were those involving patients with kissing lesions. Only studies reporting data from procedures in knee with a minimum 12 months follow-up were eligible. Animals and computational studies were not considered. Studies augmenting the intervention with less committed cells (e.g. mesenchymal stem cells) were not considered. Missing quantitative data under the outcomes of interest warranted the exclusion from this study.
Outcomes of interest
Two authors (**;**) separately performed data extraction. Study generalities (author, year, journal, type of study) and patients’ baseline demographic information were extracted (number of samples and related mean BMI and age, duration of the symptoms, duration of the follow-up, percentage of female). For every study, data concerning the International Knee Documentation Committee (IKDC) [23], Tegner Activity Scale [24] and Lysholm Knee Scoring Scale [25] at last follow-up was collected. Data regarding complications were also collected: hypertrophy, rate of failures and revisions. Failure was defined as pain and/or catching symptoms recurrence, partial or complete displaced delamination at MRI or arthroscopy [26–28].
Methodology quality assessment
The methodological quality assessment was performed by two authors (**;**). The risk of bias graph tool of the Review Manager Software (The Nordic Cochrane Collaboration, Copenhagen) was used. The following risks of bias were evaluated: selection, detection, reporting, attrition and other source of bias.
Statistical analysis
The statistical analysis was performed by the main author (**). The STATA Software/MP (StataCorporation, College Station, TX, USA) was used for the statistical analyses. To assess demographic baseline, the Shapiro-Wilk test has been performed to investigate data distribution. For parametric data, mean and standard deviation were evaluated. The baseline comparability was assessed using analysis of variance (ANOVA), with P values > 0.1 considered satisfactory. For non-parametric data, median and interquartile were evaluated. The baseline comparability was assessed by the Kruskal-Wallis test, with P values > 0.1 considered satisfactory. The network meta-analyses were performed through the STATA routine for Bayesian hierarchical random-effects model analysis. The inverse variance method was used for all the comparisons. The log odds ratio (LOR) effect measures were used for dichotomic variables, while the standardized mean difference (SMD) for the continuous variables. The overall inconsistency was evaluated through the equation for global linearity via the Wald test. If P value > 0.1, the null hypothesis could not be rejected, and the consistency assumption is accepted at the overall level of each treatment. All the variables were compared in the network analyses against a fictitious group control: no event for binary comparisons and maximal value of score for continuous endpoints. Both confidence (CI) and percentile (PrI) intervals were set at 95%. Edge plots, interval plots and funnel plots were obtained and evaluated.
Results
Search result
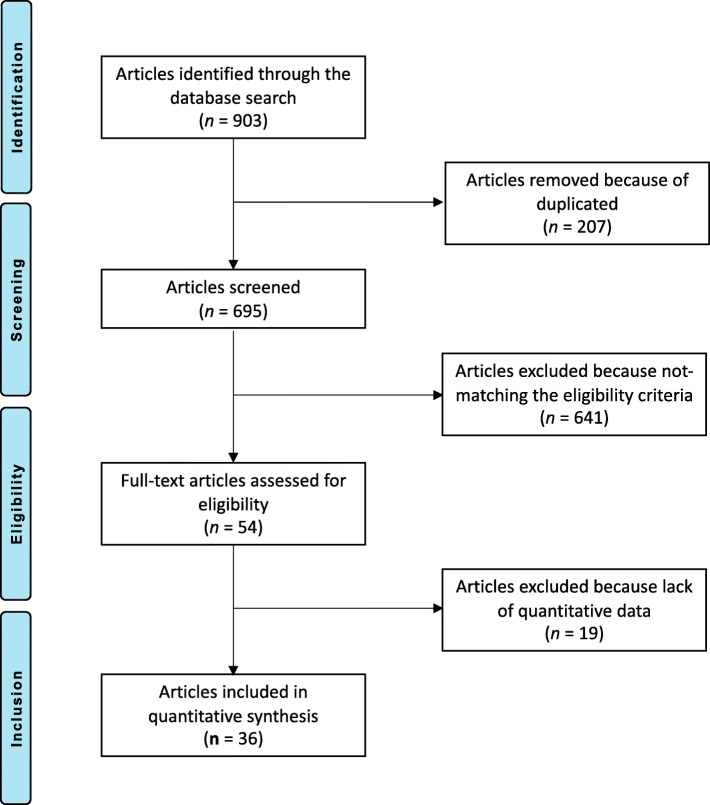
The literature search resulted in 903 articles. Of them, 207 were duplicates. A further 641 articles did not match the inclusion criteria: poor level of evidence or not comparative study (N = 407), not focused on knee (N = 197), reported short follow-up (N = 9), combined with stem cells (N = 11) and language limitations (N = 2). A further 15 articles were excluded since they did not clearly specify the surgical procedure or the eligibility criteria. A further 19 studies were not considered because they did not report quantitative data under the outcomes of interest. This left 36 comparative studies: 22 RCTs and 14 non-RCTs. The literature search results are shown in Fig. 1.
Fig. 1.
