Abstract
Background
Baricitinib is an oral selective Janus kinase 1/2 inhibitor with known anti-inflammatory properties. This study evaluates the efficacy and safety of baricitinib in combination with standard of care for the treatment of hospitalised adults with COVID-19.
Methods
In this phase 3, double-blind, randomised, placebo-controlled trial, participants were enrolled from 101 centres across 12 countries in Asia, Europe, North America, and South America. Hospitalised adults with COVID-19 receiving standard of care were randomly assigned (1:1) to receive once-daily baricitinib (4 mg) or matched placebo for up to 14 days. Standard of care included systemic corticosteroids, such as dexamethasone, and antivirals, including remdesivir. The composite primary endpoint was the proportion who progressed to high-flow oxygen, non-invasive ventilation, invasive mechanical ventilation, or death by day 28, assessed in the intention-to-treat population. All-cause mortality by day 28 was a key secondary endpoint, and all-cause mortality by day 60 was an exploratory endpoint; both were assessed in the intention-to-treat population. Safety analyses were done in the safety population defined as all randomly allocated participants who received at least one dose of study drug and who were not lost to follow-up before the first post-baseline visit. This study is registered with ClinicalTrials.gov, NCT04421027.
Findings
Between June 11, 2020, and Jan 15, 2021, 1525 participants were randomly assigned to the baricitinib group (n=764) or the placebo group (n=761). 1204 (79·3%) of 1518 participants with available data were receiving systemic corticosteroids at baseline, of whom 1099 (91·3%) were on dexamethasone; 287 (18·9%) participants were receiving remdesivir. Overall, 27·8% of participants receiving baricitinib and 30·5% receiving placebo progressed to meet the primary endpoint (odds ratio 0·85 [95% CI 0·67 to 1·08], p=0·18), with an absolute risk difference of −2·7 percentage points (95% CI −7·3 to 1·9). The 28-day all-cause mortality was 8% (n=62) for baricitinib and 13% (n=100) for placebo (hazard ratio [HR] 0·57 [95% CI 0·41–0·78]; nominal p=0·0018), a 38·2% relative reduction in mortality; one additional death was prevented per 20 baricitinib-treated participants. The 60-day all-cause mortality was 10% (n=79) for baricitinib and 15% (n=116) for placebo (HR 0·62 [95% CI 0·47–0·83]; p=0·0050). The frequencies of serious adverse events (110 [15%] of 750 in the baricitinib group vs 135 [18%] of 752 in the placebo group), serious infections (64 [9%] vs 74 [10%]), and venous thromboembolic events (20 [3%] vs 19 [3%]) were similar between the two groups.
Interpretation
Although there was no significant reduction in the frequency of disease progression overall, treatment with baricitinib in addition to standard of care (including dexamethasone) had a similar safety profile to that of standard of care alone, and was associated with reduced mortality in hospitalised adults with COVID-19.
Funding
Eli Lilly and Company.
Translations
For the French, Japanese, Portuguese, Russian and Spanish translations of the abstract see Supplementary Materials section.
Introduction
Hospitalised patients with SARS-CoV-2 infection often develop an intense hyperinflammatory state that can lead to multiple organ dysfunction, including acute respiratory distress syndrome, septic shock, and death.1, 2, 3, 4 Despite treatment advances with remdesivir, dexamethasone, and tocilizumab, reducing mortality among hospitalised patients remains a crucial unmet need.5, 6, 7, 8
Baricitinib is a selective Janus kinase (JAK)1/JAK2 inhibitor9, 10, 11 with a known anti-inflammatory profile in patients with autoimmune diseases.12, 13, 14 In February, 2020, baricitinib was identified by an artificial intelligence platform as a potential intervention for the treatment of COVID-19 because of its known anticytokine properties and potential for targeting host proteins for its antiviral mechanism.15, 16 The biochemical inhibitory effects of baricitinib on human Numb-associated kinases (AAK1, BIKE, and GAK) responsible for SARS-CoV-2 viral propagation were subsequently confirmed.17 Baricitinib was also shown to reduce multiple cytokines and biomarkers implicated in COVID-19 pathophysiology.18, 19, 20 Following the publication of these findings, several observational studies including small cohorts of hospitalised patients with COVID-19 (including older adults) were done and provided the first evidence of clinical improvement associated with baricitinib treatment.21, 22, 23, 24
Research in context.
Evidence before this study
We searched PubMed using the terms “COVID-19”, “SARS-CoV-2”, “treatment”, “baricitinib”, and “JAK inhibitor” for articles in English, published until April 31, 2020, regardless of article type. We considered previous and current clinical trials of investigational medications in COVID-19, as well as previous clinical trials of the Janus kinase (JAK)1 and JAK2 inhibitor baricitinib conducted before this study. At the time the COV-BARRIER study was designed, there were no approved therapies for the treatment of COVID-19. Management of COVID-19 was supportive, and few phase 3 randomised placebo-controlled studies had been completed. Some phase 2 and 3 data on the antimalarial hydroxychloroquine and protease inhibitor lopinavir–ritonavir were available, and trials investigating the use of the antiviral remdesivir were ongoing. Baricitinib was identified as a potential intervention for COVID-19 due to its mechanism of action as a JAK1 and JAK2 inhibitor, its known anticytokine properties, and a potential antiviral mechanism via targeting host proteins. Additionally, early case series evaluating the efficacy and safety of baricitinib in populations of hospitalised patients supported further evaluation of baricitinib as a potential treatment option for hospitalised patients with COVID-19. While the COV-BARRIER study was enrolling, ACTT-2, a phase 3 study evaluating baricitinib plus remdesivir, was completed and showed that baricitinib in combination with remdesivir improved time to recovery and other outcomes.
Added value of this study
This was the first phase 3 study to evaluate baricitinib in addition to the current standard of care, and included patients receiving antivirals, anticoagulants, and corticosteroids. After the earliest publication of the RECOVERY study in June, 2020, the treatment of hospitalised patients with COVID-19 changed with the adoption of dexamethasone as the standard of care. As a result of its design, COV-BARRIER became the first trial to evaluate the benefit and risk of baricitinib when added to the most current standard of care (dexamethasone) in these patients. This was a randomised, double-blind, placebo-controlled trial conducted globally in regions with high COVID-19 hospitalisation rates. The reduction in the composite primary endpoint of progression to non-invasive ventilation, high-flow oxygen, invasive mechanical ventilation, or death for baricitinib plus standard of care (including dexamethasone) compared with placebo plus standard of care was not statistically significant. However, analysis of a prespecified key secondary endpoint showed that treatment with baricitinib reduced 28-day all-cause mortality by 38·2% compared with placebo (HR 0·57 [95% CI 0·41–0·78], nominal p=0·0018), with one additional death prevented per 20 baricitinib-treated participants. The reduction of all-cause mortality with baricitinib was maintained up to day 60 in an exploratory analysis. The frequency of serious adverse events, serious infections, and venous thromboembolic events was similar between the baricitinib and placebo groups.
Implications of all the available evidence
In this phase 3 trial, baricitinib administered in addition to standard of care (which predominantly included dexamethasone) did not reduce the incidence of a composite endpoint of disease progression, but showed a strong effect on reduction of mortality by 28 days, an effect which was maintained up to 60 days. In the ACTT-2 study, baricitinib further reduced time to recovery above the background use of remdesivir. Taken together, these findings suggest that baricitinib has synergistic effects with other standard-of-care treatment modalities, including remdesivir and dexamethasone. Based on all available evidence, baricitinib is a potentially effective oral treatment option to decrease mortality in hospitalised patients with COVID-19.
