Abstract
Tafasitamab (MOR208), an Fc-modified, humanized, anti-CD19 monoclonal antibody, combined with the immunomodulatory drug lenalidomide was clinically active with a good tolerability profile in the open-label, single-arm, phase II L-MIND study of patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) ineligible for autologous stem-cell transplantation. To assess long-term outcomes, we report an updated analysis with ≥35 months’ follow-up. Patients were aged >18 years, had received one to three prior systemic therapies (including ≥1 CD20-targeting regimen) and Eastern Cooperative Oncology Group performance status 0-2. Patients received 28-day cycles of tafasitamab (12 mg/kg intravenously), once weekly during cycles 1-3, then every 2 weeks during cycles 4-12. Lenalidomide (25 mg orally) was administered on days 1-21 of cycles 1-12. After cycle 12, progression-free patients received tafasitamab every 2 weeks until disease progression. The primary endpoint was best objective response rate. After ≥35 months’ follow-up (data cut-off: October 30, 2020), the objective response rate was 57.5% (n=46/80), including a complete response in 40.0% of patients (n=32/80) and a partial response in 17.5% of patients (n=14/80). The median duration of response was 43.9 months (95% confidence interval [95% CI]: 26.1-not reached), the median overall survival was 33.5 months (95% CI: 18.3-not reached) and the median progression-free survival was 11.6 months (95% CI: 6.3-45.7). There were no unexpected toxicities. Subgroup analyses revealed consistent long-term efficacy results across most subgroups of patients. This extended follow-up of L-MIND confirms the long duration of response, meaningful overall survival, and well-defined safety profile of tafasitamab plus lenalidomide followed by tafasitamab monotherapy in patients with relapsed/refractory diffuse large B-cell lymphoma ineligible for autologous stem cell transplantation. ClinicalTrials.gov identifier: NCT02399085.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma, accounting for 25-45% of new cases of lymphoma each year.1 The introduction of rituximab treatment, an anti-CD20 antibody, alongside cyclophosphamide, doxorubicin, prednisone, and vincristine (R-CHOP) as an initial standard- of-care immunotherapy has improved patients’ outcomes; however, 30–40% of patients continue to experience relapse or are refractory to this first-line therapy.2 For these relapsed or refractory (R/R) patients, alternative effective and tolerable treatment options are limited and, thus, their prognosis is poor.2
Current treatment options for R/R DLBCL include salvage chemotherapy followed by high-dose chemotherapy and autologous stem-cell transplantation (ASCT).3,4 However, the majority of patients with R/R DLBCL who undergo ASCT subsequently relapse.2 More recentlydeveloped therapies, such as chimeric antigen receptor (CAR) T-cell therapy and the antibody-drug conjugate polatuzumab vedotin in combination with bendamustine and rituximab, have shown improved patients’ outcomes.5-7 However, CAR T-cell therapies have been associated with severe adverse events, including grade ≥3 cytokine release syndrome and neurotoxicity, and some can be difficult to administer safely and successfully.5,6 Thus, there remains an urgent need for novel, tolerable, and easy-to-administer treatment options for patients with R/R DLBCL, particularly those ineligible for ASCT.
The combination of tafasitamab (MOR208, previously XmAb5574), an Fc-modified, humanized anti-CD19 monoclonal antibody, with lenalidomide has been shown to be effective and well-tolerated in patients with R/R DLBCL who are ineligible for ASCT.8 The phase II study, L-MIND, demonstrated an objective response rate of 60%, with 43% of patients achieving a complete response (CR).8 Moreover, the responses were durable, with a median duration of response (DoR) of 21.7 months.8 To further determine the long-term clinical efficacy and safety of tafasitamab plus lenalidomide treatment in patients with R/R DLBCL, we provide updated data based on a minimum follow-up of 35 months. Additionally, to understand the effectiveness of this novel treatment regimen in clinically relevant subgroups of patients, we present long-term efficacy analyses stratified according to important baseline covariates of prognostic significance.
Methods
Study conduct
L-MIND was an open-label, single-arm, multicenter, phase II study (NCT02399085).8 The study was approved by the institutional review boards at each study site, and conducted in accordance with International Council for Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki; all patients provided written informed consent. We present data after 35 months of follow-up from the last patient enrolled.
Study design and patients
Details of the L-MIND study have been published elsewhere; eligibility criteria are further described in the Online Supplementary Methods.8 Patients with primary refractory disease were excluded, although until a protocol amendment in June 2016, primary refractoriness was defined as no response or progressive disease (PD) within <3 months of frontline therapy, rather than 6 months. Therefore, prior to this amendment patients with relapse or PD 3-6 months from frontline therapy were included, and form a subgroup of ‘primary refractory patients’ as per B-cell lymphoma National Comprehensive Cancer Network guidelines.3 Patients with rituximab-refractory disease had no response to or PD following a rituximab-containing regimen within <6 months of completion of therapy.
Patients received up to 12 cycles (28 days each) of tafasitamab and lenalidomide, followed by tafasitamab monotherapy in patients with stable disease or better, until PD. Tafasitamab (12 mg/kg intravenously) was administered on days 1, 8, 15, and 22 during cycles 1-3, with a loading dose on day 4 of cycle 1, and on days 1 and 15 from cycle 4 onwards. Lenalidomide (25 mg orally) was self-administered on days 1-21 of each 28-day cycle. For further details see the Online Supplementary Methods.
