Abstract
B-cell receptor signaling inhibition by targeting Bruton tyrosine kinase (BTK) is effective in treating chronic lymphocytic leukemia. The BTK inhibitor ibrutinib may be intolerable for some patients. Acalabrutinib is a more selective BTK inhibitor that may be better tolerated by patients who are intolerant to ibrutinib. A phase II study of acalabrutinib was conducted in patients with relapsed/refractory chronic lymphocytic leukemia who were ibrutinib-intolerant and had continued disease activity. Intolerance was defined as having discontinued ibrutinib due to persistent grade 3/4 adverse events or persistent/recurrent grade 2 adverse events despite dose modification/interruption. Patients received oral acalabrutinib 100 mg twice daily until disease progression or intolerance. Sixty patients were treated. The overall response rate to acalabrutinib was 73% and three patients (5%) achieved complete remission. At a median follow-up of 35 months, the median progression-free and overall survival were not reached; 24-month estimates were 72% and 81%, respectively. The most frequent adverse events with acalabrutinib were diarrhea (53%), headache (42%), contusion (40%), dizziness (33%), upper respiratory tract infection (33%), and cough (30%). The most common reasons for acalabrutinib discontinuation were progressive disease (23%) and adverse events (17%). Most patients with baseline samples (49/52; 94%) and all with on-treatment samples (3/3; 100%) had no detectable BTK and/or PLCG2 mutations. Acalabrutinib is effective and tolerable in most patients with relapsed/refractory chronic lymphocytic leukemia who are intolerant of ibrutinib. Acalabrutinib may be useful for patients who may benefit from BTK inhibitor therapy but are ibrutinib intolerant. ClinicalTrials.gov identifier: NCT02717611.
Introduction
Targeted Bruton tyrosine kinase (BTK) inhibitors are highly effective for the treatment of chronic lymphocytic leukemia (CLL).1 These agents block signaling by inhibiting BTK, a key kinase in the B-cell receptor signaling pathway.2-4 The efficacy of BTK inhibition in CLL was demonstrated by ibrutinib, the first BTK inhibitor approved for treatment of CLL.5
Ibrutinib is not always tolerated by patients with CLL. In a large, retrospective study of ibrutinib-treated CLL, toxicity was the most common reason for treatment discontinuation, accounting for 63.1% of discontinuations in the front-line setting and 50.2% of discontinuations among patients with relapsed/refractory CLL.6 The most common toxicities leading to discontinuation were arthralgia (41.6%), atrial fibrillation (25.0%), and rash (16.7%) in the front-line setting and atrial fibrillation (12.3%), infection (10.7%), and pneumonitis (9.9%) in relapsed/refractory CLL. These toxicities may be due to BTK inhibition or off-target effects of ibrutinib on other kinases.2,5,7 Some toxicities can be managed with supportive care and some require ibrutinib discontinuation, especially if more severe. For example, current guidelines recommend careful monitoring in the case of atrial fibrillation, and potential use of non-warfarin anticoagulation, although consideration should be given to alternate therapies if the atrial fibrillation is uncontrolled.8 Rates of ibrutinib discontinuation due to adverse events during extended follow-up in a clinical trial population are approximately 20%.9 In CLL patients treated outside of clinical trials at academic and community sites, discontinuation rates due to adverse events were as high as 50%, which may better capture tolerability in a general practice setting.6 This means that patients who cannot take ibrutinib due to toxicity may not be able to realize the potential benefit of BTK inhibition on their disease, thereby reducing therapeutic options available for CLL treatment.
Acalabrutinib is an oral covalent inhibitor of BTK approved for treatment of patients with CLL.10 Acalabrutinib binds to BTK at the cysteine 481 residue, which is the same binding site as that for ibrutinib.11 Compared with ibrutinib, acalabrutinib is a more selective BTK inhibitor.12,13 Fewer off-target effects potentially provide an improved safety profile compared with ibrutinib. 5,14 A low frequency of adverse events of interest, specifically atrial fibrillation and severe bleeding, has been reported with acalabrutinib.11 In a phase III trial in patients with relapsed/refractory CLL (ASCEND), atrial fibrillation occurred in eight of 154 patients (5%) receiving acalabrutinib monotherapy, seven of whom had a history of ongoing hypertension. Bleeding and infections (any grade), also events of clinical interest, occurred in 40 (26%) and 87 (57%) patients, respectively. In that trial, 11% of patients receiving acalabrutinib monotherapy discontinued this treatment because of adverse events.15
Given the improved selectivity of acalabrutinib relative to ibrutinib, we hypothesized that acalabrutinib would be effective and tolerable in patients with CLL who discontinued ibrutinib due to adverse events. This hypothesis is supported by a previous study in which the overall response rate (ORR; i.e., partial response [PR] or better) to acalabrutinib was 61% in patients with relapsed/refractory CLL who were previously unable to continue ibrutinib treatment because of adverse events.16 However, the previous study did not objectively define events classified as ibrutinib-intolerant and analyzed a cohort of patients added to the open-label, phase II, dose-expansion portion of the phase I/II study.16 We therefore conducted a dedicated phase II study of acalabrutinib in patients with relapsed/refractory CLL who were intolerant to ibrutinib treatment as defined by specific criteria, including event grade, persistence, and recurrence.