The Adaptive COVID-19 Treatment Trial 2 (ACTT-2)— a US National Institutes of Health-sponsored, double-blind, randomised, placebo-controlled, phase 3 trial in hospitalised adults with COVID-19—found that treatment with baricitinib plus remdesivir was superior to treatment with remdesivir alone in reducing time to recovery (rate ratio 1·16 [95% CI 1·01–1·32], p=0·03) and was associated with fewer serious adverse events, although 28-day mortality did not differ significantly between groups (5·1% with baricitinib and remdesivir vs 7·8% with remdesivir).6 The US Food and Drug Administration issued an emergency use authorisation for baricitinib in hospitalised patients with COVID-19 who required oxygen supplementation,25 addressing an unmet need in the treatment of COVID-19.26 Globally, however, evaluations of new treatment options to reduce mortality in hospitalised patients with COVID-19 are still urgently needed to reduce the high frequency of deaths that persists despite improvements in standards of care.
The COV-BARRIER study was designed to evaluate the efficacy and safety of baricitinib in combination with standard of care, including dexamethasone, for the treatment of hospitalised adults with COVID-19. To the best of our knowledge, this study is the first double-blind, placebo-controlled trial to evaluate mortality by day 60.
Methods
Study design and participants
This multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 3 trial included 101 centres from 12 countries in Asia, Europe, North America, and South America. Eligible participants were at least 18 years of age, were hospitalised with laboratory-confirmed SARS-CoV-2 infection, had evidence of pneumonia or active and symptomatic COVID-19, and had at least one elevated inflammatory marker (C-reactive protein, D-dimer, lactate dehydrogenase, or ferritin). Participants were excluded if, at study entry, they required invasive mechanical ventilation (National Institute of Allergy and Infectious Disease Ordinal Scale [NIAID-OS] score 7); were receiving immunosuppressants (high-dose corticosteroids, biologics, T-cell-targeted or B-cell-targeted therapies, interferon, or JAK inhibitors); had ever received convalescent plasma or intravenous immunoglobulin for COVID-19; or had neutropenia (absolute neutrophil count <1000 cells per μL), lymphopenia (absolute lymphocyte count <200 cells per μL), alanine aminotransferase (ALT) or aspartate aminotransferase concentration greater than five times the upper limit of normal, or an estimated glomerular filtration rate (eGFR) of less than 30 mL/min per 1·73 m2. Full inclusion and exclusion criteria are provided in appendix 6 (pp 6–8).
Following the disclosure of results from the ACTT-2 study showing that disease progression was unlikely in participants without baseline oxygen support, the COV-BARRIER protocol was amended on Oct 20, 2020, to limit enrolment to participants who required baseline oxygen support (NIAID-OS score 5 or 6).
The trial protocol and statistical analysis plan are available in appendix 6 (pp 33–350). The COV-BARRIER study was done in accordance with ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All sites received approval from the authorised institutional review board. All participants (or their legally authorised representatives) provided written informed consent.
Randomisation and masking
Participants were enrolled by study investigators (or a designee). Randomisation was facilitated by a computer-generated random sequence using an interactive web-response system, and was permitted by a study investigator or designee to allocate participants 1:1 to the baricitinib group or the placebo group. Participants were stratified according to the following baseline factors: disease severity (NIAID-OS 4, 5, or 6), age (<65 or ≥65 years), region (Europe, USA, or the rest of the world), and use of corticosteroids for primary study condition (yes or no). Participants, study staff, and investigators were masked to the study assignment. An independent, external data monitoring committee oversaw the study and evaluated unblinded interim data for efficacy, futility, and safety. An independent, blinded, clinical event committee adjudicated potential venous thromboembolic events and deaths.
Procedures
All participants received standard of care in keeping with local clinical practice for COVID-19 management, which could include corticosteroids, antivirals, or both. Dexamethasone use was permitted as described in the RECOVERY trial,7 but higher corticosteroid doses (>20 mg per day [or prednisone equivalent] administered for >14 consecutive days in the month before study entry) were not permitted, unless indicated per standard of care for a concurrent condition, such as asthma, chronic obstructive pulmonary disease, or adrenal insufficiency. Prophylaxis for venous thromboembolic events per local practice was required for all participants unless there was a major contraindication, such as an active bleeding event or history of heparin-induced thrombosis.
Interventions were packaged in identical bottles containing tablets of either 2 mg baricitinib or matching placebo. The baricitinib intervention consisted of baricitinib at a dose of 4 mg/day; however, 2 mg/day was given if the patient had a baseline eGFR of 30 to less than 60 mL/min/1·73 m2. Baricitinib or placebo tablets were administered orally (or crushed for nasogastric tube delivery) and given daily for up to 14 days or until discharge from hospital, whichever occurred first. The date and time of each dose administered was recorded in the source documents and the case report form, and the site investigator (or designee) was responsible for assessing study drug compliance.
For efficacy and health outcomes, baseline measurements were defined as the last non-missing assessment recorded on or before the first administration of study drug at study day 1 (randomisation).
Participants followed the study visit schedule per the protocol, which included a study visit at day 28 for assessment of the primary endpoint. Efficacy and safety were evaluated for all participants up to day 28, and and all-cause mortality was also evaluated in participants with non-missing baseline and at least one post-baseline observation up to day 60. Participants had a follow-up visit approximately 28 days after receiving their last dose of study drug.
Outcomes
The composite primary endpoint was the proportion of participants who progressed to high-flow oxygen or non-invasive ventilation (NIAID-OS score 6), invasive mechanical ventilation or extracorporeal membrane oxygenation (NIAID-OS score 7), or death (NIAID-OS score 8) by day 28, in the baricitinib group compared with the placebo group. All-cause mortality by day 28 was a prespecified key secondary endpoint, and all-cause mortality by day 60 was a prespecified exploratory endpoint. The primary, intention-to-treat analysis was done in two populations: population 1 (comprising all randomised participants) and population 2 (the subpopulation of participants who, at baseline, required oxygen supplementation and were not receiving systemic corticosteroids for COVID-19).
Prespecified key secondary outcomes also included the following and were evaluated from days 1 to 28, unless otherwise specified: all-cause mortality; proportion of participants with at least a one-point improvement on the NIAID-OS or discharge from hospital at days 4, 7, 10, and 14; number of ventilator-free days; time to recovery (NIAID-OS scores 1–3); overall improvement on the NIAID-OS, evaluated at days 4, 7, 10, and 14; duration of hospitalisation; and proportion of participants with a change in oxygen saturation from less than 94% to 94% or higher between baseline and days 4, 7, 10, and 14. All other prespecified secondary outcomes and select exploratory outcomes, including assessment of pharmacokinetics evaluating baricitinib in participants who progressed to mechanical ventilation (intubation), are described in appendix 6 (pp 9, 11–12). Adverse events were recorded on days 1–28, coded by the Medical Dictionary for Regulatory Activities (version 23.1).
Statistical analysis
The first version of the protocol specified a sample size of 400 participants. Over the course of the study (and all before unmasking), the sample size was formally increased in protocol amendments to incorporate information and data available from other COVID-19 studies. In the final amendment to the protocol, an additional primary endpoint based on population 2 was added and the sample size was updated to 1400. Power was calculated for the primary endpoint to succeed in at least one of the two primary populations, and the study included the possibility of increasing the sample size using an unblinded sample size re-estimation27 of the primary endpoint, as assessed during an interim analysis evaluated by an external data monitoring committee (completed in January, 2021, with no changes recommended). Power calculations assumed that 75% of the total α was allocated to population 1, and that 60% of the participants were taking systemic corticosteroids at baseline. Two scenarios were considered. In the first, both population 1 and population 2 had a true treatment effect size of 7·5% (power 81%). In the second, population 1 had a true effect size of 4% and population 2 had an assumed effect size of 7·5% (power 54%). In the final (pre-unmasking) version of the statistical analysis plan, the total α was amended to be allocated 99% to population 1, recognising that population 2 was much smaller than previously anticipated and unlikely to succeed.
