Abstract
Background
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has infected over 110 million individuals and led to 2.5 million deaths worldwide. As more individuals are vaccinated, the clinical performance and utility of SARS-CoV-2 serology platforms needs to be evaluated.
Methods
The ability of 4 commercial SARS-CoV-2 serology platforms to detect previous infection or vaccination were evaluated using a cohort of 53 patients who were SARS-CoV-2 PCR positive, 89 SARS-CoV-2-vaccinated healthcare workers (Pfizer or Moderna), and 127 patients who were SARS-CoV-2 negative. Serology results were compared to a cell-based SARS-CoV-2 pseudovirus (PSV) neutralizing antibodies assay.
Results
The Roche S-(spike) antibody and Diazyme neutralizing antibodies (NAbs) assays detected adaptive immune response in 100.0% and 90.1% of vaccinated individuals who received 2 doses of vaccine (initial and booster), respectively. The Roche N-(nucleocapsid) antibody assay and Diazyme IgG assay did not detect adaptive immune response in vaccinated individuals. The Diazyme NAbs assay correlated with the PSV SARS-CoV-2 median infective dose (ID50) neutralization titers (R2 = 0.70), while correlation of the Roche S-antibody assay was weaker (R2 = 0.39). Median PSV SARS-CoV-2 ID50 titers more than doubled in vaccinated individuals who received 2 doses of the Moderna vaccine (ID50, 597) compared to individuals who received a single dose (ID50, 284).
Conclusions
The Roche S-antibody and Diazyme NAbs assays robustly detected adaptive immune responses in SARS-CoV-2 vaccinated individuals and SARS-CoV-2 infected individuals. The Diazyme NAbs assay strongly correlates with the PSV SARS-CoV-2 NAbs in vaccinated individuals. Understanding the reactivity of commercially available serology platforms is important when distinguishing vaccination response versus natural infection.
Keywords: vaccines, neutralizing antibodies, COVID-19, serology, SARS-CoV-2, commercial assays
Impact Statement
For the first time, we show that commercial serology tests can differentiate natural infection from vaccination. We demonstrate that the Roche S-antibody and Diazyme neutralizing antibody (NAb) assays detect immune responses in vaccinated individuals. The Roche S-antibody assay had an observed PPA of 100% for individuals who received 2 doses of the Pfizer or Moderna vaccine. By contrast, the Roche N assay and Diazyme IgG (N/S) assay did not detect vaccine adaptive immune responses. Our findings also indicate that the Diazyme NAbs assay correlates strongly with the levels of SARS-CoV-2 ID50 neutralization titers using a pseudoviral NAb assay in vaccinated individuals.
Introduction
As of February 13, 2021, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in 110 million documented infections and nearly 2.5 million deaths worldwide (1). Recently, the United States Food and Drug Administration and European Union authorized use of SARS-CoV2 vaccines, including BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) (2, 3). The Moderna and Pfizer vaccines utilize lipid nanoparticles that encapsulate RNA encoding the prefusion stabilized spike protein (S-protein) from SARS-CoV-2 (4, 5). Previous in vitro studies have demonstrated that antibody-binding to specific S-protein epitopes disrupts viral entry and subsequent infection (6). Development of SARS-CoV-2 vaccines required amino acid substitutions in the recombinant prefusion conformation of S-protein to stabilize the structure allowing for better recognition by the humoral immune system (4, 5, 7). It was thus unclear whether commercial serology assays designed to recognize S-protein would detect antibodies generated in vaccinated patients.
Most of the original serology tests for SARS-CoV-2 were designed to detect patients who were previously exposed to the virus (8, 9). Many of these assays target antibodies produced against the SARS-CoV-2 nucleocapsid (N) protein, with several others target a combination of the N and S proteins (10, 11). Serology that exclusively detect N-protein antibodies would most likely not detect individuals vaccinated against the S-protein. The performance of serology tests that detect S-protein antibodies or a mixture of S and N-protein antibodies in response to vaccination remains incompletely understood. Clinical interpretation of commercial serology results requires an understanding of the performance of these assays in both vaccinated individuals and those previously infected with SARS-CoV-2.
The relationship between SARS-CoV-2 serology results and protective immunity against SARS-CoV-2 has been a topic of great debate (9). Some critics argue that SARS-CoV-2 serology testing may create a false sense of protection among patients testing positive (12, 13), while others argue that serology testing is an important epidemiological and clinical tool when used appropriately (14). Several studies have correlated commercial SARS-CoV-2 serology results to cell-based SARS-CoV-2 neutralizing antibody (NAb) assays (15–19). Unfortunately, these cell-based assays are generally unsuitable for use in a high-throughput clinical laboratory. However, a recently released proxy assay for SARS-CoV-2 NAbs (Diazyme) has been developed that measures the interaction between the S-protein and the human angiotensin converting enzyme 2 (ACE2) receptor; which is the virus’s mode of infection into host cells (20, 21). This high-throughput commercialized assay may provide a method to determine whether patients have NAbs against SARS-CoV-2.
Herein, we evaluate the ability of the Roche Elecsys Anti-SARS-CoV-2 S-antibody test, the Roche Elecsys Anti-SARS-CoV-2 N-antibody test, the Diazyme Anti-SARS-CoV-2 IgG assay, and the Diazyme NAbs assay to detect and discriminate previously infected, vaccinated, and noninfected individuals. We also compared these commercial serology assays to a previously validated (16) cell-based SARS-CoV-2 pseudovirus (PSV) assay to determine whether serology status predicts the level of NAbs produced by vaccinated individuals .
