Abstract
In a phase II study, the telomerase inhibitor imetelstat induced rapid hematologic responses in all patients with essential thrombocythemia who were refractory to or intolerant of prior therapies. Significant molecular responses were achieved within 3-6 months in 81% of patients with phenotypic driver mutations in JAK2, CALR and MPL. Here, we investigated the dynamics of additional somatic mutations in response to imetelstat. At study entry, 50% of patients carried one to five additional mutations in the genes ASXL1, CBL, DNMT3A, EZH2, IDH1, SF3B1, TET2, TP53 and U2AF1. Three patients with baseline mutations also had late-emerging mutations in TP53, IDH1 and TET2. Most clones with additional mutations were responsive to imetelstat and decreased with the driver mutation, including the poor prognostic ASXL1, EZH2 and U2AF1 mutations, while SF3B1 and TP53 mutations were associated with poorer molecular response. Overall, phenotypic driver mutation response was significantly deeper in patients without additional mutations (P=0.04) and correlated with longer duration of response. In conclusion, this detailed molecular analysis of heavily pretreated and partly resistant patients with essential thrombocythemia reveals a high individual patient complexity. Moreover, imetelstat demonstrates potential to inhibit efficiently co-incident mutations occurring in neoplastic clones in patients with essential thrombocythemia. (ClinicalTrials.gov number, NCT01243073).
Introduction
Imetelstat is a 13-mer lipid-conjugated oligonucleotide that targets the RNA template of hTERC and can, therefore, inhibit activity of telomerase and cell proliferation in cancer cells.1 hTERT, the catalytic subunit of telomerase that is generally not found in somatic cells, is expressed in megakaryocytes of patients with essential thrombocythemia (ET), a myeloproliferative neoplasm (MPN).2 Previously, we demonstrated a dose-dependent inhibition of megakaryocytic colony-forming units from patients with ET but not from healthy individuals in vitro.3 In a phase II study of ET patients who were refractory to or intolerant of prior treatment, imetelstat induced rapid and durable hematologic responses in all patients, and molecular responses were achieved in the majority of patients within 3-6 months.4
In ET, JAK2 V617F, CALR and MPL mutations are phenotypic driver mutations present in around 90% of patients; the remaining cases are termed “triple negative”. Non-canonical gain-of-function mutations have been identified in the JAK2 and MPL genes in a minority of triple-negative patients.5,6
Additional recurrent somatic mutations occur at lower frequencies in a number of genes in MPN, and clonality has been demonstrated.7-9 In ET, mutations in ASXL1, TET2 and DNMT3A genes are most frequent and are all involved in epigenetic regulation. Less frequent recurrent mutations are detected in EZH2, TP53, IDH1, IDH2 and CBL, as well as in genes of the splicing machinery, such as SF3B1, SRSF2, U2AF1 and ZRSF2.
So-called “adverse mutations” in SF3B1, SRSF2, U2AF1, TP53, IDH2 and EZH2 have been found to have negative effects on overall and myelofibrosis-free survival in ET, and TP53 mutations predict leukemic transformation.8-10,14 Furthermore, ASXL1 mutations have been identified as a genetic risk factor for transformation to myelofibrosis in ET patients, as they are most frequently found in post-ET myelofibrosis.11 Subsequently, genomic data were integrated in prognostic models to predict patients’ outcomes.13,14
An influence of additional non-driver mutations on treatment response in MPN has been reported for interferon- , ruxolitinib and imetelstat. In patients with CALR-mutated ET treated with interferon-, the presence of additional mutations in ASXL1, TET2, IDH2 and TP53 correlated with a poorer molecular response.15 TET2- mutated clones were resistant to interferon-therapy in JAK2-mutated patients with polycythemia vera.16 Resistance to ruxolitinib was reported in patients with myelofibrosis carrying three or more mutations.17 Furthermore, in the first clinical trial with imetelstat in myelofibrosis patients, treatment response was reported to be negatively influenced by ASXL1 mutations and favorably impacted by SF3B1 and U2AF1 mutations.18
In the present study, we assessed a panel of genes frequently mutated in MPN by next-generation sequencing at study entry and during treatment with imetelstat, and investigated the dynamics of additional mutations in ET patients and their association with hematologic and molecular response.
