Abstract
Background
Progressive difficulties with spoken language occur across the spectrum of degenerative dementia. When not a primary presenting and dominant symptom, language difficulties may be overlooked in favor of more prominent cognitive, behavior, or motor deficits. The aim of this scoping review is to examine the extent and nature of the research evidence describing (1) the spoken language impairments found in non‐language led dementias, (2) their impact on everyday living, and (3) the reported language interventions.
Methods
We searched PubMed, MEDLINE, OVID‐EMBASE, PsycINFO, and SpeechBITE using terms related to spoken language for the following dementia types: Parkinson's disease dementia (PDD), dementia with Lewy bodies (DLB), progressive supranuclear palsy (PSP), cortico‐basal syndrome (CBS), behavior variant frontotemporal dementia (bvFTD), early‐onset Alzheimer's disease (EOAD), posterior cortical atrophy (PCA), and motor neuron disease associated with FTD (MND+FTD). Risk of bias was assessed with the QualSyst tool.
Results
Seventy‐three eligible studies were included. A wide range of spoken language impairments were reported, involving both linguistic (e.g., syntactic processing) and other cognitive (e.g., sustained attention) underlying mechanisms. Although the severity of these deficits was scarcely reported, in some cases they manifested as non‐fluent, dynamic, and global aphasias. No papers in the review described either the impact of these language impairments on everyday living or language therapies to treat them.
Discussion
There is a need to understand better the level of disability produced by language impairment in people living with non–language‐led dementias. Our findings suggest three calls for action: (1) research studies should assess the clinical relevance of any spoken language deficits examined, (2) both linguistic and cognitive underlying mechanisms should be fully described (to inform the design of effective language and behavioral interventions), and (3) trials of language therapy should be conducted in those groups of individuals where significant language impairment is proved.
Keywords: aphasia, communication disorders, dementia, language therapy, spoken language
1. INTRODUCTION
The primary progressive aphasias (PPA) are a syndromic family of disorders that share the presence of language impairment as the first and most salient feature of the clinical picture.1 Currently, there are three PPA variants identified: a semantic variant characterized by fluent speech with semantic breakdown; a non‐fluent/agrammatical variant, characterized by non‐fluent speech with agrammatism and/or apraxia of speech; and a logopenic variant, characterized by word‐finding pauses and impaired sentence repetition due to phonological working memory deficits.1 This taxonomy successfully classifies more than half of all PPA cases, but up to 41% of them show overlapping features that do not fit within the three canonical phenotypes.2 Despite these difficulties in subtype classification, the recognition of progressive aphasias has been an important step in raising awareness of non–memory‐related disabilities associated with dementia. This awareness has contributed to the growth of a new area of research focused on the development of interventions to improve expressive and receptive language abilities in people living with PPA (see Cotelli et al., for a review and meta‐analysis of the 10 last years of research in the field3).
While a significant step forward, it is crucial to recognize that progressive spoken language difficulties also occur in other neurodegenerative dementia, such as early‐onset typical Alzheimer's disease (EOAD),4 Parkinson's disease dementia (PDD),5 dementia with Lewy bodies (DLB),6 posterior cortical atrophy (PCA),7 behavior variant frontotemporal dementia (bvFTD), and frontotemporal dementia with motor neuron disease (FTD+MND),8 as well as the atypical parkinsonian syndromes of progressive supranuclear palsy (PSP)9 and cortico‐basal syndrome (CBS).10 There is a current lack of systematic investigation of the language deficits in these patient groups. In this scoping review we examined and mapped the literature concerning progressive spoken language impairments in degenerative dementias wherein language is not the primary and most prominent symptom (i.e., excluding PPA syndromes). In this review we focused on spoken language rather than writing and reading because this is the most clinically salient feature, and the most commonly used in assessment of and communication with people living with dementia. The language profile (spoken and otherwise) of typical, late‐onset Alzheimer's disease is characterized by anomia (with well‐defined aphasia emerging only in late stages) and has already been well reported in the literature so it was excluded from this review.11 Studies looking at vascular dementia were also excluded due to the lack of definitional clarity and consequent heterogeneity (due to variation in lesion type and location) within this clinical identity.12
Using a scoping review methodology, we aimed to draw a map of the existing evidence regarding three specific questions: (1) What are the currently reported spoken language impairments in non–language‐led dementias? (2) What is the clinical significance of these impairments? That is, the impact on quality of life and/or activities of daily living, and (3) What are the reported language‐based interventions for people with non–language‐led dementias?
2. METHODS AND DESIGN
A protocol of this scoping review can be found in a previous publication.13 The protocol followed the Preferred Reporting Items for Systematic Reviews and Meta‐analysis Protocols (PRISMA)‐ScR guidelines.14, 15 Using the general framework outlined by Arksey and O'Malley,16 we systematically scoped the literature about language in people with common forms of dementia (excluding PPA): EOAD, PCA, PDD, DLB, bvFTD, FTD+MND (used to denote both FTD‐MND and MND‐FTD), PSP, and CBS.
2.1. Selection of studies
Studies that assess and/or treat spoken language difficulties in the target population were included. The following exclusion criteria applied:
Studies focused on late‐onset Alzheimer's disease (AD), vascular dementia, mild cognitive impairment (MCI), or a diagnosis of a non‐neurodegenerative disease.
Studies in which information regarding the nature of the language impairment was not provided or there was insufficient description of the sample, for example, lack of clarity regarding diagnostic category.
Studies that examined only writing and/or reading.
Studies that assessed motor difficulties only (i.e., motor speech, voice, prosody, dysarthria, and/or oral apraxia).