Materials and Methods
Study Design and Patient Cohort
275 serum and plasma specimens from 269 individuals were collected in BD Vacutainer collection tubes (K-EDTA, lithium-heparin plasma separator tubes, and/or serum separator tubes) (Fig. 1 in the online Data Supplement). This included serum or plasma samples from 53 patients (53 specimens) who tested positive for SARS-CoV-2 by a PCR-based assay, 89 vaccinated healthcare workers who received the Moderna (61 individuals, 66 specimens) or Pfizer (28 individuals, 29 specimens) vaccine, or 116 control patient specimens (116 patients) that were collected in 2018 and had been stored at −20 °C, and 11 patients (11 specimens) who tested positive for respiratory infections other than SARS-CoV-2 using a respiratory panel nucleic acid (RPNA) detection test. Specimens from patients with PCR-confirmed COVID-19 were collected prior to August 1, 2020 to eliminate the possibility that they had been vaccinated. The PCR-confirmed positive samples (N = 53) were separated into 2 time-frames relative to a positive SARS-CoV-2 PCR-confirmed result: <15 days postdiagnosis by PCR (n = 25) and ≥15 days postdiagnosis by PCR (n = 28). All of the PCR-confirmed positive samples came from patients with COVID-19 who had sufficient symptoms to require hospitalization. The vaccinated healthcare worker group was a convenience cohort who all were subject to weekly PCR testing for at least 1 month prior to being vaccinated. All patient specimens were collected under UCSD IRB protocol 181656.
Confirmation of SARS-CoV-2 Positive Patients
All 53 patients with SARS-CoV-2 were positively confirmed for COVID-19 by an emergency use-authorized nucleic acid test validated in our laboratory. The platforms used included the Abbott ID NOW, Abbott m2000, Abbott Alinity, Abbott RealTime, Hologic Panther, ThermoFisher TaqPath, GenMark ePlex, and the Roche Cobas 6800/8800. All patients who tested positive using any of these platforms were considered SARS-CoV-2 PCR-confirmed and for discussion simplicity are referred to as PCR positive.
Commercial Serology and Neutralization Assays
Serology assays were performed on the Roche Cobas 8000 e801/e601 and included the Elecsys Anti-SARS-CoV-2 N-antibody test and the Elecsys Anti-SARS-CoV-2 S-antibody test. The Roche N-antibody assay reports a cutoff index (COI, a signal of sample/cutoff); values ≥1.00 COI are considered reactive. The Roche S-antibody assay is semiquantitative and reports results in absorbance units per mL (AU/mL); values ≥0.8 AU/mL are considered reactive. A 1:10 dilution was performed in accordance with the manufacturer’s package insert for Roche S-antibody assay values >250 AU/mL, which extends the analytical measurement range (AMR) of the assay to 2500 AU/mL. Additional serology testing was performed using the Diazyme DZ-Lite 3000 plus clinical analyzer (Diazyme DZ-LITE 2019-nCoV IgG and Diazyme SARS-CoV-2 NAbs) in accordance with the manufacturer’s instructions. The Diazyme IgG platform reports results in absorbance units per mL (AU/mL); values ≥1.00 AU/mL are considered reactive. The Diazyme IgG assay claims to react with both N and S antibodies (10). The Diazyme NAbs assay reports results in absorbance units per mL (AU/mL); a manufacturer’s suggested cutoff is not provided with the package insert and was set at 3 standard deviations above the mean measured value of the 127 SARS-CoV-2 negative specimens (0.488 AU/mL).
GenMark ePlex Respiratory Pathogen Nucleic Acid Test
To identify patient specimens containing other PCR-confirmed microbes, a RPNA test was performed on the GenMark ePlex (GenMark Diagnostics Inc., Carlsbad, CA, USA). This panel detects adenovirus (A–F), Coronavirus (229E, HKU1, NL63, OC42), Human Metapneumovirus, Human Rhinovirus/Enterovirus, Influenza A, B, and C, Influenza 2009 H1N1, Parainfluenza (1–4), Respiratory Syncytial Virus (A and B), Chlamydia pneumoniae, and Mycoplasma pneumoniae.
Precision Studies
Precision was calculated across 5 days by running 5 batches of 5 replicates of patient-pooled plasma (Roche S-antibody assay) or quality control material (Diazyme NAbs assay) (n = 25). The positive patient pool was made by combining samples from patient who were SARS-CoV-2 PCR positive and diluting the pool with SARS-CoV-2 negative patient serum to fall within the AMR of the assay. Negative patient pools were created by combining patient specimens (Li-heparin or K-EDTA) collected from control patients in 2018, which had been stored at −20 °C.
Dilutional Linearity
Dilutional linearity for the Roche S-antibody and Diazyme NAbs assays was assessed in triplicate by mixing a positive patient pool with a negative patient pool in 10% increments prior to analysis. Patient pools for each assay were selected to ensure the pools evaluated the linear ranges of the assays.
SARS-CoV-2 Pseudovirus Neutralization Assay
SARS-CoV-2 neutralization assays were performed as previously described using a pseudovirus (PSV) (16, 22). In brief, the assay uses single cycle infectious viral particles containing firefly luciferase. The amount of luminescence in HeLa cells that stably expressed the cell surface receptor angiotensin converting enzyme 2 were measured after viral infection. Titers of 50% inhibitory dilution (ID50) were determined. For this study, the cutoff for a positive PSV neutralization result was raised to 100 (previously 50) to adjust for background changes in the assay, thus ID50 titers of greater than or equal to 100 were considered positive for PSV NAbs against SARS-CoV-2.