Flow chart of the literature search
Methodological quality assessment
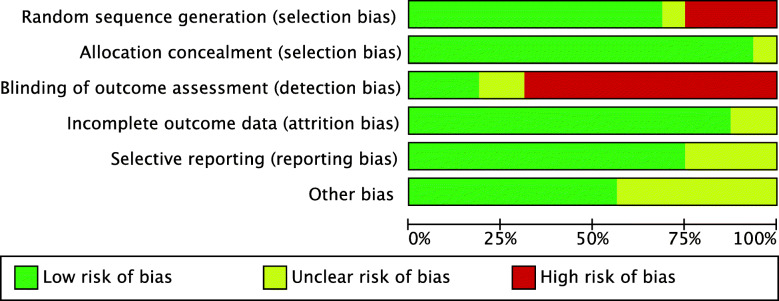
Given the predominance of RCTs (22 of 36 studies), the risk to selection bias was low. The risk of selection bias of the allocation concealment was very low. Given the overall lack of blinding, the risk of detection bias was moderate to high. The risk of attrition and reporting bias were low, as were the risks of other biases. Concluding, the overall review authors’ judgements about each risk of bias item scored low, attesting to this study a good methodological assessment. The risk of bias graph is shown in Fig. 2.
Fig. 2.
Methodological quality assessment: Cochrane risk of bias graph
Patient demographics
Data from 2220 procedures were retrieved. The mean duration of symptoms before the index surgery was 44 (25 to 86.5) months. Thirty-six percent (799 of 2210) were women. The median age of the patients was 33.9 (30 to 37) years, while the median BMI was 25.3 (25 to 26) kg/m2. The mean defect size was 3.7 ± 1.2 cm2. The median follow-up was 36 (24 to 60) months. The ANOVA test found good between studies baseline comparability in terms of mean duration of symptoms, age, BMI, gender, defect size and preoperative VAS, Tegner, Lysholm and IKDC (P > 0.0.5). Generalities of the study are shown in Table 1, while the within studies baseline is shown in greater detail in Table 2.
Table 1.
Generalities of the included studies
| Author, year | Journal | Study design | Follow-up (months) | Treatment | Procedures (n) | Female (%) | Mean age | Mean BMI |
|---|---|---|---|---|---|---|---|---|
| Anders et al., 2013 [33] | Open Orthop J | Randomized | 24 | AMIC | 8 | 12 | 35.0 | 27.4 |
| AMIC | 13 | 23 | 39.0 | 27.7 | ||||
| MFX | 6 | 33 | 41.0 | 25.2 | ||||
| Bartlett et al., 2005 [16] | J Bone Joint Surg | Randomized | 12 | cACI | 44 | 41 | 33.7 | |
| mACI | 47 | 33.4 | ||||||
| Basad et al., 2010 [62] | Knee Surg Sports Traumatol Arthrosc | Randomized | 24 | mACI | 40 | 38 | 33.0 | 25.3 |
| MFX | 20 | 15 | 37.5 | 27.3 | ||||
| Becher et al., 2017 [63] | J Orthop Surg Res | Randomized | 36 | mACI | 25 | 32 | 33.0 | 24.9 |
| mACI | 25 | 16 | 34.0 | 25.6 | ||||
| mACI | 25 | 40 | 34.0 | 25.1 | ||||
| Berruto et al., 2017 [64] | Injury | Non-Randomized | 162 | pACI | 9 | 31 | 31.6 | |
| cACI | 24 | |||||||
| Bode et al., 2013 [65] | Arch Orthop Trauma Surg | Non-Randomized | 72 | cACI | 19 | 40.2 | 25.2 | |
| cACI | 24 | 38.3 | 24.1 | |||||
| Brittberg et al., 2018 [66] | Am J Sports Med | Randomized | 60 | mACI | 65 | 38 | 35.0 | |
| MFX | 63 | 33 | 34.0 | |||||
| Chung et al., 2013 [32] | Knee Surg Sports Traumatol Arthrosc | Non-Randomized | 24 | MFX | 12 | 83 | 44.3 | |
| AMIC | 24 | 42 | 47.4 | |||||
| Cvetanovich et al., 2016 [67] | Am J Sports Med | Non-Randomized | 24 | cACI | 12 | 22 | 17.0 | 22.8 |
| mACI | 11 | 22 | 17.0 | 22.8 | ||||
| mACI | 14 | 22 | 17.0 | 22.8 | ||||
| De Girolamo et al., 2019 [68] | J Clin Med | Randomized | 100 | AMIC | 12 | 39 | 30.0 | |
| AMIC | 12 | 40 | 30.0 | |||||
| Ebert et al., 2015 [69] | Am J Sports Med | Non-Randomized | 24 | mACI | 10 | 20 | 39.0 | 25.8 |
| mACI | 13 | 7 | 36.0 | 25.6 | ||||
| mACI | 9 | 66 | 38.0 | 25.1 | ||||
| mACI | 15 | 53 | 37.0 | 25.3 | ||||