Study outcomes
The primary endpoint was the objective response rate (CR plus partial response [PR]), assessed by an independent review committee (IRC), according to the 2007 International Working Group response criteria for malignant lymphoma.9 Secondary endpoints included DoR (time from initial CR or PR to first observation of PD), progression-free survival (PFS; time from first dosing to lymphoma progression or death), overall survival (OS; time from first dosing to date of death), and incidence and severity of adverse events. Exploratory subgroup analyses were performed to evaluate DoR, PFS, and OS by refractoriness to prior treatment, as well as age, gender, International Prognostic Index (IPI) score, prior ASCT, and number of prior treatment lines. Rituximab refractoriness was defined as a response less than PR to any rituximab-containing regimen during the course of treatment or PD within ≤6 months of treatment completion. Refractoriness to last prior treatment and primary refractoriness were defined as a best response less than PR to the most recent therapy or to first-line treatment, respectively, or PD before or ≤6 months after completion of that treatment.
Statistical analyses
The previously published primary analysis for the L-MIND study (data cut-off: November 30, 2018)8 was carried out when all patients had completed a minimum of 12 months’ follow-up. The data cut-off date for the present analyses was October 30, 2020. The full analysis set comprised patients who received both tafasitamab and lenalidomide and was used to analyze efficacy outcomes. The safety analysis set comprised patients who received any study medication.
Results
Patients
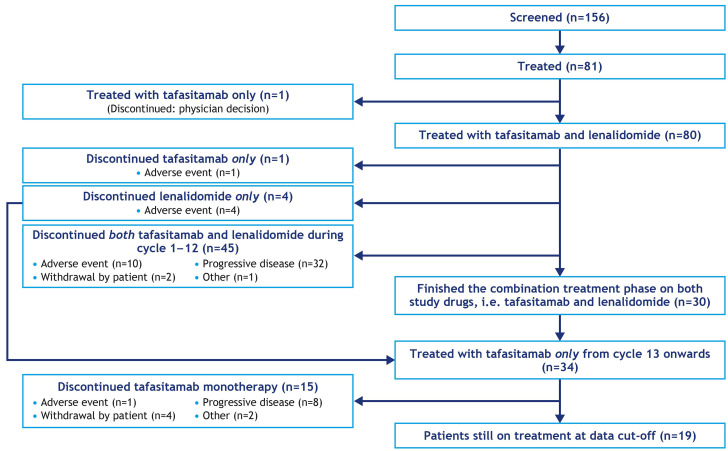
Overall, 81 patients received at least one dose of either drug and were evaluated for safety. Of those, 80 patients received ≥1 dose of both tafasitamab and lenalidomide and were evaluated for efficacy (Figure 1). A total of 34 patients received tafasitamab monotherapy after discontinuing lenalidomide (30/34 patients had completed 12 cycles of tafasitamab plus lenalidomide and 4/34 had discontinued lenalidomide prior to cycle 12 and continued tafasitamab). Fifteen of these 34 patients had discontinued tafasitamab treatment at the data cut-off for this analysis; thus, 19 patients were still receiving tafasitamab monotherapy. Of the 62/81 patients who had discontinued study treatment, 42 had died, 13 were alive and included in the survival follow-up and 7 had been lost to follow-up at the data cut-off for this report.
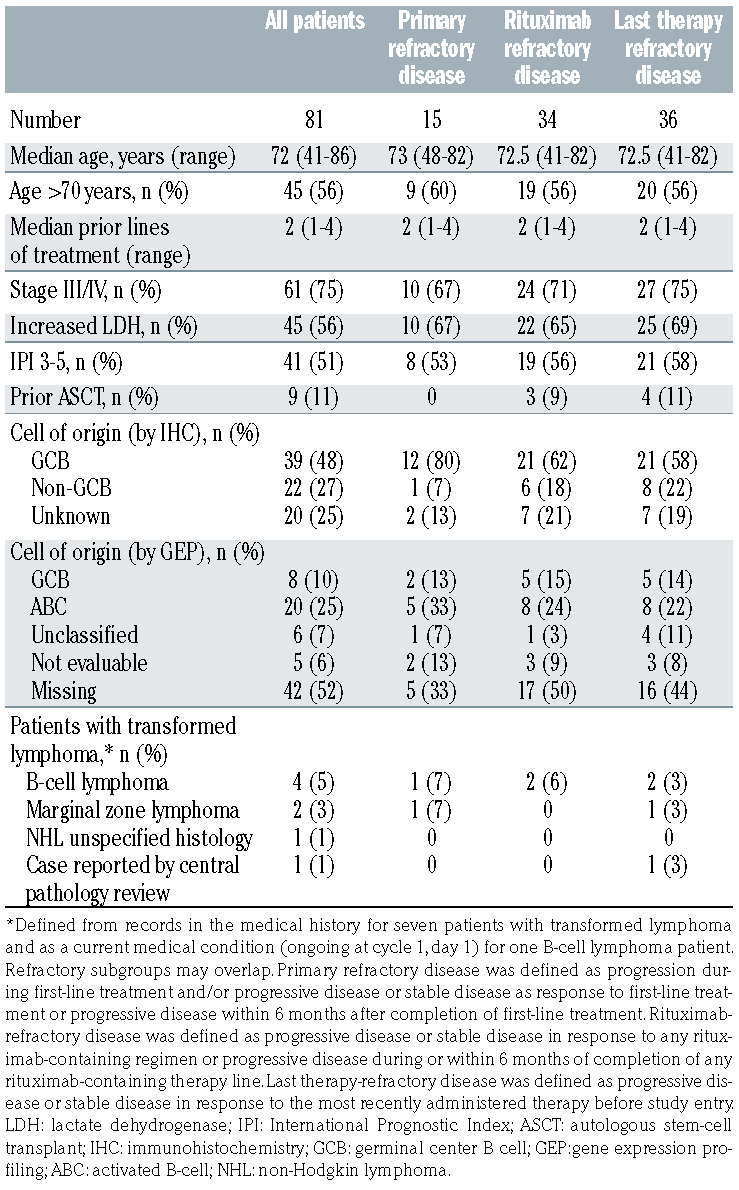
The full baseline characteristics of the patients in the LMIND study have already been published.8 Briefly, the patients had a median age of 72 years (range, 41-86) at enrollment and had received a median of two (range, 1-4) prior lines of therapy. All patients had received R-CHOP or equivalent chemoimmunotherapy prior to study entry. With the availability of additional data from a central pathology review of two patients, the baseline patients’ characteristics for cell of origin by immunohistochemistry and gene expression profiling have been updated since the primary analysis (Table 1). There was one patient each with double- and triple-hit DLBCL.