Statistical Analyses
Data were analyzed using R in Rstudio. Linear regression analysis was performed in Excel. Box plots were generated in Rstudio. Median values and interquartile ranges (IQR) are indicated for each box-plot. The upper whisker extends from the hinge to the largest value no further than 1.5 times the IQR from the hinge (where IQR is the interquartile range, or distance between the first and third quartiles). The lower whisker extends from the hinge to the smallest value at most 1.5 times the IQR of the hinge. Data beyond the end of the whiskers are called “outlying” points and are plotted individually. P values were generated using a Wilcoxon test (P < 0.05 was considered significant). Precision (%CV) was calculated by analysis of variance and total precision (%CV) was calculated by the sum of squares. Positive percentage agreement (PPA) was defined as the number of positive test results divided by the sum of positives and negatives. Negative percentage agreement (NPA) was defined as the number of negative test results divided by the sum of negatives and positives as has been described previously (15).
Results
Analytical Performance of Serology Tests
Within-run, between-run, and total precision (%CV) of the Roche S-antibody and Diazyme NAbs assays are presented in Supplemental Table 1. Within-run precision ranged from 1.4% to 2.7% for the Roche S-antibody assay and from 3.6% to 7.4% for the Diazyme NAbs assay. Between-run precision ranged from 2.4% to 3.6% for the Roche S-antibody assay and from 1.6% to 1.9% for the Diazyme NAbs assay. Total precision ranged from 2.8% to 4.5% for the Roche assay and from 4.0% to 7.7% for the Diazyme NAbs assay. Precision characteristics of the Roche N-antibody and Diazyme IgG assays have been previously published (23, 24).
Linear regression analysis was performed to illustrate the relationship between mean observed and expected values following sample dilution for the Roche S-antibody and Diazyme NAbs assays (Supplemental Fig. 2). Both the Roche S-antibody assay (R2 = 0.996, y = 1.01x −7.00) and the Diazyme NAbs assay (R2 = 0.950, y = 1.08x + 0.98) diluted in a linear fashion (Supplemental Fig. 2, A and B). Dilutional performance of the Roche N-antibody and Diazyme IgG assays have been previously reported (23, 24).
Cross-reactivity of the Roche S-antibody and Diazyme NAbs assays was evaluated using specimens from 11 patients infected with other types of respiratory pathogens, including 7 with non-COVID-19 coronavirus. No cross-reactivity was observed for the Roche or Diazyme platforms as all 11 specimens were negative (Supplemental Table 2).
Detection of SARS-CoV-2 Adaptive Immune Response in Infected Patients
The PPA between commercial assays and a positive SARS-CoV-2 PCR result was performed by dividing patients who were PCR positive into 2 different time-frames relative to the time of diagnosis by a PCR test (Table 1). The <15 day group included 25 patients who were PCR positive and the ≥15 day group included 28 patients who were PCR positive. The PPA for the <15 day group was less than 100% across all platforms: Roche S-antibody (88.0%), Roche N-antibody (84.0%), Diazyme IgG (84.0%), and Diazyme NAbs (80.0%). By contrast, the PPA observed in the ≥15 day group was 100% for all 4 assays, illustrating the time-dependent performance of serology and NAb assays following infection. The NPA for the serology assays in the PCR-negative cohort collected in 2018 was 100% for the Roche S-antibody and Diazyme IgG assays, 99.2% for the Roche N-antibody assay, and 98.4% for the Diazyme NAbs assay (Table 1).
Table 1.
PPA and NPA of commercial serology platforms with patients who were SARS-CoV-2 PCR positive.
| Positive percentage agreement (PPA) | ||||
|---|---|---|---|---|
| SARS-CoV-2 PCR (<15 days post-PCR positive) n = 25 | ||||
| Metric | Roche S | Roche N | Diazyme IgG | Diazyme NAbs |
| PCR positive | 25 | 25 | 25 | 25 |
| Positive on assay | 22 | 21 | 21 | 20 |
| PPA | 88.0% | 84.0% | 84.0% | 80.0% |
| Positive percentage agreement (PPA) | ||||
| SARS-CoV-2 PCR (≥15 days post-PCR positive) n = 28 | ||||
| Metric | Roche S | Roche N | Diazyme IgG | Diazyme NAbs |
| PCR positive | 28 | 28 | 28 | 28 |
| Positive on assay | 28 | 28 | 28 | 28 |
| PPA | 100.0% | 100.0% | 100.0% | 100.0% |
| Negative percentage agreement (NPA) | ||||
| 2018 samples (n = 116) and other respiratory pathogens (n = 11) | ||||
| Metric | Roche S | Roche N | Diazyme IgG | Diazyme NAbs |
| COVID-19 negative | 127 | 127 | 127 | 127 |
| Positive on assay | 0 | 1 | 0 | 2 |
| NPA | 100.0% | 99.2% | 100.0% | 98.4% |
The distribution of observed values on each commercial serology platform for patients who were SARS-CoV-2 PCR positive is shown in Supplemental Fig. 3. The observed values for patients who tested <15 days post-PCR positive were significantly lower compared to patients tested ≥15 days post-PCR positive (P ≤ 0.05).
