The development of novel therapies is the most important catalyst for the advancement in the treatment of patients with multiple myeloma (MM). Presently, a number of new therapies including immunotherapeutic drugs, cellular therapies and BH3 mimetics are introduced into clinical practice. This steadily increasing number of effective treatment options makes it practically impossible to compare all available regimens and treatment concepts with each other in different settings. Hence, it becomes increasingly important to obtain a deeper understanding of the mechanism of activity of individual drugs to enable the optimal selection of combination partners and treatment sequences in clinical practice.1
Figure 1.
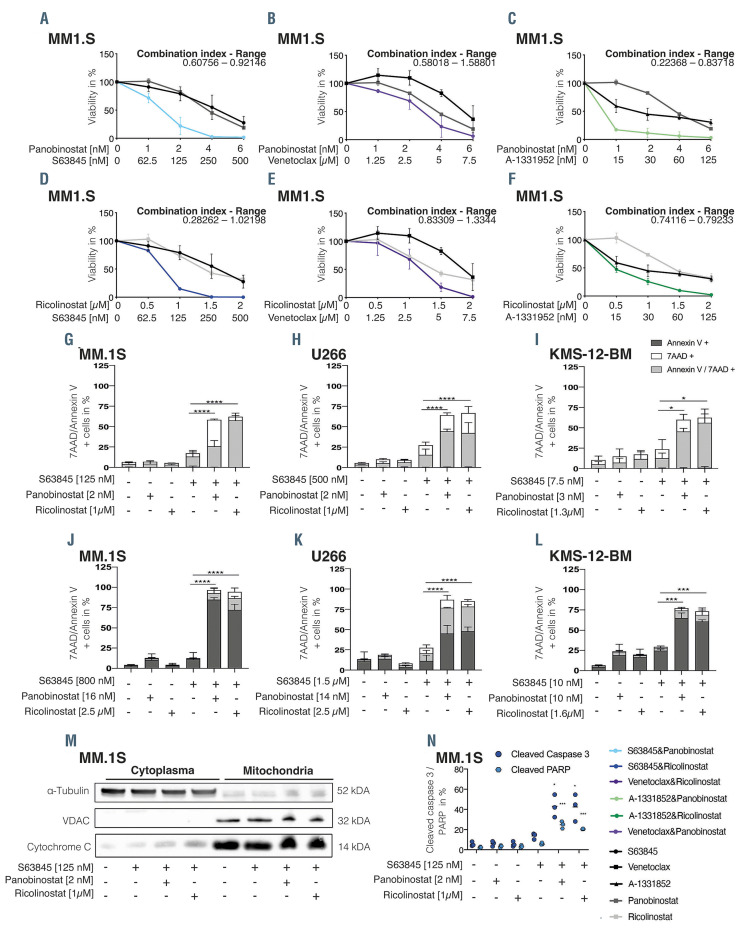
Concurrent MCL-1 and HDAC inhibition synergistically kills multiple myeloma cells in vitro via apoptosis induction. Cell viability of MM1.S cells 96 hours (h) after treatment with S63845 (A and D), venetoclax (B and E), A-1331952 (C and F) alone or in combination with either panobinostat (A to C) or ricolinostat (D to F). Results are presented relative to 0.1% dimethyl sulfoxide (DMSO) control. The combination index was calculated and stated as a range. Combination index values of <0.8, 0.8–1.2, and >1.2 were interpreted as synergistic, additive, and antagonistic drug activity, respectively. Apoptosis induction in MM.1S, U266 and KMS-12-BM cells was assessed by 7AAD/Annexin V staining 72 h after treatment in the absence (G to I) or presence of MSCT+ stromal cells (J to L). (M) Cytochrome c release assay was performed 24 h after treatment initiation at the indicated concentrations. One representative experiment of two is shown. (N) Assessment of cleaved caspase 3 and cleaved PARP via flow cytometry was performed 48 h or 72 h post treatment induction, respectively. Error bars indicate standard deviation of the mean (SDM) of triplicate experiments. Differences between groups were calculated with one-way ANOVA, corrected for multiple comparison with Bonferroni-Holm correction with ****P<0.0001, **P<0.001 and *P<0.05.
Figure 2.
