Abstract
Patients with chronic lymphocytic leukemia or small lymphocytic lymphoma whose tumors carry deletion of chromosome 17p13.1 [del(17p)] have an unfavorable prognosis and respond poorly to standard chemoimmunotherapy. Zanubrutinib is a selective next-generation Bruton tyrosine kinase inhibitor. We evaluated the safety and efficacy of zanubrutinib 160 mg twice daily in treatment-naïve patients with del(17p) disease enrolled in a dedicated, nonrandomized cohort (Arm C) of the phase III SEQUOIA trial. A total of 109 patients (median age, 70 years; range, 42–86) with centrally confirmed del(17p) were enrolled and treated. After a median of 18.2 months (range, 5.0–26.3), seven patients had discontinued study treatment due to progressive disease, four due to an adverse event, and one due to withdrawal of consent. The overall response rate was 94.5% with 3.7% of patients achieving complete response with or without incomplete hematologic recovery. The estimated 18-month progression-free survival rate was 88.6% (95% CI: 79.0–94.0) and the estimated 18-month overall survival rate was 95.1% (95% CI: 88.4–98.0). Most common all-grade adverse events included contusion (20.2%), upper respiratory tract infection (19.3%), neutropenia/ neutrophil count decreased (17.4%), and diarrhea (16.5%). Grade ≥3 adverse events were reported in 53 patients (48.6%), most commonly neutropenia (12.9%) and pneumonia (3.7%). An adverse event of atrial fibrillation was reported in three patients (2.8%). Zanubrutinib was active and well tolerated in this large, prospectively enrolled treatment cohort of previously untreated patients with del(17p) chronic lymphocytic leukemia/small lymphocytic lymphoma. This trial was registered as clinicaltrials.gov Identifier: NCT03336333.
Introduction
Patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) have historically been treated with combination chemotherapy and immunotherapy with success; however, many patients do not have sustained responses in part due to genomic aberrations that impair responsiveness to therapy. One aberration found in patients with CLL/SLL is the deletion of chromosome 17p13.1 [del(17p)]; these patients have an unfavorable prognosis and respond poorly to standard chemoimmunotherapy, with reduced rates of overall survival (OS) and worse clinical outcomes.1,2 del(17p) results in the mono or biallelic loss of the TP53 gene, which encodes the tumor suppressor p53, a multifunctional transcription factor important for cellular response to DNA damage, including cell cycle arrest and apoptosis.3 Most patients with del(17p) also have a mutation of the other TP53 allele and therefore lack wild-type TP53, leading to genomic instability and reduced responsiveness to cytotoxic chemotherapy.2 The incidence of del(17p) is approximately 5-8% of patients with CLL at diagnosis and increases with each relapse.4,5
Novel targeted therapies are therefore the preferred treatment modality for previously untreated patients whose disease bears the del(17p) mutation.6,7 Several new agents are approved by the US Food and Drug Administration and European Medicines Agency (EMA) for adult patients with CLL/SLL regardless of del(17p) status, including the BTK inhibitors ibrutinib and acalabrutinib. In the RESONATE study of ibrutinib versus ofatumumab in patients with relapsed or refractory (R/R) CLL/SLL,8 89% of ibrutinib-treated patients with del(17p) achieved an objective response with long term follow-up.9 Similar results were observed in the single-arm RESONATE- 17 study in R/R CLL10 in which 83% of patients achieved an objective response; the 24-month progression- free survival (PFS) was 63%. Acalabrutinib, a secondgeneration BTK inhibitor, has been approved recently for treatment-naïve (TN) patients with CLL/SLL regardless of del(17p) status based on results from the ELEVATE TN study.11,12 Notably, of the patients with del(17p) in that study, only 16 were assigned to the single-agent acalabrutinib arm.
Other small molecule inhibitors approved in patients with CLL/SLL and del(17p) include the BCL-2 inhibitor venetoclax13 and the phosphatidylinositide-3-kinase (PI3K)-δ inhibitor idelalisib.14 Stilgenbauer and colleagues reported the results of venetoclax treatment in 158 mostly R/R del(17p) patients,15 including a 77% overall response rate (ORR) and an estimated 24-month PFS of 50%. Notably, only five patients in this study were TN. In the CLL14 trial which compared venetoclax and obinutuzumab to chlorambucil and obinutuzumab in TN CLL, 17 patients assigned to the venetoclax arm had del(17p).16 Similarly, studies supporting the approval of idelalisib together with rituximab in the frontline setting by the EMA included few TN CLL/SLL patients with del(17p).17,18 Collectively, only limited data are available for novel targeted therapies for previously untreated patients with del(17p) CLL/SLL, and no large multi-center studies have systematically examined this specific population.
Zanubrutinib (BGB-3111) is a next-generation BTK inhibitor with favorable oral bioavailability and high specificity for BTK, exhibiting comparatively lower offtarget activity than ibrutinib for structurally related kinases such as epidermal growth factor receptor (EGFR), interleukin- 2 inducible kinase (ITK), and Src family kinases.19 The safety, pharmacokinetics, pharmacodynamics, and preliminary activity of zanubrutinib were investigated in a phase I/II study of patients with multiple B-cell malignancies in which high level, sustained BTK occupancy was noted in both peripheral blood and lymph nodes at the recommended phase II dose of 160 mg twice daily (bid).20 Encouraging activity was observed in a cohort of 78 patients with both TN and R/R CLL/SLL, including in a subset of 16 patients with del(17p) or TP53 mutation who achieved a 100% ORR.20 Activity of zanubrutinib was also observed in a separate phase II trial of 91 R/R CLL/SLL patients in China, including in a subset of 17 patients with del(17p) who achieved a 88.2% ORR.21 Zanubrutinib has recently received accelerated approval in the United States for adult patients with mantle cell lymphoma who have received at least one prior therapy22 and is currently undergoing further clinical testing in several prospective, multicenter, randomized phase III trials in CLL/SLL.
