We assessed the clinical utility of soluble CD163 (sCD163) in patients with diffuse large B-cell lymphoma (DLBCL), by measuring sCD163 levels prior to, during and after treatment in two independent cohorts. Our results demonstrate that pre-treatment sCD163 levels decrease in response to therapy and, if elevated, predict an unfavorable outcome. The findings suggest that sCD163 represents a useful and easily assessable biomarker for therapeutic monitoring in DLBCL.
Although the combination of rituximab with chemotherapy has revolutionized treatment in DLBCL,1 approximately 20-30% of patients relapse with a dismal survival.2 International Prognostic Index-based classifications are used to predict outcomes,3 but the composition of the tumor microenvironment has also been recognized to have prognostic impact on survival.4 Macrophages infiltrating into the tumor microenvironment are usually polarized as tumor-associated macrophages (TAM), which strongly express CD163,5 and CD163 expression in the tumor microenvironment is regarded as a marker of TAM. Elevated levels of CD163 ectodomain, a sCD163, have been detected in serum, and associated with adverse outcome in lymphoid malignancies.6,7 While TAM content predicts survival in DLBCL4,8 the clinical relevance of sCD163 is unknown.
We examined the clinical utility of sCD163 in two independent cohorts of patients with DLBCL to gain further understanding of the biological role of macrophages during the clinical course of DLBCL. A prospective clinical trial cohort and a population-based cohort were used to reach generalizable results.
The trial cohort included patients <65 years with highrisk DLBCL treated with dose-dense immunochemotherapy in the Nordic NLG-LBC-05 phase II trial.9 Available samples included 119 pre-treatment and 94 paired midtreatment samples. Samples from five healthy volunteers formed a control group. The trial was registered at www.ClinicalTrials.gov as NCT01325194. All patients gave written informed consent to the study. The Institutional Review Boards, National Medical Agencies, and Ethics Committees in Finland, Norway, Denmark, and Sweden approved the protocol and sampling.
The population-based cohort was obtained from the Swedish biobank U-CAN10 and included 125 pre-treatment samples collected between 2010 and 2016. Available paired samples included 30 mid-treatment samples, 71 post-treatment samples from patients in complete remission, and 11 samples taken during primary progressive or relapsing disease. A majority of the patients (93%) were treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP)-based therapy. Complete remission was defined in clinical routine. All patients gave written informed biobank consent. The study was approved by the Regional Board of the Ethical Committee in Uppsala, Sweden.
In the trial cohort, sCD163 was measured using a Quantikine enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA). In the population-based cohort, levels were measured as previously described.6 Measurements were performed independently in different laboratories. The median pre-treatment sCD163 values in the respective cohorts were defined a priori as a cutoff for testing prognostic implications.
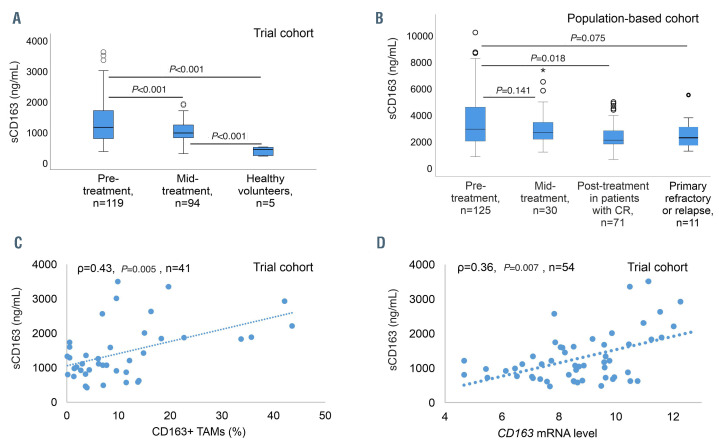
Figure 1.
sCD163 levels in two cohorts and correlation between pre-treatment sCD163 levels, CD163+ tumor-associated macrophages and CD163 mRNA levels in the trial cohort. (A) Comparison of sCD163 levels in pre-treatment and mid-treatment samples (after three courses) in the trial cohort, and in healthy volunteers. (B) sCD163 levels in pre-treatment samples compared to those in paired mid-treatment samples and paired post-treatment samples from patients in complete remission, and at primary progressive or relapsing disease in the population- based cohort. For graphical reasons the two extreme outliers with pre-treatment sCD163 levels over 11,000 ng/mL are not shown in the figure, but are included in the statistical analyses. (C) Correlation of pre-treatment sCD163 levels and CD163+ tumor-associated macrophages (TAM) in the tumor tissue in the trial cohort. (D) Correlation of pre-treatment sCD163 levels and CD163 gene expression levels from the matching tumor tissue in the trial cohort.