Patient subgroups of clinical interest included 15 patients (18.5%) with primary refractory disease, 33 patients (41.3%) with rituximab-refractory disease, and 35 patients (43.8%) who were refractory to their last therapy. Most patients who were refractory to their last line of therapy had received two prior lines of treatment (71.4%), and the last prior line included chemotherapy in 94.4% and rituximab in 80.0% of cases. The baseline characteristics of patients in the refractory subgroups were generally comparable with those of the overall population (Table 1), although patients in refractory subgroups were more likely to have increased lactate dehydrogenase and germinal center B cell of origin by immunohistochemistry.
Prior treatment regimens for patients refractory to their last treatment are shown in Online Supplementary Table S1.
Efficacy outcomes
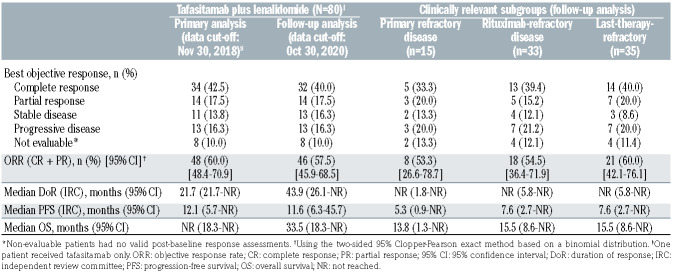
After the primary analysis, the best responses for three patients were revised based on an IRC re-adjudication due to a disagreement between the two primary radiologists. At this long-term data cut-off after at least 35 months’ follow-up, the IRC-assessed objective response rate was 57.5% (46/80; 95% confidence interval [95% CI]: 45.9-68.5), the CR rate was 40.0% (32/80) and the PR rate was 17.5% (14/80) (Table 2). Additionally, 16.3% of patients (13/80) had stable disease. The median time to response was 2.1 months (range, 1.7-34.7) and the median time to CR was 6.8 months (range, 1.7-46.3). Thirty patients had completed the combination treatment phase of 12 cycles on both study drugs and achieved a best response of CR (n=24), PR (n=3), or stable disease (n=3) as per IRC.
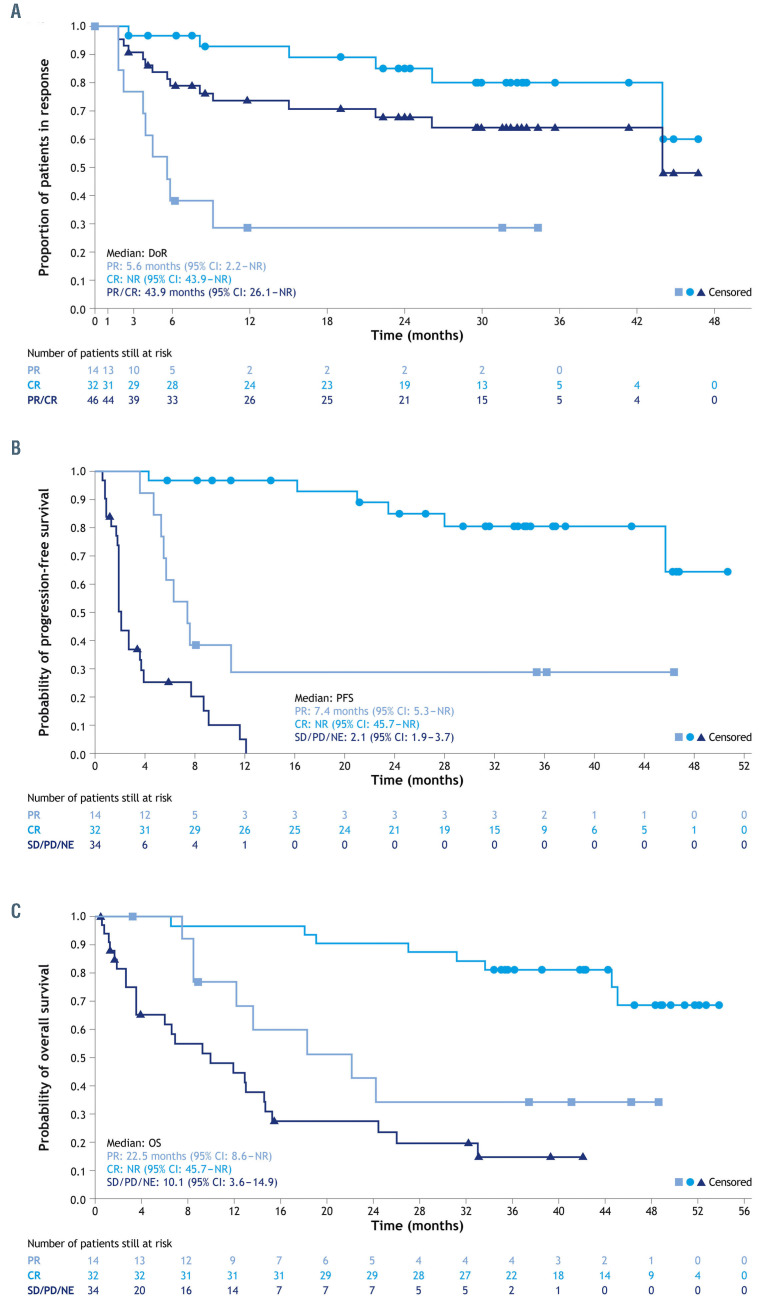
Time-to-event endpoints are shown in Table 2 with Kaplan-Meier plots in Figure 2. The median IRC-assessed DoR was 43.9 months (95% CI: 26.1-not reached [NR]), and was not reached in patients who achieved a CR (95% CI: 43.9-NR). The median IRC-assessed PFS was 11.6 months (95% CI: 6.3-45.7) with a median follow-up for PFS of 33.9 months. A total of 38 patients were censored at data cut-off; 21/38 patients (55.3%) were ongoing on PFS follow-up. The Kaplan-Meier plot of PFS suggests a plateau at around 18 months (Figure 2B). The median OS had not been reached at the primary analysis and was 33.5 months (95% CI: 18.3-NR) in this analysis, with a median survival follow-up of 42.7 months. Figure 2C shows the impact of response quality on OS; among the patients with a CR, the median OS was not reached, and OS estimates were 96.9% (95% CI: 79.8-99.6) at 18 months, 90.6% (95% CI: 73.7-96.9) at 24 months, and 81.3% (95% CI: 62.9-91.1) at 36 months. Among patients with a PR, the median OS was 22.5 months (95% CI: 8.6- NR), and OS estimates were 59.8% (95% CI: 28.5-81.0) at 18 months, 42.7% (95% CI: 15.9-67.5) at 24 months and 34.2% (95% CI: 10.7-59.8) at 36 months. In