Methods
Patients and response criteria
A total of 18 patients with ET diagnosed according to the World Health Organization (WHO) 2008 criteria were treated with imetelstat in a phase II study.4 The study was approved by the institutional review board at each participating site. All patients provided written informed consent. Diagnoses were re-evaluated according to the WHO 2016 classification.19 Sequential blood samples were taken at baseline and at up to eight time-points during treatment with imetelstat through cycle 26 (28-day cycles), with approximately 12 weeks between samples. Mutational analysis was performed on all collected samples.
Clinical and hematologic responses were assessed according to the European LeukemiaNet criteria.20 Molecular responses of phenotypic driver mutations were defined as follows: a major molecular response (MMR) was achieved when the mutant allele burden reduction was >50% from baseline value, and a partial molecular response (PMR) was present when a 25% to 49% reduction of the mutant allele burden was observed.
Genetic analysis
DNA was extracted from granulocytes or leukocytes from peripheral blood samples. The molecular response of JAK2 V617F, CALR and MPL mutations was assessed using allele-specific realtime polymerase chain reaction, sequencing and fragment length analysis, respectively, as previously described.4
Targeted next-generation sequencing of all relevant exons and adjacent intronic sequences of 15 recurrently mutated genes (ASXL1, CBL, DNMT3A, EZH2, IDH1, IDH2, JAK2, MPL, SOCS1, TET2, TP53, SF3B1, SRSF2, U2AF1 and ZRSR2) was performed using Ion Torrent™ semiconductor chip technology on the Ion Personal Genome Machine® PGM™ (Thermo Fisher Scientific Inc.). Genes were covered by two custom-designed amplicon libraries comprising 511 and 307 amplicons. In addition, a commercial panel for TP53 was used for confirmation of TP53 variants (Ion AmpliSeqTM Community Panel TP53, Thermo Fisher). For each primer pool, 10 ng of DNA were processed using the AmpliSeqTM chemistry for selective amplification of target sequences and library preparation according to the manufacturer’s instructions. Libraries were diluted and combined according to the Ion PGM chip size to obtain a minimum coverage of 500x for all amplicons. Templates were prepared on the Ion Chef™ and sequencing was performed on the Ion PGM instrument. Variants were called using IonTorrent VariantCaller v4.3 software based on the human reference genome (GRCh37/hg19). Analysis of TP53 was performed according to the manufacturer’s instruction. Annotation was done using the Mutalyzer, dbSNP, COSMIC, ClinVar, UniProt and IARC TP53 databases and the functional in silico prediction algorithms PolyPhen-2 and SIFT.21
Fragment analysis was used to screen for insertions and deletions in ASXL1 exon 12 (NG_027868.1), which are frequently missed by next-generation sequencing. Primers were designed according to Pratcorona et al.22 with small adaptations, and analysis was performed on a 3130 Genetic Analyzer using peak scanner software (Thermo Fisher). Sequences were confirmed by Sanger sequencing.
The limits of detection for real-time polymerase chain reaction analysis of JAK2 and MPL mutations were 0.5%, whereas those for CALR and ASXL1, determined by fragment analysis, and all variants detected by next-generation sequencing were set at 2%.
Validation of genetic variants
All novel variants were confirmed by Sanger sequencing. For ASXL1 and TET2 analysis, published primers were used,23,24 and primers for other genes were designed using Oligo7 and Primer3 software.25,26 Low-level variants (<10%) were confirmed by a second round of next-generation sequencing analysis.
Statistics
Categorical patients’ characteristics were summarized by frequencies and percentages and continuous characteristics by medians, means and an unpaired Student t-test. The efficiency of imetelstat treatment was analyzed by a paired Student t-test comparing percentages of mutant allele burdens before treatment and at best response. Smooth estimates of allele burdens over time were generated using running medians and smoothing splines. Standard errors and confidence intervals were computed by bootstrap.