Methods
Study design and participants
This multicenter, single-agent, phase II study (ClinicalTrials.gov identifier NCT02717611; ACE-CL-208) enrolled adults with CLL who were intolerant to ibrutinib and for whom purine analogue-based therapy was not an option. Ibrutinib intolerance was defined as: (i) having discontinued ibrutinib treatment due to grade 3 or 4 adverse events that persisted despite optimal supportive care; or (ii) having experienced grade 2 adverse events related to ibrutinib treatment that persisted for at least 2 weeks or recurred at least twice, whether the dose of ibrutinib was reduced or interrupted, despite optimal supportive care. Patients had to have had at least one prior attempt at ibrutinib treatment for CLL and not be appropriate for treatment or retreatment with purine analogue-based therapy (e.g., fludarabine). After discontinuing ibrutinib, patients had to meet the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) 2008 criteria for progressive disease (PD)17 as a sign of continued disease activity and not have received other CLL therapy.
To meet the eligibility criteria, patients’ most recent systemic anticancer therapy was required to be ibrutinib; those who received an alternative anticancer therapy after ibrutinib discontinuation were excluded. Patients were excluded if they had an ongoing grade 3 or 4 adverse event attributed to ibrutinib. Other patients who were excluded were those with evidence of active Richter transformation or any evidence of PD on ibrutinib; patients who had previously received a BCL-2 inhibitor; patients who had significant cardiovascular disease, such as uncontrolled or symptomatic untreated arrhythmias, congestive heart failure, or myocardial infarction within 6 months of screening, or any class 3 or 4 cardiac disease as defined by the New York Heart Association functional classification or QTc >480 ms at screening (except for controlled, asymptomatic atrial fibrillation during screening, which was allowed); and patients who were receiving anticoagulation with warfarin or equivalent vitamin K antagonists within 7 days of the first study drug dose. Patients taking other anticoagulants could be included.
All patients signed written informed consent before enrollment into the study, which was approved by the institutional review board/independent ethics committee of each participating institution and conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice.
Procedures
Eligible patients were treated with acalabrutinib 100 mg orally twice a day on days 1 to 28 of 28-day cycles until disease progression, as long as treatment was tolerated. Response was assessed according to modified iwCLL 2008 criteria,17 with the first assessment occurring 3 months after starting acalabrutinib. Adverse events were collected and graded according to Common Terminology Criteria for Adverse Events version 4.03.
An exploratory analysis of molecular resistance to BTK inhibitors was performed retrospectively using deep sequencing of BTK and PLCG2 in patients with pretreatment samples18,19 (details in the Online Supplementary Methods).
Outcomes
The primary endpoint was investigator-assessed ORR according to iwCLL 2008 criteria.17 ORR was defined as the proportion of patients achieving a best overall response of either complete remission (CR), complete remission with incomplete bone marrow recovery (CRi), nodular partial remission (nPR), or PR at or before initiation of subsequent anticancer therapy. Secondary efficacy endpoints were duration of response (DOR; defined as the time from the initial response [CR, CRi, nPR, or PR] until documented PD), progression-free survival (PFS; defined as the time from first dose to first documented PD or death), time to next treatment (TTNT; defined as the time from first dose to institution of subsequent anticancer therapy for CLL or death), and overall survival (OS; defined as the time from first dose to death). Safety was assessed via laboratory assessments and adverse events by their frequency, and causal attribution.
Details of the statistical analysis can be found in the Online Supplementary Methods.
Results
Patients, treatment, and disposition
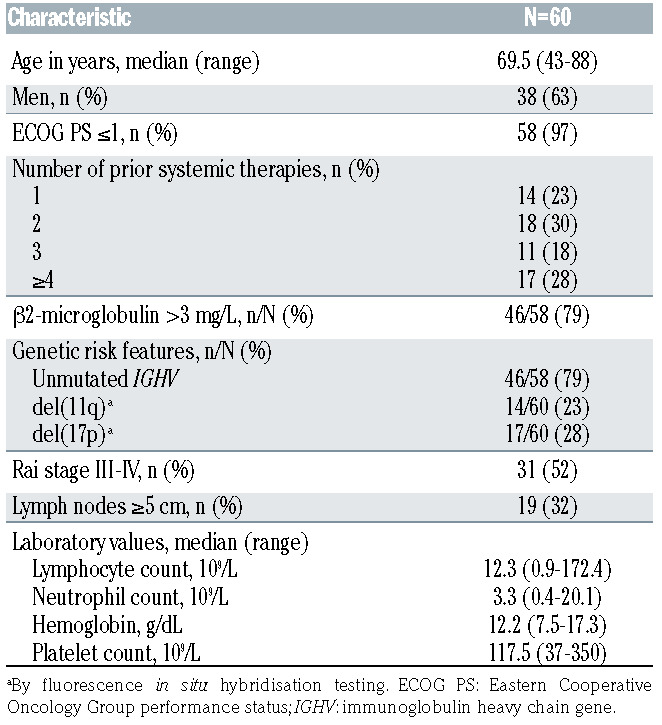
In total, 60 patients were enrolled between March 23, 2016, and August 2, 2017. Their median age was 69.5 years (range, 43-88 years) and the median time from diagnosis to first dose of study drug was 103.2 months (range, 10.3-307.9 months). Seventeen (28%) patients had del(17p) and 31 (52%) had Rai stage III or IV disease. The baseline patient and disease characteristics are shown in Table 1.
The median number of prior therapies was two (range, 1-10). All patients had taken ibrutinib previously, with 50 (83%) having received ibrutinib as monotherapy and ten (17%) having received ibrutinib in combination with another agent (Online Supplementary Table S1). Forty-three (72%) patients had been exposed to an anti-CD20 monoclonal antibody and 36 (60%) had received prior systemic chemotherapy (Online Supplementary Table S1).