To control the overall family-wise type I error rate at a two-sided α level of 0·05, a graphical testing procedure was used to test the primary and key secondary endpoint results in a hierarchical manner. For example, in order for the first key secondary analysis in the hierarchy (the proportion of participants with ≥1-point improvement on the NIAID-OS or live discharge from hospital at day 14) to be considered multiplicity-controlled significant, it was necessary to achieve statistical significance in the population 1 primary endpoint analysis. Each subsequent test relied on succeeding in the preceding test in the hierarchy (appendix 6 p 14). In this report we use the term “nominal p value” when referring to key secondary endpoints for which p values were direct from the prespecified statistical models and were unadjusted for multiplicity.
Efficacy data were analysed in the intention-to-treat population, defined as all randomly allocated participants. Logistic regression was used for dichotomous endpoints, proportional odds models were used for ordinal endpoints, ANOVA was used for continuous endpoints, and mixed-effects models of repeated measures were used to assess continuous endpoints over time. Log-rank tests and hazard ratios (HRs) from Cox proportional hazard models were used for time-to-event analyses. These statistical models were adjusted for treatment and baseline stratification factors. Prespecified subgroup analyses for the primary and selected key secondary endpoints evaluated treatment effect across the following subgroups: baseline severity (NIAID-OS score 4, 5, or 6), baseline systemic corticosteroid use (yes or no), baseline remdesivir use (yes or no), geographical region (Europe, USA, or the rest of the world), sex, disease duration at baseline (<7 days or ≥7 days), and age at baseline (<65 years or ≥65 years).
Safety analyses included all randomly allocated participants who received at least one dose of study drug and who were not lost to follow-up before the first post-baseline visit. Adverse events were inclusive of the 28-day treatment period. Statistical tests of treatment effects were done at a two-sided significance level of 0·05, unless otherwise stated (ie, for the graphical multiple testing strategy). Statistical analyses were done with SAS (version 9.4 or higher) or R. Further details are described in appendix 6 (pp 9–10).
This trial is registered with ClinicalTrials.gov, NCT04421027.
Role of the funding source
COV-BARRIER was designed jointly by consultant experts and representatives of the sponsor, Eli Lilly and Company. Data were collected by investigators and analysed by the sponsor. All authors participated in the interpretation of the data analysis, draft, and final manuscript review, and provided critical comment, including the decision to submit the manuscript for publication with medical writing support provided by the sponsor. The authors had full access to the data and authors from the sponsor verified the veracity, accuracy, and completeness of the data and analyses as well as the fidelity of this report to the protocol.
Results
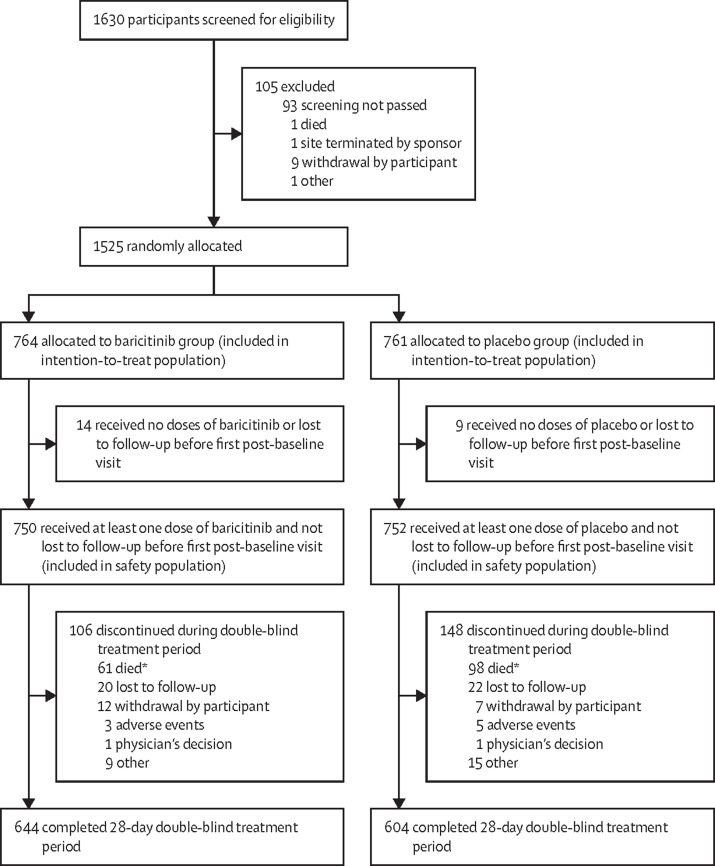
Between June 11, 2020, and Jan 15, 2021, 1630 participants were screened for eligibility and 1525 (93·6%) were randomly allocated to receive either 4 mg baricitinib once daily plus standard of care (n=764) or placebo plus standard of care (n=761). 23 (1·5%) participants received no dose of either baricitinib or placebo or were lost to follow-up before the first post-baseline follow-up visit and were therefore excluded from the safety analyses. Of the remaining 1502 participants, 1248 (83·1%) completed the 28-day treatment period and 254 (16·9%) discontinued the treatment during this period, of whom 159 (62·6%) died (figure 1 ). No randomly allocated participants were excluded from the intention-to-treat population; however, some participants were excluded from specific analyses because of the various information requirements of the different statistical methods as outlined in appendix 6 (p 20).
Figure 1.
Trial profile
*159 deaths were reported by day 28; an additional three deaths occurred after the treatment period disposition but within 28 days.
Baseline demographics and disease characteristics were similar between study groups (table 1 ). The mean age of the participants was 57·6 years (SD 14·1) and 963 (63·1%) participants were male. The greatest proportions of participants were enrolled in Brazil (337 [22·1%] participants), the USA (320 [21·0%]), Mexico (281 [18·4%]), and Argentina (208 [13·6%]), and the remaining participants were enrolled across several European countries as well as India, Japan, Korea, and Russia. Of 1493 participants with available data on race, 920 (61·6%) were White, 316 (21·2%) were American Indian or Alaskan Native, 174 (11·7%) were Asian, 75 (5·0%) were Black or African American (including 33 [10·3%] of 320 US participants), five (0·3%) were of Native Hawaiian or other Pacific Islander races, and three (0·2%) were of multiple races.
Table 1.