Results
Characteristics of patients and phenotypic driver mutations
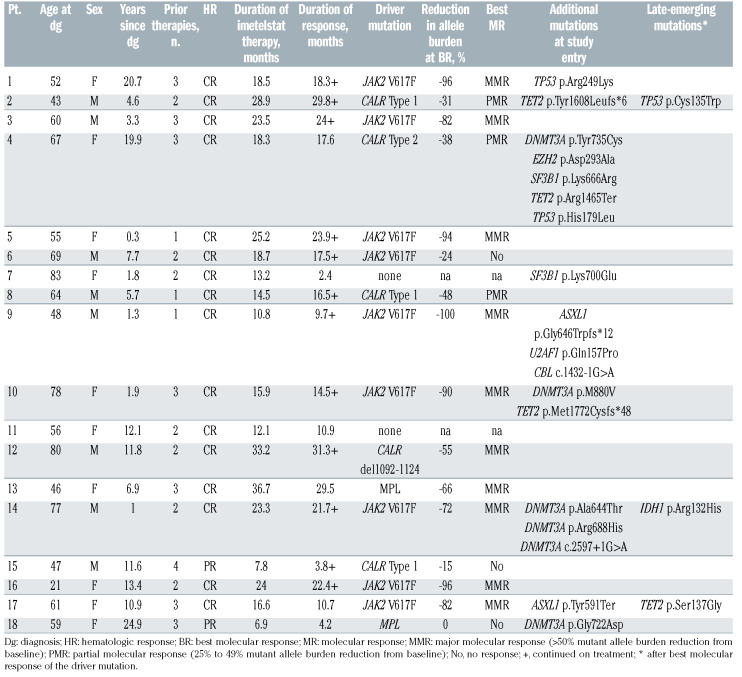
Of 18 patients with ET enrolled in the study, nine (50%) were refractory and 14 (78%) were intolerant of at least one prior therapy. Thirteen patients had received more than one prior therapy and the median time since diagnosis was 7.2 years (range, 0.3-24.9) (Table 1). The median age of patients at study entry was 59.5 years (range, 21-83) (Online Supplementary Table S1). Upon treatment with imetelstat, all patients had a hematologic response, with 16 patients achieving complete hematologic responses.4
Table 1.
Characteristics of patients.
Figure 1.
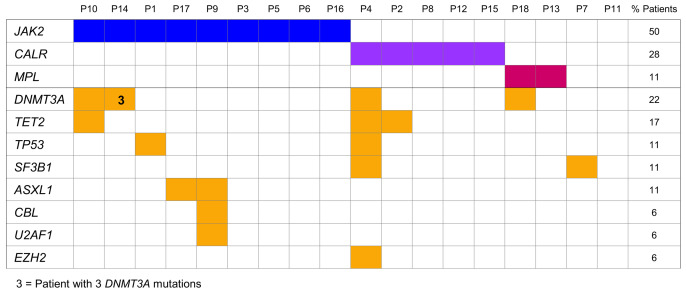
Frequency and distribution of mutations by patient at study entry.
Figure 2.
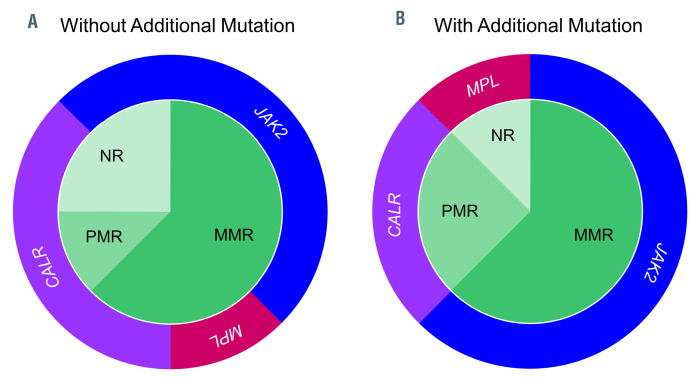
Distribution of molecular response and driver mutations among patients with or without additional mutations. (A) Patients without additional mutations (n=8). (B) Patients with additional mutations (n=8). MMR: major molecular remission; PMR: partial molecular remission; NR: no remission; JAK2: JAK2 V617F; CALR: CALR mutation; MPL: MPL mutation.