| Ferruzzi et al., 2008 [70] | J Bone Joint Surg | Non-Randomized | 60 | pACI | 48 | 38 | 32.0 | |
| mACI | 50 | 28 | 31.0 | |||||
| Fossum et al., 2019 [30] | Orthop J Sports Med | Randomized | 24 | AMIC | 20 | 60 | 38.3 | 27.9 |
| cACI | 21 | 33 | 37.2 | 25.7 | ||||
| Gooding et al., 2006 [71] | Knee | Randomized | 24 | pACI | 33 | 51 | 31.0 | |
| cACI | 35 | |||||||
| Gudas et al., 2006 [72] | Knee Surg Sports Traumatol Arthrosc | Randomized | 37 | MFX | 28 | 43 | 24.3 | |
| OAT | 29 | 35 | 24.6 | |||||
| Gudas et al., 2009 [73] | J Pediatr Orthop | Randomized | 24 | OAT | 25 | 40 | 15.0 | |
| MFX | 22 | 40 | 14.0 | |||||
| Gudas et al., 2012 [74] | Am J Sports Med | Randomized | 120 | OAT | 28 | 32 | 25.0 | |
| MFX | 29 | 41 | 24.0 | |||||
| Hoburg et al., 2019 [28] | Orthop J Sports Med | Non-Randomized | 63 | mACI | 29 | 48 | 16.0 | 21.3 |
| 48 | mACI | 42 | 29 | 27.0 | 24.1 | |||
| Horas et al., 2003 [75] | J Bone Joint Surg | Non-Randomized | 124 | pACI | 20 | 60 | 31.4 | |
| OAT | 20 | 25 | 35.4 | |||||
| Knutsen et al., 2016 [76] | J Bone Joint Surg | Randomized | 180 | pACI | 40 | |||
| MFX | 40 | |||||||
| Kon et al., 2009 [42] | Am J Sports Med | Non-Randomized | 60 | mACT | 40 | 17 | 29.0 | |
| MFX | 40 | 32 | 31.0 | |||||
| Kon et al., 2011 [43] | Am J Sports Med | Non-Randomized | 61 | mACT | 22 | 32 | 46.0 | 24.7 |
| 58 | mACI | 39 | 35 | 45.0 | 25.6 | |||
| Lim et al., 2012 [77] | Clin Orthop Rel Res | Randomized | 60 | MFX | 30 | 40 | 33.0 | |
| OAT | 22 | 45 | 30.0 | |||||
| pACI | 18 | 44 | 25.0 | |||||
| Macmull et al., 2012 [78] | Int Orthop | Non-Randomized | 66 | cACI | 24 | 29 | 16.0 | |
| mACI | 7 | |||||||
| Macmull et al., 2012 [79] | Am J Sports Med | Non-Randomized | 45 | cACI | 25 | 80 | 35.0 | |
| 35 | mACI | 23 | 61 | 35.0 | ||||
| Niemeyer et al., 2016 [80] | Am J Sports Med | Randomized | 12 | mACI | 25 | 33 | 33.0 | 24.9 |
| mACI | 25 | 16 | 34.0 | 25.6 | ||||
| mACI | 25 | 40 | 34.0 | 25.1 | ||||
| Niemeyer et al., 2019 [27] | Orthop J Sports Med | Randomized | 24 | mACI | 52 | 36 | 36.0 | 25.7 |
| MFX | 50 | 44 | 37.0 | 25.8 | ||||
| Saris et al., 2009 [81] | Am J Sports Med | Randomized | 36 | pACI | 57 | 39 | 33.9 | |
| MFX | 61 | 33 | 33.9 | |||||
| Saris et al., 2014 [82] | Am J Sports Med | Randomized | 24 | mACI | 72 | 37 | 35.0 | 26.2 |
| MFX | 72 | 33.0 | 26.4 | |||||
| Schneider et al., 2016 [83] | J Orthop Surg | Randomized | 12 | MFX | 13 | 50 | 47.0 | |
| MFX | 4 | 37.0 | ||||||
| Skowronski et al., 2013 [55] | Orthop Traumatol Rehab | Non-Randomized | 60 | cACI | 21 | 42 | 26.0 | |
| cACI | 25 | 44 | 26.0 | |||||
| Van Assche et al., 2010 [84] | Knee Surg Sports Traumatol Arthrosc | Randomized | 24 | pACI | 33 | 33 | 31.0 | 24.0 |
| MFX | 34 | 10 | 31.0 | 25.0 | ||||
| Vanlauwe et al., 2011 [85] | Am J Sports Med | Randomized | 60 | MFX | 61 | 20 | 34.0 | |
| pACI | 51 | 43 | 34.0 | |||||
| Volz et al., 2017 [31] | Int Orthop | Randomized | 60 | AMIC | 17 | 29 | 34.0 | 27.4 |
| AMIC | 17 | 11 | 39.0 | 27.6 | ||||
| MFX | 13 | 23 | 40.0 | 25.0 | ||||
| Wolf et al., 2018 [86] | Cartilage | Non-Randomized | 24 | MFX | 18 | 55 | 38.0 | |
| MFX | 3 | 50.0 | ||||||
| Zeifang et al., 2010 [87] | Am J Sports Med | Randomized | 24 | mACI | 11 | 45 | 29.0 | |
| pACI | 10 | 0 | 30.0 |
Table 2.