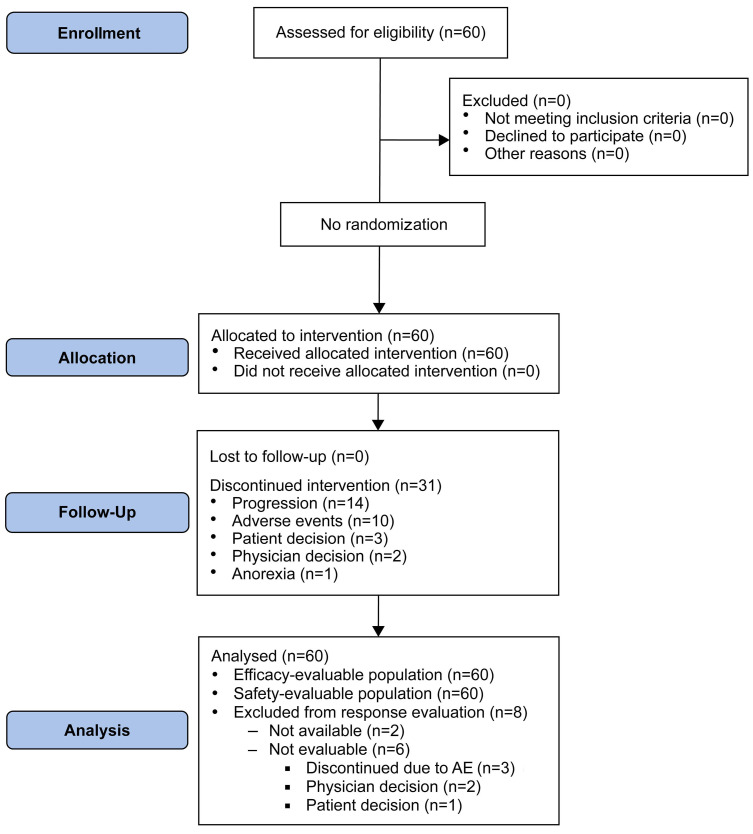
The median duration of ibrutinib treatment was 5.7 months (range, <1-55.5). Out of total of 60 patients, 15 (25%) received ibrutinib for <2 months. Of these 15 patients, only two discontinued acalabrutinib (due to squamous cell carcinoma of the lung and endometrial cancer [n=1 each]). As ibrutinib treatment occurred before study entry, treatment response to ibrutinib was not fully captured for the entire patient population (safety, however, was captured). The most common adverse events leading to ibrutinib discontinuation were atrial fibrillation (23%), diarrhea (12%), arthralgia (10%), and rash (10%) (Online Supplementary Table S2). After ibrutinib discontinuation, the median time from taking the last dose of ibrutinib to starting acalabrutinib was 7.5 months (range, 0.8-31.1). At a median follow-up of 34.6 months (range, 1.1-47.4), 29 (48%) patients remained on acalabrutinib; 45 patients (75%) had at least 1 year of treatment. The median time exposed to acalabrutinib was 32 months (range, 0.3-47.4). Of the 31 patients who discontinued acalabrutinib, the most common reason for discontinuation was disease progression (n=14, 23%) followed by adverse events (n=10, 17%); other reasons were patient or physician decision (n=3 and n=3, respectively), and comorbid anorexia (n=1) (Figure 1). For the 14 patients who discontinued due to disease progression, 11 patients had an Eastern Cooperative Oncology Group performance status of 1, seven had Rai stage III-IV disease, and their median age was 72 years. Four of these 15 patients had del(17p), four had del(11q), and 12 had unmutated IGHV.
Efficacy
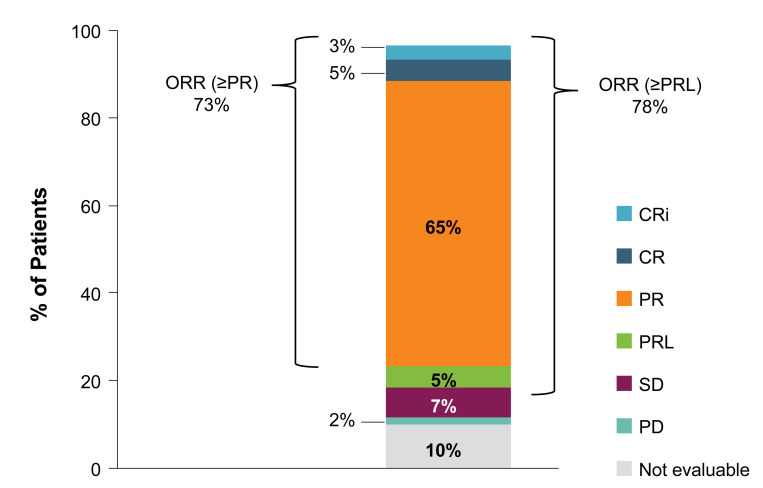