The SEQUOIA trial (clinicaltrialsgov. Identifier: NCT03336333) is an open-label, multi center, randomized phase III study of TN patients with CLL/SLL. Patients without centrally confirmed del(17p) were randomized to receive either zanubrutinib 160 mg bid until unacceptable toxicity or disease progression (PD), or six cycles of rituximab and bendamustine. Considering the poor outcomes associated with any standard chemoimmunotherapy regimen in patients with del(17p), those with centrally confirmed del(17p) during screening for SEQUOIA were not randomized but assigned to single-agent zanubrutinib in a separate cohort (Arm C). This is the first report of the safety and efficacy results in this high-risk del(17p) patient cohort.
Methods
Study design and population
Eligible patients had confirmed CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia (iwCLL) definition.23 TN adults were eligible if either aged ≥65 years or unsuitable for treatment with fludarabine, cyclophosphamide, and rituximab (FCR) and an Eastern Cooperative Oncology Group (ECOG) performance status ≤2.24 Centrally confirmed del(17p) by fluorescence in situ hybridization with >7% aberrant nuclei present was required. Patients had adequate endorgan function, including absolute neutrophil count (ANC) ≥1,000/mm3 and platelet count ≥75,000/mm3. For patients with bone marrow involvement, ANC ≥750/mm3 and platelet count ≥50,000/mm3 were allowed. Patients with history of atrial fibrillation and/or long-term anticoagulation, or those requiring moderate or strong CYP3A inhibitors, could enroll.25 All Arm C patients were assigned to receive zanubrutinib (160 mg bid) until intolerance or PD.
This study was conducted according to principles of the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice and approved by the Institutional Review Board/Ethics Committee at each participating site. All patients provided written informed consent. This study adhered to CONSORT-10 guidelines for reporting.26
Objectives and assessments
Key objectives for Arm C included assessments of ORR, PFS, duration of response (DOR), and safety (frequency and severity of all treatment-emergent adverse events [AE]). ORR was assessed per modified iwCLL criteria for CLL23,27 and per Lugano criteria for SLL.28 Measurable disease, defined as ≥1 lymph node >1.5 cm in the longest diameter and measurable in two perpendicular diameters, was assessed by computed tomography/magnetic resonance imaging. Response assessments were performed every 12 weeks after the first dose day for 96 weeks, then every 24 weeks until PD or initiation of new, nonprotocol therapy, whichever came first. Patients underwent bone marrow examination at baseline and for confirmation of complete response (CR), or CR with incomplete hematologic recovery (CRi), or if PD was suspected due to cytopenia.
All treatment-emergent AE, including AE of interest (AEI) based on the known toxicity profile for BTK inhibitors occurring on or after day 1 until 30 days after treatment discontinuation were summarized. AEI were categorized in accordance with predefined MedDRA search criteria (Online Supplementary Table S1). AE severity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 and the Grading Scale for Hematologic Toxicity in CLL Studies.23 Biomarkers were assessed at baseline and optionally at progression.
Statistical analyses
Primary efficacy and safety analyses included all patients with centrally confirmed del(17p) CLL/SLL receiving ≥1 dose of zanubrutinib. ORR was summarized as percentage of responders (CR, CRi, nodular partial response [nPR], partial response [PR], or PR with lymphocytosis [PR-L]) with corresponding 95% Confidence Interval (CI). An evaluation of ORR in subgroups defined by key demographic and baseline disease characteristics was conducted and summarized in a forest plot. DOR was defined as time from first response until PD or death due to any cause. PFS was measured from time of first dose to PD or death due to any cause. Median DOR, PFS, and event-free survival rates were estimated using Kaplan-Meier methodology with corresponding 95% CI.
Results
Patient characteristics and disposition
Between February 3, 2018 and February 20, 2019, 109 patients with centrally confirmed del(17p) CLL/SLL were enrolled from 59 sites in 13 countries (Online Supplementary Table S2) and received ≥1 dose of zanubrutinib. Two additional patients without del(17p) were assigned in error to this study arm and are not included in the analysis. At the data cutoff date of April 15, 2020, the median duration of study follow-up was 18.2 months (range, 5.0–26.3). Median age at study entry was 70.0 years (range, 42–86); the majority of patients presented with CLL (90.8%). Reported reasons for treatment per iwCLL criteria in order of frequency included progressive marrow failure (41.3%); massive, progressive, or symptomatic lymphadenopathy (41.3%); significant fatigue (33.0%); night sweats (32.1%); progressive lymphocytosis with increase of > 50% over 2 months or doubling time of <6 months (28.4%); massive, progressive, or symptomatic splenomegaly (23.9%); and unintentional weight loss (14.7%). More than one reason for treatment may have been given, with a median of two reasons given for each patient. Many patients had other high-risk disease characteristics, including 40 patients with CLL who presented as Binet stage C (40.4%), 42 patients with bulky disease ≥5 cm (38.5%), 78 patients with elevated β2-microglobulin (78.8% of 99 patients with available data), and 67 patients with an unmutated immunoglobulin heavy chain variable (IGHV) locus (65.0% of 103 patients with sufficient RNA for testing). Furthermore, 32 (37.2%) of 86 patients with sufficient metaphases available for analysis had at least three distinct karyotypic abnormalities defined as complex karyotype (Table 1).