Baseline demographics and clinical characteristics
| Baricitinib group (n=764) | Placebo group (n=761) | |||
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 57·8 (14·3) | 57·5 (13·8) | ||
| <65 | 508/764 (66%) | 518/761 (68%) | ||
| ≥65 | 256/764 (34%) | 243/761 (32%) | ||
| Sex | ||||
| Male | 490/764 (64%) | 473/761 (62%) | ||
| Female | 274/764 (36%) | 288/761 (38%) | ||
| Race | ||||
| American Indian or Alaskan Native* | 148/752 (20%) | 168/741 (23%) | ||
| Asian | 80/752 (11%) | 94/741 (13%) | ||
| Black or African American | 39/752 (5%) | 36/741 (5%) | ||
| Native Hawaiian or other Pacific Islander | 3/752 (<1%) | 2/741 (<1%) | ||
| White | 480/752 (64%) | 440/741 (59%) | ||
| Multiple | 2/752 (<1%) | 1/741 (<1%) | ||
| Ethnicity† | ||||
| Hispanic or Latino | 54/162 (33%) | 46/158 (29%) | ||
| Not Hispanic or Latino | 92/162 (57%) | 94/158 (59%) | ||
| Not reported | 16/162 (10%) | 18/158 (11%) | ||
| Region and country | ||||
| Europe | 73/764 (10%) | 70/761 (9%) | ||
| Germany | 9/764 (1%) | 11/761 (1%) | ||
| Italy | 15/764 (2%) | 10/761 (1%) | ||
| Spain | 45/764 (6%) | 42/761 (6%) | ||
| UK | 4/764 (1%) | 7/761 (1%) | ||
| USA (including Puerto Rico) | 162/764 (21%) | 158/761 (21%) | ||
| Rest of world | 529/764 (69%) | 533/761 (70%) | ||
| Argentina | 107/764 (14%) | 101/761 (13%) | ||
| Brazil | 172/764 (23%) | 165/761 (22%) | ||
| India | 19/764 (2%) | 31/761 (4%) | ||
| Japan | 19/764 (2%) | 19/761 (2%) | ||
| South Korea | 16/764 (2%) | 20/761 (3%) | ||
| Mexico | 138/764 (18%) | 143/761 (19%) | ||
| Russia | 58/764 (8%) | 54/761 (7%) | ||
| Body-mass index (kg/m2) | 30·4 (6·4) | 30·6 (6·6) | ||
| Duration of disease symptoms before enrolment, days | ||||
| <7 | 137/762 (18%) | 116/756 (15%) | ||
| ≥7 | 625/762 (82%) | 640/756 (85%) | ||
| Score on NIAID-OS | ||||
| 4 (hospitalised, not requiring supplemental oxygen) | 89/762 (12%) | 97/756 (13%) | ||
| 5 (hospitalised, requiring supplemental oxygen) | 490/762 (64%) | 472/756 (62%) | ||
| 6 (hospitalised, receiving non-invasive ventilation or high-flow oxygen) | 183/762 (24%) | 187/756 (25%) | ||
| Concomitant medications of interest | ||||
| Remdesivir | 140/762 (18%) | 147/756 (19%) | ||
| Systemic corticosteroids | 612/762 (80%) | 592/756 (78%) | ||
| Dexamethasone | 566/612 (92%) | 533/592 (90%) | ||
| Pre-existing comorbidities of interest | ||||
| Obesity | 250/764 (33%) | 253/761 (33%) | ||
| Diabetes (types 1 and 2) | 224/764 (29%) | 233/761 (31%) | ||
| Chronic respiratory disease | 34/764 (4%) | 36/761 (5%) | ||
| Hypertension | 365/764 (48%) | 366/761 (48%) | ||
Data are mean (SD) or n/N (%). NIAID-OS=National Institute of Allergy and Infectious Disease Ordinal Scale.
Includes participants from Mexico and Latin America.
Reporting required in the USA only.
Of 1518 participants with available data, 1265 (83·3%) had symptoms for at least 7 days before enrolment. Baseline NIAID-OS scores (indicating clinical status) were 4 for 186 (12·3%) participants, 5 for 962 (63·4%) participants, and 6 for 370 (24·4%) participants. 1204 (79·3%) participants were receiving systemic corticosteroids at baseline, of whom 1099 (91·3%) were on dexamethasone. 287 (18·9%) participants were receiving remdesivir, of whom 263 (91·6%) were also receiving corticosteroids (table 1; appendix 6 p 21).
1520 (99·7%) of 1525 participants had at least one pre-existing comorbidity of interest (table 1). Median baseline C-reactive protein concentration was elevated (65·0 mg/L) and was similar in the baricitinib group (67·5 mg/L) and placebo group (62·0 mg/L). Select baseline demographics and clinical characteristics by baseline systemic corticosteroid use are presented in appendix 6 (p 22).
In population 1 (all randomly allocated participants), the proportion of patients who progressed to high-flow oxygen, non-invasive ventilation, invasive mechanical ventilation, or death by day 28 (the composite primary endpoint) was 27·8% in the baricitinib group and 30·5% in the placebo group (odds ratio [OR] 0·85 [95% CI 0·67–1·08], p=0·18; table 2 ). The absolute risk difference was −2·7 percentage points (95% CI −7·3 to 1·9). Of the 434 participants who progressed, 95 (22%) progressed by day 1 (the day of randomisation) and 246 (57%) progressed by day 3 (appendix 6 p 23).
Table 2.
Primary and key secondary outcomes in the intention-to-treat population
| Baricitinib group (n=764) | Placebo group (n=761) |
Baricitinib vs placebo |
|||
|---|---|---|---|---|---|
| Point estimate (95% CI) | p value* | ||||
| Primary outcome | |||||
| Progression to high-flow oxygen, non-invasive ventilation, invasive mechanical ventilation (including ECMO), or death, by day 28† | |||||
| Population 1‡ | 27·8% | 30·5% | OR 0·85 (0·67 to 1·08) | 0·18 | |
| Population 2§ | 28·9% | 27·1% | OR 1·12 (0·58 to 2·16) | 0·73 | |
| Key secondary outcomes | |||||
| All-cause mortality | 62/764 (8%) | 100/761 (13%) | HR 0·57 (0·41 to 0·78) | 0·0018 | |
| Likelihood of overall improvement on the NIAID-OSঠ| |||||
| Day 4 | .. | .. | OR 1·21 (1·00 to 1·47) | 0·046 | |
| Day 7 | .. | .. | OR 1·25 (1·04 to 1·49) | 0·017 | |
| Day 10 | .. | .. | OR 1·17 (0·97 to 1·41) | 0·092 | |
| Day 14 | .. | .. | OR 1·28 (1·05 to 1·56) | 0·017 | |
| ≥1-point improvement on NIAID-OS or live discharge from hospital†‡ | |||||
| Day 4 | 25·2% | 21·1% | OR 1·26 (0·98 to 1·61) | 0·067 | |
| Day 7 | 49·8% | 45·8% | OR 1·18 (0·95 to 1·46) | 0·13 | |
| Day 10 | 65·0% | 63·5% | OR 1·07 (0·86 to 1·34) | 0·54 | |
| Day 14 | 75·6% | 72·3% | OR 1·21 (0·95 to 1·55) | 0·13 | |
| Median time to recovery (NIAID-OS), days | 10·0 (9·0 to 11·0) | 11·0 (10·0 to 12·0) | RR 1·11 (0·99 to 1·24) | 0·15 | |
| Number of ventilator-free days‡ | 24·5 (0·39) | 23·7 (0·39) | LSMD 0·75 (−0·0 to 1·5) | 0·059 | |
| Duration of hospitalisation‡ | 12·9 (0·40) | 13·7 (0·40) | LSMD −0·76 (−1·6 to 0·0) | 0·063 | |
| Change from baseline in oxygen saturation from <94% to ≥94% | |||||
| Day 4 | 133/282 (47%) | 119/282 (42%) | OR 1·20 (0·86 to 1·69) | 0·29 | |
| Day 7 | 146/282 (52%) | 146/282 (52%) | OR 0·97 (0·69 to 1·37) | 0·88 | |
| Day 10 | 160/282 (57%) | 148/282 (52%) | OR 1·15 (0·81 to 1·63) | 0·43 | |
| Day 14 | 166/282 (59%) | 166/282 (59%) | OR 0·95 (0·66 to 1·37) | 0·79 | |
Group data are %, n/N (%), median (95% CI), or least squares mean (SE). Population 1 includes all randomised participants. Population 2 includes participants who, at baseline, required oxygen supplementation and were not receiving dexamethasone or other systemic corticosteroids for the primary study condition. Data were assessed from days 1 to 28, unless otherwise indicated. Dichotomous endpoints were analysed with a logistic regression model. Ordinal efficacy endpoints were analysed with a proportional odds model. Continuous endpoints were analysed by ANOVA. All of these analyses had baseline randomisation factors and treatment group in the model, except in cases where the factor was redundant in the model (eg, for population 2, baseline corticosteroid use [yes or no] could not be included in the model because no participants in this population were on corticosteroids at baseline). For time-to-event endpoints, p values were calculated using an unstratified log-rank test. The HR (and corresponding 95% CI) was calculated using a Cox proportional hazards model. ECMO=extracorporeal membrane oxygenation. OR=odds ratio. HR=hazard ratio. NIAID-OS=National Institute of Allergy and Infectious Disease Ordinal Scale. RR=rate ratio. LSMD=least squares mean difference.