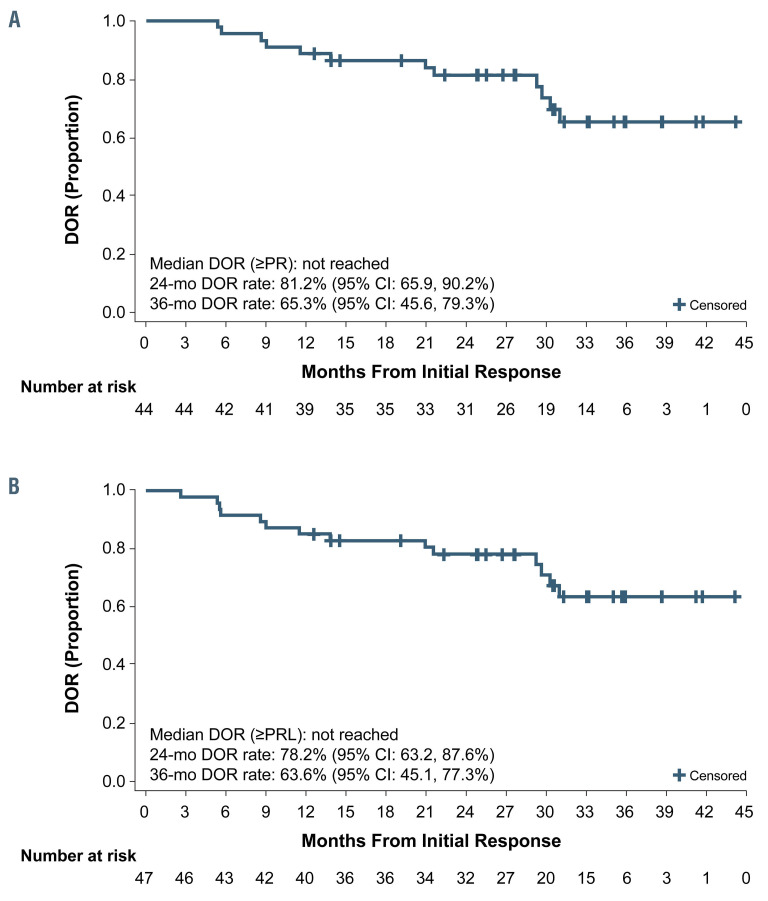
The ORR to acalabrutinib treatment was 73% (n=44/60; 95% confidence interval [95% CI]: 60-84%) (Figure 2). The ORR in patients with del(17p) was similar (71% [n=12/17]; 95% CI: 44-90%). The ORR including patients with PR with lymphocytosis (PRL) was 78% (n=47/60; 95% CI: 66-88%), comprising three (5%) patients with a CR, two (3%) with CRi, 39 (65%) with a PR, and three (5%) with a PRL. Of the 13 patients not achieving a response, four (7%) had stable disease (SD), one (2%) had PD; six (10%) patients were not evaluable for response due to discontinuing treatment before the first response assessment at 3 months, and two (3%) were not available for response assessment. For the six patients who were not evaluable, three discontinued due to adverse events and three discontinued due to patient or physician decision (1 and 2 patients, respectively) (Figure 1). The median DOR was not reached; the estimated 24-month DOR was 81% (n=44, 95% CI: 66-90%) and 78% (n=47, 95% CI: 63-88%) when patients with PRL were included, and the estimated 36-month DOR was 65% (95% CI: 46-79%) and 64% (95% CI: 45-77%) when patients with PRL were included (Figure 3A and B, respectively).
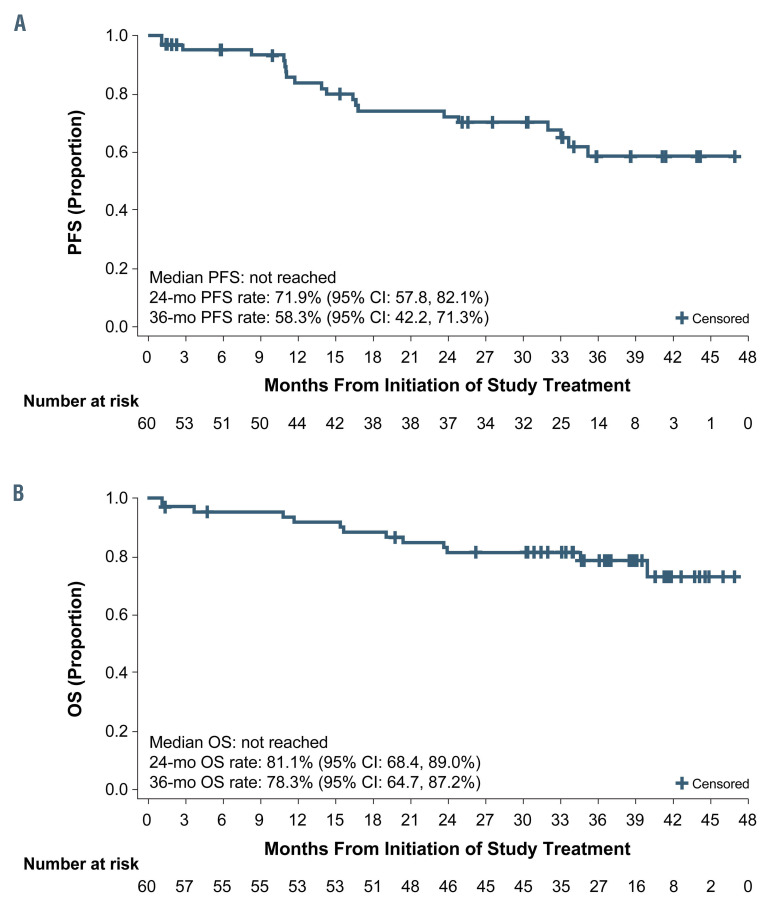
The median PFS was not reached; estimated 24-month and 36-month PFS rates were 72% (95% CI: 58-82%) and 58% (95% CI: 42-71%), respectively (Figure 4). The median OS was not reached. Estimated 24-month and 36- month OS rates were 81% (95% CI: 68-89%) and 78% (95% CI: 65-87%), respectively (Figure 4). Sixteen (27%) patients started a subsequent treatment for CLL, and the median TTNT was 44 months (95% CI: 27-not estimable) (Online Supplementary Figure S1).