Efficacy
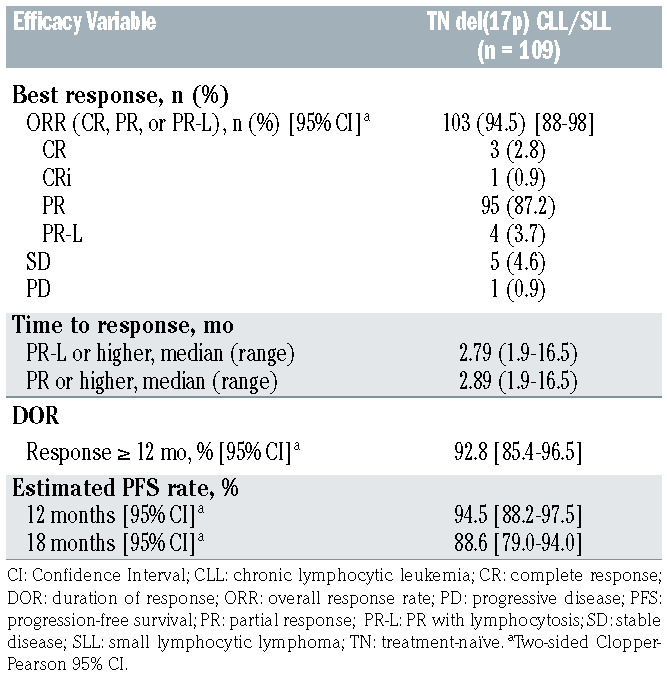
The ORR was 94.5%, which included three patients (2.8%) with CR, one patient (0.9%) with CRi, 95 (87.2%) with PR, and four (3.7%) with PR-L (Table 2). Five (4.6%) patients had a best response of stable disease (SD). One patient (0.9%) had PD at the first response assessment. Five (4.6%) patients met clinical CR criteria but did not undergo bone marrow biopsy. Ninety-seven patients (89.0%) remained on treatment at the time of analysis. Nine patients with an initial response (8.3%) progressed on study, four of whom had histologically confirmed Richter transformation. Median time to transformation was 13.7 months (time to transformation for each patient: 3.9, 13.6, 13.8, and 15.7 months). Two other patients had new lesions seen on CT with positron emission tomography (PET) avidity for which biopsy could not definitively confirm transformation, while one other patient had accelerated CLL. Seven patients who have progressed have discontinued treatment; two other patients with progression remained on treatment at time of data cutoff. Four patients who progressed have died; two due to progression, one due to an adverse event after progression (acute kidney injury), and one after progression due to septic shock. Four patients (3.7%) discontinued treatment due to AEs, of whom 2 have died (see Safety below), while one patient discontinued treatment after withdrawal of consent and was lost to follow up.
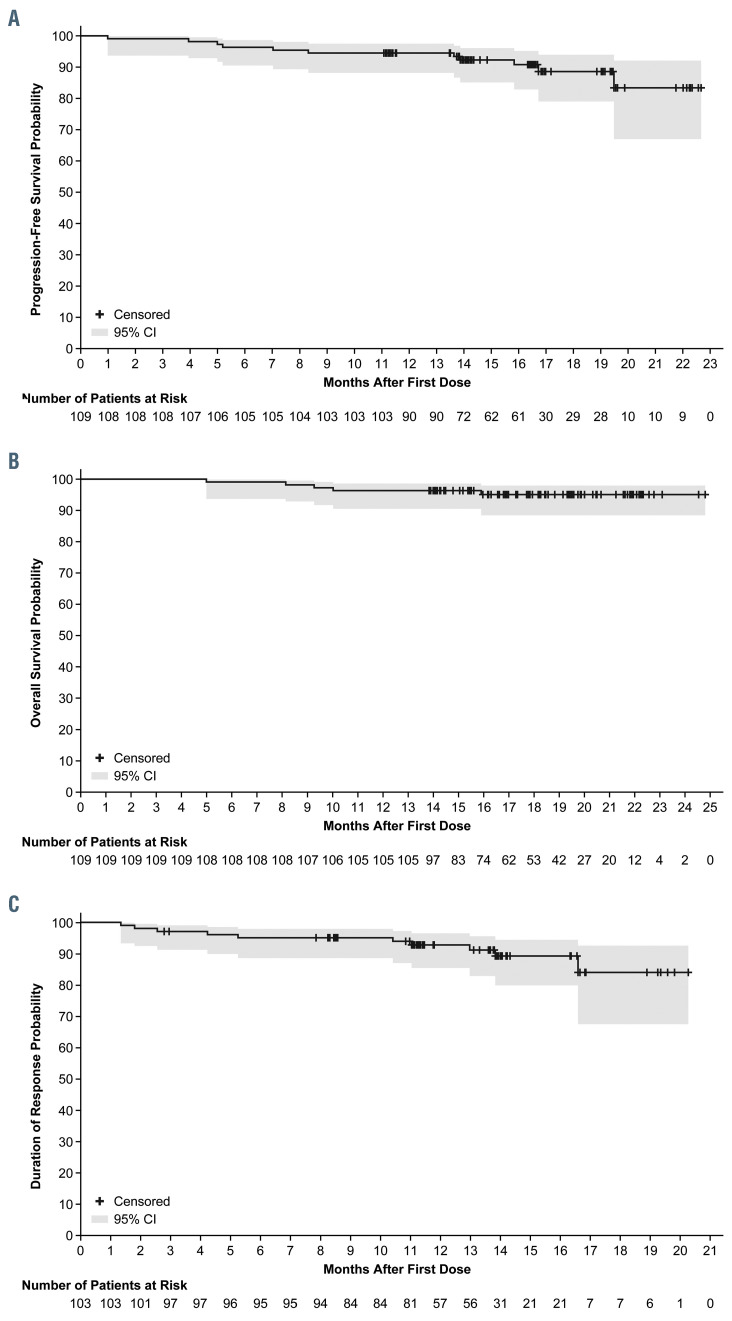
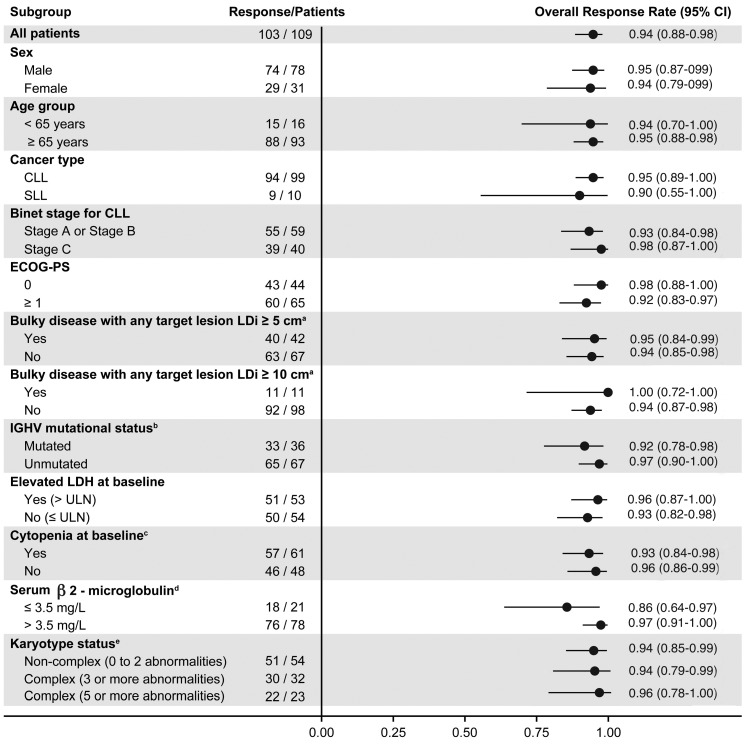
Median PFS and OS were not reached. The estimated 18-month PFS rate was 88.6% (95% CI: 79.0–94.0) (Figure 1A), while the estimated 18-month OS rate was 95.1% (95% CI: 88.4–98.0) (Figure 1B). The median time to response was 2.8 months (range, 1.9–16.5) The median DOR was not reached; 92.8% of patients had a DOR ≥ 12 months (Figure 1C). ORR was consistent across all prespecified demographic and baseline disease characteristics (Figure 2).