Articles for which the full text was not readily available or was not written in English.
Studies where no original findings were presented (e.g., reviews/editorials/letters to the editor).
Non‐peer reviewed material.
Conference abstracts.
Errata/correction of no significance to required data.
Gray literature.
2.2. Information sources, search, and identification of evidence
The search was conducted on PubMed, MEDLINE, OVID‐EMBASE, PsycINFO, and SpeechBITE with an unlimited starting date, up until July 2019. These databases were selected as the most relevant to the topic of the review. The literature included was indexed and written in English. Database‐specific conventions and use of multiple search fields and filters were customized for individual databases. Reference lists from key articles and reviews were scrutinized and relevant articles included. The search terms were developed in consultation with experienced researchers and librarians and piloted before conducting the actual search.
The specific electronic search strategy can be found in the study protocol published by Savage et al.13 The search for PubMed comprised the following terms: (Early‐onset Alzheimer's disease OR early onset Alzheimer's disease OR young‐onset Alzheimer's disease OR young onset Alzheimer's disease OR Parkinson* dementia OR Parkinson* disease dementia OR dementia with Lewy bodies OR Lewy body dementia OR Posterior Cortical Atrophy OR frontotemporal dementia OR Pick's disease OR Progressive Supranuclear Palsy OR Cortico‐basal Syndrome OR cortico‐basal degeneration OR corticobasal degeneration OR motor neuron disease OR FTD‐MND OR MND‐FTD OR ALS‐FTD OR FTD‐ALS) AND (language impairment OR communication disorder OR aphasia).
All references retrieved were exported into EndNote software version X9, with duplicates removed via the “Find duplicates” function and through visual inspection of title and author. The screening and selection of the candidate articles was conducted using a multi‐level title‐first method.17 The primary reviewer (ASG) independently inspected all the citations, while reviewers two and three (AC and RG) independently inspected 50% of the citations each, so that every item was considered by at least two independent reviewers. Screening took place first by title, and then by abstract. Any differences in the agreement were discussed. A 10% sample of the full‐text articles selected by the primary reviewer were also screened by reviewers two and three to ensure reliability.
2.3. Data charting and critical appraisal
Information extracted from each article was recorded in a tailored data‐charting form by ASG and discussed with SS. Information items included the study design, diagnoses, reported language impairments and their severity (e.g., as measured by standardized tools or clinician's judgment), clinical significance (i.e., measures of quality of life or impact of aphasia on everyday living), and language‐based interventions. Risk of bias within the reported studies was evaluated using the Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields (QualSyst)18 to assist in determining the feasibility of a future systematic review. A score of 75 in QualSyst is the threshold for a paper to meet quality criteria to be included in a review.19
RESEARCH IN CONTEXT
Systematic Review: We reviewed the literature regarding spoken language impairments in eight non‐language led dementias (i.e., excluding primary progressive aphasias [PPA]) using PubMed, MEDLINE, OVID‐EMBASE, PsycINFO, and SpeechBITE.
Interpretation: Results showed a wide range of deficits reported in non‐PPA dementias, including difficulties in single‐word processing, sentence processing, and narrative building. In some cases, very severe impairments were reported (e.g., global aphasia). Little mention was found, however, regarding the clinical significance of such deficits or of any behavioral or language therapies to treat them.
Future Directions: Our findings highlight the need for future studies to examine how the language deficits impact everyday functioning and quality of life. In addition, to guide effective development of interventions, there is a need for improved understanding of the underlying linguistic and cognitive mechanisms. Importantly, trials of language therapy should be initiated in those groups of individuals in which a significant language impairment is present.
2.4. Synthesis of results
Studies were grouped by phenotype and summarized in Tables 1 and 2.
TABLE 1.
Characteristics of papers included
| Author | Design | Sample | Language impairments found | Associated mechanisms | Severity of language impairment | Impact of impairments | Intervention study? | QualSyst (%) |
|---|---|---|---|---|---|---|---|---|
| Parkinson disease dementia (PDD) | ||||||||
| Cummings et al. (1988) | Case‐control | Sixteen people with PDD (compared to 35 PD without dementia) | Reduced phrase length, information content, phrase repetition, comprehension, and naming (compared to PD without dementia) | NR | NR | NR | NO | 60 |
| Piat et al. (1999) | Case‐control | Twenty people with PDD (compared to 59 healthy control and 57 with PD) | PDD impaired in fluency tasks (particularly action naming) | NR | NR | NR | NO | 93 |
| Ash et al. (2017) | Case‐control | Three PDD/five DLB (among a group of 23 people with PDD) | Slower speech rate, higher mean of length of utterance and poorer report of content | No relationship found between language function and changes in overall cognitive or motor function | NR | NR | NO | 54 |