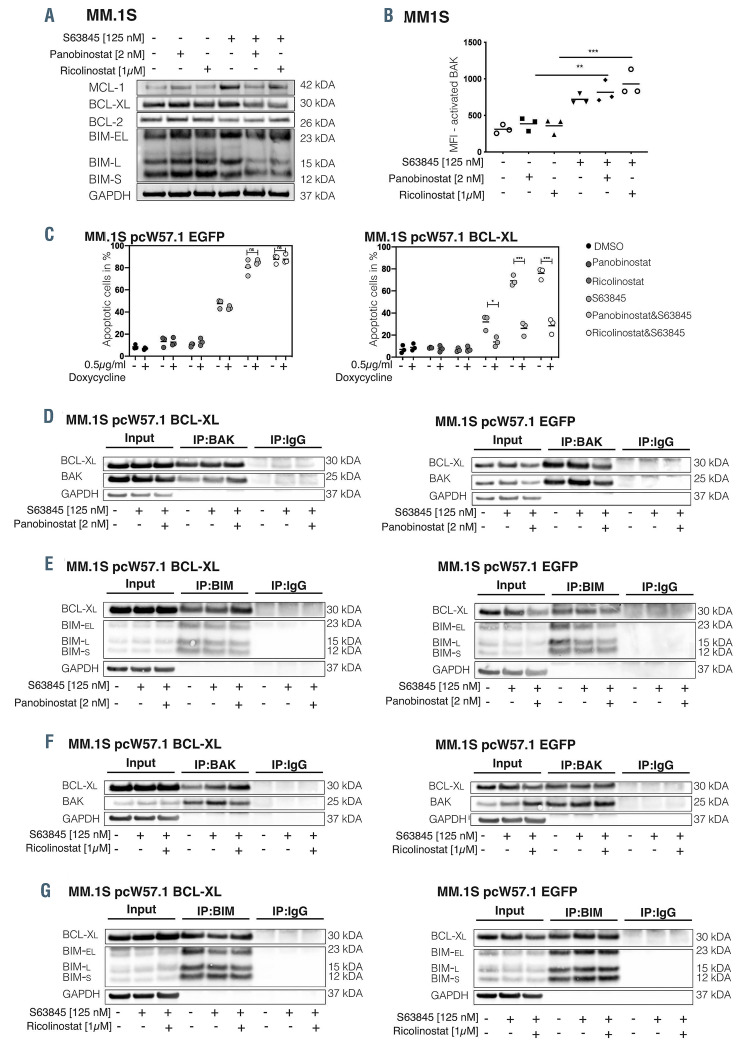
BCL-XL overexpression sequesters BIM and BAK and impairs S63845 mediated apoptosis induction. (A) Immunoblot analysis of the indicated BCL-2 family members was performed 24 hours (h) post-treatment initiation. One representative experiment of three independent biological replicates is shown. (B) BAK activation in MM.1S was determined by staining with antibodies against its active form. (C) MM.1S cells transduced with pcW57.1 EGFP (left panel) or pcW57.1 BCL-XL (right panel) were treated for 24 h with 0.5 μg/mL doxycycline to induce protein overexpression and afterwards exposed to the indicated treatments. Apoptotic cells were assessed 24 h post- treatment induction. Results indicate the mean +/- standard deviation of the mean (SDM) of three independent experiments. Differences between groups were calculated with one-way ANOVA, corrected for multiple comparison with Bonferroni-Holm correction with ****P<0.0001, **P<0.001 and *P<0.05. (D and G) Co-immunoprecipitation experiments in MM.1S cells transduced with either pcW57.1-EGFP (right panels) or pcW57.1-BCL-XL (left panels) were performed after 24 h pretreatment with 0.5 μg/mL doxycycline to induce protein overexpression and subsequent drug exposure for 24 h.
In MM, the anti-apoptotic BH3 family member MCL- 1 was shown to act as a master regulator of cell survival and resistance to therapy.2,3 Accordingly, several MCL-1 inhibitors, such as S63845, S64315, AMG176 and AZD5991 are currently under evaluation in clinical trials. 4 However, elevated expression of either BCL-XL or BCL-2 may affect the activity of MCL-1 inhibitors (MCL-1i).5 This suggests that the simultaneous targeting of multiple anti-apoptotic proteins might significantly enhance the activity of BH3 mimetics and overcome intrinsic as well as acquired drug resistance. In this context, a deregulation of BH3 protein family members by HDAC inhibitors (HDACi) was reported in MM6 making these compounds attractive combination partners for BH3 mimetics.