Transient treatment-related lymphocytosis was observed (Online Supplementary Figure S1A), but there was a significant reduction in target lesion size by the first scheduled response assessment, consistent with the short median time to response (Online Supplementary Figure S1A). The peak median change in absolute lymphocyte count (ALC) and time to resolution of lymphocytosis both appeared to be decreased from previous experience with zanubrutinib.19 In an exploratory analysis, changes in ALC were compared between patients with mutated and unmutated IGHV locus. Patients with unmutated IGHV showed a slight trend towards less treatment-related lymphocytosis (Online Supplementary Figure S1B), similar to previous reports.9,29
Sixty-one patients (56.0%) began the study with at least one cytopenia (Table 1), including 43 patients with anemia (39.4%), eight patients with neutropenia (7.3%), and 28 patients with thrombocytopenia (25.7%) (Online Supplementary Table S3). Eleven patients (10.1%) received at least one dose of a granulocyte colony-stimulating factor, while one patient (0.9%) received at least one dose of an erythrocyte-stimulating growth factor. Of those patients with baseline anemia, 86.0% of patients demonstrated sustained improvement in hemoglobin; 75.0% of patients with baseline neutropenia also demonstrated sustained improvement in ANC, and 85.7% of patients with baseline thrombocytopenia demonstrated sustained improvement in platelet count (Online Supplementary Table S3).
In an exploratory post hoc analysis, baseline characteristics and response rate were compared between patients with a percentage of del(17p)-positive nuclei of ≥20% del(17p) high] versus patients with a percentage of >7% to <20% [del(17p) low] (Online Supplementary Table S4). Patients in the del(17p) high category were observed to have a higher rate of unmutated IGHV (75% vs. 56.4% of patients with a resulted test; P=0.0478) and complex karyotype status (56.8% vs. 22.4% of patients with sufficient metaphases for analysis; P=0.0011); no other differences in baseline characteristics were observed. Best ORR and estimated 18-month PFS were 98% and 89%, respectively, in the del(17p) high category and 92% and 88%, respectively, in the del(17p) low category.
Figure 1.