| Frank et al. (1996) | Case‐control | Twelve PDD (part of a group of 42 individuals with dementia due to AD, HD, and PD and age‐matched controls) | Decline in confrontation naming in moderate dementia PD group | Naming may be reduced due to reduced access to semantic referents | NR | NR | NO | 45 |
| Magdalinou et al. (2018) | Case‐control | Eighteen people living with PDD (and seven PSP, four CBS) | Reduced verbal fluency and sentence generation (in the three patient groups) | No associated language deficits: executive function performance appeared to play a role; authors suggest a specific effect on verbal output of dopaminergic stimulation | NR | NR | NO | 66 |
| Azuma et al. (1997) | Case‐control | Fifteen PDD (also 99 PD and 46 healthy controls) | Reduced semantic fluency; some reduction in letter fluency and fluency for proper names in the PDD group | NR | NR | NR | NO | 69 |
| Bayles et al. (1993) | Case‐control | Twenty people with PDD (among a group of 88 with PD, 21 AD, and 43 healthy controls) | Reduced semantic and letter fluency in the PDD group (scoring similar to the AD group) | NR | NR | NR | NO | 83 |
| Lewis et al. (1998) | Case‐control | Eight PDD (among a group of 20 PD) | Reduced naming, word definition, ability to interpret ambiguity and figurative language, sentence construction, and semantic verbal fluency compared to PD and normal score in cognitive functioning | NR | NR | NR | NO | 83 |
| Suhr et al. (1998) | Case‐control | Twenty‐six PDD with mild dementia (compared to 31 AD and 14 HD) | Reduced semantic and verbal fluency; less repetition errors in PDD group | NR | NR | NR | NO | 83 |
| Dementia with Lewy bodies/PDD | ||||||||
| Ash et al. (2012) | Case‐control | Fourteen DLB/PDD (out of a group including 21 patients with PD and 21 healthy controls) | Speech rate reduced (wpm) in half compared to seniors and significantly reduced compared to PD. More silent pauses and difficulties in grammatical production | Reduced speech rate correlated with measures of between‐utterance pauses, executive function and grammatical comprehension (frontal lobe mediated) | NR | NR | NO | 80 |
| Ash et al. (2012)b | Case‐control | Twenty‐nine DLB/PDD (compared to 26 healthy controls) | Deficit in both narrative comprehension and narrative expression | Deficits partially due to material‐neutral deficit in organizational executive resources (correlated with prefrontal cortical atrophy) | NR | NR | NO | 79 |
| Ash et al. (2011) | Case‐control | Fourteen DLB/PDD (compared to 18 PD) | Significant narrative production deficit: impairment in connecting one scene to the next and poor ability to maintain the theme | Deficit related to impairment on measures of executive function and speech fluency | NR | NR | NO | 73 |
| Grossman et al. (2012) | Case‐control | Seventeen DLB/PDD (compared to 26 PD and 19 healthy controls) | Sentence processing deficit for syntactic ambiguities | Deficit correlates with impairment in working memory | NR | NR | NO | 73 |
| Gross et al. (2012) | Case‐control | Eight PDD/nine DLB (compared to 16 PD) | Impaired sentence processing | Increased difficulty processing sentences with increase working memory demands | NR | NR | NO | 73 |
| Gross et al. (2013) | Case‐control | Twelve DLB/PDD (compared to 30 PD and 12 healthy controls) | Impaired script comprehension | Deficits associated to executive impairment | NR | NR | NO | 73 |
| Grossman et al. (2017) | Case‐control | Fourteen PDD/12 DLB (compared to 30 PD) | Difficulty appreciating narrative organization | Associated with frontal grey matter atrophy | NR | NR | NO | 67 |
| Atypical parkinsonisms PSP and CBS | ||||||||
| Santos‐Santos et al. (2016) | Cohort (longitudinal) | Five PSP/nine CBS (and 10 controls) | Both groups showed impairment in verbal fluency, naming and sentence comprehension; fewer words per minute; greater syntactical errors; reduced proportion of words in sentences. CBS but not PSP produced fewer narrative words than controls. Worsening in longitudinal assessment without new differences between groups | Phonetic and syntactic levels affected | NR | NR | NO | 100 |
| Rosser and Hodges (1994) | Case‐control | Ten PSP (and 10 with AD, 10 with HD) | Impaired fluency: letter fluency more impaired than category fluency | Poorer performances in the PSP group were associated to initiation and retrieval problems secondary to disruption in frontostriatal circuits | NR | NR | NO | 79 |
| Lebrun (1986) | Case study | One PSP | Reduced fluency (apart from voice and articulation deficits) | NR | NR | NR | NO | 100 |
| Podoll et al. (1991) | Cross‐sectional | Six PSP | High rate of self‐correction and errors in object naming (errors referring to visually similar objects) | Visual processing defect/misperception likely related to naming errors as no word‐finding difficulty was evident (indicated by word‐finding pauses, or semantic paraphasias) | NR | NR | NO | 83 |
| Robinson et al. (2006) | Case report | One PSP | Dynamic aphasia. Propositional language impaired (preserved naming, repetition and comprehension) | Impairment interpreted as being underpinned by a deficit in the generation of a fluent sequence of novel thought in discourse generation | NR | NR | NO | 70 |
| Barker et al. (2018) | Case‐control | Five PSP (compared to a group of 30 controls, three AD and two svFTD) | Four PSP showed initial periods of responding followed by periods of poorer performance for both tasks of verbal fluency and spontaneous speech | Decreased energization, defined as the attentional process of initiating and sustaining a response over time | NR | NR | NO | 100 |