With regard to phenotypic driver mutations, nine (50%) patients had a JAK2 V617F mutation, five (28%) patients had CALR mutations (type 1, n=3; type 2, n=1; a novel 33 bp deletion at position 1092, n=1) and two patients had MPL mutations (1 with W515L, 1 with W515K). Two patients (11%) were triple negative. Overall, there was a significant reduction of driver mutant allele burden, with a median decrease of 69% at best response during treatment (P<0.001). In detail, of 16 patients with a phenotypic driver mutation, ten (63%) reached a MMR (8 JAK2- mutated, 1 CALR-mutated, 1 MPL-mutated), three (19%) reached a PMR (3 CALR-mutated), and three (19%) patients did not reach a PMR (1 JAK2-mutated, 1 CALRmutated, 1 MPL-mutated).
Additional mutations at study entry
At study entry, a total of 18 different additional somatic mutations (11 missense, 3 frameshift, 2 nonsense, 2 splice site) were identified in nine patients (50%), affecting the DNMT3A (n=6), TET2 (n=3), ASXL1 (n=2), TP53 (n=2), SF3B1 (n=2), CBL (n=1), EZH2 (n=1) and U2AF1 (n=1) genes (Figure 1). Details on mutations and variant allele frequencies at diagnosis and best response are given in Online Supplementary Table S2. Among the patients with any driver mutation, 40-56% carried up to five additional mutations (5/9 patients with JAK2 V617F, 2/5 with CALR mutation, 1/2 with MPL mutation), and of two triple-negative patients one had an additional mutation.
Impact of additional mutations on molecular response and dynamics of mutant clones
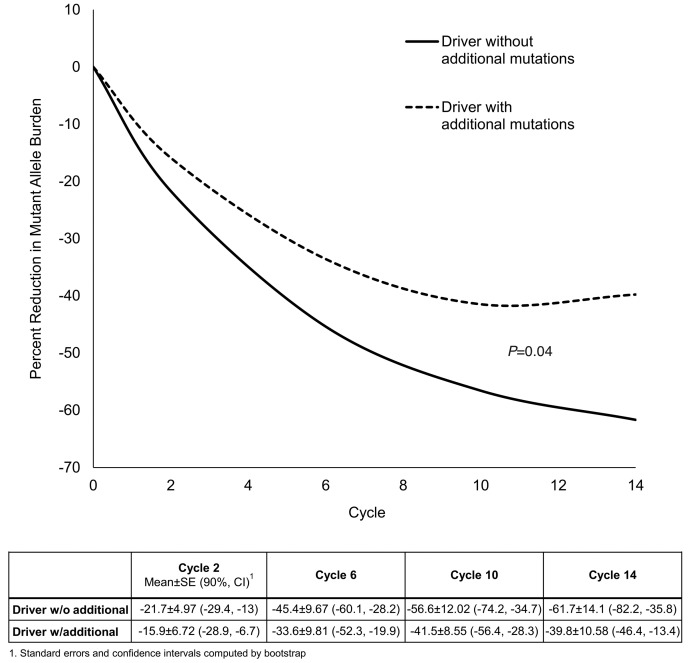
Patients with or without additional mutations had similar molecular responses to imetelstat therapy with five (63%) patients reaching MMR in each group; one and two patients without and with additional mutations reached PMR, respectively (Figure 2). All patients with additional mutations who reached MMR had a JAK2 V617F driver mutation. Regarding the reduction in mutant allele burden, phenotypic driver mutation response was significantly deeper in patients without additional mutations (P=0.04) (Figure 3).