Survival and response analyses using the Kaplan-Meier method. (A) Progression-free survival as determined by investigator assessment. Shaded area indicates 95% Confidence Interval (CI). (B) Overall survival. Shaded area indicates 95% CI. (C) Duration of response as determined by investigator assessment. Shaded area indicates 95% CI.
Table 1.
Key patient and disease characteristics.
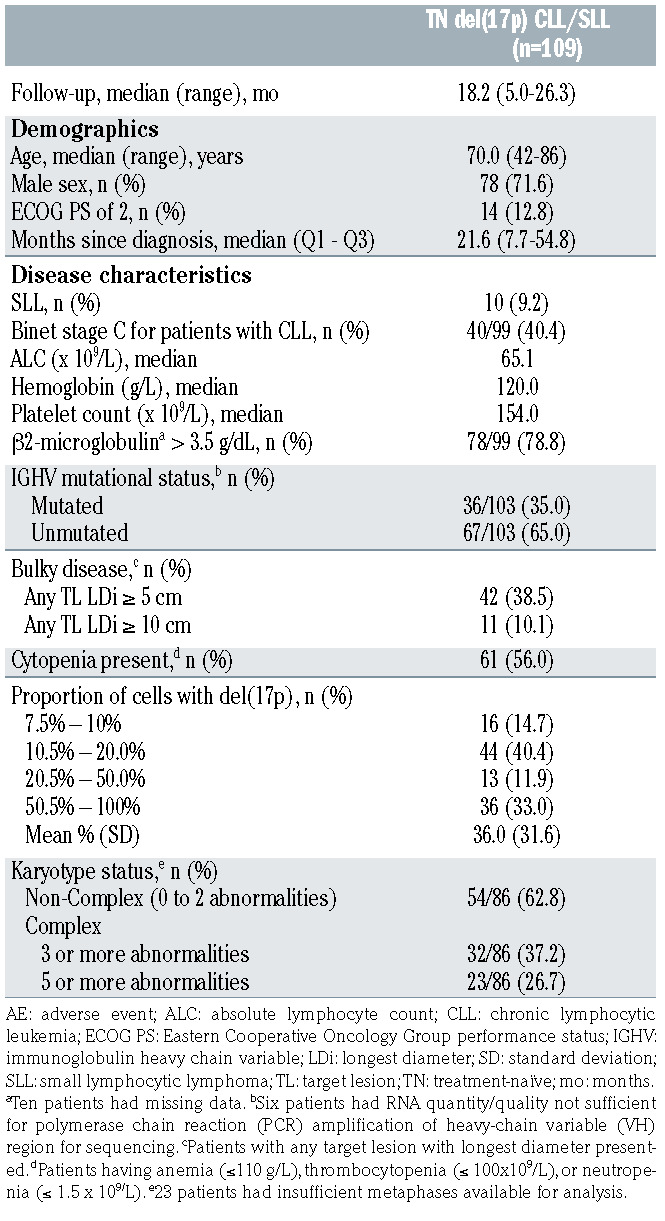
Table 2.
Summary of investigator-assessed efficacy.
Safety
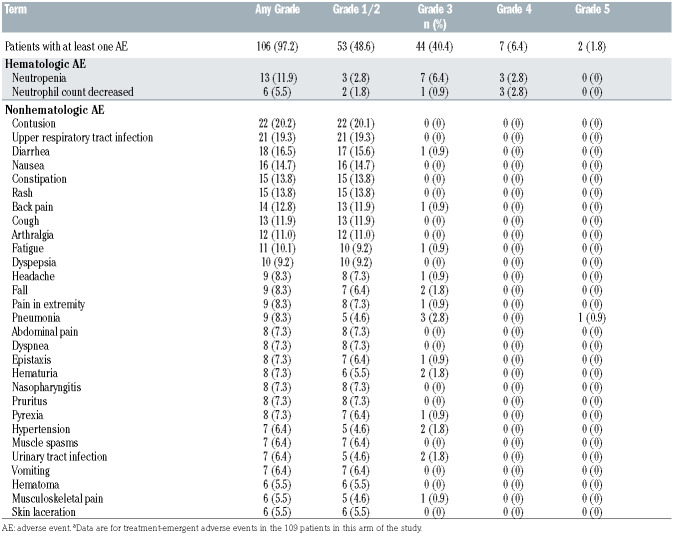
AE of any grade reported in ≥10% of patients included contusion (20.2%), upper respiratory tract infection (19.3%), neutropenia/neutrophil count decreased (17.4%), diarrhea (16.5%), nausea (14.7%), rash (13.8%), constipation (13.8%), back pain (12.8%), cough (11.9%), arthralgia (11.0%), and fatigue (10.1%) (Table 3). Grade ≥3 events were reported in 53 patients (48.6%), with neutropenia/decreased neutrophil count (12.9%) and pneumonia (3.7%) being the most common. Serious AE (SAE) were reported in 36.7% of patients, with pneumonia (3.7%) being the most common. AE led to dose reduction in 6 (5.5%) patients and included thrombocytopenia, diarrhea, gastritis, blood bilirubin increased, arthralgia, myalgia, and headache. All six patients remain on treatment.