| Robinson et al. (2015) | Case report experimental | One PSP | Dynamic aphasia | Aphasia not underpinned by a language‐specific deficit in selection or planning, but by a domain‐general deficit in fluent sequencing of novel thoughts | NR | NR | NO | 100 |
| Boeve et al. (2003) | Case report | One PSP | Initial anomia, paragrammatism, verbal hesitancy and slowly produced speech that evolved to full non‐fluent aphasia over time | NR | NR | NR | NO | 100 |
| Esmonde et al. (1996) | Case series | Three PSP | Dynamic aphasia | Impairment in planning and initiating language output; impairment in letter and category fluency pointed toward a deficit in initiation and retrieval processes | NR | NR | NO | 100 |
| Roher et al. (2010) | Cross‐sectional | Four PSP | Non‐fluent aphasia characterized by marked reduction in propositional speech, fewer speech errors | NR | NR | NR | NO | 75 |
| Daniele et al. (2013) | Case‐control | Ten PSP (and 10 healthy controls) | Poorer verbs/actions compared to nouns/objects in several lexical‐semantic tasks: confrontation naming, auditory and visual word‐picture matching | Deficits in the comprehension of action‐verbs from a specific semantic impairment in the category of actions | NR | NR | NO | 100 |
| Josephs et al. (2005) | case series | Four PSP with apraxia of speech | C1–Comprehension: mildly impaired. Expression: poor letter fluency. Agrammatism and semantic errors (non‐fluent) C2–Expression: poor letter fluency (only participant with no obvious aphasia) C3–Expression: poor letter fluency. Agrammatism and semantic errors (non‐fluent) C4–Expression: poor letter fluency. Paragrammatism (anomic aphasia with paragramatic errors) | NR | NR | NR | NO | 100 |
| Burrell et al. (2018) | Case‐control | Twenty‐two PSP (compared to 29 PNFA and 93 healthy controls) | Language impairment was similar in PSP than in patients with non‐fluent progressive aphasia (naming, word comprehension, semantic association and syntactic comprehension) | No significant correlation between performance on the SYDBAT subtests and performance on executive or working memory tasks, suggesting not due to executive dysfunction | SYDBAT: PSP performing similar to PNFA in naming, comprehension and semantic association domains and better in repetition | NR | NO | 100 |
| Catricala et al. (2019) | Case‐control | Seventeen PSP with movement disorder presentation (compared to 21 PD and 27 healthy controls) | More than 50% showed impairment in picture naming, semantic fluency, and sentence and single word comprehension. More than 40% of PSP showed impaired non‐word repetition | Affected linguistic levels identified were phonological, lexico‐semantic, and discourse‐pragmatic | NR | NR | NO | 100 |
| Brito‐Marques et al. (2011) | case report | One CBS | Difficulties for verbal and semantic fluency | NR | NR | NR | NO | 100 |
| Troiani et al. (2011) | Case‐control | Eleven CBS (among 32 with neurodegenerative diseases and 14 healthy controls) | Impaired verbal comprehension of quantifiers | Not attributable to visuospatial processing impairment or a primary language deficit but to a specific quantifier comprehension deficit related to damage of the parietal cortex | NR | NR | NO | 73 |
| Graham et al. (2003) | Cross‐sectional | Ten CBS | Prevalent phonologic and spelling impairments (a minority of patients also showed semantic memory and naming deficits) | NR | NR | NR | NO | 100 |
| Ferrer et al. (2003) | Cross‐sectional | Three CBS | C2–loss of fluency and anomia and deficits in comprehension of complex orders C3–no description of the characteristics of language disorder C4–no description of the characteristics of language disorder | NR | NR | NR | NO | 100 |
| McMillan et al. (2006) | Case‐control | Sixteen CBS (also 23 FTD, 25 AD, and 17 healthy controls) | Quantifier comprehension is impaired in CBS, particularly for first order quantifiers (e.g., at least three flowers) | Comprehension impairment at least partially due to number knowledge impairment in CBS (not to language impairment in the verbal representation of the number or object knowledge) and partially due to working memory deficits | NR | NR | NO | 70 |
| Morgan et al. (2011) | Case‐control | Thirteen CBS, three PCA, 14 bvFTD | People with CBS and PCA showed impairment in comprehension of cardinal quantifiers. bvFTD patients showed impaired in their comprehension of logical quantifiers | CBS and PCA impairments were partially due to deficits in quantity knowledge; bvFTD impairments correlated with deficits in executive measures | NR | NR | NO | 84 |
| Levin et al. (2015) | Cross‐sectional | Thirty‐eight CBS | Sixteen with aphasia | NR | NR | NR | NO | 91 |
| Donovan et al. (2007) | Case report | One CBS | Mild aphasia with abnormal social language usage: lack of awareness of the parameters of conversational turn taking, topic cohesion, and listener feedback | The reported frontal executive dysfunction described in CBS is suggested as the underlying cause of this abnormal language usage | WAB: 89 (mild aphasia) | NR | NO | 80 |
| McMonagle et al. (2006) | Case‐control | Fifty‐five CBS: 19 with motor onset and 36 with cognitive/behavioral onset CBS (PPA and AD group control) | Aphasia | NR | WAB: 88 (mild aphasia) in the motor onset group. WAB: 66 (moderate aphasia) in the cognitive onset group | NR | NO | 91 |
| Takao et al. (2006) | Case report | One CBS | Non‐fluent aphasia | NR | NR | NR | NO | 80 |
| Gross et al. (2010) | Case‐control | Twenty CBS (eight healthy controls) | Impaired discourse, with deficits in narrative theme, global connectedness (impaired in 16/20 subjects with CBS) and reductions in local connectedness | Discourse impairment was related to higher‐order integration of visual material (the task was based on a picture story) but not basic visuoperceptual, language, or memory functions | NR | NR | NO | 80 |