Patient demographic at baseline
| Treatment | AMIC (N = 103) | cACI (N = 253) | mACI (N = 761) | MFX (N = 619) | OAT (N = 124) | pACI (N = 319) |
|---|---|---|---|---|---|---|
| Follow-up (months) | 56.0 ± 34.1 | 59.7 ± 42.0 | 44.9 ± 18.2 | 45.7 ± 40.6 | 73.0 ± 46.6 | 75.4 ± 58.7 |
| Female (%) | 29.2 ± 14.9 | 43.0 ± 20.1 | 33.8 ± 14.4 | 37.2 ± 17.4 | 35.3 ± 7.6 | 37.7 ± 16.7 |
| Mean age | 36.3 ± 6.1 | 29.0 ± 9.3 | 32.7 ± 7.3 | 34.9 ± 8.4 | 26.0 ± 7.6 | 31.1 ± 2.6 |
| Mean BMI | 27.5 ± 0.2 | 24.0 ± 1.2 | 24.8 ± 1.2 | 25.8 ± 0.9 | 26.1 ± 1.1 | 24.0 ± 1.3 |
| defect size (cm2) | 3.4 ± 0.9 | 4.8 ± 0.7 | 4.2 ± 1.1 | 2.7 ± 0.9 | 3.1 ± 0.4 | 3.9 ± 1.4 |
| Symptoms | 83.6 ± 31.0 | 64.8 ± 30.2 | 30.6 ± 10.1 | 23.5 | 47.4 ± 27.1 | |
| VAS (0–10) | 6.1 ± 0.5 | 5.9 ± 0.5 | 6.3 ± 0.4 | 6.1 | 4.8 | |
| Tegner score | 4.5 ± 0.3 | 3.1 ± 1.6 | 2.4 ± 0.6 | 2.7 | 3.4 ± 1.0 | |
| Lysholm score | 68.8 ± 5.0 | 53.6 ± 1.6 | 61.7 ± 13.7 | 53.5 ± 2.2 | 53.2 | 56.9 ± 6.3 |
| IKDC score | 47.0 | 36.3 | 37.7 ± 6.9 | 36.0 ± 6.5 | 46.2 ± 8.3 |
Outcomes of interest
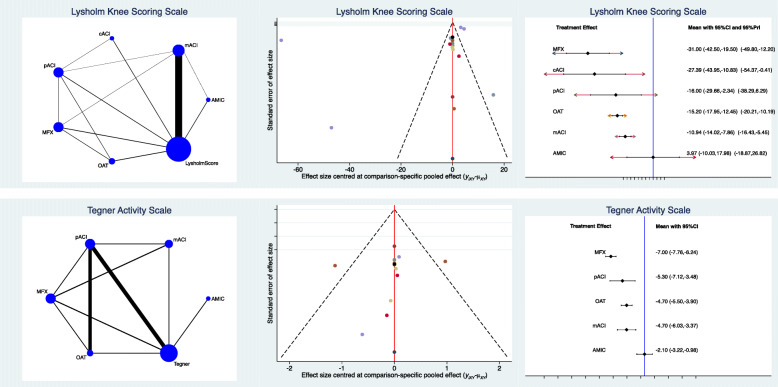
AMIC reported higher Lysholm score (SMD 3.97; 95% CI −10.03 to 17.98) and Tegner score (SMD 2.10; 95% CI −3.22 to −0.98). No statistically significant heterogeneity was found concerning these two endpoints (P > 0.1). Statistically significant inconsistency was found for the comparison IKDC; therefore, no further considerations can be inferred. Edge, funnel and interval plots of the Lysholm and Tegner scores are shown in Fig. 3.
Fig. 3.
Results of Tegner and IKDC scores. The edge plot (left) showed direct and indirect comparisons; greater asymmetries of estimated effects in the funnel plot (middle) correlated with higher risk of bias; the interval plot (right) ranked the final effects of the network comparisons
Complications
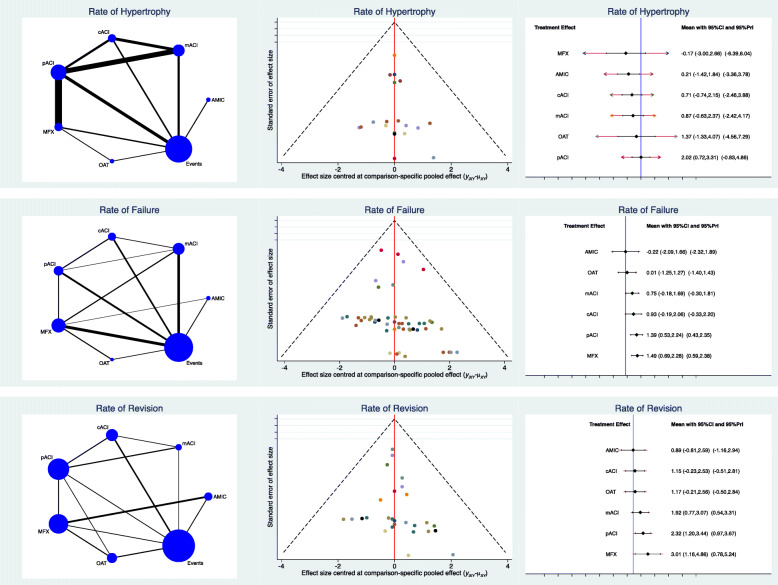
AMIC demonstrated the lowest rate of failures (LOR −0.22; 95% CI −2.09 to 1.66) and the lowest rate of revisions (LOR 0.89; 95% CI −0.81 to 2.59). As expected, MFX showed the lowest rate of hypertrophy (LOR −0.17; 95% CI −3.00 to 2.66) followed by AMIC (LOR 0.21; 95% CI −1.42 to 1.84). No statistically significant inconsistency was found concerning these two endpoints (P > 0.1). Edge, funnel and interval plots of complications are shown in detail in Fig. 4.
Fig. 4.
Results of complications. The edge plot (left) showed direct and indirect comparisons; greater asymmetries of estimated effects in the funnel plot (middle) correlated with higher risk of bias; the interval plot (right) ranked the final effects of the network comparisons
Discussion
According to the present Bayesian network meta-analysis, AMIC procedure for the management for chondral defects of the knee performed better overall at approximately 3 years’ follow-up. Among the ACI procedures, mACI performed better. Patients undergoing pACI reported the highest rate of graft hypertrophy, while MFX performed worst overall.