Four patients discontinued treatment due to AE; a 77- year-old male patient had grade 4 pseudomonal sepsis associated with grade 4 neutropenia and atrial fibrillation who recovered, while another 72-year-old male patient had grade 3 melanoma requiring surgery and adjuvant therapy. One grade 5 event was reported in a 84-year-old female patient with health-care associated pneumonia diagnosed 8 days after the last dose of zanubrutinib which was held for an unrelated procedure. This case was complicated by the development of sepsis which was assessed as related to zanubrutinib. Finally, one grade 5 event was reported in a 72-year-old male patient who developed disease progression at the week 36 response assessment including massive enlargement of intra-abdominal lymph nodes associated with hypercalcemia. Prior to discontinuation of study drug, the patient shortly thereafter had renal failure requiring dialysis and subsequently died due to pulmonary edema. No sudden or unknown deaths were reported.
AEI known to be associated with BTK inhibitors were characterized in greater detail, including grouping of similar AE by category (Online Supplementary Table S1). AEI reported in ≥10% of treated patients included infections (64.2%; 13.8% grade ≥3), minor bleeding (26.6%), bruising (24.8%; 0% grade ≥3), neutropenia (18.3%; 13.8% grade ≥3), diarrhea (15.6%; 0.9% grade ≥3), nausea (13.8%; 0% grade ≥3), arthralgia (11.0%; 0% grade ≥3), fatigue (10.1%; 0.9% grade ≥3) (Online Supplementary Table S5). The most common infections reported in ≥ 5% of patients included upper respiratory tract infection (19.3%), pneumonia (8.3%), nasopharyngitis (7.3%), and urinary tract infection (6.4%); most of these were grade 1 or 2 events. Prophylaxis against opportunistic infections was allowed per local standard of care but not required; no opportunistic infections were reported.
Figure 2.
Subgroup analysis of overall response rate. Overall response rate presented as of November 1, 2019. Two-sided Clopper-Pearson 95% Confidence Interval (CI) are used. CLL: chronic lymphocytic lymphoma; ECOG PS: Eastern Cooperative Oncology Group performance status; LDH: lactate dehydrogenase; LDi: longest diameter; SLL: small lymphocytic leukemia. aPatients with any target lesion with longest diameter presented. bSix patients had RNA quantity/quality not sufficient for polymerase chain reaction amplification of immunoglobulin heavy chain variable (VH) region for sequencing. cPatients having anemia (≤110 g/L), thrombocytopenia (≤100x109/L), or neutropenia (≤1.5x109/L). d10 patients had missing data. e23 patients had insufficient metaphases available for analysis.
Of other malignancies reported on study, most were dermatological malignancies reported in ten patients (9.2%). Other than two patients with melanoma, all were basal and squamous cell carcinomas of the skin (grade 1/2) reported from patients in Australia and New Zealand, where skin cancers are frequent, especially in patients with CLL/SLL.30,31 One patient developed grade 3 melanoma requiring surgery and adjuvant therapy with pembrolizumab leading to discontinuation of zanubrutinib. Five patients (4.6%) reported a non-dermatologic other malignancy. One patient developed localized breast cancer for which axillary lymph node biopsy indicated disease transformation to DLBCL, while one other patient developed lung cancer for which interlobar lymph node biopsy also indicated disease transformation to DLBCL. Three patients reported a transitional cell carcinoma of the bladder or ureter, all of whom underwent resection without known residual disease and remain on study drug treatment.
The usage of therapeutic anticoagulation was not restricted on this study; 20 patients (18.3%) were reported to have taken a therapeutic anticoagulant including warfarin, direct-acting oral anticoagulants, and heparins during the study, while 27 patients (24.8%) were reported to have taken an oral platelet aggregation inhibitor, including aspirin, during the study. Bleeding of any type was reported in 47.7% of patients (4.6% grade ≥3). Major bleeding events were defined as any bleeding event with grade ≥3, any SAE, or any bleed affecting the central nervous system (CNS); these occurred in six patients (5.4%). A description of each event, including any confounding factors, is presented in Online Supplementary Table S6; in two patients, bleeding occurred in the setting of a surgical procedure without dose hold as advised per protocol. All patients continued study treatment after dose interruption. No bleeding events affecting the CNS were reported.
Table 3.
Most common adverse events regardless of causality. Adverse events of any grade occurring in ≥ 5% of patients and all grade ≥3 adverse events occurring in ≥2% of patients are shown.
A history of atrial fibrillation or flutter was reported in seven patients (6.4%); four patients (3.7%) entered the study with controlled and hemodynamically stable atrial fibrillation or flutter. An AE of atrial fibrillation or flutter was reported in three patients (2.8%). One grade 2 event was reported in a 71-year-old male patient with hypertension and prior history of atrial fibrillation, while a grade 3 event was reported in a 78-year-old male patient who was septic from cholecystitis. Both of these events resolved and did not require discontinuation of study treatment. Finally, one grade 4 event was reported in a 77-year-old male patient with grade 3 hypertension at baseline, who discontinued treatment due to sepsis secondary to Pseudomonas; atrial fibrillation resolved following recovery from sepsis.