| Tree & Kay (2008) | Case‐report longitudinal | One CBS | Non‐fluent aphasia | NR | NR | NR | NO | 80 |
| Kertesz et al. (2000) | Cohort (longitudinal) | Thirty‐five CBS (15 presented with motor symptoms and 20 with cognitive onset) | Twenty‐one with aphasia | NR | Measured with the WAB. The aphasia quotient ranged from 28 to 99 in the group presenting with cognitive disorders and 59 to 99 in the group presenting with movement disorders | NR | NO | 100 |
| Grossman et al. (2004) | Case‐control | Nine CBS | Naming deficits | Confrontation naming deficits correlate with lexical retrieval and also with visual‐spatial function | NR | NR | NO | 76 |
| Behavioral variant FTD | ||||||||
| Ash et al. (2019) | Case‐control | Fourteen people with bvFTD | Baseline: reduced fluency (reduced wpm but nearly free of speech errors); follow‐up: impaired dependent clause production and production of well‐formed sentences, slower speech that became simplified with disease progression | NR | NR | NR | NO | 80 |
| Cotelli et al. (2006) | Case‐control | Sixteen bvFTD, 10 PSP, 10 CBS | Action naming more impaired than object naming for all groups. The discrepancy was larger in PSP and CBS | Suggestive of involvement of action knowledge and action representations | NR | NR | NO | 69 |
| Davis et al. (2010) | Case‐control | Twenty‐two bvFTD (among a group of 20 healthy controls, 144 NPH and 15 PNFA). BvFTD and PNFA groups were analyzed together | bvFTD/PNFA showed impairment in animal and action naming fluency | NR | NR | NR | NO | 73 |
| Pakhomov et al. (2010) | Case‐control | bvFTD (N not specified; among a total of 38 with PNFA, svFTD, and lvPPA) | Impaired naming and fluency | NR | NR | NR | NO | 69 |
| Saxon et al. (2017) | Retrospective case control study | 185 people with bvFTD and 56 ALS‐FTD | Agrammatism and impaired sentences comprehension, naming, word finding difficulties in conversation, impaired comprehension and repetition, word repetition, presence of phonological errors, agrammatism, reduced speech output, verbal perseveration and echolalia | NR | NR | NR | NO | 83 |
| Snowden et al. (2019) | Case‐control | Seventy‐one bvFTD (compared to 32 svFTD) | Around 50% bvFTD showed impaired naming and 17% impaired word‐picture matching | Compared possible semantic deficit versus executive contribution as basis for naming difficulties in bvFTD. Findings support the view that anomia can arise independently of executive impairment | NR | NR | NO | 100 |
| Yunusova et al. (2015) | Case‐control | Nine bvFTD (among a group of 33 healthy controls, 85 people with ALS and nine PNFA) | Although total pause time and number of pauses were elevated when reading out loud in bvFTD, phrase duration was normal in this group | Reference to pauses being longer because of cognitive‐language impairments | NR | NR | NO | 69 |
| d'Honincthun & Pillon (2008) | Case‐report | One bvFTD | Verb naming impaired when assessed using static depictions of actions but normal when using videotaped actions or verbal stimuli | Authors make a call for caution about stimuli selection for studies | NR | NR | NO | 91 |
| Hardy et al. (2015) | Case‐control | Twenty‐four bvFTD (compared to 14 svFTD and 18 nfvPPA and 24 healthy individuals) | Deficits in noun and verb naming and single word comprehension and diminished propositional speech | Isolated linguistic components, like verbal semantics were identified (authors corrected by nonverbal executive performance) | NR | NR | NO | 69 |
| Silvery et al. (2003) | Case‐control | Seventeen bvFTD (compared to 42 AD | Impaired action naming | Noun and verb naming and the gap in between showed correlation with executive tasks | NR | NR | NO | 92 |
| Peelle et al. (2008) | Case‐control | Thirty‐two SOC/EXEC (compared to 28 PNFA and 28 svFTD) | 28% of SOC/EXEC impaired in grammatically complex sentence comprehension | Deficits were attributable to decline in executive cognitive resources | NR | NR | NO | 62 |
| Grossman et al. (2005) | Case‐control | Eight FTD with executive deficits (compared to five PNFA and three svFTD) | Sentence comprehension difficulties | Correlation with measures of working memory, planning, and inhibition control; speculation that a material‐neutral executive resources deficit not dedicated to grammatical processing may play a role in the sentence comprehension deficit | NR | NR | NO | 62 |
| Peelle et al. (2007) | Case‐control | Seven SOC/EXEC (six people with PNFA and 20 healthy controls) | Difficulties processing sentences–partial sensitivity to grammatical errors but insensitivity to thematic violations | Results were consistent with impairment in the formation of a coherent thematic matrix | NR | NR | NO | 83 |
| Ash et al. (2006) | Case‐control | Twelve people with FTD SOC/EXEC | Profound difficulty organizing narratives: impaired WPM, accuracy of reported information, global connectedness, and maintenance of theme | Correlated with poor performances on measures of executive resources requiring and organized mental speech | NR | NR | NO | 77 |
| Cotelli et al. (2007) | Case‐control | Nine bvFTD, 15 PSP, 11 CBS (compared to 4 svFTD, 10 AD, and 10 healthy controls) | Syntactic knowledge impairment found in CBS but not PSP or bvFTD | NR | Performed within normal range in Aachener Aphasia Test | NR | NO | 75 |
| Early onset AD | ||||||||