Because the primary outcome was not statistically significant in the prespecified hierarchical graphical testing procedure, none of the key secondary outcomes could be considered statistically significant using this same procedure; therefore, nominal, non-multiplicity-controlled p values are shown for all secondary outcomes.
Percentages were calculated with a multiple imputation method, which does not support a meaningful reporting of n because it is an average of 100 imputed datasets.
Multiple imputation included N=756 for placebo and N=762 for baricitinib.
Multiple imputation included N=109 for placebo and N=96 for baricitinib.
Results are represented for the overall OR compared with placebo because this was derived from each individual contributing NIAID-OS scores (1–8) at each timepoint.
In population 2 (the subpopulation on oxygen and not receiving steroids at baseline), 28·9% participants in the baricitinib group and 27·1% in the placebo group met the composite primary endpoint (OR 1·12 [95% CI 0·58–2·16], p=0·73; table 2). Because statistical significance was not found in the primary analysis for population 1 per the prespecified graphical testing scheme, none of the key secondary endpoints were considered statistically significant after adjusting for multiplicity. Subsequent p values reported are nominal and non-multiplicity-controlled.
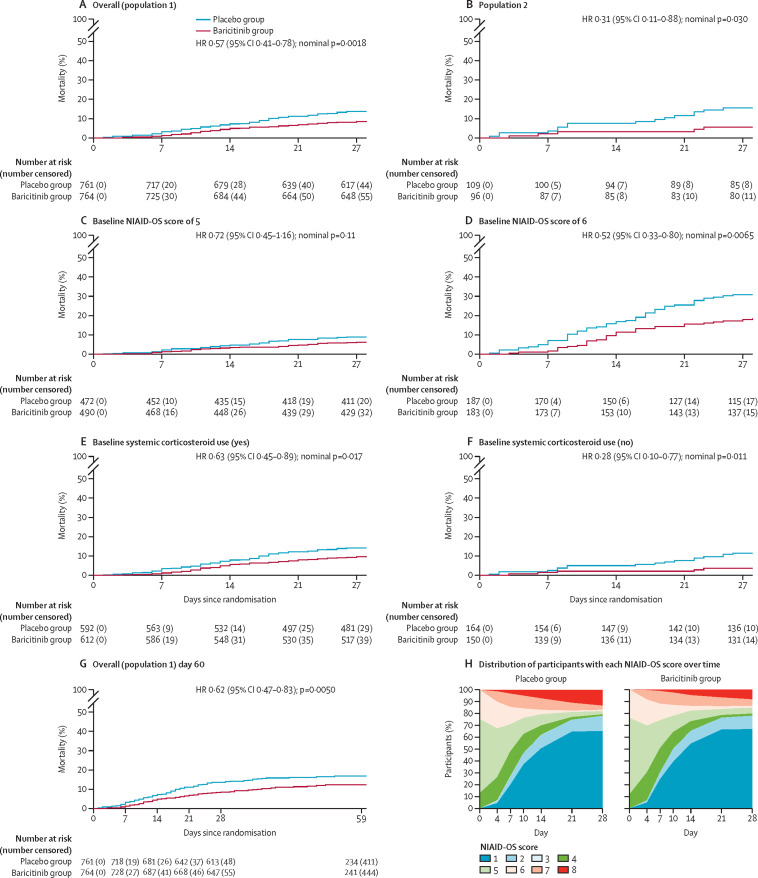
In population 1, by day 28, 162 participants had died (62 [8%] of 764 in the baricitinib group and 100 [13%] of 761 in the placebo group). 28-day all-cause mortality was 38% lower in the baricitinib group than in the placebo group (HR 0·57 [95% CI 0·41–0·78], nominal p=0·0018; table 2; figures 2A, 3) and showed an absolute risk difference of −5·0 percentage points. Overall, one additional death was prevented per 20 baricitinib-treated participants.
In population 2, 28-day all-cause mortality was 5% (five of 96 participants) in the baricitinib group and 15% (16 of 109) in the placebo group, equating to a 65% relative reduction (HR 0·31 [95% CI 0·11–0·88], nominal p=0·030; figures 2B, 3).
Other key secondary outcomes are described in table 2 and other secondary outcomes are described in appendix 6 (p 11, 18, 27–29).
All-cause mortality by day 60 was evaluated in a prespecified exploratory analysis. Between days 28 and 60, 33 additional deaths occurred in the overall population (17 in the baricitinib group and 16 in the placebo group). 60-day mortality remained significantly lower in the baricitinib group (79 [10%] of 764 patients) than in the placebo group (116 [15%] of 761; HR 0·62 [95% CI 0·47–0·83], p=0·0050; figure 2G ), with an absolute risk difference of −4·9 percentage points, similar to that observed for 28-day mortality. Population 2 also showed reduced mortality by day 60 with baricitinib treatment (five [5%] of 96) compared with placebo (19 [18%] of 108; HR 0·27 [95% CI 0·10–0·75], p=0·0080; appendix 6 p 16).
Figure 2.
Kaplan-Meier estimates of 28-day and 60-day all-cause mortality, and distribution of participants with each NIAID-OS score over time
(A–F) 28-day all-cause mortality in population 1, the overall population (A); population 2, comprising participants who, at baseline, required oxygen supplementation and were not receiving dexamethasone or other systemic corticosteroids for the primary study condition (B); populations with baseline NIAID-OS scores of 5 (C) or 6 (D); and populations with (E) and without (F) baseline systemic corticosteroid use. The number at risk at day 27 represents the number of participants with available data at day 28. (G) 60-day all-cause mortality in population 1. The number at risk and number censored before day 28 differ slightly between panels A and G because the day 60 database contained further information on eight participants who were censored at the day 28 database lock but were known to be alive at the day 60 database lock. The number at risk at day 59 represents the number of participants with available data at day 60. For time-to-event endpoints, the p value for baricitinib versus placebo was calculated using an unstratified log-rank test, and HRs and 95% CIs were calculated using a Cox proportional hazards model. The treatment effect was adjusted by all baseline randomisation factors, except when redundant (ie, for baseline corticosteroid use in population 2). (H) Distribution of participants in each NIAID-OS category over time, among patients in the intention-to-treat population with available baseline NIAID-OS scores and at least one post-baseline NIAID-OS score, using last observation carried forward. An NIAID-OS score of 5 represents patients who are hospitalised and require supplemental oxygen, and a score of 6 represents patients who are hospitalised and receiving oxygen support via high-flow oxygen devices or non-invasive ventilation. HR=hazard ratio. NIAID-OS=National Institute of Allergy and Infectious Disease Ordinal Scale.
Figure 2H shows the distribution of participants in each NIAID-OS category over time to day 28. The majority of patients were recovered (NIAID-OS score 1, 2 or 3) in the baricitinib and placebo groups at day 28. Fewer participants died in the baricitinib treatment group by day 28 than in the placebo group.
The exploratory results of pharmacokinetic analyses in participants who progressed to mechanical ventilation (intubation) and received baricitinib as a solution of crushed tablets via nasopharyngeal tube are shown in appendix 6 (pp 11–12, 17). These pharmacokinetic data were consistent with those previously reported for baricitinib in other populations, such as in healthy participants in phase 1 studies (without baseline comorbidities) and participants with rheumatoid arthritis (appendix 6 pp 11–12, 17).