Discussion
In this report, we have shown the activity and safety of zanubrutinib in a large non-randomized cohort of treatment- naïve CLL/SLL patients with centrally confirmed del(17p), enrolled as part of the global SEQUOIA trial. As expected, these results compare favorably with those from previous studies of TN patients treated with chemoimmunotherapy, including the CLL8 trial of FCR.1,2,32 At the present time, prospective clinical trial data from BTK inhibitor-treated patients with TN del(17p) CLL/SLL are limited. Patients with del(17p) were not eligible for enrollment in RESONATE-233 and the ECOGE191234 trials evaluating ibrutinib in the TN setting. The Alliance A041702 study, comparing chemotherapy, ibrutinib, or ibrutinib and rituximab in older patients with TN CLL/SLL, did allow patients with del(17p) to enroll; of these, 6% had del(17p), and only nine patients were assigned to receive ibrutinib alone.35 Two studies examining combinations of novel targeted therapies with anti- CD20 antibodies also enrolled a small number of TN patients with del(17p), including the iLLUMINATE study, where 14 patients with del(17p) received combination ibrutinib and obinutuzumab.36 In a single-center, phase 2 study, single-agent ibrutinib was evaluated in 35 patients with TN CLL; notably, this population was selected based on cytogenetics or TP53 sequencing and allowed younger and/or fit patients, leading to enrollment of a population with a median age of 62.37,38 At a median follow-up time of 15 months, ORR was reported as 97%, including 12% CR, 70% PR, and 15% PR-L.
The ORR with zanubrutinib observed in the present study appears at least comparable with the reported ibrutinib experience. Median time to response essentially was defined by the first scheduled response assessment (Figure 1C), consistent with reduction in target lesion size (Online Supplementary Figure S1A) and resolution of cytopenias (Online Supplementary Table S3). Consistent with previous studies of other BTK inhibitors,12,32,37 CR were uncommon with short follow-up; longer follow-up will be needed to more precisely define the CR rate. Response rates appear to be similarly high regardless of coincident risk factors, including IGHV mutational status and complex karyotype status, though the low number of non-responders may limit the ability to detect meaningful differences between subgroups.
In the UK LRF CLL4 trial, del(17p) in >20% of nuclei was found to be independently associated with shorter PFS in patients treated with chemoimmunotherapy.39,40 As expected, patients with a higher burden of del(17p) were associated with a higher rate of poor prognostic factors such as unmutated IGHV status and complex karyotype. When examining ORR and progression events in the current study, ORR appeared to be similar in patients without and with del(17p) in ≥20% of nuclei (Online Supplementary Table S4), suggesting that zanubrutinib has preserved activity in high-risk patients with enrichment of malignant cells for del(17p). This is comparable to the activity seen in the RESONATE-17 trial of R/R patients with del(17p) treated with ibrutinib.10
Ten patients progressed on study. In addition to presence of del(17p), seven patients who progressed had an unmutated IGHV locus. Karyotype analysis was available for eight patients who had progressed, of whom two had complex karyotype (number of abnormalities: 5 and 6). Four patients had histologically confirmed transformation to aggressive lymphoma, while two patients had suspected transformation. The present results compare favorably to those reported with chemoimmunotherapy, where 23% of patients with del(17p) treated in the first line experienced disease transformation, with a median time to transformation of 12 months.41 In the RESONATE-17 trial of R/R CLL patients, 44% of progression events were due to transformation, most within the first 6 months of treatment. 10 Similarly, both early progression events in the TN del(17p) or TP53 populations treated with ibrutinib, as reported by Farooqi and colleagues, occurred due to Richter transformation.37 These data are in line with the known association of del(17p) and transformation to aggressive lymphomas.42 Long term outcomes for patients with del(17p) treated with ibrutinib were reported by Ahn and colleagues,38 showing an approximate 5-year PFS of 75%. Further follow-up will be required to demonstrate the durability of responses to zanubrutinib. Additional analyses, including correlation of response and progression with concurrent genomic abnormalities and other genetic mutations (e.g., TP53, NOTCH1, BTK, and PLCG2 mutations), both at baseline and at the time of progression, are currently in progress.
The clinically meaningful activity noted in this patient series appears to be associated with a favorable toxicity profile and is consistent with that reported in other studies of zanubrutinib to date.20,43 Despite enrolling a more elderly and comorbid population and allowing for therapeutic anticoagulation on study, the incidence of grade ≥3 AE or SAE leading to major bleeding was 5.6% with no CNS events reported, and all patients able to continue study drug after dose interruption. Consistent with its greater selectivity for BTK and less inhibition of kinases such as EGFR, Src, and others, the incidence of grade 3 AE such as diarrhea, arthralgia, and myalgia were all ≤1%. Importantly, only three patients on this study reported treatment-emergent atrial fibrillation, six patients required ongoing dose reduction, and four patients discontinued zanubrutinib due to an AE. Two phase III randomized studies in patients with R/R CLL/SLL44 and Waldenström macroglobulinemia45 are ongoing to directly compare the efficacy and safety profiles of ibrutinib and zanubrutinib.
Limitations of this study include the relatively short duration of follow-up and its single-arm design. A retrospective analysis for baseline TP53 mutations is currently being performed for this study arm and the larger randomized arms. Analogous to other BTK inhibitors, the singleagent activity of zanubrutinib is not expected to induce a deep response with eradication of minimal residual disease (MRD). Several studies have recently reported achievement of undetectable MRD in patients with TN CLL/SLL, including those patients with del(17p), by combining a BTK inhibitor with obinutuzumab or venetoclax. 36,46,47 The SEQUOIA trial is currently enrolling patients in Arm D, which will evaluate the safety and activity of a combination of zanubrutinib with venetoclax in TN CLL/SLL patients with del(17p). In summary, these results indicate that single-agent zanubrutinib is active and generally well tolerated in this very high-risk population.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in the study, their supporters, and the investigators and clinical research staff from the study centers. Medical writing and editorial assistance were provided, under the direction of the authors, by Bio Connections.