Here, we aimed to evaluate synergistic or additive combination approaches for selected BH3 mimetics. We assessed the activity of the pan-HDACi panobinostat or HDAC6i ricolinostat in combination with inhibitors targeting either BCL-2 (venetoclax), BCL-XL (A-1331952) or MCL-1 (S63845) in a panel of MM cell lines (MM.1S, KMS-12-BM, MOLP-8, U266, SKMM-1, RPMI-8226, OPM-2, NCI-H929). Interestingly, in six of eight MM cell lines (KMS-12-BM, MM.1S, U266, MOLP-8, NCIH929, OPM-2) a synergistic or additive effect was observed when combining S63845+HDACi (Figure 1A to D; Online Supplementary Figure S1A to H). The combination of either venetoclax or A-1331952 with HDACi led to synergistic or additive activity in three and four cell lines, respectively (Figure 1B and E; Online Supplementary Figure S1I to N).
The observed synergism was confirmed with alternative MCL-1 inhibitors (AZD5991, AMG-701) (data not shown) and translated into a significant increase in apoptosis in MM.1S, U266 and KMS-12-BM monoculture experiments using non-lethal concentrations of S63845, panobinostat and ricolinostat (Figure 1G to I). Similar effects were observed in co-culture experiments with MSCT+ stromal cells (Figure 1J to L).
Additional validation experiments confirmed the observed augmentation of the apoptotic signaling cascade including an enhanced release of cytochrome c (Figure 1M), cleavage of caspase 3 and PARP (Figure 1N; Online Supplementary Figure S1O and P) in all cell lines analyzed. No cell cycle alterations were observed upon single agent or combinational therapy (Online Supplementary Figure S2C to E).
The combination of S63845+HDACi proved to be particularly pronounced in the BCL-XL (co)-dependent MM cells MM1.S and U266,3,7 which otherwise did either not respond at all or only barley responded to single- agent MCL-1 inhibition.8 Moreover, BCL-XL is not only a major driver of intrinsic but also acquired MCL-1 inhibitor resistance,9 as well as dual MCL1/BCL2 inhibition. 10 Hence, concurrent BCL-XL inhibition seems to optimally augment the efficacy of MCL-1 inhibitors, but prior clinical trials aiming to directly inhibit BCL-XL failed due to untoward toxicity.11,12
Based on these results we evaluated whether deregulation of pro- or anti-apoptotic BCL-2 family proteins by HDAC inhibitors explains the observed synergism. Single-agent treatment with S63845 monotherapy led to the accumulation of MCL-1 and BCL-XL in MM.1S (Figure 2A) and U266 (Online Supplementary Figure S2A), but not in KMS-12-BM cells (Online Supplementary Figure S2B). Conversely, combined MCL-1+HDAC inhibition led to the downregulation of BCL-XL and MCL-1 protein levels in all tested cell lines prior to the onset of apoptosis (Figure 2B; Online Supplementary Figure S2A and B). In addition, a significant increase in BAK activation in KMS-12-BM cells (Online Supplementary Figure S1R) was noted. In MM.1S and U266 cell lines BAK is already fully activated by S63845 alone and the combination of MCL-1i and HDACi did not augment it any further (Figure 2B; Online Supplementary Figure S1M to Q), suggesting that active BAK is kept under control by alternative anti-apoptotic family members – most likely BCL-XL.