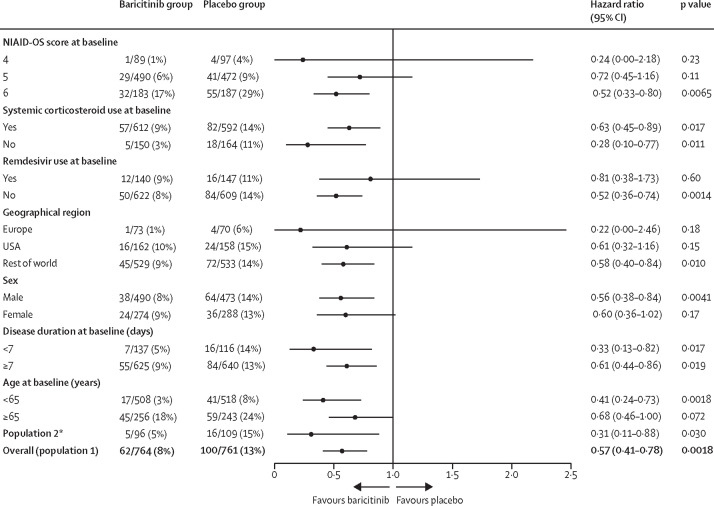
We did efficacy analyses across prespecified subgroups by baseline severity (NIAID-OS score), baseline systemic corticosteroid use (yes or no), baseline remdesivir use (yes or no), geographical region (Europe, USA, or the rest of the world), sex, disease duration at baseline (<7 days or ≥7 days), and age at baseline (<65 years or ≥65 years). The results of the primary composite endpoint across subgroups by region are reported in appendix 6 (p 25); a numerical reduction in progression with baricitinib compared with placebo was observed for participants from the USA and the rest of the world but not for participants from Europe. Trends observed across all prespecified baseline NIAID-OS subgroups were similar to those observed in the overall population (ie, numerically but not significantly lower progression in the baricitinib group than in the placebo group; appendix 6 p 24).
Analyses of 28-day all-cause mortality in subgroups of population 1 (Figure 2, Figure 3 ) showed a numerical (but not significant) reduction in mortality with baricitinib compared with placebo in subgroups of patients with baseline NIAID-OS scores of 4 (figure 3) or 5 (Figure 2, Figure 3). However, in the subgroup of patients with a baseline NIAID-OS score of 6, 28-day mortality was significantly lower with baricitinib than with placebo (Figure 2, Figure 3); in this subgroup, one additional death was prevented per nine baricitinib-treated participants. Baricitinib was also associated with a significant reduction in 28-day mortality in subgroups of participants with systemic corticosteroid treatment at baseline (Figure 2, Figure 3), without systemic corticosteroid treatment at baseline (Figure 2, Figure 3), or without remdesivir treatment at baseline (figure 3; appendix 6 p 15). Among the 287 participants with concomitant remdesivir treatment at baseline (263 [92%] of whom also received corticosteroids), a numerical (but not significant) reduction in mortality was observed (figure 3; appendix 6 p 15). Significant reductions in 28-day mortality associated with baricitinib were also observed in participants younger than 65 years and those located in the rest of the world region, with numerical (but not significant) reductions observed in participants aged 65 years or older and those located in Europe or the USA (figure 3; appendix 6 p 26).
Figure 3.
28-day all-cause mortality by subgroup
HRs and 95% CIs were calculated with a Cox proportional hazards model. The treatment effect was adjusted by all baseline randomisation factors, except when redundant (eg, for age group [<65 or ≥65 years] in the age subgroup analyses). HR=hazard ratio. NIAID-OS=National Institute of Allergy and Infectious Disease Ordinal Scale. *Participants who, at baseline, required oxygen supplementation and were not receiving dexamethasone or other systemic corticosteroids for the primary study condition.
In population 2, for participants with a baseline NIAID-OS score of 5, 28-day mortality was numerically (but not significantly) lower (HR 0·45 [95% CI 0·13–1·54], nominal p=0·31) in the baricitinib group (four [5%] of 79 patients) than in the placebo group (eight [9%] of 88). In participants with a baseline NIAID-OS score of 6, 28-day mortality was significantly lower (HR 0·20 [95% CI 0·02–1·62], nominal p=0·040) in the baricitinib group (one [6%] of 17) than in the placebo group (eight [38%] of 21).
With regard to the subgroup analyses of 60-day all-cause mortality, the observed reduction with baricitinib was not significant in the NIAID-OS score 5 subgroup (34 [7%] of 490 in the baricitinib group vs 49 [10%] of 472 in the placebo group; HR 0·70 [95% CI 0·45–1·09], p=0·071) but was statistically significant in the NIAID-OS score 6 subgroup (42 [23%] of 183 vs 63 [34%] of 187; 0·58 [0·39–0·86], p=0·014; appendix 6 p 16). Significant reductions in 60-day mortality were also observed with baricitinib compared with placebo in the subgroups of participants with systemic corticosteroid use at baseline (73 [12%] of 612 vs 95 [16%] of 593; 0·69 [0·51–0·94], p=0·044) and without systemic corticosteroid use at baseline (six [4%] of 150 vs 21 [13%] of 163; 0·30 [0·12–0·75], p=0·0072; appendix 6 p 16).
334 (45%) of 750 participants in the baricitinib group and 334 (44%) of 752 in the placebo group had at least one treatment-emergent adverse event, and serious adverse events were observed in 110 (15%) participants in the baricitinib group and 135 (18%) in the placebo group (table 3 ). The most common serious adverse events are described in appendix 6 (p 30). The frequencies of deaths reported as being due to adverse events (ie, rather than disease progression, 12 [2%] participants in the baricitinib group vs 31 [4%] in the placebo group) and of discontinuation of study treatment due to adverse events (56 [7%] vs 70 [9%]) were numerically lower with baricitinib than placebo. Serious infections were reported in 64 (9%) baricitinib-treated participants and 74 (10%) placebo-treated participants (table 3). Among participants using corticosteroids at baseline, serious infections occurred at a similar frequency between groups (58 [10%] of 605 vs 63 [11%] of 590; appendix 6 p 31). There were similar distributions of positively adjudicated venous thromboembolic events (20 [3%] vs 19 [3%]) and major adverse cardiovascular events (eight [1%] vs nine [1%]) in the baricitinib and placebo groups and no reports of gastrointestinal perforations (table 3). Safety data are described further in table 3 and appendix 6 (pp 11, 31).
Table 3.
Adverse events in the safety population
| Baricitinib group (n=750) | Placebo group (n=752) | |||
|---|---|---|---|---|
| Treatment-emergent adverse event | 334 (45%) | 334 (44%) | ||
| Mild | 133 (18%) | 115 (15%) | ||
| Moderate | 90 (12%) | 89 (12%) | ||
| Severe | 111 (15%) | 130 (17%) | ||
| Death due to adverse event* | 12 (2%) | 31 (4%) | ||
| Serious adverse event | 110 (15%) | 135 (18%) | ||
| Discontinuation from study treatment due to adverse event (including death) | 56 (7%) | 70 (9%) | ||
| Treatment-emergent infection | 119 (16%) | 123 (16%) | ||
| Serious infections | 64 (9%) | 74 (10%) | ||
| Herpes simplex | 1 (<1%) | 4 (1%) | ||
| Herpes zoster | 1 (<1%) | 4 (1%) | ||
| Tuberculosis | 1 (<1%) | 0 | ||
| Opportunistic infections | 6 (1%) | 7 (1%) | ||
| Candida infection | 1 (<1%) | 0 | ||
| Eye infection, fungal | 0 | 1 (<1%) | ||
| Fungal retinitis | 1 (<1%) | 0 | ||
| Herpes zoster | 1 (<1%) | 3 (<1%) | ||
| Listeriosis | 0 | 1 (<1%) | ||
| Oropharyngeal candidiasis | 0 | 1 (<1%) | ||
| Pulmonary tuberculosis | 1 (<1%) | 0 | ||
| Systemic Candida | 2 (<1%) | 0 | ||
| Varicella zoster virus infection | 0 | 1 (<1%) | ||
| Venous thromboembolic event† | 20 (3%) | 19 (3%) | ||
| Deep vein thrombosis | 4 (1%) | 2 (<1%) | ||
| Pulmonary embolism | 13 (2%) | 9 (1%) | ||
| Other peripheral venous thrombosis | 8 (1%) | 10 (1%) | ||
| Major adverse cardiovascular event | 8 (1%) | 9 (1%) | ||
| Cardiovascular death | 1 (<1%) | 3 (<1%) | ||
| Myocardial infarction | 4 (1%) | 4 (1%) | ||
| Stroke | 4 (1%) | 4 (1%) | ||
| Gastrointestinal perforation | 0 | 0 | ||
Data are n (%). Data were assessed from days 1–28.