In the trial cohort, CD163 mRNA levels in 54 tumor samples were measured with NanoString nCounter (Nanostring Technologies, Seattle, WA, USA).11 Proportions of CD163+ cells in 41 tumor samples were analyzed by multiplex immunohistochemistry11 (Online Supplementary Figure S1A, B). Blood monocyte counts were available for 103 patients.
Statistical analyses were performed with IBM SPSS Statistics v.25.0 (IBM, Armonk, NY, USA) and STATA/IC 12.1 (StataCorp LP, Texas, TX, USA). The Wilcoxon signed ranks test was used to evaluate changes in sCD163 levels during treatment. The Mann-Whitney Utest was used to compare sCD163 levels between patient and control groups. Spearman rank analysis was used in correlation analyses. The χ2 test and the Fisher-Freeman- Halton test were used to evaluate differences in frequency of prognostic factors. The Kaplan-Meier method was used to estimate differences in outcome between the subgroups. The degree of significance was calculated using a log-rank test. Cox regression analysis (with 95% confidence intervals [95% CI]) was used and adjusted for gender and the variables in the International Prognostic Index, and in the trial cohort also for molecular subtype. P-values <0.05 were considered statistically significant. All statistical tests were two-tailed.
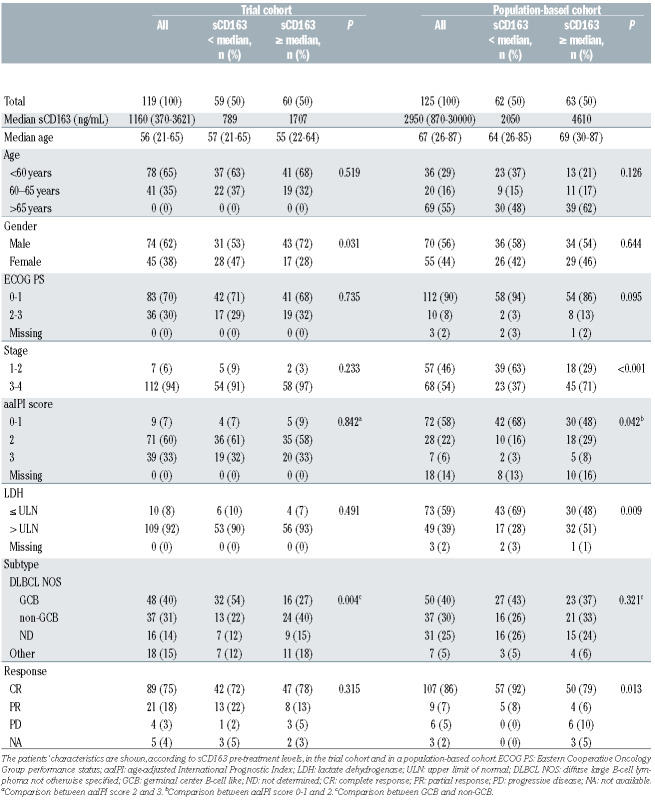
Table 1.
The characteristics of all patients and patients divided by sCD163 pre-treatment levels above and below the median.
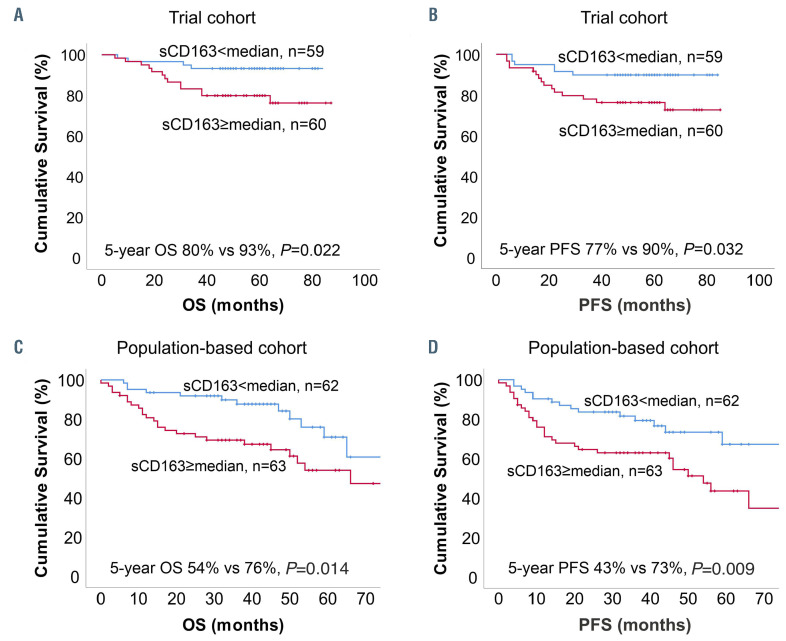
Figure 2.