Funding Statement
Funding: This work, including medical writing and editorial assistance, was supported by BeiGene USA, Inc. BeiGene was involved in the study design, compilation of data, and statistical analysis.
References
- 1.Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30(26):3209-3216. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164-1174. [DOI] [PubMed] [Google Scholar]
- 3.Kastenhuber ER, Lowe SW. Putting p53 in context. Cell. 2017;170(6):1062-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910-1916. [DOI] [PubMed] [Google Scholar]
- 5.Yu L, Kim HT, Kasar S, et al. Survival of del17p CLL depends on genomic complexity and somatic mutation. Clin Cancer Res. 2017;23(3):735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aitken MJL, Lee HJ, Post SM. Emerging treatment options for patients with p53- pathway-deficient CLL. Ther Adv Hematol. 2019;10:2040620719891356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brander D, Islam P, Barrientos JC. Tailored treatment strategies for chronic lymphocytic leukemia in a rapidly changing era. Am Soc Clin Oncol Educ Book. 2019;39:487-498. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JR, Hillmen P, O'Brien S, et al. Extended follow-up and impact of highrisk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018; 32(1):83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409-1418. [DOI] [PubMed] [Google Scholar]
- 11.Calquence® [package insert]. Gaithersburg, MD: AstraZeneca; November 2019. [Google Scholar]
- 12.Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venclyxto® [summary of product characteristics]. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co. KG; April 2020. [Google Scholar]
- 14.Zydelig® [summary of product characteristics]. County Cork, Ireland: Gilead Sciences, Inc.; April 2019. [Google Scholar]
- 15.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol. 2018;36(19): 1973-1980. [DOI] [PubMed] [Google Scholar]
- 16.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225-2236. [DOI] [PubMed] [Google Scholar]
- 17.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014; 370(11):997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015; 126(25):2686-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y, Liu Y, Hu N, et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton's tyrosine kinase. J Med Chem. 201962(17):7923-7940. [DOI] [PubMed] [Google Scholar]
- 20.Tam CSL, Trotman J, Opat S, et al. Phase 1 study of selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019; 134(11):851-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W, Yang S, Zhou K, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brukinsa® [package insert]. Beijing, China: BeiGene Co. Ltd.; November 2019. [Google Scholar]
- 23.Hallek M, Cheson BD, Catovsky D, et al. iwCLL Guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745-2760. [DOI] [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. [PubMed] [Google Scholar]
- 25.Mu S, Tang Z, Novotny W, et al. Effect of rifampin and itraconazole on the pharmacokinetics of zanubrutinib (a Bruton's tyrosine kinase inhibitor) in Asian and non- Asian healthy subjects. Cancer Chemother Pharmacol. 2020;85(2):391-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Int Med. 2010;152:726-732. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30(23):2820-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman SE, Niemann CU, Farooqui M, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia. 2014;28(11):2188-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hock BD, McIntosh ND, McKenzie JL, et al. Incidence of cutaneous squamous cell carcinoma in a New Zealand population of chronic lymphocytic leukaemia patients. Intern Med J. 2016;46(12):1414-1421. [DOI] [PubMed] [Google Scholar]
- 31.Royle JA, Baade PD, Joske D, et al. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105(7):1076-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer K, Bahlo J, Fink AM, et al. Longterm remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208-215. [DOI] [PubMed] [Google Scholar]
- 33.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018; 379:2517-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43-56. [DOI] [PubMed] [Google Scholar]
- 37.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16(2):169-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn IE, Farooqui MZH, Tian X, et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood. 2018;131(21):2357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oscier DG, Wade R, Orchard J, et al. Prognostic factors in the UK LRF CLL4 trial. Blood. 2006;108(11):299. [Google Scholar]
- 40.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007; 370(9583):230-239. [DOI] [PubMed] [Google Scholar]
- 41.Strati P, Keating MJ, O'Brien SM, et al. Outcomes of first-line treatment for chronic lymphocytic leukemia with 17p deletion. Haematologica. 2014;99(8):1350-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabbri G, Khiabanian H, Holmes AB, et al. Genetic lesions associated with chronic lymphocytic leukemia transformation to Richter syndrome. J Exp Med. 2013; 210(11):2273-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tam CS, Opat S, Zhu J, et al. Pooled analysis of safety data from monotherapy studies of the Bruton tyrosine kinase (BTK) inhibitor, zanubrutinib (BGB-3111) in B-cell malignancies. Presented at: 24th European Hematology Association Congress; June 13-16, 2019; Amsterdam, the Netherlands. [Google Scholar]
- 44.Hillmen P, Brown JR, Eichhorst BF, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2020;16(10):517-523. [DOI] [PubMed] [Google Scholar]
- 45.Tam CS, LeBlond V, Novotny W, et al. A head-to-head phase III study comparing zanubrutinib versus ibrutinib in patients with Waldenström macroglobulinemia. Future Oncol. 2018;14(22):2229-2237. [DOI] [PubMed] [Google Scholar]
- 46.Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019; 380(22):2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lampson BL, Tyekucheva S, Crombie JL, et al. Preliminary safety and efficacy results from a phase 2 study of acalabrutinib, venetoclax and obinutuzumab in patients with previously untreated chronic lymphocytic leukemia (CLL). Blood. 2019; 134(Suppl 1):S32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.