Detection of Adaptive Immune Response in Vaccinated Individuals
To evaluate whether the commercial serology assays detect SARS-CoV-2 vaccinated individuals (Pfizer or Moderna), the PPA between vaccinated individuals and a positive result on each commercial assay was determined (Table 2). Vaccinated individuals were divided into 2 groups based on whether an individual had or had not received their booster shot (pre- vs post-booster). The median number of days postinitial injection for pre-booster individuals was 22.5 days with an interquartile range (IQR) of 16.25–28.75 days. The median number of days postinitial injection for post-booster individuals was 37 days with an IQR of 34–38 days, and the median number of days post-booster was 8 days with an IQR of 5–13 days. The Roche S-antibody assay had an observed PPA of 87.5% for pre-booster individuals and an observed PPA of 100% for post-booster individuals. The Diazyme NAbs assay was less sensitive to detecting adaptive immune response related to vaccination status and had an observed PPA of 66.6% for pre-booster individuals and an observed PPA of 94.4% for post-booster individuals. The Roche N-antibody assay and Diazyme IgG (N/S) assay did not react with vaccinated individuals. All vaccinated individuals depicted in Table 2 that were reactive on the Roche N-antibody and Diazyme IgG (N/S) assays were previously infected with SARS-CoV-2 (PCR-confirmed).
Table 2.
PPA of commercial serology platforms with SARS-CoV-2 vaccinated individuals.
| Vaccine positive percentage agreement (PPA) | ||||
|---|---|---|---|---|
| Specimens from vaccinated individuals (pre-booster) | ||||
| Median days post initial injection: 22.5 days | ||||
| IQR: 16.25–28.75 days | ||||
| Metric | Roche S | Roche N | Diazyme IgG | Diazyme NAbs |
| Vaccinated | 24 | 24 | 24 | 24 |
| Positive on assay | 21 | 1 | 1 | 16 |
| PPA | 87.5% | 3.8% | 3.8% | 66.6% |
| Specimens from vaccinated individuals (post-booster) | ||||
| Median days post initial injection: 37 days | ||||
| IQR: 34–38 days | ||||
| Metric | Roche S | Roche N | Diazyme IgG | Diazyme NAbs |
| Vaccinated | 71 | 71 | 71 | 71 |
| Positive on assay | 71 | 2 | 1 | 67 |
| PPA | 100.0% | 2.8% | 1.4% | 94.4% |
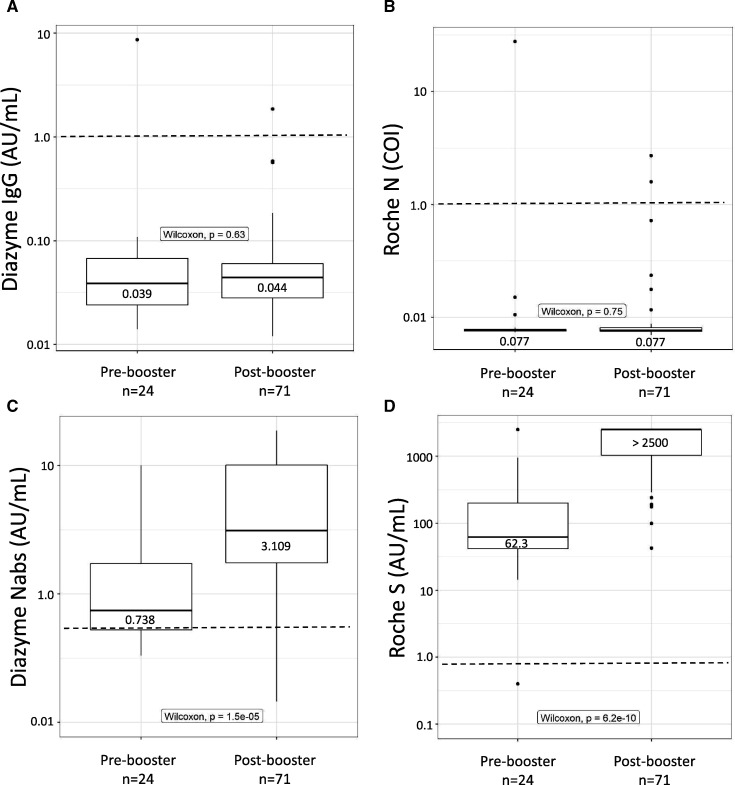
The median value observed for vaccinated individuals on the Diazyme IgG assay was 0.039 AU/mL (IQR: 0.024–0.069) for pre-booster individuals and 0.044 AU/mL (IQR: 0.028–0.060) for post-booster individuals (Fig. 1, A). The median value observed on the Roche N-antibody assay was 0.077 COI (IQR: 0.076–0.079) for pre-booster individuals and 0.077 COI (IQR: 0.075–0.081) for post-booster individuals (Fig. 1, B). The median value observed for vaccinated individuals on the Diazyme NAbs assay was 0.738 AU/mL (IQR: 0.478–1.769) for pre-booster individuals and 3.109 AU/mL (IQR: 1.659–10.43) for post-booster individuals (Fig. 1, C). The median value observed for vaccinated individuals on the Roche S-antibody assay was 62.3 AU/mL (IQR: 38.07–210.88) for pre-booster individuals and >2500 AU/mL (IQR: 1009–>2500) for post-booster individuals (Fig. 1, D). Median values observed for the Diazyme IgG and Roche N-antibody assays were well below the cutoff for a positive test result with no significant difference between pre- and post-booster individuals. In contrast, for both pre- and post-booster individuals, the median values observed for the Diazyme NAbs and Roche S-antibody assays were above the cutoff for a positive result and were significantly different (pre- to post-booster P < 0.05).