patients who received tafasitamab plus lenalidomide as second-line treatment (n=40), the median PFS was 23.5 months (95% CI: 7.4-NR), the median DoR was 43.9 months (95% CI: 9.1-NR) and the median OS was 45.7 (95% CI: 24.6-NR). In patients receiving tafasitamab plus lenalidomide as third- or later-line treatment (n=40), the median PFS was 7.6 months (95% CI: 2.7-NR), the median DoR was not reached (95% CI: 15.0-NR) and the median OS was 15.5 months (95% CI: 8.6-NR).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram of the L-MIND study at the October 30, 2020 data cut-off.
Following the discontinuation of treatment in L-MIND, 33 patients received subsequent salvage therapies, which included stem cell transplant in two patients and CAR Tcell therapy in two other patients, following further chemotherapy (see Online Supplementary Results). Additionally, five patients who achieved a CR in L-MIND but discontinued the treatment for reasons other than disease progression were alive at the data cut-off date for this analysis, without further therapeutic intervention.
Subgroup analyses
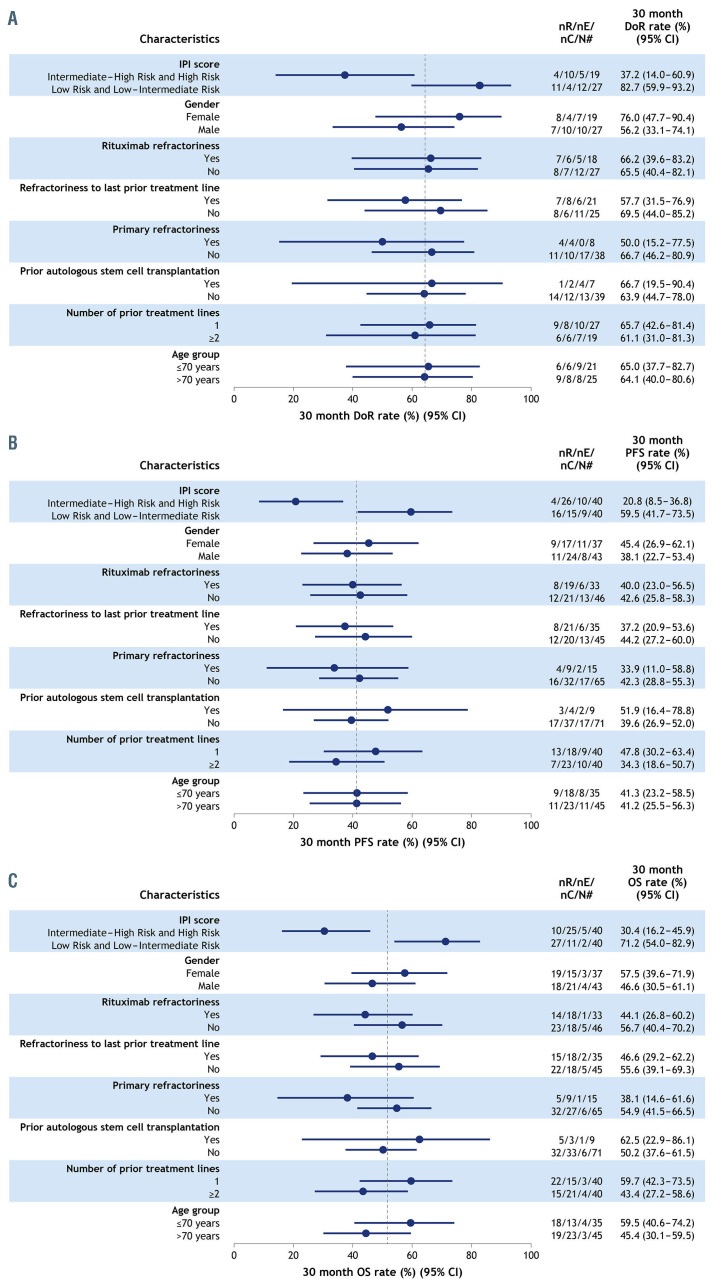
Overall response and CR rates were consistent regardless of refractoriness in patient subgroups of clinical interest although, as expected, the median PFS and OS were short in patients with primary refractory disease (5.3 months and 13.8 months, respectively), rituximab-refractory and last-line refractory disease (both 7.6 months and 15.5 months, respectively) (Table 2). Forest plots for Kaplan-Meier estimates of 30-month time-to-event endpoints are shown in Figure 3. Across DoR, PFS and OS, the only patient subgroup that consistently had a significantly poorer prognosis than the overall group was that of patients with an intermediate-high and high-risk IPI score. Patients in the rituximab-refractory (n=33 evaluable) and last-line-refractory (n=35 evaluable) subgroups had similar 30-month DoR and PFS rates to the rest of the population (DoR: 66.2% vs. 65.5% and 57.7% vs. 69.5%; PFS: 40.0% vs. 42.6% and 37.2% vs. 44.2%, respectively), whereas 30-month DoR and PFS rates were lower in patients with primary refractory disease (n=15; DoR: 50.0% vs. 66.7%; PFS: 33.9% vs. 42.3%) (Figure 3A, B). In all refractory subgroups, the 30-month OS rate was lower compared with that of the rest of the population (Figure 3C). Kaplan-Meier plots for PFS in the refractory subgroups are shown in Online Supplementary Figure S1.