The efficacy (ORR, DOR, PFS) of acalabrutinib was also assessed by duration of previous ibrutinib treatment and by duration of treatment hold (time from ibrutinib discontinuation to start of acalabrutinib). These assessments were exploratory, and no statistical analyses were performed. The ORR was 64% (n=20/31; 95% CI: 45-81%) in patients who received prior ibrutinib treatment for ≥6 months and 83% (n=24/29; 95% CI: 64-94%) in those who received ibrutinib for <6 months. DOR and PFS on acalabrutinib in patients who received prior ibrutinib treatment for ≥6 months trended towards being shorter (no statistical analyses were performed) (Online Supplementary Figure S2 A, C, and E). Duration of treatment hold did not appear to affect ORR, DOR, or PFS during acalabrutinib treatment (Online Supplementary Figure S2 B, D, and F).
Table 1.
Patient’s baseline.
Safety
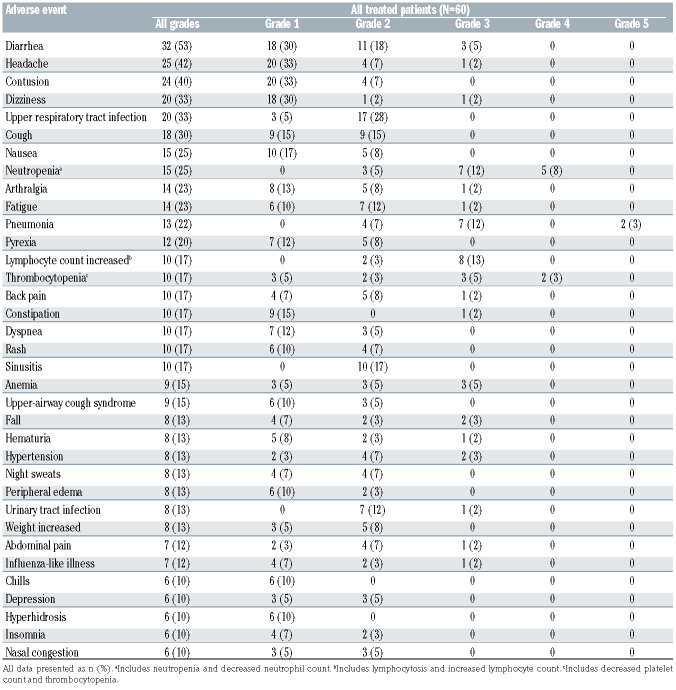
The most frequent adverse events of any grade occurring with acalabrutinib were diarrhea (n=32, 53%), headache (n=25, 42%), contusion (n=24, 40%), dizziness (n=20, 33%), upper respiratory tract infection (n=20, 33%), and cough (n=18, 30%) (Table 2 and Online Supplementary Table S3). The most frequent grade ≥3 adverse events were pneumonia (n=9, 15%), neutropenia (n=7, 12%), increased lymphocyte count (including lymphocytosis and lymphocyte count increased; n=8, 13%), and thrombocytopenia (including platelet count decreased and thrombocytopenia; n=5, 8%) (Table 2). Serious adverse events of any grade were experienced by 31 (52%) patients. Treatment-related severe adverse events of any grade that were deemed related to acalabrutinib by the investigator were experienced by ten (17%) patients. There were five dose reductions in four patients; one patient had two dose reductions due to vaginal yeast infection. All four patients had successful adverse event management with dose reduction and continued on study. However, one of these patients later discontinued acalabrutinib due to adverse events.
Ten (16.7%) patients had an adverse event leading to acalabrutinib discontinuation, including pneumonia (n=2, 1 grade 3 event and 1 death), diarrhea (n=1, grade 2), headache (n=1, grade 1), endometrial cancer (n=1, grade 3), stomatitis (n=1, grade 2), subdural hematoma (n=1, grade 2), cerebrovascular accident (n=1, grade 2), increased transaminases (n=1, grade 4), and squamous cell carcinoma of lung (n=1, grade 2). Among these events, the investigator considered diarrhea, headache, stomatitis, and subdural hematoma to be related to the acalabrutinib treatment. Only one patient discontinued acalabrutinib due to the same adverse event (diarrhea) that resulted in prior ibrutinib discontinuation; grade 3 or 4 diarrhea led to ibrutinib discontinuation and grade 2 diarrhea led to acalabrutinib discontinuation.
To better understand acalabrutinib tolerability following ibrutinib discontinuation, the incidence of ibrutinibintolerance adverse events was examined during acalabrutinib treatment. Among 60 enrolled patients, 27 ibrutinib- intolerance adverse events occurred in 24 (40%) patients during acalabrutinib treatment. Of these, most events (67% [n=18/27 events in 18 patients) were lower grade on acalabrutinib than on prior ibrutinib treatment; 30% (n=8/27 events in 6 patients) were of an unchanged grade (Online Supplementary Table S4). Only one event (4% [n=1/27] events in 1 patient) was of a higher grade during acalabrutinib treatment than during prior ibrutinib treatment. This event was increased liver function test (grade 2 on ibrutinib and grade 3 during acalabrutinib treatment). Two patients treated with acalabrutinib had recurrence of the same ibrutinib-intolerance adverse event of atrial fibrillation, both of whom had atrial fibrillation events with a lower severity grade on acalabrutinib treatment (grade 3/2 and grade 2/1 during ibrutinib/acalabrutinib treatment). One of the two patients had a medical history of atrial fibrillation and hypertension and discontinued acalabrutinib treatment due to pneumonia that started the same day as atrial fibrillation. The second patient had a medical history of atrial fibrillation; at the time of data analysis, treatment with acalabrutinib was ongoing in the presence of ongoing atrial fibrillation (the dose was not changed and no other action was taken).