Association of survival outcomes with pre-treatment sCD163 levels. (A) Overall survival (OS) and (B) progression-free survival (PFS) according to pre-treatment sCD163 level (< vs. ≥ median, 1,160 ng/mL) in the trial cohort. (C) OS and (D) PFS according to pre-treatment sCD163 level (< vs. ≥ median, 2,950 mg/mL) in the population-based cohort.
The baseline characteristics of the patients in the trial cohort are presented in Table 1. The median follow-up for the patients alive was 61 months (range, 40-87). In total, 16 (13%) patients died and 18 (15%) relapsed during follow-up. Five-year overall survival and progressionfree survival rates were 87% and 83%, respectively. Pretreatment sCD163 levels were higher in these patients than in healthy volunteers (median 1,160 ng/mL [range, 370-3,621] vs. 437 ng/mL [range, 220-518]; P<0.001), and higher in the subgroup with non-germinal center B-cell like (non-GCB) lymphoma than in the GCB subgroup (Table 1, Online Supplementary Figure S1C), declining in response to therapy (median 975 ng/mL [range, 299-1,923], P<0.001) (Figure 1A, Online Supplementary Figure S1E, G). Pre-treatment sCD163 levels correlated with CD163+ TAM (Figure 1C) and with CD163 mRNA levels (Figure 1D), whereas no correlation was found with blood monocyte counts (ρ=0.0, P=1.00). High pre-treatment sCD163 levels (above the median) translated into poor outcome (Figure 2A, B). Relative risks of death and progression were, respectively, 3.4-fold (95% CI: 1.12-10.51, P=0.031) and 2.7-fold (95% CI: 1.05-6.94, P=0.04) higher. In Cox regression analysis including International Prognostic Index factors, gender and molecular subtype, sCD163 remained the only significant prognostic factor for progression-free survival (hazard ratio [HR]=4.40, 95% CI: 1.09-17.83; P=0.038) (Online Supplementary Table S1). A similar trend was seen for poor overall survival (HR=5.08, 95% CI: 0.98-26.39; P=0.053) (Online Supplementary Table S1). The mean pre-treatment sCD163 levels in patients stratified by their later response to therapy are presented in Online Supplementary Figure S1I.
The baseline characteristics of the population-based cohort are presented in Table 1. During a median followup of 40 months (range, 0-94), 42 (34%) patients died and 29 (23%) relapsed. The estimated 5-year overall and progression-free survival rates were 65% and 57%, respectively. The median pre-treatment sCD163 level was 2,950 ng/mL (range, 870-30,000 ng/mL). All cases later developing progressive disease had a diagnostic sCD163 value above the median (Table 1). Pre-treatment levels did not differ according to subtype (Online Supplementary Figure S1D). In the 71 patients with paired pre-treatment and post-treatment samples in complete response, sCD163 levels declined significantly (median 2,510 ng/mL [range 870-30,000] to 2,120 ng/mL [range 670-5,000], P=0.018) (Figure 1B, Online Supplementary Figure S1F). In 30 patients with paired pre- and mid-treatment samples, levels also declined, although not statistically significantly (Figure 1B, Online Supplementary Figure S1F, H). sCD163 levels in 11 patients with primary progressive or relapsing disease did not differ from the paired pre-treatment levels (Figure 1B). The distribution of pretreatment sCD163 levels in groups of patients stratified by their later response to treatment are presented in Online Supplementary Figure S1J.
The outcomes in the subgroup with pre-treatment sCD163 levels above the median were worse than those with levels below the median (Figure 2C, D). The relative risks of death and progression were, respectively, 2.2-fold (95% CI: 0.99-4.94; P=0.052) and 2.2-fold (95% CI: 1.05-4.48; P=0.037) higher. In Cox regression analysis, sCD163 remained an independent prognostic factor for poor progression-free survival (HR=2.16, 95% CI: 1.05-4.48; P=0.037) (Online Supplementary Table S2) together with age, poor performance status, elevated lactate dehydrogenase concentration and male gender. A similar trend was seen for poor overall survival (HR=2.21, 95% CI: 0.99-4.94, P=0.052) (Online Supplementary Table S2).
Taken together, we measured sCD163 levels in two independent DLBCL cohorts. Pre-treatment sCD163 levels correlated with CD163+ TAM and CD163 mRNA levels in the lymphoma tissue, while no correlation with monocyte counts was seen, suggesting that circulating sCD163 predominantly arises from the lymphoma tissue and that the elevated levels reflect host response to an aggressive lymphoma presentation. Pre-treatment sCD163 levels were elevated compared to those in healthy controls, and high levels were associated with unfavorable outcomes. We observed a decline in sCD163 levels in response to therapy, which in turn suggests that sCD163 could be used as a disease response biomarker in DLBCL. Similar observations have been previously made in chronic lymphocytic leukemia and multiple myeloma. 6,7 The few samples at relapse prevented us from drawing firm conclusions, but the levels seemed in line with diagnostic values.