Based on medical history and central pathology diagnosis, eight patients had DLBCL arising from transformation of low-grade lymphoma, and there was one patient each with double- and triple-hit lymphoma. Of the eight patients with transformed lymphoma, four experienced PR, three experienced CR and one had stable disease as best response. The patient with double-hit lymphoma (MYC and BCL2 translocations) was refractory to his last line of therapy before L-MIND (rituximab-dexamethasone- cytarabine-cisplatin) and achieved a PR to tafasitamab and lenalidomide, and was progression-free for >6 months. The patient with triple-hit lymphoma (MYC, BCL2 and BCL6 translocations) had previously experienced a CR for 4.5 months in response to R-CHOP and started tafasitamab plus lenalidomide 1 month after relapse. This patient experienced a CR in L-MIND with sustained remission for >30 months. Swimmer plots for all of these patients are shown in Online Supplementary Figure S2.
Table 1.
Updated baseline characteristics and patient subgroups of clinical interest.
Safety outcomes
As of October 30, 2020, the median duration of exposure to study treatment (either lenalidomide or tafasitamab) was 9.2 months (range, 0.2-54.7). The median duration of exposure to tafasitamab monotherapy (following discontinuation of lenalidomide at any time [n=52]) was 13.9 months (range, 0.2-43.4), compared with a median of 4.1 months’ exposure to tafasitamab monotherapy in the primary analysis (range, 0.1-20.8 months; data cut-off November 30, 2018).8 However, with the exception of one patient with recurrence of a previously diagnosed marginal zone lymphoma that was documented as an adverse event (Figure 1), no patients discontinued the study due to adverse events during the tafasitamab extended monotherapy phase.
Table 2.
Efficacy outcomes in the primary and follow-up analyses.
Overall, 64 (79.0%) patients required a temporary interruption of tafasitamab, of which 73.4% cases were due to adverse events. During combination therapy, 43 (53.1%) patients required no dose reduction of lenalidomide from the starting dose of 25 mg. Lenalidomide interruptions were required by 28 (34.6%) patients, being due to adverse events in 89.3% of cases, and 37 patients (45.7%) required a lenalidomide dose reduction. The most frequent treatment-emergent adverse event (TEAE) leading to treatment interruption for tafasitamab (± lenalidomide) and lenalidomide (± tafasitamab) was neutropenia (28 [34.6%] patients and 24 [29.6%] patients, respectively). During the extended tafasitamab monotherapy phase, 21 (52.5%) patients had an interruption of tafasitamab treatment due to at least one TEAE, the most common reasons being neutropenia or leukopenia (9 patients) and respiratory tract infections (6 patients).
At the current analysis, 42 patients (51.9%) had died. There were eight deaths (9.9%) on treatment (5 related to PD, plus 1 stroke, 1 sudden death and 1 respiratory failure), and 34 deaths (42.0%) after treatment (26 related to PD, plus 1 intracerebral hemorrhage, 1 pulmonary edema due to heart failure, 1 pneumonia, 1 end-stage marrow failure, 1 progressive multifocal leukoencephalopathy, 1 congestive heart failure and 1 acute myeloid leukemia considered by the investigator to be secondary to past chemotherapy, and 1 unknown cause).