Fig. 1.
Commercialized platforms detect vaccinated individuals. (A–D), Box plots illustrating the distribution of values observed on the Diazyme IgG, Roche N-antibody, Diazyme NAbs, and Roche S-antibody assays. Vaccinated individuals are stratified based on whether or not they had received a booster shot (pre- vs post-booster). Observed values are plotted on the y axis on a log10 scale. The dashed line indicates the cutoff for each platform with values above representing positive results and values below indicating negative results. Median values and interquartile ranges are indicated for each box-plot. Whiskers are up to 1.5 times the interquartile range.
Correlation of Commercial Serology Results to SARS-CoV-2 ID50 Neutralization Titers
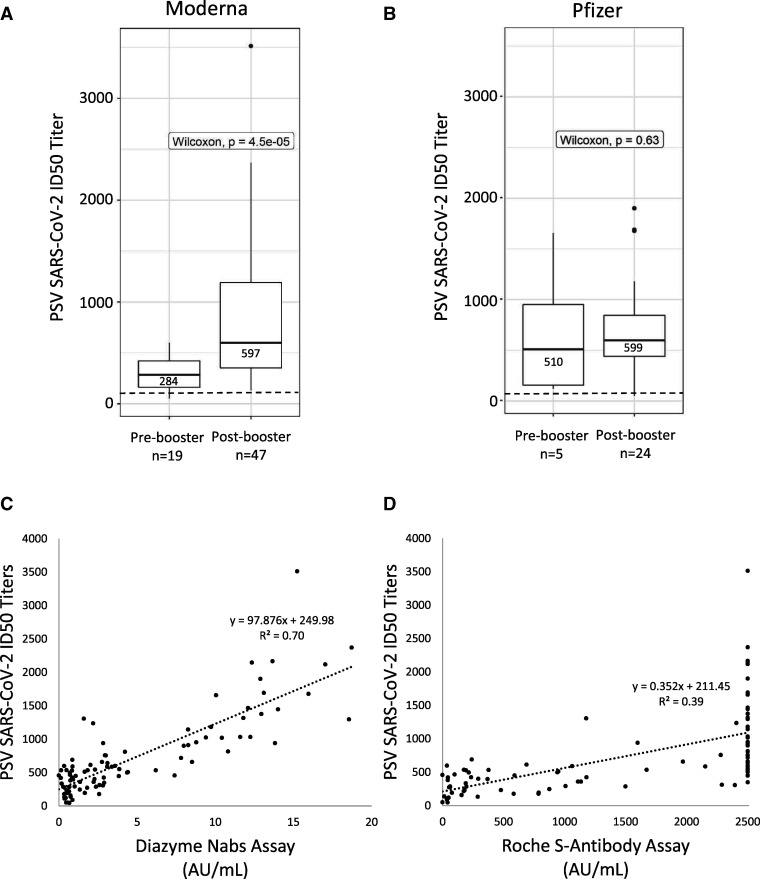
Utilizing a clinically validated cell-based PSV assay (15), we measured NAb titers (ID50) against SARS-CoV-2 in vaccinated individuals. The PPA between vaccinated individuals and the SARS-CoV-2 PSV assay was 87.5% for pre-booster individuals and 98.6% for post-booster individuals (Table 3). Furthermore, the median ID50 titer observed in pre-booster individuals was 285 (IQR: 141–485), while the median ID50 titer more than doubled to 597 (IQR: 359–1032) for post-booster individuals. Stratifying post-booster individuals based on vaccine manufacturer reveals that Moderna vaccinated individuals’ median ID50 titers were not significantly different than Pfizer vaccinated individuals; 597 for Moderna and 599 for Pfizer (Fig. 2, A and B). There was a clear difference between pre- and post-booster ID50 titers for Moderna vaccinated individuals (P < 0.05) (Fig. 2, A). Significant difference was not observed between ID50 titers produced in pre- vs post-booster Pfizer vaccinated individuals (Fig. 2, B), where a robust response was seen after the first dose, although the number of participants in this group was small (N = 5). A strong correlation to SARS-CoV-2 ID50 neutralization titers was observed with the Diazyme NAbs assay (R2 = 0.70), while a weaker correlation was observed for the Roche S-antibody assay (R2 = 0.39) (Fig. 2, C and D). The median SARS-CoV-2 PSV ID50 for vaccinated individuals that were positive on the Diazyme NAbs assay was 543 with an IQR of 330–1022, while the median for vaccinated individuals who were negative on the Diazyme NAbs assay was 226 with an IQR of 117–476. The median ID50 titer observed in individuals who were PCR positive was 1813 as compared with a median of 597 and 599 for the Moderna and Pfizer vaccines, respectively.
Table 3.