Figure 1.
Trial profile. AE: adverse events.
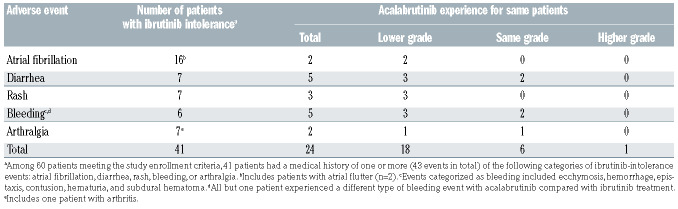
Among 60 enrolled patients, during ibrutinib treatment, 41 patients had the following ibrutinib-intolerance adverse events: arthralgia, atrial fibrillation, bleeding, diarrhea, or rash (Table 3, Online Supplementary Table S5). Of the 74 ibrutinib-intolerance adverse events in the 60 enrolled patients, 42 (57%) did not recur during acalabrutinib treatment. Eighteen (30%) patients treated with acalabrutinib had recurrence of the same ibrutinib-intolerance adverse event. The most common ibrutinib-intolerance adverse events recurring with acalabrutinib were diarrhea (n=5) and bleeding events (n=5), all of which had the same or lower severity grade with acalabrutinib treatment. Among the five patients with recurrent bleeding events, only one patient had the same type of bleeding event reported with ibrutinib and acalabrutinib (recurrent hematuria); the other four patients had bleeding events with ibrutinib that were different from those reported on acalabrutinib (Online Supplementary Table S4).
Eleven deaths occurred during the study; the causes included pneumonia (n=3), Richter transformation (n=2), bronchopulmonary aspergillosis, ventricular fibrillation, squamous cell carcinoma of lung, multiple organ dysfunction syndrome, disease progression, and death (n=1 each). Of the seven deaths due to adverse events, two occurred while the patient was receiving study treatment (1 each, pneumonia and subdural hematoma), and the remainder occurred following acalabrutinib discontinuation. Of note, an 85-year-old male patient with multiple cardiac morbidities died of ventricular fibrillation 27 days after acalabrutinib was discontinued due to stomatitis. The other event of interest which resulted in death occurred in a 76-year-old female patient with a medical history of herpes zoster who died of bronchopulmonary aspergillus 7 days after discontinuation of acalabrutinib due to pneumonia.
Analysis of mutations associated with resistance to BTK inhibitors
To determine whether mutations associated with resistance to BTK inhibitors were present before acalabrutinib treatment, purified B-cell samples at the start of acalabrutinib treatment were tested for mutations in BTK and PLCG2. Mutations in BTK and PLCG2 have been associated with clinical disease progression during ibrutinib treatment and with resistance to acalabrutinib because both bind to BTK at the same site.18-22
Samples were available for 55 of 60 (92%) patients. Pretreatment samples were available for 52 patients; samples were taken at later time-points for three patients (cycle 1, day 28, n=1; cycle 6, day 28, n=2). Three (5%) were found to have a mutation in at least one gene. Two patients had multiple mutations associated with ibrutinib resistance in BTK or BTK and PLCG2, while one patient had a PLCG2 mutation of uncertain significance (Online Supplementary Table S6).18,19,23,24 All mutations were found in pretreatment samples.
Of the two patients with mutations associated with BTK and/or PCLG2, one was electively taken off acalabrutinib after just over 2 months of exposure due to the presence of these mutations and was not evaluable for evaluation of response to acalabrutinib. The other patient received acalabrutinib and had PD after 15 months, having achieved a best response of SD during acalabrutinib treatment and at CLL progression, the major BTK C481S clone identified at baseline expanded from 30.7% to 90.2% allele fraction (Online Supplementary Tables S6 and S7).
Figure 2.
Response to acalabrutinib. Patients who discontinued study treatment before evaluation for response (n=6) or who were not available for response assessment (n=2) were classified as not evaluable. CR: complete remission; Cri: CR with incomplete bone marrow recovery; ORR: overall response rate; PD: progressive disease; PR: partial remission; PRL: partial remission with lymphocytosis; SD: stable disease.
The patient with the D993N missense variant in PLCG2 (which has been associated with ibrutinib resistance, but not shown to alter drug sensitivity in vitro to date), achieved a CR with acalabrutinib at treatment cycle 18 and had remained on therapy for 25 months at the time of data cutoff.
At the time of this analysis, five patients had relapsed on acalabrutinib and peripheral blood mononuclear cell samples were collected at treatment termination. Three of the five patients were confirmed to have no BTK or PLCG2 mutations; one of these patients experienced a best overall response of PRL on acalabrutinib treatment (DOR, 11.53 months), the second patient had a best overall response of PR (DOR, 15.67 months), and the third patient had SD (Online Supplementary Table S7). The fourth patient who achieved a PR (DOR, 14.29 months) had low levels of BTK C481S and T474I mutations as well as a predominant PLCG2 1140N mutation at treatment termination, none of which was detectable at baseline. The fifth patient, described above, had a pre-existing clone with BTK C481S expansion during treatment that was detectable at progression (Online Supplementary Table S7).