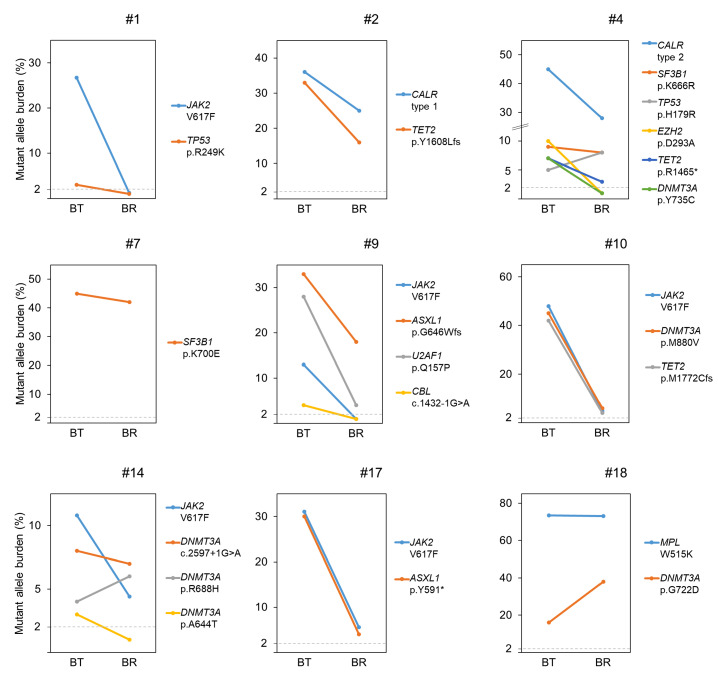
Different dynamics of mutations in response to imetelstat were observed in individual patients (Figure 4). In five patients (#1, #2, #9, #10, #17), additional mutant allele burdens decreased with the driver mutation. In contrast, in two patients with three and five additional mutations (#4, #14), differential responses to imetelstat treatment were observed; i.e., the allele burden of some additional mutations decreased in parallel with the driver mutation while others persisted or increased (i.e., mutations in TP53, SF3B1, and DNMT3A) despite driver mutation response, suggesting the presence of at least two clones or subclones. Lack of response was observed in two patients: in patient #18, a MPL mutation did not respond while the DNMT3Amutated clone expanded, and in patient #7 without a driver mutation (triple negative), a known hotspot mutation in SF3B1 persisted at a high level. In total, non-responsive mutations were detected in TP53, DNMT3A and SF3B1 genes.
Three patients (#2, #14, #17) acquired additional mutations in TP53, IDH1 and TET2 with low allele burden (mean 5%) after best molecular response of the driver mutation, at 10, 9 and 13 months of imetelstat treatment, respectively (Table 1). All three patients already had one to three preexisting additional mutations in other genes at study entry.
Clinical outcome in relation to additional mutations
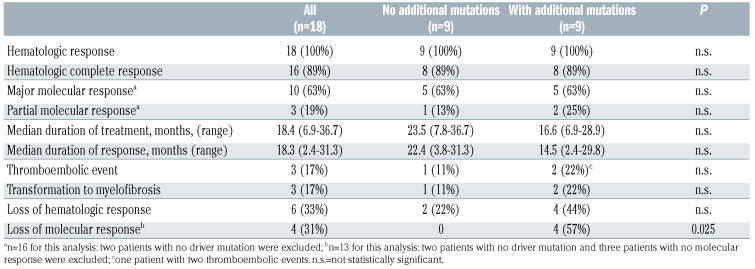
Hematologic and molecular responses were equally reached independently of the presence of additional mutations (Table 2). Loss of response was, however, more frequent in patients with additional mutations. Namely, four patients with additional mutations lost their molecular response, including three patients with DNMT3A mutations and one patient with a TET2 mutation, but none of the patients without additional mutations lost their molecular response (P=0.025). Patients with a higher burden of additional mutations at study entry had a shorter duration of clinical response compared to patients with no or a lower burden of additional mutations (10.2 vs. 22.1 months, median; cut-off at 10% mutant allele burden, P=0.053).
Table 2.
Clinical outcome data.
Figure 3.
Mean phenotypic driver mutant allele burden over time. The solid line represents the driver mutant allele burden in patients without additional mutations (n=8). The dashed line represents the driver mutant allele burden in patients with additional mutations. Patients without additional mutations reached significantly lower mutant allele burdens (P=0.04).
Loss of response was associated with thromboembolic events (3/4 events unrelated to therapy), resistance to imetelstat treatment or progression to myelofibrosis. Transformation to myelofibrosis occurred in two patients with additional mutations during follow-up and 6 months after treatment termination (#4 and #17, respectively). The former, a CALR-mutated patient, carried five additional mutations in DNMT3A, EZH2, SF3B1, TET2 and TP53, and the latter, a JAK2-mutated patient, had an ASXL1 and a late-emerging TET2 mutation. On retrospective evaluation, none of the patients fulfilled the criteria for prefibrotic myelofibrosis according to the newer WHO 2016 criteria.