| Imamura et la. (1998) | Cross‐sectional | N: 150 AD (using age at onset as a continuous variable) | EOAD worse on word comprehension and sequential commands and rapid decline of naming ability | NR | Measured with WAB. The earlier the age at onset the lower the AQ on the WAB | NR | NO | 83 |
| Sa et al. (2012) | Case‐control | 109 EOAD (compared to 171 LOAD) | No language impairment described | NR | NR | NR | NO | 72 |
| Borges et l. (2018) | Case‐report | One person with EOAD | Marked difficulties in comprehension and sentence repetition that progressed to global aphasia | NR | Global aphasia 3 years after symptoms onset | NR | NO | 30 |
| Selnes et la. (1988) | Case‐control | Sixty‐one EOAD (compared to 72 LOAD) | No language impairment and no differences in language dysfunction between groups found | NR | NR | NR | NO | 77 |
| Posterior cortical atrophy | ||||||||
| Fitzpatrick et al. (2019) | Case report | One PCA | Logopenic aphasia | NR | NR | NR | NO | 40 |
| Steeb et al. (2018) | Case‐report longitudinal | One PCA | Baseline: impaired fluency for nouns; follow‐up: impaired fluency for nouns and verbs | NR | NR | NR | NO | 80 |
| Shebani et al. (2017) | Case‐control | Ten PCA (compared to one svFTD) | Greater deficits on word processing than for spatial prepositions in PCA (e.g., under, through) | NR | NR | NR | NO | 75 |
| Crutch et al. (2013) | Case‐control | 15 PCA (compared to 7 LPA and 18 healthy individuals) | Impairment across all language domains, more prominent for anomia, reduced fluency and speech rate. PCA performed better than LPA on tasks of comprehension and spontaneous speech | NR | NR | NR | NO | 69 |
| Magnin et al. (2012) | Case‐control | Nine PCA | 8/9 PCA participants showed a logopenic profile, with anomia, reductions in fluency, and a length‐dependent deficit | NR | NR | NR | NO | 83 |
| Suárez‐González et al. (2019) | Case‐control | Eight PCA (compared to 21 AD and 18 healthy controls) | Impaired comprehension of measurement units (e.g., grams) | NR | NR | NR | NO | 69 |
| Motor neuron disease with FTD | ||||||||
| Bak et al. (2001) | Cross‐sectional | 2/6 MND‐dementia‐aphasia | In one case, severe reduction in spontaneous speech, semantic paraphasia, communicating in a “telegraphic” way. The other case shows echolalia and naming inaccuracies. | NR | NR | NR | NO | 73 |
| Rakowic & Hodges (1998) | Case‐control | Three MND‐FTD (compared to two MND with aphasia and 13 MND) | Only 2/3 patients were formally assessed–deficits on verbal fluency, picture naming, word‐picture matching, semantics, and grammar were found | NR | NR | NR | NO | 79 |
| Bak & Hodges (2004) | Cross‐sectional | Seven MND‐dementia | Poverty of spontaneous speech, severe impairment on syntactic comprehension, a consistently large impairment noticed for verbs (both naming and comprehension) | NR | described as severe | NR | NO | 81 |
| Kamminga et al. (2016) | Case‐control | Fifteen FTD‐ALS (compared to 20 ALS, 27 PNFA, and 23 controls) | Impaired syntactic comprehension | NR | NR | NR | NO | 79 |
Abbreviations: AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; AQ, aphasia quotient; bvFTD, behavioral variant frontotemporal dementia; CBS, corticobasal syndrome; DLB, dementia with Lewy bodies; EOAD, early‐onset Alzheimer's disease; FTD, frontotemporal dementia; HD, Huntington's disease; LOAD, late‐onset Alzheimer's disease; LPA, logopenic aphasia; lvPPA, language variant primary progressive aphasia; MND, motor neuron disease; nfvPPA, non‐fluent variant of primary progressive aphasia; NPH, normal pressure hydrocephalus; NO, xxxxxxxxx; NR, no reported; PCA, posterior cortical atrophy; PDD, Parkinson's disease dementia; PNFA, progressive non‐fluent aphasia; PPA, primary progressive aphasia; PSP, progressive supranuclear palsy; QualSyst, Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; SOC/EXEC, disorder of social comportment and executive processing (this is the term used by the authors of the corresponding papers); svFTD, semantic variant frontotemporal dementia; SYDBAT, Sydney Language Battery; WAB, Western Aphasia Battery; WPM, words per minute.
TABLE 2.
Summary of language impairments reported on each patient subgroup
| Language impairment described | PDD (N = 16) | DLB (N = 8) | PSP (N = 17) | CBS (N = 18) | bvFTD (N = 16) | EOAD (N = 4) | PCA (N = 7) | FTD‐MND (N = 5) |
|---|---|---|---|---|---|---|---|---|
| Single word processing | ||||||||
| Naming | 3 (18%) | – | 4 (23%) | 7 (38%) | 9 (56%) | 1 (25%) | 3 (42%) | 2 (40%) |
| Verbal fluency | 6 (37%) | – | 6 (35%) | 6 (33%) | 1 (6%) | – | 4 (57%) | 1 (20%) |
| Verb and/or noun processing | 1 (6%) | – | 2 (11%) | 1 (5%) | 4 (25%) | – | 1 (14%) | 1 (20%) |
| Semantic processinga | 2 (12%) | – | – | 3 (16%) | 1 (6%) | – | 3 (42%) | 1 (20%) |
| Sentence level processing and narrative | ||||||||
| Syntactic processing | 3 (18%) | 2 (25%) | 4 (23%) | 1 (5%) | 3 (18%) | – | – | 3 (60%) |
| Verbal expressive languageb | 13 (81%) | 8 (100%) | 3 (17%) | 1 (5%) | 3 (18%) | 1 (25%) | 3 (42%) | 2 (40%) |
| Auditory comprehension | 4 (25%) | 5 (62%) | 4 (23%) | 5 (27%) | 6 (37%) | 1 (25%) | 3 (42%) | 1 (20%) |
| Aphasia syndromes | ||||||||
| Aphasia (as reported by authors)c | – | – | 3 (17%) | 4 (22%) | 1 (25%) | – | – | |
| Profile compatible with non‐fluent/agrammatic aphasia | – | – | 3 (17%) | 2 (11%) | – | – | – | |
| Profile compatible with logopenic aphasia | – | – | 3 (42%) | – |
Abbreviations: bvFTD, behavioral variant frontotemporal dementia; CBS, corticobasal syndrome; DLB, dementia with Lewy bodies; EOAD, early‐onset Alzheimer's disease; FTD, frontotemporal dementia; MND, motor neuron disease; N, total number of papers; PCA, posterior cortical atrophy; PDD, Parkinson's disease dementia; PSP, progressive supranuclear palsy.