Included in overall mortality together with deaths due to disease progression.
Positively adjudicated by an independent external blinded clinical event committee.
Discussion
COV-BARRIER is the first international, multicentre, double-blind, randomised, placebo-controlled trial designed to evaluate the potential benefit and safety of baricitinib plus standard of care (which included systemic corticosteroids and remdesivir) for the treatment of hospitalised adults with COVID-19, and is the first to report 60-day outcomes in this population from a double-blind, randomised, controlled trial. This study addresses an important knowledge gap related to the optimisation of treatment strategies for hospitalised patients with COVID-19. In this study, baricitinib plus standard of care (including dexamethasone) did not significantly reduce progression to increased oxygen support or death (the composite primary endpoint) when compared with placebo plus standard of care. However, the group of patients allocated to receive baricitinib did show absolute risk reductions of 5 percentage points in all-cause mortality at 28 days and 4·9 percentage points in all-cause mortality at 60 days, resulting in a number-needed-to-treat of 20 to yield one additional survivor at these two timepoints.
We also report the largest set of randomised, placebo-controlled, safety data from hospitalised patients with COVID-19 treated with an immunomodulatory agent in addition to corticosteroids. The frequencies of treatment-emergent adverse events, serious adverse events, infections, and venous thromboembolic events were similar between the baricitinib and placebo groups, and no new safety signals were detected. Notably, despite COVID-19 being considered a risk factor for thrombosis, treatment with baricitinib was not associated with increased venous thromboembolic events in this setting of short-term use. Additionally, baricitinib in combination with standard of care (predominantly corticosteroids) was not associated with an increase in infections, including serious infections or opportunistic infections, in this hospitalised patient population. This dataset provides clinically relevant safety information for the acute care of these patients in the context of administration of baricitinib with concomitant corticosteroids.
During the COV-BARRIER study, the standard of care changed significantly to include routine use of corticosteroids, and guidelines28, 29 were updated following the disclosure of results from the open-label RECOVERY trial in June, 2020,30 in which a 10·9% relative reduction in mortality was observed with dexamethasone (mortality 22·9%) compared with standard of care alone (25·7%; age-adjusted rate ratio 0·83 [95% CI 0·75–0·93], p<0·001).7 By comparison, treatment with baricitinib reduced 28-day all-cause mortality by 38·2% compared with placebo (HR 0·57 [95% CI 0·41–0·78], nominal p=0·0018). The RECOVERY trial also showed a relative risk reduction of 11·4% in 28-day mortality with the anti-IL-6 receptor antibody tocilizumab (31%) versus standard of care (35%; HR 0·85 [95% CI 0·76–0·94], p=0·0028). This benefit was not maintained in the absence of corticosteroid use, but interpretation was limited by small numbers.8 In the ACTT-2 trial, the 28-day mortality was 5·1% with baricitinib plus remdesivir and 7·8% with remdesivir alone; however, the study was not powered to detect a difference in mortality between the groups and the use of corticosteroids was limited at baseline and during the trial.6 In COV-BARRIER, baricitinib plus standard of care showed a 38·2% relative reduction in 28-day mortality compared with placebo plus standard of care and most included patients were being treated with dexamethasone at baseline. Reduction in mortality was also found in our subpopulation of participants who, at baseline, required oxygen supplementation and were not receiving dexamethasone or systemic corticosteroids for the primary study condition. The JAK inhibitors ruxolitinib and tofacitinib have also been associated with reductions in mortality in small, single-country, multicentre, randomised controlled trials.31, 32 To our knowledge, baricitinib showed the largest effect size on mortality of any COVID-19 treatment reported in other randomised trials in hospitalised patients, and showed a benefit in addition to the use of standard of care (corticosteroids) alone.5, 6, 7, 8
The enrolment timeline of COV-BARRIER is also relevant considering the evolving standard of care and the heterogeneity of treatments across different geographical regions. All-cause mortality is the most relevant outcome in trials of patients hospitalised for COVID-19, and baricitinib plus standard of care showed a meaningful reduction in mortality compared with placebo plus standard of care, most notably for participants receiving high-flow oxygen or non-invasive ventilation.
Limitations of this study included the choice of disease progression, as measured by clinical status including oxygen support levels, as the primary outcome. As the primary endpoint did not achieve statistical significance, none of the key secondary endpoints were considered to have achieved statistical significance after adjusting for multiple comparisons. Nevertheless, both 28-day and 60-day all-cause mortality were highly significant on the basis of nominal p value assessments, which were derived directly from the statistical models without any adjustment for multiplicity. Measuring progression on the basis of NIAID-OS reflects treatment decisions (such as the use of specific oxygen delivery devices) and might be influenced by the heterogeneity of clinical practice across different geographical regions. Another reason why a statistically significant outcome was not found for the composite primary endpoint that included progression is that patients with COVID-19 can undergo very rapid deterioration. Nearly 22% of COV-BARRIER participants progressed on the first day. It is unlikely that a treatment would act so quickly as to meaningfully reduce progression to higher oxygen needs within the first 24 h of treatment. Although baricitinib showed a consistent reduction in progression versus placebo in the three components of the composite primary endpoint, the difference was not statistically significant. By contrast, death is an objective and definitive patient outcome that does not change across geographical regions. Baricitinib might prevent death without a significant difference in the primary endpoint because death as an outcome integrates the multiorgan effects of COVID-19, which include but are not limited to pulmonary effects. The anti-inflammatory effects of baricitinib probably affect organ systems that are not evaluated by the NIAID-OS.20 Additionally, the significantly lower mortality by day 60 in the baricitinib group versus the placebo group confirms that the reduction in mortality with baricitinib persists. Future research on baricitinib in patients with COVID-19 should include assessment of the effect of baricitinib at higher doses or with a loading dose to prevent progression events.33
In summary, our results suggest that baricitinib reduces 28-day and 60-day mortality when used in addition to the current standard of care. The safety profile was similar between the baricitinib group and the placebo group. As such, baricitinib plus standard of care could be a treatment option to reduce overall deaths in the context of the global burden of mortality during the COVID-19 pandemic.