Discussion
This phase II study of acalabrutinib in patients who were ibrutinib-intolerant demonstrated that acalabrutinib is effective and tolerable in a large proportion of this population. The ORR of 73% with a median PFS that was not reached demonstrates durable disease control in this population of relapsed/refractory CLL patients. A similar response rate was reported with ibrutinib in the front-line setting in a population of elderly patients with a similar median age (71 years) and follow-up duration (22.1 months).25 In this study, 10% of patients were not evaluable for response because they discontinued treatment before the first response assessment. As responses to BTK inhibitors tend to improve with longer treatment duration, it is possible that with additional follow-up, the ORR will increase and additional CR will be observed.9 It was not unexpected that acalabrutinib was effective in these patients, as the prior ibrutinib exposure was short for most patients (median, <6 months) and most were assumed to have a response to BTK inhibition (based on disease progressing after discontinuing ibrutinib due to adverse events).
The safety of acalabrutinib in this study is perhaps more helpful than the observed response rate in understanding the impact of this agent. At a median follow-up of 35 months, 48% of patients remained on acalabrutinib. The most common reason for discontinuation was disease progression (23%) and the rate of acalabrutinib discontinuation due to adverse events was 17%. This rate of discontinuation due to adverse events is low considering that 100% of patients had discontinued ibrutinib due to adverse events, suggesting that acalabrutinib is tolerable in a large proportion of patients who are intolerant of ibrutinib.
Figure 3.
Duration of response to acalabrutinib. (A, B) The median duration of response was not reached when patients with partial remission with lymphocytosis were excluded (A) or when they were included (B). CI: confidence interval; DOR: duration of response; PR: partial remission; PRL: partial remission with lymphocytosis.
Comparing the full spectrum of adverse events between ibrutinib and acalabrutinib in this study is difficult because the ibrutinib experience was not captured prospectively. The study was not intended to compare toxicity between two drugs, but rather to determine acalabrutinib tolerability in patients who discontinued ibrutinib due to toxicity. When reviewing events of arthralgia, atrial fibrillation, bleeding, diarrhea, and rash leading to ibrutinib intolerance, 24/41 patients experienced recurrence during acalabrutinib treatment, and recurrence was at a similar (25%) or lower (75%) grade of severity in all patients. Most adverse events (64%) limiting ibrutinib treatment were not experienced during acalabrutinib treatment. Additionally, all adverse events causing ibrutinib intolerance and recurring with acalabrutinib treatment (27 events in total) were reviewed to determine differences in maximal severity grade experienced. Of these adverse events, only one occurred at a higher grade, while 18 occurred at a lower grade with acalabrutinib, demonstrating that the severity of intolerance adverse events during acalabrutinib treatment may be decreased. This reduction in rate includes hemorrhage events, which have previously been observed to be a class effect of BTK inhibitors,26 but in this study were observed to occur at a lower grade with acalabrutinib than with ibrutinib. Only one patient discontinued acalabrutinib for the same adverse event (diarrhea) that was also the cause reported for ibrutinib discontinuation.
Clinical strategies for patients with ibrutinib intolerance, such as switching to alternative kinase inhibitors or combining different therapeutic agents, have been evaluated. Real-world data have suggested that ibrutinib-intolerant patients could be treated successfully with an alternative kinase inhibitor.27 Early phase clinical trial data have also demonstrated the efficacy and safety of an alternative kinase inhibitor, umbralisib, in ibrutinib-intolerant patients. There is a potential clinical benefit in switching patients with ibrutinib intolerance to another BTK inhibitor so that venetoclax remains a future treatment option. However, depending on the type and severity of the adverse events and their potential for harm of recurrence, switching to another drug class such as venetoclax should be considered.28 The safety profile and efficacy of different therapeutic agents and combination strategies has been evaluated in head-to-head trials. One such trial is ASCEND, a phase III study of acalabrutinib monotherapy versus rituximab plus idelalisib (I-R) or rituximab plus bendamustine (B-R), which demonstrated improved PFS for acalabrutinib compared with either rituximab combination; there were fewer serious adverse events and fewer adverse events leading to discontinuation with acalabrutinib monotherapy compared with IR. 29 In that study, fatal adverse events occurred in 6/154 (4%), 5/118 (4%), and 2/35 (6%) patients receiving acalabrutinib monotherapy, I-R, and B-R, respectively.
Figure 4.
Progression-free survival and overall survival with acalabrutinib. (A, B) The medians were not reached for progression-free survival (A) or overall survival (B). CI: confidence interval; OS: overall survival; PFS: progression-free survival.
This study was not designed to test whether acalabrutinib is effective in patients with therapeutic resistance to ibrutinib and the analysis of mutations associated with resistance to ibrutinib was exploratory. Among patients with evaluable samples at baseline (92%), most (95%) had no mutation in BTK/PLCG2, as determined by deep sequencing of sorted B cells. One patient harboring a PLCG2 D993N mutation at baseline achieved a response to acalabrutinib. This is an uncommon PLCG2 potentially gain-of-function mutation and may not confer resistance to acalabrutinib or ibrutinib. However, acalabrutinib may not be effective in patients who develop progression on ibrutinib with typical resistance mutations.
Of the five patients with paired baseline and progression samples, only one acquired mutations in BTK and PLCG2 after a best overall response of PR and a DOR of 14.29 months. One patient had low levels of the BTK C481S and the T4741 gatekeeper resistance mutation at baseline as well as the PLCG2 D1140N C2 domain mutation detected at progression. The PLCG2 D1140N mutation was predominant (indicating many CLL cells in the sample were without a BTK mutation), whereas with ibrutinib, treatment resistance mutations in this PLCG2 domain were more commonly secondary mutations after BTK C481X development.19
Table 2.