PPA of the SARS-CoV-2 PSV assay with vaccinated individuals.
| Vaccine positive percentage agreement (PPA) | |
|---|---|
| Specimens from vaccinated individuals (pre-booster) | |
| Median days postinitial injection: 22.5 days | |
| IQR: 16.25–28.75 days | |
| Median SARS-CoV-2 ID50 titer: 285 | |
| ID50 IQR: 141–485 | |
| Metric | SARS-CoV-2 PSV |
| Vaccinated | 24 |
| Positive on assay | 21 |
| PPA | 87.5% |
| Specimens from vaccinated individuals (post-booster) | |
| Median days postinitial injection: 37 days | |
| IQR: 34–38 days | |
| Median SARS-CoV-2 ID50 titer: 597 | |
| ID50 IQR: 359–1032.3 | |
| Metric | SARS-CoV-2 PSV |
| Vaccinated | 71 |
| Positive on assay | 70 |
| PPA | 98.6% |
Fig. 2.
Nabs in vaccinated individuals and correlation to commercial platforms. (A), Box-plot describing the distribution of SARS-CoV-2 ID50 titers (y axis) across vaccinated individuals receiving the Moderna vaccine. (B), Box-plot describing the distribution of SARS-CoV-2 ID50 titers (y axis) across vaccinated individuals receiving the Pfizer vaccine. The positive cutoff for NAbs on the PSV assay is indicated by a dashed line. Median values and interquartile ranges are indicated for each box-plot. Whiskers are up to 1.5 times the interquartile range. (C, D), Linear regression analysis of SARS-CoV-2 ID50 titers (y axis) observed in vaccinated individuals compared to values observed on the Diazyme NAbs or Roche S-antibody assays (x axis). Linear regression analysis with corresponding R2 values and y = b + mx equations are indicated within each plot.
Temporal Evaluation of SARS-CoV-2 NAbs and Serology in Vaccinated Individuals
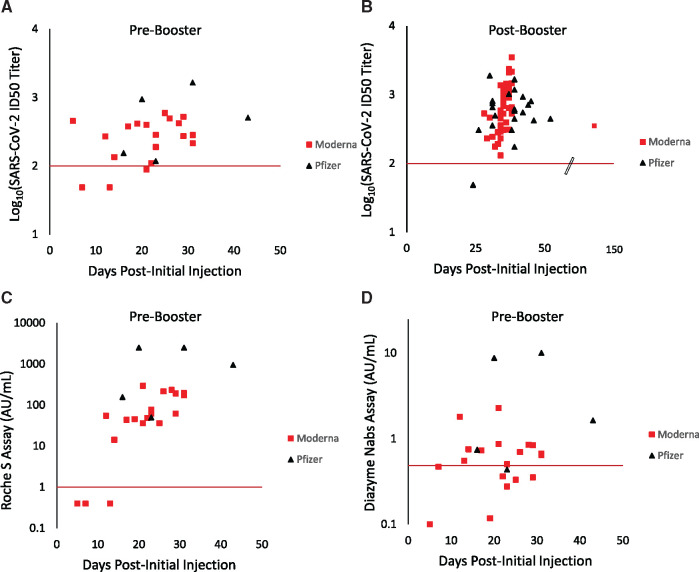
The appearance of PSV SARS-CoV-2 NAbs is shown relative to an individual’s initial vaccination date (Fig. 3, A and B). For pre-booster vaccinated individuals, ID50 titers ranged from 49 to 952 (Fig. 3, A). As expected, the highest ID50 titers were observed in post-booster individuals (Fig. 3, B), with PSV ID50 titers ranging from 49 to 3512. Three pre-booster individuals were negative for PSV SARS-CoV-2 NAbs. These individuals received the Moderna vaccine and were 7, 13, and 21 days postinitial injection. A single post-booster Pfizer vaccinated individual had PSV ID50 titers of <100 and was tested 24 days after their initial vaccination date and 3 days after receiving their booster. The earliest positive serology result for a vaccinated individual on either the Roche S-antibody or Diazyme NAbs assays was 12 days following initial injection of the vaccine (Fig. 3, C and D), this patient had a PSV ID50 titer of 272.
Fig. 3.
Kinetics of SARS-CoV-2 NAbs and serology in vaccinated individuals. (A), log10(SARS-CoV-2 ID50 neutralization titers) are shown (y axis) for pre-booster vaccinated individuals. (B), log10(SARS-CoV-2 ID50 neutralization titers) are shown (y axis) for post-booster vaccinated individuals. The scale break-point on the x axis is indicated for when the axis is no longer to scale. (C), Values on the Roche S-antibody assay (y axis) for pre-booster vaccinated individuals. (D), Values on the Diazyme NAbs assay (y axis) for pre-booster vaccinated individuals. For all graphs, individuals receiving the Moderna vaccine are indicated by red squares while individuals receiving the Pfizer vaccine are indicated with black triangles. The number of days following an individual’s initial vaccination date is plotted on the x axis. The red x axis indicates the positive cutoff for each assay.
Discussion
The goal of the current study was to determine the potential clinical utility of various SARS-CoV-2 antibody detection assays to identify and distinguish adaptive immune responses from prior SARS-CoV2 infection and vaccination. We compared 4 commercially available in vitro diagnostic serology assays and a cell-based assay, applicable mainly for research purposes. Each assay emerged as useful in specific contexts.
The Roche S-antibody test and the Diazyme NAbs assay had appropriate precision for clinical use (Supplemental Table 1). Both assays diluted in a linear fashion, with R2 values of greater than 0.95 (Supplemental Fig. 2). Previously, we reported that the Abbott IgG and Roche N-antibody assays dilute in a nonlinear fashion (23). Linear dilution is particularly critical when using the Roche S-antibody assay because a large portion of the values observed on this platform were above the AMR (250 AU/mL) in both vaccinated individuals and patients infected with SARS-CoV-2 .