At a median follow-up for OS of 42.7 months, compared with 19.6 months at the primary analysis (an additional follow-up duration of 23.1 months), TEAE were consistent in incidence and severity with the those of the primary analysis (Table 3), with the most common TEAE (all grades) at extended follow-up remaining neutropenia (51%) and anemia (37%). The adverse event burden, expressed in terms of number of adverse events per patient-year of exposure to study medication, decreased greatly during the tafasitamab monotherapy phase compared with that during the combination therapy phase (Table 4). Consistent with the safety profile of tafasitamab monotherapy in other studies,10,11 the most common adverse events during the monotherapy phase were neutropenia, cough, diarrhea, anemia, nasopharyngitis, and pyrexia, and the majority of adverse events were of grade 1 or 2. Similar to the primary analysis, the most common grade ≥3 TEAE were neutropenia (49%), thrombocytopenia (17%) and febrile neutropenia (12%).
Treatment-emergent serious adverse events (SAE) were reported in 43 patients (53.1%). The most common SAE were pneumonia (7 patients [8.6%]), febrile neutropenia (5 patients [6.2%]), pulmonary embolism (3 patients [3.7%]), bronchitis, lower respiratory tract infection, atrial fibrillation and congestive cardiac failure (all 2 patients [2.5%]). Of these, pneumonia and lower respiratory tract infection had been reported in an additional two and one patients, respectively, compared with the primary analysis, while the rest remained unchanged. Overall, ten patients (12.3%) experienced febrile neutropenia (grade 3 or 4). Five of these patients also developed infections whose timing was associated with febrile neutropenia (urinary tract infection [grade 3 adverse event]; sepsis and urinary tract infection [both grade 4 SAE]; Enterobacter bacteremia [grade 3 SAE]; staphylococcal skin infection [grade 2 adverse event]; rhinitis [grade 1 adverse event] and respiratory syncytial virus infection [grade 3 SAE]), and all recovered within 3-24 days; the other five patients developed no infections at all or their timing was not associated with febrile neutropenia.
Between the primary analysis and this update, there were few new adverse events reported related to infection and rash (Online Supplementary Table S2). This observation is consistent with the low incidence of these events associated with tafasitamab monotherapy.
Eleven patients (13.6%) experienced 13 TEAE of special interest, including tumor flare (3 events in 3 patients [3.7%]), allergic dermatitis (3 events in 3 patients [3.7%]), basal cell carcinoma (4 events in 2 patients [2.5%]), myelodysplastic conditions (2 events in 2 patients [2.5%]), and Bowen disease (1 event in 1 patient [1.2%]). There were no cases of grade ≥3 infusion-related reactions, tumor lysis syndrome (of any grade), or cytokine release syndrome (of any grade) during the study.
Figure 2.
Proportion of patients in remission. (A-C) Kaplan-Meier plots of duration of response (A), progression-free survival. (B) and overall survival (C) after 35 months of follow-up. 95% CI. 95% confidence interval; CR: complete response; DoR: duration of response; NE: not evaluable; NR: not reached; OS: overall survival; PD: progressive disease; PFS: progression- free survival; PR: partial response; SD: stable disease.
Figure 3.
Kaplan-Meier estimates of 30-month time-toevent endpoints. (A) Duration of response,* (B) progression-free survival and (C) overall survival rates. *Based on patients who achieved an objective response (CR or PR) in the respective subgroups. 95% CI: 95% confidence interval; DoR: duration of response; IPI: International Prognostic Index; nC: number of patients censored; nE: number of patients with event; nR: number of patients at risk; n#: number of responders within each subgroup (A: DoR), or number of overall patients within each subcategory (B: PFS; C: OS); OS: overall survival; PFS: progression- free survival. The vertical line indicates the 30- month DoR (A), PFS (B) and OS (C) rates across all responders/patients.
Table 3.
Treatment-emergent adverse events occurring in ≥10% of patients, or grade 3-5 treatment-emergent adverse events in >1 patient, reported at the updated L-MIND analysis.
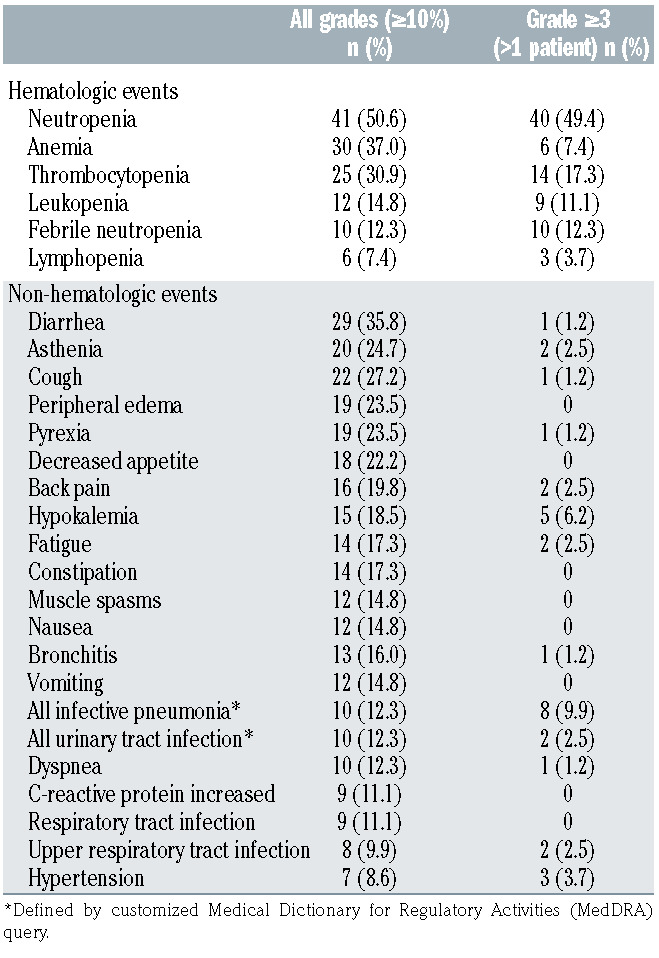
Table 4.