To the best of our knowledge, only Riboth et al. in 2016 [29] conducted a Bayesian network meta-analysis on surgical strategies for chondral defect of knee. Their study was based on 15 RCTs, involving 855 procedures. In the present study, the number of procedures was greater, as we identified for analysis 21 RCTs and 14 prospective cohort studies with level of evidence II. Differently to Riboth et al. [29], we also implemented the analyses including the rate of failure, included AMIC procedures and analysed separately the results of the Tegner and Lysholm scores. The current literature lacks head-to-head studies that compared AMIC with other surgical techniques for the management of knee chondral defects. AMIC is a single stage technique that avoids the harvesting of non-weightbearing cartilage, cells culture and expansion, exploiting the potential of autologous bone marrow-derived mesenchymal stem cells (MSCs). The nature of the membrane used for AMIC is the same of mACI. Fossum et al. [30] comparing 20 patients treated with AMIC versus 21 patients with cACI, at 2 years’ follow-up, reported no significant differences between the two techniques in terms of Knee injury and Osteoarthritis Outcome Score (KOOS), Lysholm, VAS and rate of TKA. Previous studies have compared AMIC versus MFX for knee chondral defects. Volz et al. [31] compared AMIC versus MFX at 5 years postoperatively. AMIC was an effective cartilage repair procedure with stable clinical results and significantly greater outcome scores than the MFX group [31]. Similar results were found by Chung et al. [32] and Anders et al. [33] at 2 years’ follow-up.
The present Bayesian network meta-analysis certainly has limitations. The limited number of studies and consequently procedures is an important limitation. Chondrocyte culture and expansion methods for ACI among the included studies are heterogeneous. We included all types of surgical approach (arthroscopy, mini-open, arthrotomy), membrane type (collagenic or hyaluronic) and fixation (glue, fibrin, both, none). The influence of these factors has not been yet fully clarified, and further studies are required. Several comparative trials concerning MSCs augmentation for knee chondral defects have been published [34–38]. While MSCs seem to hold great potential for musculoskeletal systems [39–41], to overcome current limitations to clinical translation is still challenging and a deeper understanding of the biological background to optimize tissue neogenesis is required. Thus, given these limitations, studies concerning MSC augmentation were not considered for inclusion. Two studies [42, 43] performed membrane-assisted autologous chondrocyte transplantation (mACT). In the mACT technique, chondrocytes are cultivated and expanded into a membrane in the same fashion of mACI. The chondrocyte-loaded membrane is then carefully transplanted to fill the defect with custom-made instruments in a full-arthroscopic fashion [44, 45]. We included data from this technique in the mACI group and did not analyse them separately. Given the lack of data, it was not possible to analyse the aetiology of chondral defects as separate data sets. Moreover, almost all the included studies did not analyse primary and revision surgeries as separate events. Similarly, most of studies reported data over multiple locations, without differentiation between patella, trochlear, condylar and tibial defects. Finally, many authors combined these techniques with other surgical intervention, such as osteotomy, tibial tubercle transfer and meniscal procedures, and data were not presented separately. Given these limitations, results from the present study should be interpreted with caution. Current evidence concerning chondral procedures augmented with mesenchymal stem cells (MSCs) is still very limited [34–38, 46–55]. The best delivery protocol is still debated, and several different procedures are described through different methodologies with a variable degree of invasiveness, from arthroscopy to mini arthrotomy, or formal arthrotomy [34–36, 46–50, 52–54]. Most articles investigating chondral procedures augmented with MSCs referred to a small sample size and limited length of the follow-up, and the size and location of the chondral defect and the cell delivery protocol are heterogeneous, precluding statistical analysis [1, 56–61]. Moreover, meniscectomy, synovectomy, anterior cruciate ligament repair and high tibial osteotomy were often performed concomitantly [34–38, 46, 50, 52, 53]. Several MSCs sources, culture, expansion and implantation modalities have been described, but seldom compared to one another. Thus, given these limitations, chondral procedures augmented with MSCs were not included. Future studies should overcome these limitations to give new insights and more reliable results.
Conclusion
AMIC procedure as management for focal chondral defects of the knee performed better overall at approximately 3 years’ follow-up.
Acknowledgements
None
Abbreviations
- MFX
Microfractures
- OAT
Osteochondral autograft transplantation
- AMIC
Autologous matrix-induced chondrogenesis
- ACI
Autologous chondrocyte implantation
- pACI
Periosteal autologous chondrocyte implantation
- cACI
Collagen-membrane autologous chondrocyte implantation
- mACI
Matrix-induced autologous chondrocyte implantation
- LOR
Log odds ratio
- SMD
Standardized mean difference
Authors’ contributions
FM: literature search, data extraction, methodological quality assessment, statistical analyses, writing; NM: supervision, revision, final approval; AB: literature search, data extraction, methodological quality assessment; JE: revision; HS, MT: supervision. The authors read and approved the final manuscript.
Funding
No external source of funding was used. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
This study does not contain any third material.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All the authors approved the manuscript.