The Roche S-antibody test and the Diazyme NAbs assay detect SARS-CoV-2 infected patients with similar sensitivity (PPA) and specificity (NPA) as previously validated SARS-CoV-2 serology assays (23–25); all 4 commercial serology platforms had PPAs of 100% for patients tested ≥15 days a post-PCR positive result. Since the Roche S-antibody and Roche N-antibody assays are performed on the same platform, clinical laboratories can use a single analyzer to implement the CDC’s recommended screen and confirm approach when testing low disease prevalence populations for past exposure to SARS-CoV-2 (26).
With the arrival of SARS-CoV-2 vaccines, a major concern is whether commercial SARS-CoV-2 serology assays can distinguish vaccinated individuals from those with natural infections. This is particularly important for patients with symptoms of “long haul” COVID-19 (27) and when diagnosing previously asymptomatic infections. The Roche S-antibody assay detected 100% of post-booster vaccinated individuals, making it an excellent choice for determining whether vaccinated individuals have mounted an immune response (Table 2). However, at the present time, antibody testing is not recommended by the CDC to confirm seroconversion following routine vaccinations (28). The Diazyme NAbs assay detected 94.4% of post-booster individuals, suggesting that it is either less sensitive than the Roche S-antibody assay or that some individuals produce antibodies to the S-protein that are not neutralizing. The Roche N-antibody assay and Diazyme IgG (N/S) assay did not detect vaccinated individuals, as all positive results from vaccinated individuals had a previous SARS-CoV-2 PCR-confirmed infections. A combination of S-antibody and N-antibody assays can be used to differentiate naturally infected individuals from vaccinated individuals, as naturally infected individuals are positive on both the S and N-antibody assays we evaluated. Furthermore, this strategy would likely also be useful for differentiating natural infection from vaccination with adenovirus-based vaccines such as vaccines manufactured by AstraZeneca (29) and Johnson and Johnson (30), as these vaccines are also based on expression of recombinant prefusion stabilized S-protein. As expected the Roche S assay detected 100% of fully vaccinated individuals, however, the Diazyme N/S assay only detected naturally infected individuals. It is unclear why the Diazyme N/S assay did not react with vaccinated individuals. This unexpected finding emphasizes the need for clinical laboratories to fully evaluate these types of assay in the intended use population. The distribution of values observed on the Diazyme NAbs and Roche S-antibody assays were significantly different between pre- and post-booster vaccinated individuals (P < 0.0001), which is the anticipated response.
The high PPAs observed between individuals receiving a vaccine and the SARS-CoV-2 PSV assay (87.5% for pre-booster individuals and 98.6% for post-booster individuals), indicates that 98.6% of vaccinated individuals developed NAbs against SARS-CoV-2 (Table 3). The median PSV SARS-CoV-2 ID50 titers were not significantly different between post-booster individuals that received the Moderna (597) or Pfizer (599) vaccines (Fig. 2, A and B).
One limitation of our study is that the ID50 NAb titer that corresponds to immunity against SARS-CoV-2 infection is not known and may be different for NAbs targeting different epitopes (31). Previous studies of the relationship between serology and protection from non-SARS-CoV-2 coronaviridae have yielded varying results with high antiviral IgG titers generally correlating with protection from severe clinical disease and reduced transmission (32). A commercially available assay that does not require a pseudoviral component and detects the presence and titer of NAbs will be important. A previous study indicates that the Roche N-antibody and Diazyme IgG assays had weak correlations with results from SARS-CoV-2 PSV NAb assay, with correlation coefficients of 0.27 and 0.40, respectively (16). The Diazyme NAbs assay strongly correlated with the level of SARS-CoV-2 ID50 PSV neutralization titers (R2 = 0.70) (Fig. 2, C), demonstrating the importance of functional assays and that not all antibodies made in response to vaccination have neutralizing activity.
As more individuals become vaccinated, it is important to understand the timeframe for when a vaccinated individual may be detected by these commercial assays. Our observations suggest that a healthcare provider should wait at least 2 and preferably 3 weeks after an individual has received a SARS-CoV-2 vaccine before attempting to determine whether they have produced antibodies, as the earliest observed seroconversion for individuals receiving the Moderna or Pfizer vaccines was 12 days and 16 days, respectively (Fig. 3, C and D). These results correlate well with the kinetics of ID50 neutralization titers that vaccinated individuals generate in response to vaccination (Fig. 3, A and B).
In summary, our study demonstrates that the Roche S-antibody and Diazyme NAbs assays detect responses to the Moderna and Pfizer SARS-CoV-2 vaccines as well as from natural infections. Both assays perform well compared to previously validated commercial SARS-CoV-2 serology platforms and correlate with the presence of NAbs against SARS-CoV-2, as determined by a cell-based PSV assay.
Supplemental Material
Supplemental material is available at The Journal of Applied Laboratory Medicine online.
Supplementary Material
Acknowledgment
We would like to acknowledge all the staff at the UC San Diego Health clinical laboratories for their help identifying specimens for assay validation and testing. We would also like to thank Amy Rockefeller and Ernestine Ferrer for valuable technical expertise.