This online publication has been corrected. The corrected version first appeared at thelancet.com/respiratory on September 8, 2021
Data sharing
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU or after the trial is completed, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents (including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms) will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Declaration of interests
VCM received research grants from the US Centers for Disease Control and Prevention (CDC), Gilead Sciences, the US National Institutes of Health (NIH), Veterans Affairs, and ViiV; received honoraria from Eli Lilly and Company; served as an advisory board member for Eli Lilly and Company and Novartis; and participated as a study section chair for the NIH. AVR received research grants from Eli Lilly and Company; and served as a speaker or consultant for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Roche, Sobi, and Union Chimique Belge. SB, CEK, VK, RL, MLBP, AC, SC, BC, PR, XZ, and DHA are employees and shareholders of Eli Lilly and Company. JDG received research support from Eli Lilly and Company, Regeneron Pharmaceuticals, and Gilead Sciences; grants from Eurofins Viracor and the Biomedical Advanced Research and Development Authority (administered by Merck); speaker fees from Eli Lilly and Company, Gilead Sciences, and Mylan Pharmaceuticals; and advisory board fees from Gilead Sciences. JAA served as a speaker and scientific advisor for AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly and Company, Foundation Medicine, Novartis, MSD, Roche, and Takeda. VE received a research grant from Eli Lilly and Company. MS received research grants from Eli Lilly and Company, NIAID, and Novartis; and served as a board member for NBOME, Osteopathic Founders Foundation, and COGMED. EWE received research grants from the CDC, NIH, and Veterans Affairs; and served as an unpaid consultant for Eli Lilly and Company. RDP declares no competing interests
Acknowledgments
Acknowledgments
This study was sponsored by Eli Lilly and Company, under license from Incyte Corporation. The authors would like to thank the participants and study investigators and staff, including the COV-BARRIER Study Group (appendix 6 pp 2–5) who participated in the study, and the following colleagues from Eli Lilly and Company: Douglas Schlichting (formerly with Eli Lilly and Company), Maher Issa, Jennifer L Milata, Theodore Spiro, and Zhongkai Wang for scientific input; Nicole Byers for medical writing support and manuscript preparation; Praveen Shetty for assistance with figure development; and Roisin McCarthy for assisting with manuscript process support. VM would like to thank his colleagues Christina Gavegnano, Raymond F Schinazi, and Boghuma Titanji (Emory University School of Medicine, Atlanta, GA, USA) for their expertise and support. VM and JG wish to dedicate this manuscript to Francisco Marty, who died this year and was an incredible champion of infectious diseases research and clinical care, with a substantial impact on COVID-19 treatment.
Contributors
All authors contributed to the concept and design of the trial, data analysis and interpretation, critical revision of the publication, and final approval to submit, and were accountable for the accuracy and integrity of the publication. CEK, RL, SC, and BC have accessed and verified the underlying data in this report.
Contributor Information
COV-BARRIER Study Group:
Mi-Young Ahn, Miriam Akasbi, Jorge Alatorre-Alexander, Javier David Altclas, Federico Ariel, Horacio Alberto Ariza, Chandrasekhar Atkar, Anselmo Bertetti, Meenakshi Bhattacharya, Maria Luisa Briones, Akshay Budhraja, Aaliya Burza, Adrian Camacho Ortiz, Roberto Caricchio, Marcelo Casas, Valeria Cevoli Recio, Won Suk Choi, Emilia Cohen, Angel Comulada-Rivera, Paul Cook, Dora Patricia Cornejo Juarez, Carnevali Daniel, Luiz Fernando Degrecci Relvas, Jose Guillermo Dominguez Cherit, Todd Ellerin, Dmitry Enikeev, Suzana Erico Tanni Minamoto, Vicente Estrada, Elie Fiss, Motohiko Furuichi, Kleber Giovanni Luz, Jason D. Goldman, Omar Gonzalez, Ivan Gordeev, Thomas Gruenewald, Victor Augusto Hamamoto Sato, Eun Young Heo, Jung Yeon Heo, Maria Hermida, Yuji Hirai, David Hutchinson, Claudio Iastrebner, Octavian Ioachimescu, Manish Jain, Maria Patelli Juliani Souza Lima, Akram Khan, Andreas E. Kremer, Thomas Lawrie, Mark MacElwee, Farah Madhani-Lovely, Vinay Malhotra, Michel Fernando Martínez Resendez, James McKinnell, Patrick Milligan, Cesar Minelli, Miguel Angel Moran Rodriguez, Maria Leonor Parody, Priscila Paulin, Rita de Cassia Pellegrini, Priscilla Pemu, Ana Carolina Procopio Carvalho, Massimo Puoti, Joshua Purow, Mayur Ramesh, Alvaro Rea Neto, Alvaro Rea Neto, Philip Robinson, Cristhieni Rodrigues, Gustavo Rojas Velasco, Jose Francisco Kerr Saraiva, Morton Scheinberg, Stefan Schreiber, Dario Scublinsky, Anete Sevciovic Grumach, Imad Shawa, Jesus Simon Campos, Nidhi Sofat, Mousumi Som, Christoph D. Spinner, Eduardo Sprinz, Roger Stienecker, Jose Suarez, Natsuo Tachikawa, Hasan Tahir, Brian Tiffany, Alexander Vishnevsky, Adilson Westheimer Cavalcante, and Kapil Zirpe
Supplementary Materials
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184:5298–5307. doi: 10.4049/jimmunol.0902819. [DOI] [PubMed] [Google Scholar]
- 10.Shi JG, Chen X, Lee F, et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol. 2014;54:1354–1361. doi: 10.1002/jcph.354. [DOI] [PubMed] [Google Scholar]
- 11.McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21:183. doi: 10.1186/s13075-019-1964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez GAM, Reinhardt A, Ramsey S, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. 2018;128:3041–3052. doi: 10.1172/JCI98814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y, Emoto K, Cai Z, et al. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: a 12-week, double-blind, randomized placebo-controlled study. J Rheumatol. 2016;43:504–511. doi: 10.3899/jrheum.150613. [DOI] [PubMed] [Google Scholar]
- 14.Dörner T, Tanaka Y, Petri MA, et al. Baricitinib-associated changes in global gene expression during a 24-week phase II clinical systemic lupus erythematosus trial implicates a mechanism of action through multiple immune-related pathways. Lupus Sci Med. 2020;7 doi: 10.1136/lupus-2020-000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stebbing J, Krishnan V, de Bono S, et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130:6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrone L, Petruccioli E, Alonzi T, et al. In-vitro evaluation of the immunomodulatory effects of Baricitinib: implication for COVID-19 therapy. J Infect. 2021;82:58–66. doi: 10.1016/j.jinf.2021.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sims JT, Krishnan V, Chang CY, et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol. 2021;147:107–111. doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titanji BK, Farley MM, Mehta A, et al. Use of baricitinib in patients with moderate to severe coronavirus disease 2019. Clin Infect Dis. 2021;72:1247–1250. doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stebbing J, Sánchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7 doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Food & Drug Administration Letter of authorization: EUA for baricitinib (Olumiant) for treatment of coronavirus disease 2019 (COVID-19) https://www.fda.gov/media/143822/download
- 26.Goletti D, Cantini F. Baricitinib therapy in COVID-19 pneumonia—an unmet need fulfilled. N Engl J Med. 2021;384:867–869. doi: 10.1056/NEJMe2034982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao P, Ware JH, Mehta C. Sample size re-estimation for adaptive sequential design in clinical trials. J Biopharm Stat. 2008;18:1184–1196. doi: 10.1080/10543400802369053. [DOI] [PubMed] [Google Scholar]
- 28.WHO COVID-19 clinical management: living guidance. Jan 25, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
- 29.National Institutes of Health Coronavirus disease 2019 (COVID-19) treatment guidelines. April 8, 2021. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 30.Horby P, Lim WS, Emberson JR, et al. Effect of dexamethasone in hospitalized patients with COVID-19—preliminary report. medRxiv. 2020 doi: 10.1101/2020.06.22.20137273. published online June 22. (preprint). [DOI] [Google Scholar]
- 31.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137. doi: 10.1016/j.jaci.2020.05.019. 46.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;385:406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasan MJ, Rabbani R, Anam AM, et al. Impact of high dose of baricitinib in severe COVID-19 pneumonia: a prospective cohort study in Bangladesh. BMC Infect Dis. 2021;21:427. doi: 10.1186/s12879-021-06119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU or after the trial is completed, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents (including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms) will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.