Competing interests
Professor Maffulli is Editor in Chief of the Journal of Orthopaedic Surgery and Research. The other authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Migliorini F, Berton A, Salvatore G, Candela V, Khan W, Longo UG, Denaro V. Autologous chondrocyte implantation and mesenchymal stem cells for the treatments of chondral defects of the knee- a systematic review. Curr Stem Cell Res Ther. 2020;15(6):547–556. doi: 10.2174/1574888X15666200221122834. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Cho H, Young K, Park J, Lee J, Suh D. In vivo animal study and clinical outcomes of autologous atelocollagen-induced chondrogenesis for osteochondral lesion treatment. J Orthop Surg Res. 2015;10(1):82. doi: 10.1186/s13018-015-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg A, Mitchell K, Soans J, Kim L, Zaidi R. The use of mesenchymal stem cells for cartilage repair and regeneration: a systematic review. J Orthop Surg Res. 2017;12(1):39. doi: 10.1186/s13018-017-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andjelkov N, Hamberg H, Bjellerup P. No outgrowth of chondrocytes from non-digested particulated articular cartilage embedded in commercially available fibrin matrix: an in vitro study. J Orthop Surg Res. 2016;11(1):23. doi: 10.1186/s13018-016-0355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farr J, Gomoll AH. 2016 barriers to cartilage restoration. J Clin Orthop Trauma. 2016;7(3):183–186. doi: 10.1016/j.jcot.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewan AK, Gibson MA, Elisseeff JH, et al. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res Int. 2014;2014:272481. doi: 10.1155/2014/272481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chubinskaya S, Haudenschild D, Gasser S, Stannard J, Krettek C, Borrelli J. Articular Cartilage Injury and Potential Remedies. J Orthop Trauma. 2015;29(Suppl 12):S47–S52. doi: 10.1097/BOT.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1456–1464. doi: 10.1007/s00167-010-1042-3. [DOI] [PubMed] [Google Scholar]
- 9.Chow JC, Hantes ME, Houle JB, et al. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2- to 5-year follow-up study. Arthroscopy. 2004;20(7):681–690. doi: 10.1016/S0749-8063(04)00590-0. [DOI] [PubMed] [Google Scholar]
- 10.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. doi: 10.1016/S0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 11.Drongowski RA, Coran AG, Wojtys EM. Predictive value of meniscal and chondral injuries in conservatively treated anterior cruciate ligament injuries. Arthroscopy. 1994;10(1):97–102. doi: 10.1016/S0749-8063(05)80299-3. [DOI] [PubMed] [Google Scholar]
- 12.Arshi A, Fabricant PD, Go DE, Williams RJ, McAllister DR, Jones KJ. Can biologic augmentation improve clinical outcomes following microfracture for symptomatic cartilage defects of the knee? A systematic review. Cartilage. 2018;9(2):146–155. doi: 10.1177/1947603517746722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman SL, Thyssen E, Nuelle CW. Osteochondral autologous transplantation. Clin Sports Med. 2017;36(3):489–500. doi: 10.1016/j.csm.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 15.Minas T, Ogura T, Bryant T. Autologous chondrocyte implantation. JBJS Essent Surg Tech. 2016;6(2):e24. doi: 10.2106/JBJS.ST.16.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 17.Ebert JR, Robertson WB, Lloyd DG, Zheng MH, Wood DJ, Ackland T. A prospective, randomized comparison of traditional and accelerated approaches to postoperative rehabilitation following autologous chondrocyte implantation: 2-year clinical outcomes. Cartilage. 2010;1(3):180–187. doi: 10.1177/1947603510362907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC): combining microfracturing and a collagen I/III matrix for articular cartilage resurfacing. Cartilage. 2010;1(1):65–68. doi: 10.1177/1947603509360044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. 2019;47(1):222–231. doi: 10.1177/0363546517740575. [DOI] [PubMed] [Google Scholar]
- 20.Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC) Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2109–2115. doi: 10.1007/s00167-011-1840-2. [DOI] [PubMed] [Google Scholar]
- 21.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JPA, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 22.Howick JCI, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M. The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine. 2011. [Google Scholar]
- 23.Higgins LD, Taylor MK, Park D, Ghodadra N, Marchant M, Pietrobon R, Cook C, International Knee Documentation Committee Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine. 2007;74(6):594–599. doi: 10.1016/j.jbspin.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890–897. doi: 10.1177/0363546508330143. [DOI] [PubMed] [Google Scholar]
- 25.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 26.Ogura T, Merkely G, Bryant T, et al. Autologous chondrocyte implantation “segmental-sandwich” technique for deep osteochondral defects in the knee: clinical outcomes and correlation with magnetic resonance imaging findings. Orthop J Sports Med. 2019;7:2325967119847173. doi: 10.1177/2325967119847173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niemeyer P, Laute V, Zinser W, et al. A prospective, randomized, open-Label, multicenter, phase III noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus srthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. 2019;7:2325967119854442. doi: 10.1177/2325967119854442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoburg A, Loer I, Korsmeier K, et al. Matrix-associated autologous chondrocyte implantation is an effective treatment at midterm follow-up in adolescents and young adults. Orthop J Sports Med. 2019;7:2325967119841077. doi: 10.1177/2325967119841077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riboh JC, Cvetanovich GL, Cole BJ, Yanke AB. Comparative efficacy of cartilage repair procedures in the knee: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3786–3799. doi: 10.1007/s00167-016-4300-1. [DOI] [PubMed] [Google Scholar]