In order to test our assumption that BCL-XL inhibits apoptosis induction by S63845, we transduced MM cell lines with the Tet-on pcW57.1 vector harboring either full length BCL-XL or EGFP (control) and assessed apoptotic cells via flow cytometry. In MM.1S-BCL-XL cells, apoptosis significantly decreased upon treatment with S63845+HDACi (left panel of Figure 2C) compared to MM.1S-EGFP cells (right panel of Figure 2C). Similar findings were obtained in U266 and KMS-12-BM cells (Online Supplementary Figure 2F and G). In order to explore this rescue mechanism further, we examined the binding kinetics of BAK and BIM to BCL-XL via coimmunoprecipitations. Upon doxycycline-mediated induction of BCL-XL or EGFP protein, we treated the cells for 24 hours (h) with S63845 alone or in combination with either panobinostat or ricolinostat. Strikingly, BCL-XL overexpression in S63845+HDACi-treated cells resulted in an increased association of BCL-XL and BAK as well as BIM in all investigated cell lines (left panel of Figure 2D to G; Online Supplementary Figures 2H and I and S3A to D). On the contrary, in EGFP expressing cells, BIM and/or BAK binding to BCL-XL strongly decreased upon combined S63845+HDACi exposure as compared to S63845 treatment alone (right panel of Figure 2D to G; Online Supplementary Figures S2H and I and S3A to D).
Noteworthy, we also observed cell line-specific and combination-specific effects such as an exclusive impact of ricolinostat or panobinostat on BIM-BCL-XL, but not BAK-BCL-XL binding, in MM.1S-EGFP and U266-EGFP cells, respectively (right panel of Figure 2F and G; right panel of Online Supplementary Figure 2H and I). Furthermore, in KMS-12-BM-EGFP cells BIM was not associated with BCL-XL (right panel of Online Supplementary Figure S3C), as BIM is rather sequestered by BCL-2 (data not shown) in line with the MCL-1/BCL-2 dependency of this t(11;14) carrying cell line.3 In conclusion, these results strengthen a model where BCL-XL is capable of sequestering BAK and BIM released by S63845, thus prohibiting the onset of the apoptotic signaling cascade.
Figure 3.
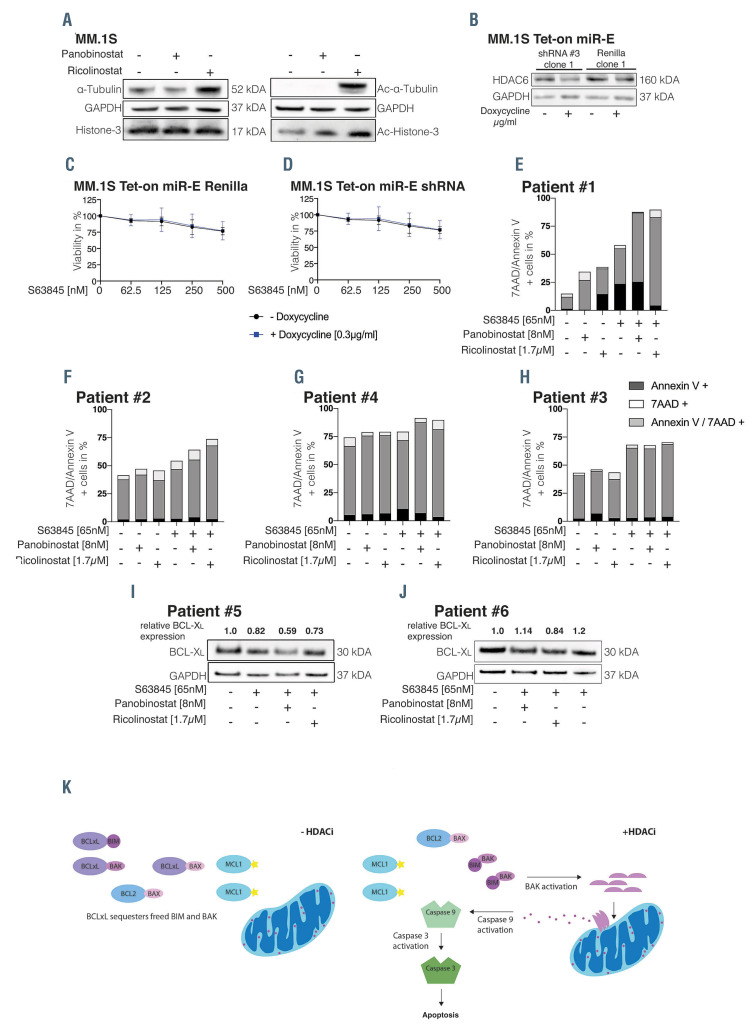
Ricolinostat promotes the activity of S63845 independent of HDAC6 inhibition. (A) MM.1S cells were either treated with panobinostat or ricolinostat for 24 hours (h), then whole-cell lysates were blotted for the indicated proteins. (B) Single-cell clones of MM.1S cells harboring a Tet-on miR-E vector expressing either a short hairpin RNA (shRNA) targeting Renilla (control) or an shRNA targeting HDAC6 were exposed to 0.3 μg/mL doxycycline for 48 h and whole-cell lysates were blotted for the indicated proteins. Western blots are representative for three independent experiments. (C and D) Cells were pretreated with doxycycline for 48 h and viability was assessed after an additional 48-h treatment with increasing concentrations of S63845 as indicated in the Figure. Results show the mean +/- standard deviation (SD) of three independent experiments performed in triplicates. (E and J) CD138 purified plasma cells sorted from patients with multiple myeloma (MM) were exposed to S63845, panobinostat or ricolinostat the respective combinations for 20 h (E and H) or 10 h (I and J). (E and H) Apoptotic cells were determined via Annexin V/7AAD positive staining. (I and J) Whole-cell lysates of primary MM cells were blotted for the indicated proteins. Short-term exposure of primary patient samples was chosen to avoid spontaneous cell death. (K) Proposed model of the underlying mechanism of the observed synergism between MCL-1i and HDACi in MM. In S63845 treated cells BCL-XL is capable to sequester BAK, hence inhibiting the apoptotic signaling cascade resulting in diminished MM cell death. In combination with HDACi, BCL-XL protein is downregulated and thereby BAK, released by MCL-1i, can be activated, can oligomerize and can insert into the MOMP which in turn releases cytochrome c and activates initiator as well as effector caspases, leading to PARP cleavage and cell death.