Author Declaration: A version of this article was previously posted as a preprint on medRxiv as https://www.medrxiv.org/content/10.1101/2021.03.10.21253299v1.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest:Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:Employment or Leadership: None declared. Consultant or Advisory Role: None declared. Stock Ownership: None declared. Honoraria: None declared. Research Funding: Waters Corporation and Roche Diagnostics are acknowledged for funding the clinical chemistry fellowships for R.T. Suhandynata and M.A. Hoffman, respectively. D. Huang and D. Nemazee received support from NIH grants R37AI059714 and R01AI132317. Diazyme provided evaluation kits for the proxy neutralizing antibody assay free of charge. Expert Testimony: None declared. Patents: None declared. Other Remuneration: M.A. Hoffman, personal fees from Roche, outside the submitted work; N.J. Bevins, personal fees from Thermo Fisher, outside the submitted work.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
References
- 1.Worldometer. Coronavirus Update (Live): 110,211,925 Cases and 2,434,029 Deaths from COVID-19 Virus Pandemic – Worldometer. 2021.. https://www.worldometers.info/coronavirus/ (Accessed February 2021).
- 2.US Food and Drug Administration. COVID-19 vaccines. FDA. FDA; 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (Accessed February2021).
- 3.European Commission. Safe COVID-19 vaccines for Europeans. 2021. https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/safe-covid-19-vaccines-europeans_en (Accessed February2021).
- 4.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouwer PJM, Caniels TG, Aldon Y, Bangaru S, Torres JL, Okba NMA, et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 2020;369: 643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh C-L, Goldsmith JA, Schaub JM, DiVenere AM, Kuo H-C, Javanmardi K, et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 2020;369:1501–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vashist SK.In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics 2020;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theel ES, Slev P, Wheeler S, Couturier MR, Wong SJ, Kadkhoda K.. The role of antibody testing for SARS-CoV-2: is there one? J Clin Microbiol 2020;58:e00797–20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7383527/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. EUA authorized serology test performance. FDA; 2020. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance (Accessed July 2020).
- 11.European Union. COVID-19 In vitro diagnostic medical devices. COVID-19 in vitro diagnostic devices and test methods database. 2021.. https://covid-19-diagnostics.jrc.ec.europa.eu/devices (Accessed February 2021).
- 12.Abbasi J.The promise and peril of antibody testing for COVID-19. JAMA 2020;323:1881–3. [DOI] [PubMed] [Google Scholar]
- 13.Torres R, Rinder HM.. Double-edged Spike. Are SARS-CoV-2 serologic tests safe right now? Am J Clin Pathol 2020;153:709–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein MC, Freedberg KA, Hyle EP, Paltiel AD.. Waiting for certainty on COVID-19 antibody tests — at what cost? N Engl J Med 2020;383:e37. [DOI] [PubMed] [Google Scholar]
- 15.Jääskeläinen A, Kuivanen S, Kekäläinen E, Ahava M, Loginov R, Kallio-Kokko H, et al. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J Clin Virol 2020;129:104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suhandynata RT, Hoffman MA, Huang D, Tran JT, Kelner MJ, Reed SL, et al. Commercial serology assays predict neutralization activity against SARS-CoV-2. Clin Chem 2021;67:404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luchsinger LL, Ransegnola BP, Jin DK, Muecksch F, Weisblum Y, George PJ, et al. Serological assays estimate highly variable SARS-CoV-2 neutralizing antibody activity in recovered COVID-19 patients. J Clin Microbiol 2020;58:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang MS, Case JB, Franks CE, Chen RE, Anderson NW, Henderson JP, et al. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin Chem 2020;66:1538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muecksch F, Wise H, Batchelor B, Squires M, Semple E, Richardson C, et al. Longitudinal serological analysis and neutralizing antibody levels in coronavirus disease 2019 convalescent patients. J Infect Dis 2021;223:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D.. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020;181:281–292. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He W, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020;369:eabc7520–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suhandynata RT, Hoffman MA, Kelner MJ, McLawhon RW, Reed SL, Fitzgerald RL.. Multi-platform comparison of SARS-CoV-2 serology assays for the detection of COVID-19. J Appl Lab Med 2020;5:1324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suhandynata RT, Hoffman MA, Kelner MJ, McLawhon RW, Reed SL, Fitzgerald RL.. Longitudinal monitoring of SARS-CoV-2 IgM and IgG seropositivity to detect COVID-19. J Appl Lab Med 2020;5:908–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard JA, Geno KA, Khan J, Szczepiorkowski ZM, de Gijsel D, Ovalle AA, et al. Comparison of two automated immunoassays for the detection of SARS-CoV-2 nucleocapsid antibodies. J Appl Lab Med 2021;6:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC. Information for Laboratories about Coronavirus (COVID-19). Cent Dis Control Prev. 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html (Accessed June 2020).
- 27.Rubin R.As their numbers grow, COVID-19 “Long Haulers” stump experts. JAMA 2020;324:1381–3. [DOI] [PubMed] [Google Scholar]
- 28.CDC. Interim clinical considerations for use of mRNA COVID-19 vaccines. 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html (Accessed March 2021).
- 29.European Medical Agency. DIMITROVA EK. COVID-19 Vaccine AstraZeneca. Eur. Med. Agency. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-astrazeneca (Accessed March 2021).
- 30.Janssen COVID-19 emergency use authorization (EUA) Official Website. 2021. Available from: https://www.janssencovid19vaccine.com/ (Accessed March 2021).
- 31.Mor M, Werbner M, Alter J, Safra M, Chomsky E, Lee JC, et al. Multi-clonal SARS-CoV-2 neutralization by antibodies isolated from severe COVID-19 convalescent donors. PLOS Pathog 2021;17:e1009165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.