- 30.Fossum V, Hansen AK, Wilsgaard T, et al. Collagen-covered autologous chondrocyte implantation versus autologous matrix-induced chondrogenesis: a randomized trial comparing 2 methods for repair of cartilage defects of the knee. Orthop J Sports Med. 2019;7:2325967119868212. doi: 10.1177/2325967119868212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years. Int Orthop. 2017;41(4):797–804. doi: 10.1007/s00264-016-3391-0. [DOI] [PubMed] [Google Scholar]
- 32.Chung JY, Lee DH, Kim TH, Kwack KS, Yoon KH, Min BH. Cartilage extra-cellular matrix biomembrane for the enhancement of microfractured defects. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1249–1259. doi: 10.1007/s00167-013-2716-4. [DOI] [PubMed] [Google Scholar]
- 33.Anders S, Volz M, Frick H, Gellissen J. A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC(R)) to microfracture: analysis of 1- and 2-year follow-Up data of 2 Centers. Open Orthop J. 2013;7(1):133–143. doi: 10.2174/1874325001307010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teo BJ, Buhary K, Tai BC, et al. Cell-based therapy improves function in adolescents and young adults with patellar osteochondritis dissecans. Clin Orthop Relat Res. 2013;471(4):1152–1158. doi: 10.1007/s11999-012-2338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 36.Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32(1):97–109. doi: 10.1016/j.arthro.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Gobbi A, Scotti C, Karnatzikos G, Mudhigere A, Castro M, Peretti GM. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2494–2501. doi: 10.1007/s00167-016-3984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akgun I, Unlu MC, Erdal OA, Ogut T, Erturk M, Ovali E, Kantarci F, Caliskan G, Akgun Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135(2):251–263. doi: 10.1007/s00402-014-2136-z. [DOI] [PubMed] [Google Scholar]
- 39.Longo UG, Rizzello G, Berton A, Ciuffreda M, Migliorini F, Khan W, Denaro V. Potential of adipose derived stem cells in orthopaedic surgery. Curr Stem Cell Res Ther. 2013;8(6):418–421. doi: 10.2174/1574888X1130800058. [DOI] [PubMed] [Google Scholar]
- 40.Migliorini F, Rath B, Colarossi G, et al. Improved outcomes after mesenchymal stem cells injections for knee osteoarthritis: results at 12-months follow-up: a systematic review of the literature. Arch Orthop Trauma Surg. 2019. [DOI] [PubMed]
- 41.Migliorini F, Rath B, Tingart M, Baroncini A, Quack V, Eschweiler J. Autogenic mesenchymal stem cells for intervertebral disc regeneration. Int Orthop. 2019;43(4):1027–1036. doi: 10.1007/s00264-018-4218-y. [DOI] [PubMed] [Google Scholar]
- 42.Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37(1):33–41. doi: 10.1177/0363546508323256. [DOI] [PubMed] [Google Scholar]
- 43.Kon E, Filardo G, Condello V, Collarile M, di Martino A, Zorzi C, Marcacci M. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med. 2011;39(8):1668–1675. doi: 10.1177/0363546511404675. [DOI] [PubMed] [Google Scholar]
- 44.Filardo G, Kon E, Andriolo L, di Matteo B, Balboni F, Marcacci M. Clinical profiling in cartilage regeneration: prognostic factors for midterm results of matrix-assisted autologous chondrocyte transplantation. Am J Sports Med. 2014;42(4):898–905. doi: 10.1177/0363546513518552. [DOI] [PubMed] [Google Scholar]
- 45.Filardo G, Kon E, Di Martino A, et al. Arthroscopic second-generation autologous chondrocyte implantation: a prospective 7-year follow-up study. Am J Sports Med. 2011;39(10):2153–2160. doi: 10.1177/0363546511415658. [DOI] [PubMed] [Google Scholar]
- 46.Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92(Suppl 2):2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 47.Buda R, Baldassarri M, Perazzo L, Ghinelli D, Pagliazzi G. A useful combination for the treatment of patellofemoral chondral lesions: realignment procedure plus mesenchymal stem cell-retrospective analysis and clinical results at 48 months of follow-up. Eur J Orthop Surg Traumatol. 2019;29(2):461–470. doi: 10.1007/s00590-018-2310-z. [DOI] [PubMed] [Google Scholar]
- 48.de Windt TS, Vonk LA, Slaper-Cortenbach IC, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35(1):256–264. doi: 10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
- 49.de Windt TS, Vonk LA, Slaper-Cortenbach ICM, Nizak R, van Rijen MHP, Saris DBF. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: a first-in-man trial in 35 patients. Stem Cells. 2017;35(8):1984–1993. doi: 10.1002/stem.2657. [DOI] [PubMed] [Google Scholar]
- 50.Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Kaps C, Gigante A. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee. 2013;20(6):562–569. doi: 10.1016/j.knee.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Gigante A. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee. 2015;22(1):30–35. doi: 10.1016/j.knee.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286–299. doi: 10.1177/1947603510392023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648–657. doi: 10.1177/0363546513518007. [DOI] [PubMed] [Google Scholar]
- 54.Haleem AM, Singergy AA, Sabry D, et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1(4):253–261. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skowronski J, Rutka M. Osteochondral lesions of the knee reconstructed with mesenchymal stem cells - results. Ortop Traumatol Rehabil. 2013;15:195–204. doi: 10.5604/15093492.1058409. [DOI] [PubMed] [Google Scholar]
- 56.Cavinatto L, Hinckel BB, Tomlinson RE, Gupta S, Farr J, Bartolozzi AR. The role of bone marrow aspirate concentrate for the treatment of focal chondral lesions of the knee: a systematic review and critical analysis of animal and clinical studies. Arthroscopy. 2019;35(6):1860–1877. doi: 10.1016/j.arthro.2018.11.073. [DOI] [PubMed] [Google Scholar]
- 57.Chahla J, Dean CS, Moatshe G, et al. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4:2325967115625481. doi: 10.1177/2325967115625481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717–1729. doi: 10.1007/s00167-012-2329-3. [DOI] [PubMed] [Google Scholar]