Discussion
This is the first report on the mutational repertoire of refractory and/or intolerant ET patients after one to four prior therapies. Following treatment with imetelstat, a first-in-class, specific telomerase inhibitor, all patients achieved hematologic responses, and significant molecular responses were seen within 3-6 months, i.e., 63% and 19% of patients with driver mutations reached MMR or PMR, respectively.
At study entry, 50% of patients carried one to five somatic mutations in addition to the phenotypic driver mutation, including one triple-negative case. This frequency is higher than mutation rates reported from other cohorts of ET patients,7,8,12 although still lower than the 86% and 98% overall rates of additional mutations detected in patients with myelofibrosis.17,18 The high frequency of additional mutations in our ET cohort might reflect the concept of genetic instability in MPN and subsequent clonal evolution in a subset of these highly pretreated and partially resistant patients who had been diagnosed a median of 7.2 years previously. This concept is further supported by the finding that more than half of patients carried more than one additional mutation, as has also been reported by others.27 In addition, only patients with additional mutations at study entry acquired even more somatic mutations late during treatment (n=3).
Figure 4.
Best molecular response of patients with additional mutations. Mutant allele burdens of each mutation before imetelstat treatment and at best response. Driver mutations are depicted in blue. Limit of detection at 2%, dashed line. BT: before treatment; BR: at best response.
The most frequently mutated gene was DNMT3A followed by TET2. DNMT3A mutations co-occurred with other somatic mutations in three of four patients, in line with published data.17 In ET, DNMT3A and TET2 mutations are often early events involved in disease initiation, which may precede the JAK2 V617F mutation and influence the phenotype.9,28,29 Of the other genes mutated at study entry, TP53, SF3B1, U2AF1 and EZH2 are part of a group of “adverse risk mutations” for ET, based on their significantly poor impact on overall, leukemia-free and myelofibrosis-free survival, and ASXL1 mutations are known as molecular risk factors for transformation to myelofibrosis.12,14
Patients with or without additional mutations had similar molecular responses to imetelstat with 63% of MMR in both groups; however, the presence of additional mutations had a negative effect on the depth of response, as mutant allele burden reductions were significantly deeper in patients without additional mutations. Of interest, all patients with additional mutations who gained a deep response (MMR) had JAK2 V617F driver mutations while patients with CALR or MPL driver mutations had poorer responses. In contrast, response depth in patients without additional mutations was not assigned to a specific driver mutation type. Further evidence from larger cohorts of patients is needed to support this observation.
The majority of cells with additional mutations were suppressed by imetelstat, and additional mutations tracked with the driver mutation. Of note, ASXL1 mutations were also responsive to imetelstat treatment, although one ASXL1-mutated patient later lost response and transformed to myelofibrosis with an acquired TET2 mutation. This is in contrast to a study on imetelstat in myelofibrosis patients that reported a lack of response among patients with ASXL1 mutations.18
With regard to the additional mutations, we observed several patterns of response. The parallel decrease of one or more mutations with the driver mutation in five of nine patients suggests that coexistence of mutations in the same clone or subclone was frequent in our cohort of patients. Unfortunately, we were not able to track the clonal architecture or coexistence of mutations within a cell due to the lack of additional cell material.