Concepts mostly related to quantity and/or space.
For example, pauses, phrase length, ability to maintain topic, building narrative, etc.
For example, dynamic, global, or non‐specified aphasia.
3. RESULTS
Of the 4672 records identified, 196 full‐text articles were assessed, with 73 of them meeting inclusion criteria (see Figure 1 for PRISMA flowchart of study selection).
FIGURE 1.
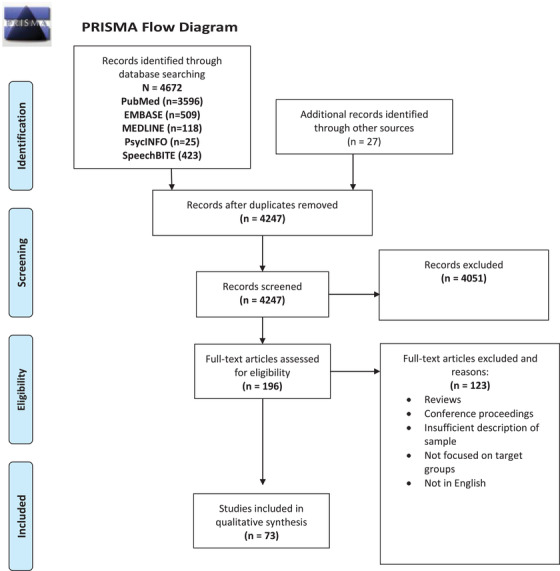
PRISMA flow diagram
3.1. Characteristics of sources of evidence
An overview of the distribution of studies according to design and quality is shown in Figure 2. Table 1 illustrates the characteristics of the studies, including the critical appraisal score produced by QualSyst with 64% of studies scoring 75 and above (good quality).
FIGURE 2.
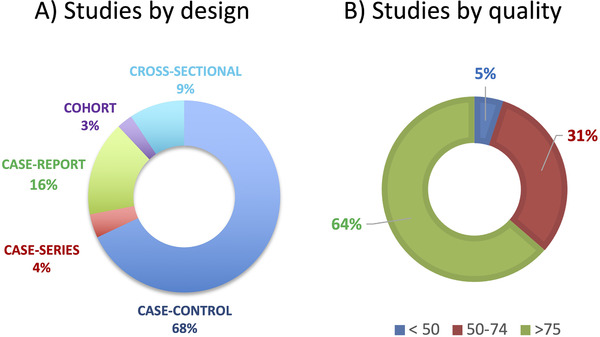
Overview of the distribution of studies according to design and quality. A, Studies by design. Case‐report are considered studies where n = 1; case series are studies in which the paper contains multiple case reports; cohort studies were considered longitudinal studies of a group of patients (a case series followed over time); cross‐sectional studies were defined as a group of patients examined at one point in time (or multiple groups of patients at one point in time); case‐control studies corresponded to cross‐sectional study that compared to a healthy group or other disease group. B, Studies by quality. The definition of quality based on the summary score (SS) obtained on Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields (QualSyst) is strong (SS of >80), good (SS of 71–79), adequate (SS of 50–70) and limited (SS of <50)19
3.2. Synthesis of results
3.2.1. Language impairments reported
A summary of the spoken language impairments reported for every phenotype is available in Table 2.
Parkinson's disease dementia
There were 16 articles describing spoken language impairments in PDD. These included difficulties at both basic and complex levels of language processing. At a single word level, three of them reported difficulties with naming20, 21, 22 and six with verbal fluency,22, 23, 24, 25, 26, 27 with deficits most commonly found in semantic fluency22, 24 and action fluency.27
For phrase‐ and sentence‐level processing, two studies identified reduced words per minute,28, 29 while another study described reduced phrase length and impaired phrase repetition.20 People with PDD also were found to make more pauses during speech,29 show a higher mean length of utterance,28 and have poorer topic maintenance.30 Reduced sentence generation23 and construction22 were also reported. Receptive language difficulties were also found with respect to grammar,22, 29, 34 sentence processing,35 and semantics.22, 34 Three studies reported impaired comprehension of sentences20, 32, 33 or scripts.33
Nine studies examined different elements of the narrative process in PDD. These included deficits in narrative organization,31 production,30 and conveying content.20, 28, 32
Severity of language impairment
None of the PDD studies reported the severity of the language impairments identified.
Dementia with Lewy bodies
We identified eight articles describing language impairments in DLB, which included deficits in both comprehension and expression,30, 31, 33, 35 with specific reductions in speech rate associated with pausing and grammar difficulties,29, 34 as well as topic maintenance.30
Severity of language impairment
None of the DLB studies reported measures of severity for the language impairments found.
Progressive supranuclear palsy
We found 17 studies describing spoken language difficulties in PSP. These included reports of dynamic36, 37, 38 and non‐fluent aphasias39, 40, 41 along with a wide range of discrete deficits in expressive and receptive language.