We next aimed to confirm the impact of panobinostat and ricolinostat on histone and tubulin acetylation as mechanism of synergy with MCL-1 inhibitors. Panobinostat is expected to strongly increase histone acetylation, while ricolinostat, which is selectively targeting HDAC6,13,14 should increase acetylated α-tubulin without altering acetylated histone levels.15 We treated MM.1S, U266 and KMS-12-BM cells with both HDACi and assessed acetylated and total protein expression levels of α-tubulin and histone-3 after 24 h. Panobinostat strongly increased acetyl-histone-3 in all three cell lines (Figure 3A; Online Supplementary Figure S3E and F). Contrary to our expectations, ricolinostat treatment likewise led to a strong increase in acetyl-histone- 3 besides elevating acetyl-α-tubulin levels (Figure 3A; Online Supplementary Figure S3E and E), indicating that ricolinostat has off-target effects on additional HDAC family members.
Accordingly, we aimed to investigate whether the synergism of ricolinostat and S63845 is facilitated via HDAC6 inhibition or via the epigenetic off-target effect. To this end, we performed viability assays with MM.1S and U266 cells transduced with a Tet-on miR-E plasmid harboring a control short hairpin RNA (shRNA) (Renilla), or HDAC6 shRNA. HDAC6 knockdown was confirmed 48 h after induction with 0.3 μg/mL doxycycline (Figure 3B; Online Supplementary Figure S3G). However, S63845 significantly decreased cell viability regardless of the presence of HDAC6 knockdown (Figure 3C and D; Online Supplementary Figure S3H to J). These results suggest that the synergism between S63845 and ricolinostat is due to the unspecific epigenetic effect of ricolinostat.
In order to validate our findings in primary MM cells we treated CD138-selected MM cells ex vivo for 20 h with single-agent HDACi and S63845 as well as the corresponding combinations before evaluating apoptosis induction. This demonstrated an increase of apoptotic cells upon combination treatment with S63845+HDACi in three of four samples tested, whereas the magnitude was highly variable (Figure 3E to H). Unfortunately, we were unable to collect sufficient cell material to establish a link between BCL-2 family dependencies and combination activity. However, we investigated whether the synergism was accompanied by a downregulation of BCL-XL in MM patient samples ex vivo. For this purpose, we treated patient samples for 10 h with S63845 alone or in combination with HDACi and determined BCL-XL protein expression. In both analyzed patient samples, downregulation of BCL-XL protein expression was observed in the MM cell lines (approximately 30% vs. S63845 single-agent treatment) upon S63845 combination with either panobinostat or ricolinostat, respectively. (Figure 3I to J). This suggests that the combination of MCL-1 inhibitors with HDACi is capable to tackle both, MCL-1 and BCL-XL, in MM patient cells. However, our findings need to be confirmed in enlarged patient cohorts and advanced in vivo models (i.e., carrying humanized MCL- 1)4 to better evaluate the clinical potential of our results as well as to define patient stratification markers.