- 59.Anderson JA, Little D, Toth AP, Moorman CT, III, Tucker BS, Ciccotti MG, Guilak F. Stem cell therapies for knee cartilage repair: the current status of preclinical and clinical studies. Am J Sports Med. 2014;42(9):2253–2261. doi: 10.1177/0363546513508744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38(6):1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- 61.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519–527. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 63.Becher C, Laute V, Fickert S, Zinser W, Niemeyer P, John T, Diehl P, Kolombe T, Siebold R, Fay J. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J Orthop Surg Res. 2017;12(1):71. doi: 10.1186/s13018-017-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berruto M, Ferrua P, Pasqualotto S, Uboldi F, Maione A, Tradati D, Usellini E. Long-term follow-up evaluation of autologous chondrocyte implantation for symptomatic cartilage lesions of the knee: a single-centre prospective study. Injury. 2017;48(10):2230–2234. doi: 10.1016/j.injury.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Bode G, Schmal H, Pestka JM, Ogon P, Südkamp NP, Niemeyer P. A non-randomized controlled clinical trial on autologous chondrocyte implantation (ACI) in cartilage defects of the medial femoral condyle with or without high tibial osteotomy in patients with varus deformity of less than 5 degrees. Arch Orthop Trauma Surg. 2013;133(1):43–49. doi: 10.1007/s00402-012-1637-x. [DOI] [PubMed] [Google Scholar]
- 66.Brittberg M, Recker D, Ilgenfritz J, Saris DBF, on behalf of the SUMMIT Extension Study Group Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46(6):1343–1351. doi: 10.1177/0363546518756976. [DOI] [PubMed] [Google Scholar]
- 67.Cvetanovich GL, Riboh JC, Tilton AK, Cole BJ. Autologous chondrocyte implantation improves knee-specific functional outcomes and health-related quality of life in adolescent patients. Am J Sports Med. 2017;45(1):70–76. doi: 10.1177/0363546516663711. [DOI] [PubMed] [Google Scholar]
- 68.de Girolamo L, Schonhuber H, Vigano M, et al. 2019. Autologous matrix-induced chondrogenesis (AMIC) and AMIC enhanced by autologous concentrated bone marrow aspirate (BMAC) allow for stable clinical and functional improvements at up to 9 years follow-up: results from a randomized controlled study. J Clin Med 8. [DOI] [PMC free article] [PubMed]
- 69.Ebert JR, Fallon M, Smith A, Janes GC, Wood DJ. Prospective clinical and radiologic evaluation of patellofemoral matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2015;43(6):1362–1372. doi: 10.1177/0363546515574063. [DOI] [PubMed] [Google Scholar]
- 70.Ferruzzi A, Buda R, Faldini C, et al. Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique. Comparison at a minimum follow-up of five years. J Bone Joint Surg Am. 2008;90(Suppl 4):90–101. doi: 10.2106/JBJS.H.00633. [DOI] [PubMed] [Google Scholar]
- 71.Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee. 2006;13(3):203–210. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 72.Gudas R, Stankevicius E, Monastyreckiene E, et al. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834–842. doi: 10.1007/s00167-006-0067-0. [DOI] [PubMed] [Google Scholar]
- 73.Gudas R, Simonaityte R, Cekanauskas E, et al. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop. 2009;29(7):741–748. doi: 10.1097/BPO.0b013e3181b8f6c7. [DOI] [PubMed] [Google Scholar]
- 74.Gudas R, Gudaite A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499–2508. doi: 10.1177/0363546512458763. [DOI] [PubMed] [Google Scholar]
- 75.Horas U, Pelinkovic D, Herr G, et al. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 76.Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Ludvigsen TC, Løken S, Solheim E, Strand T, Johansen O. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am. 2016;98(16):1332–1339. doi: 10.2106/JBJS.15.01208. [DOI] [PubMed] [Google Scholar]
- 77.Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470(8):2261–2267. doi: 10.1007/s11999-012-2304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macmull S, Jaiswal PK, Bentley G, Skinner JA, Carrington RWJ, Briggs TWR. The role of autologous chondrocyte implantation in the treatment of symptomatic chondromalacia patellae. Int Orthop. 2012;36(7):1371–1377. doi: 10.1007/s00264-011-1465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macmull S, Parratt MT, Bentley G, et al. Autologous chondrocyte implantation in the adolescent knee. Am J Sports Med. 2011;39(8):1723–1730. doi: 10.1177/0363546511404202. [DOI] [PubMed] [Google Scholar]
- 80.Niemeyer P, Laute V, John T, Becher C, Diehl P, Kolombe T, Fay J, Siebold R, Niks M, Fickert S, Zinser W. The effect of cell dose on the early magnetic resonance morphological outcomes of autologous cell implantation for articular cartilage defects in the knee: a randomized clinical trial. Am J Sports Med. 2016;44(8):2005–2014. doi: 10.1177/0363546516646092. [DOI] [PubMed] [Google Scholar]
- 81.Saris DB, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10S–19S. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 82.Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, Emans P, Podskubka A, Tsuchida A, Kili S, Levine D, Brittberg M, on behalf of the SUMMIT study group. Paša L, Trc T, Slynarski K, Sanson BJ, Bezuidenhoudt M. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42(6):1384–1394. doi: 10.1177/0363546514528093. [DOI] [PubMed] [Google Scholar]
- 83.Schneider U. Controlled, randomized multicenter study to compare compatibility and safety of ChondroFiller liquid (cell free 2-component collagen gel) with microfracturing of patients with focal cartilage defects of the knee joint. J Ortop Surg. 2016;1:1–8. [Google Scholar]
- 84.Van Assche D, Staes F, Van Caspel D, et al. Autologous chondrocyte implantation versus microfracture for knee cartilage injury: a prospective randomized trial, with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):486–495. doi: 10.1007/s00167-009-0955-1. [DOI] [PubMed] [Google Scholar]
- 85.Vanlauwe J, Saris DB, Victor J, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39(12):2566–2574. doi: 10.1177/0363546511422220. [DOI] [PubMed] [Google Scholar]
- 86.Wolf MT, Zhang H, Sharma B, Marcus NA, Pietzner U, Fickert S, Lueth A, Albers GHR, Elisseeff JH. Two-year follow-up and remodeling kinetics of ChonDux hydrogel for full-thickness cartilage defect repair in the knee. Cartilage. 2020;11(4):447–457. doi: 10.1177/1947603518800547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38(5):924–933. doi: 10.1177/0363546509351499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study does not contain any third material.