Summary of hematologic and non-hematologic treatmentemergent adverse events (any grade) by patient-years of exposure to tafasitamab.
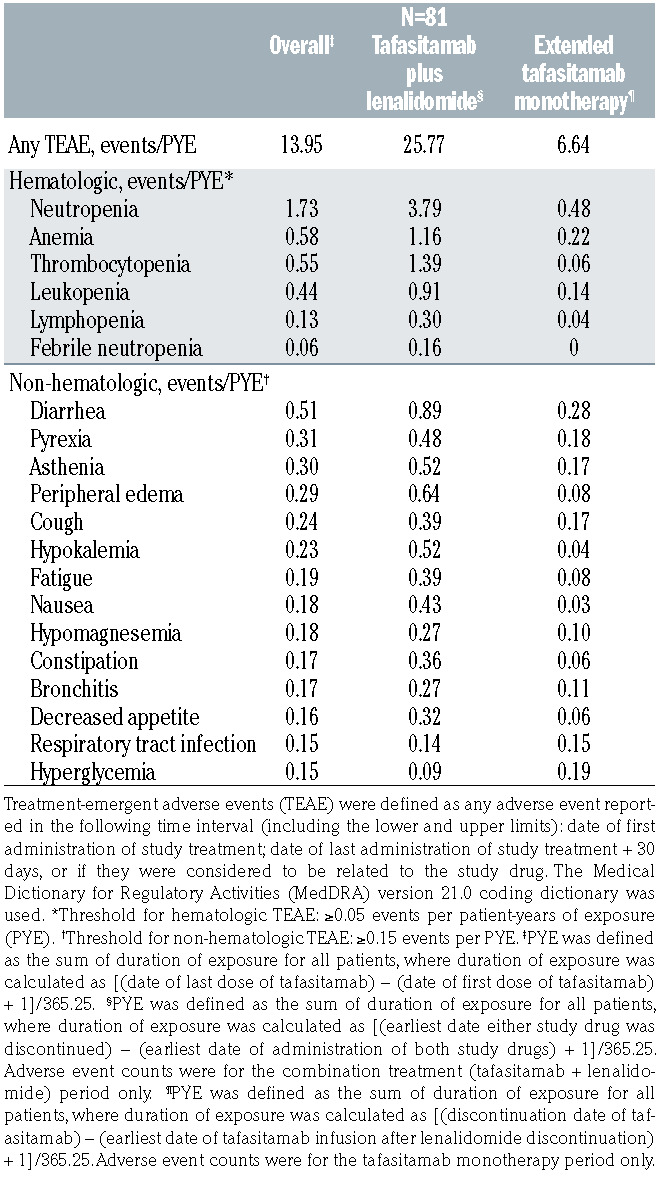
The median duration of common adverse events (all grades) was longest for opportunistic infections12 (20 days; 1 event each of progressive multifocal leukoencephalopathy, grade 5; hepatitis B reactivation, grade 2; Clostridium difficile colitis, grade 2; and skin candida, grade 1; 7 events of herpes viral infection, grade 1-4, including 1 event of grade 4 disseminated varicella zoster virus infection in blood, gut, lungs and liver), followed by pneumonia and fatigue or asthenia (18 and 15 days, respectively) and shortest for nausea and vomiting (2 days).
Discussion
The primary analysis of the L-MIND study, at a median follow-up of 13.2 months, showed that combination therapy with tafasitamab and lenalidomide resulted in a promising response, including durable CR in a significant proportion of patients, and was well tolerated in transplant- ineligible patients with R/R DLBCL.8 With followup of at least 35 months, these long-term data confirm and extend the results of the primary analysis and provide more information on the consolidation tafasitamab monotherapy phase of the study, with an objective response rate of 57.5%. This regimen was granted accelerated approval by the US Food and Drug Administration for patients with R/R DLBCL not eligible for ASCT, based on a high response rate to therapy and prolonged DoR.13
This long-term follow-up analysis shows clinically significant durable responses for combination therapy followed by tafasitamab monotherapy. The median DoR was nearly 44 months with a median OS of 33.5 months; neither the median DoR nor the median OS was reached in patients with a CR, with 80.1% and 81.3% of patients with a CR in response or alive at 36 months, respectively (Figure 2A, C). The median PFS was notable in patients who received tafasitamab plus lenalidomide as secondline therapy compared with those who received the combination third-line or later (23.5 months vs. 7.6 months [n=40, both groups]). The corresponding median OS were 45.7 months vs. 15.5 months, suggesting that patients derive more benefit from this regimen when it is given in an earlier treatment setting.
Good response rates were also achieved with combination therapy in the subgroups of patients with primary refractory, rituximab-refractory and last-therapy-refractory disease, especially given that these patients are considered difficult to treat, and those responses were durable. The median PFS and OS were, however, shorter than those for the overall population, especially in primary refractory patients, so there is still room for improvement in outcomes for difficult-to-treat patients. Notably, two patients with double- and triple-hit lymphoma and seven out of eight patients with transformed lymphoma responded to therapy.