In conclusion, our findings support a model where BCL-XL sequesters BAK/BIM released in response to MCL-1 inhibition, particularly in tumor clones with baseline BCL-XL functionality such as MM.1S and U266 cells. By combining MCL-1i with HDACi, BCL-XL protein is downregulated, leading to unrestrained BAK activation and initiation of the apoptotic signaling cascade (Figure 3K). Previous efforts to pharmacologically target BCL-XL failed due to its role in megakaryopoiesis.12,11 Hence, our findings point towards an alternative opportunity to indirectly tackle BCL-XL/MCL-1 co-dependent MM cells by combining MCL-i1 and HDACi and highlight the importance of exploring various options of apoptosis induction for designing new treatment concepts for clinical evaluation
Supplementary Material
Acknowledgments
The authors would like to thank Waltraud Scherbler and Martin Schreder for providing primary patient material and Anna Walzl for excellent assistance.
Funding Statement
Funding: this study was funded by the Wilhelminen Cancer Research Institute, the Austrian Forum Against Cancer and the Austrian Academy of Science (# 25542). AS is the recipient of a DOC Fellowship of the Austrian Academy of Sciences at the Wilhelminen Cancer Research Institute.
References
- 1.Rajkumar SV. Multiple Myeloma: 2016 update on diagnosis, riskstratification and management. Am J Hematol. 2016;91(7):719-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robillard N, Gomez P, Moreau P, Le Gouill S, Harousseau J, Amiot M.Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia. 2005;19(7):1248-1252. [DOI] [PubMed] [Google Scholar]
- 3.Gong J, Khong T, Segal D, et al. Hierarchy for targeting prosurvival BCL2 family proteins in multiple myeloma : pivotal role of MCL1. Blood. 2016;128(14):1834-1844. [DOI] [PubMed] [Google Scholar]
- 4.Bolomsky A, Vogler M, Köse MC, et al. MCL - 1 inhibitors, fast - lane development of a new class of anti - cancer agents. J Hematol Oncol. 2020;13(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hockings C, Alsop AE, Dewson G, et al. Mcl-1 and Bcl-x L sequestration of Bak confers differential resistance to BH3-only proteins. Cell Death Differ. 2018;25(4):721-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramakrishnan VG, Miller KC, Macon EP, et al. Histone deacetylase inhibition in combination with MEK or BCL-2 inhibition in multiple myeloma. Haematologica. 2019;104(10):2061-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touzeau C, Ryan J, Guerriero J, et al. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia. 2016;30(3):761-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotschy A, Szlavik Z, Murray J, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477-482. [DOI] [PubMed] [Google Scholar]
- 9.Patricia Gomez-Bougie, Sophie Maïga, Benoit Tessoulin, et al. Respective place of venetoclax and MCL1 BH3 mimetics in multiple myeloma treatment. Blood. 2018;132(25):2656-2669. [DOI] [PubMed] [Google Scholar]
- 10.Seiller C, Maiga S, Touzeau C, et al. Dual targeting of BCL2 and MCL1 rescues myeloma cells resistant to BCL2 and MCL1 inhibitors associated with the formation of BAX / BAK heterocomplexes. Cell Death Dis. 2020;11(5):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson WH, Connor OAO, Czuczman MS, et al. Navitoclax, a targeted high-affi nity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010; 11(12):1149-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128(6):1173-1186. [DOI] [PubMed] [Google Scholar]
- 13.Santo L, Hideshima T, Kung AL, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2019;119(11):2579-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carew JS, Espitia CM, Zhao W, et al. Rational cotargeting of HDAC6 and BET proteins yields synergistic antimyeloma activity. Blood Adv. 2019;3(8):1318-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubbert C, Guardiola A, Shao R.HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455-458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.