Discrepant patterns of response were seen in patients with multiple mutations that were responsive or persistent, with DNMT3A, SF3B1 and TP53 mutations persisting or increasing over time, suggesting the presence of independent clones. It has been reported that DNMT3A mutations are often present in preleukemic clones and persist during therapy in myeloid malignancies, e.g., in acute myeloid leukemia.30,31 In this study, the four patients with DNMT3A mutations were 78, 80, 84 and 87 years old at study entry, and had had ET for 1, 2, 25 and 20 years, respectively. Since they were significantly older than the patients without DNMT3A mutations (mean age at study entry 82 years vs. 64 years, P<0.05), antecedent age-related clonal hematopoiesis (ARCH/CHIP) may be a contributing factor.32-34 Individuals with ARCH/CHIP have a high risk of developing a hematologic malignancy. Experiments in mouse models carrying loss-of-function mutations in DNMT3A or TET2 suggest a competitive advantage and enhanced self-renewal capacity of the mutant stem cells leading to clonal expansion.34
Of the other non-responsive mutations in this study, mutations affecting the splicing factor SF3B1 are uncommon events in ET, reported to occur in 5% or fewer.12,14,35 They have been considered as “adverse mutations” based on their negative impact on myelofibrosis-free and overall survival.12,14 TP53 mutations in MPN were described to be present for several years at low allelic burden and, after loss of the wild-type TP53 allele, clones expanded rapidly resulting in leukemic transformation.8,36 Hence, the presence of TP53 mutations may be a warning of leukemic transformation in MPN.10
The presence of additional mutations per se, specific mutations and the total number of additional mutations have been associated with inferior response to treatment with interferon-and ruxolitinib.15-18,37 In contrast, imetelstat treatment led to a high proportion of MMR in patients with or without additional mutations, although the latter patients had more reduction of mutant allele burden. Furthermore, initial mutant allele burden may have an impact on response as high-level additional mutations at study entry correlated with shorter duration of response.
Overall, this detailed molecular analysis of heavily pretreated and resistant ET patients reveals high individual patient complexity, with half of the patients harboring up to five additional somatic mutations at study entry. These results raise the question of whether additional mutations were acquired prior to diagnosis or whether mutational events were induced during treatment with prior therapies. Additional studies are needed to address this question.
In conclusion, treatment with imetelstat led to rapid and sustained hematologic and molecular responses and additional mutant allele burdens were also reduced. However, additional mutations significantly reduced the depth of response and had an impact on duration of response. Of acquired mutations with known adverse prognosis and/or risk for transformation to myelofibrosis or acute myeloid leukemia, ASXL1, EZH2 and U2AF1 mutations were responsive to imetelstat, while SF3B1 and one of two TP53 mutations persisted. These data emphasize imetelstat’s potential to inhibit neoplastic clones in patients with ET.
Supplementary Material
Acknowledgments
The authors would like to thank the patients, caregivers and staff who participated in this study, and Ingrid Helsen, Dania Hiltbrunner and Barbara Hügli for technical assistance at the Laboratory of Hematopoiesis and Molecular Genetics, Department of BioMedical Research, University of Bern.
Funding Statement
Funding: This investigator-initiated and -driven study was supported by research funding from Geron to GMB and EOL.
References
- 1.Ouellette MM, Wright WE, Shay JW. Targeting telomerase-expressing cancer cells. J Cell Mol Med. 2011;15(7):1433-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florena AM, Tripodo C, Di Bernardo A, et al. Different immunophenotypical apoptotic profiles characterise megakaryocytes of essential thrombocythaemia and primary myelofibrosis. J Clin Pathol. 2009;62(4): 331-338. [DOI] [PubMed] [Google Scholar]
- 3.Baerlocher GM, Haubitz M, Braschler TR, et al. Imetelstat inhibits growth of megakaryocyte colony-forming units from patients with essential thrombocythemia. Blood Adv. 2019;3(22):3724-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baerlocher GM, Oppliger Leibundgut E, et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. N Engl J Med. 2015;373(10):920-928. [DOI] [PubMed] [Google Scholar]
- 5.Milosevic Feenstra JD, Nivarthi H, Gisslinger H, et al. Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple-negative myeloproliferative neoplasms. Blood. 2016;127(3):325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabagnols X, Favale F, Pasquier F, et al. Presence of atypical thrombopoietin receptor (MPL) mutations in triple-negative essential thrombocythemia patients. Blood. 2016;127(3):333-342. [DOI] [PubMed] [Google Scholar]