The two different ELISA methods implemented in the cohorts have been compared in the past and their results showed a strong correlation (r2=0.97), but a systematic bias due to different calibration levels.12 This likely explains the difference in sCD163 levels between the two subsets, with higher levels observed in the population- based cohort even though the trial cohort had a larger number of patients with advanced disease. Another contributing factor may be the larger number of patients >60 years in the population-based cohort.
An advantage of sCD163 as a potential biomarker is its stability in plasma, simplifying sample collection and handling.12 While absolute levels might differ between individuals for reasons other than tumor burden, levels could also be used as a patient-specific measure of response, indicated by declining levels in patients achieving complete remission. Indeed, the intraindividual biological variation in sCD163 is low, supporting the use of sCD163 for monitoring.12 While several prognostic factors are already used in clinical routine, disease monitoring tools in DLBCL are less common. Interim fluorodeoxyglucose positron emission tomography/computed tomography13 and down-modulation of circulating tumor DNA14 are useful and promising for disease monitoring, but the impact of host-related factors such as the tumor microenvironment should not be ignored.
Our results show that sCD163 is an indicator of biologically aggressive DLBCL. The findings were similar in two independent cohorts despite differences in clinical variables, implying that the prognostic impact of sCD163 is not limited to a particular population of patients. Therefore, these results suggest that sCD163 represents a useful and easily assessable biomarker for therapeutic monitoring of patients with DLBCL.
Supplementary Material
Acknowledgments
The authors would like to thank Anne Aarnio and Marika Tuukkanen for technical assistance and Sara Ekberg for statistical support.
Funding Statement
Funding: the study was supported by grants from the Academy of Finland (to SL), Finnish Cancer Foundation (to SL), Juselius Foundation (to SL), Ida Montin Foundation (to HV), Finnish Society for Oncology (to HV), University of Helsinki (to SL), Helsinki University Hospital (to SL), Swedish Cancer Society (19 0123 Pj 01 H and 19 0109 SCIA) (to IG), Swedish Society of Medicine (to IG) and Lions Research Cancer Fund, Uppsala Sweden (to IG).
References
- 1.Salles G, Barrett M, Foà R, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34(10):2232-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrysson S, Eloranta S, Ekberg S, et al. Incidence of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) including CNS relapse in a population-based cohort of 4205 patients in Sweden. Blood Cancer J. 2021; 11(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International Prognostic Index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(14):2373-2380. [DOI] [PubMed] [Google Scholar]
- 4.Kridel R, Steidl C, Gascoyne RD. Tumor-associated macrophages in diffuse large B-cell lymphoma. Haematologica. 2015;100(2):143-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosser DM.The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209-212. [DOI] [PubMed] [Google Scholar]
- 6.Andersen MN, Abildgaard N, Maniecki MB, Møller HJ, Andersen NF. Monocyte/macrophage-derived soluble CD163: a novel biomarker in multiple myeloma. Eur J Haematol. 2014;93(1):41-47. [DOI] [PubMed] [Google Scholar]
- 7.Nederby L, Roug AS, Knudsen SS, et al. Soluble CD163 as a prognostic biomarker in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56(11):3219-3221. [DOI] [PubMed] [Google Scholar]
- 8.Wada N, Zaki MAA, Hori Y, et al. Tumour-associated macrophages in diffuse large B-cell lymphoma: a study of the Osaka Lymphoma Study Group. Histopathology. 2012;60(2):313-319. [DOI] [PubMed] [Google Scholar]
- 9.Leppä S, Jørgensen J, Tierens A, et al. Patients with high-risk DLBCL benefit from dose-dense immunochemotherapy combined with early systemic CNS prophylaxis. Blood Adv. 2020;4(9):1906-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glimelius B, Melin B, Enblad G, et al. U-CAN: a prospective longitudinal collection of biomaterials and clinical information from adult cancer patients in Sweden. Acta Oncol. 2018;57(2):187-194. [DOI] [PubMed] [Google Scholar]
- 11.Autio M, Leivonen S, Brück O, et al. Immune cell constitution in the tumor microenvironment predicts the outcome in diffuse large B-cell lymphoma. Haematologica. 2021; 106(3):718-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Møller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72(1):1-13. [DOI] [PubMed] [Google Scholar]
- 13.Sun N, Zhao J, Qiao W, Wang T.Predictive value of interim PET/CT in DLBCL treated with R-CHOP: meta-analysis. Biomed Res Int. 2015;2015:648572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtz DM, Scherer F, Jin MC, et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J Clin Oncol. 2018;36(28):2845-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.