In the exploratory subgroup analysis, the only disease characteristic that appeared to have a negative effect on prognosis was IPI score ≥3 (i.e., intermediate-high- or high-risk disease); a high IPI score has long been recognized as a risk factor for poor outcomes in DLBCL.14
In regard to safety, there was little change in the adverse event profile since the primary analysis, which indicates a good tolerability profile for tafasitamab monotherapy. There was a reduction in the burden of common hematologic and non-hematologic adverse events as patients transitioned from combination therapy to tafasitamab monotherapy, with a residual tolerability profile similar to that in previous studies of tafasitamab monotherapy.10,11 This observation is of considerable importance for frail or elderly patients, who may prefer treatment with limited effects on their quality of life. In particular, the low incidence of infusion-related reactions (all of which were grade 1) and absence of cytokine release syndrome with tafasitamab plus lenalidomide is an important consideration for therapy in frail patients, given the occurrence of these events with CAR T-cell and other antibody therapies.
Subsequent treatment, including ASCT and CAR T cells, was not precluded by previous administration of tafasitamab and lenalidomide in patients who experienced disease progression during this combination regimen.
In this trial, lenalidomide was given for a limited time of up to 12 months, which is in line with the median DoR of lenalidomide monotherapy in R/R non-Hodgkin lymphoma of 10.5 months,15,16 and the observation that the best responses with tafasitamab plus lenalidomide typically occur within this time window. Treatment until progression with tafasitamab is a novel concept, and although the exact contribution of the monotherapy phase cannot be delineated in this trial, it deserves further investigation. The excellent durability of CR achieved raises the question of whether cure is possible with tafasitamab plus lenalidomide; longer follow-up data will be needed to assess this.
Patients with R/R DLBCL who are not eligible for ASCT have few options. In patients who had previously received rituximab, cytotoxic chemotherapy with six to eight cycles of rituximab plus gemcitabine and oxaliplatin was associated with a CR/unconfirmed CR rate of 42% with a median PFS of 4 months and median OS of 8 months, and an overall high incidence of grade ≥3 neutropenia (73%) and thrombocytopenia (44%), requiring transfusions of blood (33%) and platelets (23%).17
In patients with third- or later-line disease, the median PFS of 7.6 months and median OS of 15.5 months with tafasitamab plus lenalidomide are comparable with those achieved with other options such as polatuzumab plus bendamustine and rituxiamb (approved for R/R DLBCL in the European Union)7 and CAR T-cell therapy.6,18 The median DoR has not been reached, with more than 80% of patients with a best response of CR still in remission after 3.5 years. The L-MIND regimen is readily available to administer in an outpatient setting, with oral lenalidomide self-administered by the patient and weekly tafasitamab infusions (fortnightly after the first 3 months of therapy).
In conclusion, combination therapy with tafasitamab plus lenalidomide followed by tafasitamab monotherapy provided clinically significant durable responses in patients with R/R DLBCL who were not eligible for ASCT, including those with refractory disease, with manageable toxicity during combination treatment and a reduced adverse event burden during tafasitamab monotherapy. These long-term data further validate tafasitamab plus lenalidomide followed by extended tafasitamab monotherapy as a valuable option for patients with R/R DLBCL who are not eligible for ASCT.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and their families, clinical researchers, and their teams and hospitals that participated in this study. The authors thank Günter Fingerle-Rowson and Emanuel Lohrmann of MorphoSys AG for their input to this manuscript. This study was sponsored by MorphoSys AG. Medical writing assistance was provided by Rebecca Hurst, PhD of Syneos Health and funded by MorphoSys AG.
References
- 1.World Health Organization. World Cancer Report 2020: Cancer Research for Cancer Prevention. Cancer Control. 2020;199. [Google Scholar]
- 2.Sarkozy C, Sehn LH. New drugs for the management of relapsed or refractory diffuse large B-cell lymphoma. Ann Lymphoma. 2019;3:10. [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines: B-Cell Lymphomas V3.2021. Published 2021. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed February 7, 2021. [Google Scholar]
- 4.Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116-v125. [DOI] [PubMed] [Google Scholar]
- 5.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 7.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single- arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 10.Woyach JA, Awan F, Flinn IW, et al. A phase 1 trial of the Fc-engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood. 2014;124(24):3553-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurczak W, Zinzani PL, Hess G, et al. A phase IIa, open-label, multicenter study of single-agent tafasitamab (MOR208), an Fcoptimized anti-CD19 antibody, in patients with relapsed or refractory B-cell non- Hodgkin’s lymphoma: long-term followup, final analysis. Blood. 2019;134 (Suppl_1):4078. [Google Scholar]
- 12.Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Published 2020. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Adult_OI.pdf. Accessed July 21, 2020. [Google Scholar]
- 13.US Food & Drug Administration. FDA grants accelerated approval to tafasitamabcxix for diffuse large B-cell lymphoma. Published online 2020:1-2. https://www.fda.gov/drugs/drugapprovals-and-databases/fda-grants-accelerated-approval-tafasitamab-cxix-diffuselarge-b-cell-lymphoma. Accessed February 7, 2021. [Google Scholar]
- 14.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non- Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987-994. [DOI] [PubMed] [Google Scholar]
- 15.Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011;22(7):1622-1627. [DOI] [PubMed] [Google Scholar]
- 16.Zinzani PL, Rigacci L, Cox MC, et al. Lenalidomide monotherapy in heavily pretreated patients with non-Hodgkin lymphoma: an Italian observational multicenter retrospective study in daily clinical practice. Leuk Lymphoma. 2015;56(6):1671-1676. [DOI] [PubMed] [Google Scholar]
- 17.Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica. 2013;98(11):1726-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.