- 7.Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220-2228. [DOI] [PubMed] [Google Scholar]
- 9.Vainchenker W, Kralovics R.Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017; 129(6):667-679. [DOI] [PubMed] [Google Scholar]
- 10.Harutyunyan A, Klampfl T, Cazzola M, Kralovics R.p53 lesions in leukemic transformation. N Engl J Med. 2011;364(5):488-490. [DOI] [PubMed] [Google Scholar]
- 11.Cerquozzi S, Tefferi A.Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J. 2015;5(11):e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tefferi A, Lasho TL, Guglielmelli P, et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 2016;1(1):21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grinfeld J, Nangalia J, Baxter EJ, et al. Classification and personalized prognosis in myeloproliferative neoplasms. N Engl J Med. 2018;379(15):1416-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tefferi A, Guglielmelli P, Lasho TL, et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br J Haematol. 2020;189(2):291-302. [DOI] [PubMed] [Google Scholar]
- 15.Verger E, Cassinat B, Chauveau A, et al. Clinical and molecular response to interferon- alpha therapy in essential thrombocythemia patients with CALR mutations. Blood. 2015;126(24):2585-2591. [DOI] [PubMed] [Google Scholar]
- 16.Kiladjian JJ, Masse A, Cassinat B, et al. Clonal analysis of erythroid progenitors suggests that pegylated interferon alpha-2a treatment targets JAK2V617F clones without affecting TET2 mutant cells. Leukemia. 2010;24(8):1519-1523. [DOI] [PubMed] [Google Scholar]
- 17.Patel KP, Newberry KJ, Luthra R, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015;126(6):790-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tefferi A, Lasho TL, Begna KH, et al. A Pilot Study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med. 2015; 373(10):908-919. [DOI] [PubMed] [Google Scholar]
- 19.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 20.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113(20):4829-4833. [DOI] [PubMed] [Google Scholar]
- 21.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratcorona M, Abbas S, Sanders MA, et al. Acquired mutations in ASXL1 in acute myeloid leukemia: prevalence and prognostic value. Haematologica. 2012;97(3):388-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelsi-Boyer V, Trouplin V, Adelaide J, et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145(6):788-800. [DOI] [PubMed] [Google Scholar]
- 24.Langemeijer SM, Kuiper RP, Berends M, et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet. 2009;41(7):838-842. [DOI] [PubMed] [Google Scholar]
- 25.Rychlik W. OLIGO 7 primer analysis software. Methods Mol Biol. 2007;402:35-60. [DOI] [PubMed] [Google Scholar]
- 26.Untergasser A, Cutcutache I, Koressaar T, et al. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones AV, Cross NC. Inherited predisposition to myeloproliferative neoplasms. Ther Adv Hematol. 2013;4(4):237-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nangalia J, Nice FL, Wedge DC, et al. DNMT3A mutations occur early or late in patients with myeloproliferative neoplasms and mutation order influences phenotype. Haematologica. 2015; 100(11): e438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortmann CA, Kent DG, Nangalia J, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med. 2015; 372(7):601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debarri H, Lebon D, Roumier C, et al. IDH1/2 but not DNMT3A mutations are suitable targets for minimal residual disease monitoring in acute myeloid leukemia patients: a study by the Acute Leukemia French Association. Oncotarget. 2015; 6(39):42345-42353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeziskova I, Musilova M, Culen M, et al. Distribution of mutations in DNMT3A gene and the suitability of mutations in R882 codon for MRD monitoring in patients with AML. Int J Hematol. 2015;102(5):553-557. [DOI] [PubMed] [Google Scholar]
- 32.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130(6):742-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366(6465):eaan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boiocchi L, Hasserjian RP, Pozdnyakova O, et al. Clinicopathological and molecular features of SF3B1-mutated myeloproliferative neoplasms. Hum Pathol. 2019;86:1-11. [DOI] [PubMed] [Google Scholar]
- 36.Kubesova B, Pavlova S, Malcikova J, et al. Low-burden TP53 mutations in chronic phase of myeloproliferative neoplasms: association with age, hydroxyurea administration, disease type and JAK2 mutational status. Leukemia. 2018;32(2):450-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montalban-Bravo G, Takahashi K, Patel K, et al. Impact of the number of mutations in survival and response outcomes to hypomethylating agents in patients with myelodysplastic syndromes or myelodysplastic/ myeloproliferative neoplasms. Oncotarget. 2018;9(11):9714-9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.