Adverse events occurring in ≥10% of patients (all grades) or ≥5% of patients (grade ≥3 in severity).
Table 3.
Ibrutinib-intolerance adverse events and recurrence after acalabrutinib treatment.
This study was designed to determine whether acalabrutinib is effective in patients intolerant to ibrutinib or unable to continue ibrutinib treatment due to adverse events. However, it is acknowledged that the study had a few limitations, the most significant being that the ibrutinib experience was not prospectively or rigorously captured. This not only means that a significant portion of these patients’ responses to ibrutinib were unknown, but also that not all of the details were captured for the adverse events on ibrutinib. In addition, subjective reporting of adverse events by patients prior to enrollment who sought to have access to the study drug could have influenced the patients’ enrollment. It is therefore possible that some adverse events occurring at a low grade with ibrutinib may have occurred at a greater severity with acalabrutinib. To partially overcome this limitation, we applied two different approaches to assessing the occurrence of known adverse events with ibrutinib during acalabrutinib treatment. However, only a prospective or randomized study could fully capture differences in toxicities between the two drugs. The other important limitation is in understanding differential CLL resistance to acalabrutinib. PD was the most common reason for acalabrutinib discontinuation, with a relatively high rate of 23%. The direct comparison of acalabrutinib with ibrutinib is ongoing via a phase III randomized non-inferiority clinical trial in patients with previously treated, high-risk CLL (NCT02477696).
In summary, the results of this study demonstrate that acalabrutinib is a safe and effective option for patients with relapsed/refractory CLL who are not able to tolerate ibrutinib. Acalabrutinib is an important therapeutic option in this population and will allow more CLL patients to benefit from BTK inhibitor treatment.
Supplementary Material
Acknowledgments
The authors acknowledge the Acerta Pharma study team for their commitment to this study. They also thank the patients who participated in this study as well as their friends and family who supported them.
Funding Statement
Funding: KAR is a scholar in clinical research of and received grant support from the Leukemia & Lymphoma Society. This grant provides salary support for clinical research and for writing and other study activities. The study was funded by Acerta Pharma (South San Francisco, CA, USA) a member of the AstraZeneca Group. Acerta Pharma provided the study drug. Medical writing assistance, funded by Acerta Pharma, was provided by Tracy Diaz, PhD, and Cindy Gobbel, PhD, of Peloton Advantage, LLC, an OPEN Health company.
References
- 1.Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton's tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287-6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woyach JA, Bojnik E, Ruppert AS, et al. Bruton's tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014;123(8):1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman SEM, Montraveta A, Niemann CU, et al. The Bruton tyrosine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(11): 2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinibtreated patients in the United States: a realworld analysis. Haematologica. 2018;103(5): 874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bose P, Gandhi VV, Keating MJ. Pharmacokinetic and pharmacodynamic evaluation of ibrutinib for the treatment of chronic lymphocytic leukemia: rationale for lower doses. Expert Opin Drug Metab Toxicol. 2016;12(11):1381-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma version 2.2021. December 3, 2020. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed December 21, 2020. [Google Scholar]
- 9.O'Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calquence approved in the US for adult patients with chronic lymphocytic leukaemia [press release]. 2019. Available from: https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2019/calquence-approved-in-theus-for-adult-patients-with-chronic-lymphocytic-leukaemia-21112019.html. Accessed December 21, 2020. [Google Scholar]
- 11.Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bye AP, Unsworth AJ, Desborough MJ, et al. Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017;1(26):2610-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363(2):240-252. [DOI] [PubMed] [Google Scholar]
- 14.Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849-2861. [DOI] [PubMed] [Google Scholar]
- 16.Awan FT, Schuh A, Brown JR, et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3(9): 1553-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12): 5446-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woyach JA, Ruppert AS, Guinn D, et al. BTK(C481S)-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol. 2017;35(13):1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones D, Woyach JA, Zhao W, et al. PLCG2 C2 domain mutations co-occur with BTK and PLCG2 resistance mutations in chronic lymphocytic leukemia undergoing ibrutinib treatment. Leukemia. 2017;31(7):1645-1647. [DOI] [PubMed] [Google Scholar]
- 20.Byrd JC, Wierda WG, Schuh A, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;135(15):1204-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woyach J, Huang Y, Rogers K, et al. Resistance to acalabrutinib in CLL is mediated primarily by BTK mutations. Blood. 2019;134(Suppl 1):504. [Google Scholar]
- 23.Albitar A, Ma W, DeDios I, et al. Using highsensitivity sequencing for the detection of mutations in BTK and PLCgamma2 genes in cellular and cell-free DNA and correlation with progression in patients treated with BTK inhibitors. Oncotarget. 2017;8(11): 17936-17944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu TM, Woyach JA, Zhong Y, et al. Hypermorphic mutation of phospholipase C, gamma2 acquired in ibrutinib-resistant CLL confers BTK independency upon Bcell receptor activation. Blood. 2015;126(1): 61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Series J, Garcia C, Levade M, et al. Differences and similarities in the effects of ibrutinib and acalabrutinib on platelet functions. Haematologica. 2019;104(11):2292-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128(18): 2199-2205. [DOI] [PubMed] [Google Scholar]
- 28.Mato AR, Schuster SJ, Lamanna N, et al. A phase 2 study to assess the safety and efficacy of umbralisib (TGR-1202) in patients with chronic lymphocytic leukemia (CLL) who are intolerant to prior BTK or PI3Kδ inhibitor therapy. Hematol Oncol. 2019;37(s2):88-89. [Google Scholar]
- 29.Ghia P, Pluta A, Wach M, et al. ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849-2861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.