At a single word level, some of the deficits found included difficulties with comprehension and non‐word repetition,43 reduced verbal fluency,23, 40, 43, 44, 45, 58 anomia,43, 46, 48, 49 and the use of verbs/actions.47
For phrase and sentence level, processing difficulties with syntactic knowledge50 have been reported, as well as the presence of paragrammatism,40, 46 agrammatism,40 and impaired sentence comprehension.43 Breakdown in verbal production was also described in studies reporting decreased energization (loss of sustained attention over time that affects verbal communication)42 and hesitant and slow speech production.46
Severity of language impairment
One paper examined the severity of language impairment in a group of 22 people with PSP as compared to a group of people with the non‐fluent variant of PPA (nfvPPA)41 finding that the severity of impairment did not differ between groups (although there was no indication of whether this level was mild, moderate, or severe)
Cortico‐basal degeneration
We found 18 studies describing spoken language difficulties in CBS. Four papers reported an aphasia syndrome in CBS without providing further information regarding the details of the aphasia profile,10, 51, 52, 53, 65 with two further studies identifying a language impairment resembling nfvPPA.54, 55 Six other papers reported difficulties with fluency and/or anomia,23, 56, 57, 58, 59 with an additional study identifying impairments specifically in action naming.49 Phonologic impairments,60 impairment of syntactic knowledge,50 abnormal use of the pragmatics of language,52 and deficits in global connectedness of narrative during discourse61 were shown in different samples.
In addition, difficulties in verbal comprehension were also found in relation to comprehending sentences,58 complex orders,56 and quantifiers.62, 63, 64
Severity of language impairment
Three studies reported the aphasia quotient (AQ) in CBS as calculated by the Western Aphasia Battery (WAB), with patients scoring in the mild or moderate range.52, 53, 65
Behavioral variant frontotemporal dementia
Sixteen studies examined language impairment in bvFTD. Nine studies reported impairments in naming49, 66, 67, 68, 69, 70, 71, 72 with four of these studies specifically identifying action naming as a deficit.49, 66, 71, 72 Other impairments included errors in word repetition, phonological errors, reduced speech output, verbal perseverations and echolalia,69 and difficulties with organizing narratives73 and with grammar.69, 74
Several studies also describe impaired comprehension for single words,66, 69 sentences,69, 75, 76, 77 or logical quantifiers.64 Total pause time and number of pauses in speech was elevated in the bvFTD group studied by Yunusova et al.78
Severity of language impairment
Pakhomov et al.,70 found that the bvFTD patients in their study were similarly impaired to people with nfvPPA in the category fluency tasks. While this study compared performances between bvFTD and nfvPPA, no studies specifically reported severity levels as measured by standardized tools.
Early‐onset Alzheimer's disease
Four studies examined the language deficits in EOAD. Two of them did not find language impairment in EOAD.79, 80 In contrast, another found that people with younger onset showed poorer comprehension of words, sentences, and sequential commands than those with later onset AD.81 The fourth study described a patient who developed a “full aphasia syndrome” over time.82
Severity of language impairment
Although no papers were found describing the severity of language impairments in this group, Imamura et al.81 suggest that earlier age of onset may be associated with greater severity of aphasia.
Posterior cortical atrophy
Seven studies examined spoken language impairments in people with PCA. A language profile similar to that seen in people with logopenic aphasia was reported in three studies,83, 84, 85 but with milder impact on comprehension and spontaneous speech.84 One paper highlighted impaired fluency for nouns and verbs.86 The remaining three studies identified additional impairments in comprehending words related to space and quantity.64, 87, 88
Severity of language impairment
No papers were found describing the severity of language impairments in this group.
Motor neuron disease combined with frontotemporal dementia
Five studies reported spoken language difficulties in people with a dual diagnosis of FTD and MND. In the largest study to date,69 involving 56 people with FTD and MND, impairments were identified in: word finding, confrontational naming, single‐word and sentence‐level comprehension, with reduced speech production, perseverations, and echolalia. Additional studies have also identified deficits in verbal fluency, confrontational naming, semantic and grammar89 syntactic comprehension,90, 91 and naming and comprehension of verbs.91 Two studies reported poverty of spontaneous speech.91, 92
Severity of language impairment
One paper91 described the deficits in their patient as severe, evolving to mutism “within a few weeks.”
Clinical significance of language impairments: impact on daily activities or quality of life
No papers selected for review reported impact of language impairments on activities of daily living or quality of life.
Reported language‐based interventions
No papers selected for review reported language‐based interventions.
4. DISCUSSION
This scoping review shows evidence of a wide range of spoken language impairments within the eight degenerative dementia syndromes examined. The clinical significance of these language deficits was not reported across studies (i.e., their impact on the quality of life or activities of daily living of the patients). Likewise, we did not find studies describing language‐based interventions for these patients. The most frequently studied patients in this review were those with the atypical parkinsonism syndromes, PSP and CBS. While the relatively high numbers of studies on PSP and CBS may seem surprising considering the low prevalence of these disorders, the presence of non‐fluent aphasia in both people with PSP and CBS has been clearly demonstrated in the literature and incorporated into diagnostic guidelines.94, 95 This knowledge may have contributed to raised awareness about the language impairments in this population, resulting in a larger number of research studies in this group. Although a large volume of language research exists for people who have PD without dementia,93 the relatively modest number of studies in people with PDD/DLB could be due to a tendency to focus on more prominent cognitive deficits (e.g., executive difficulties) and neuropsychiatric features in these conditions. Likewise, the limited attention given to EOAD may reflect an overshadowing by other cognitive and behavioral symptoms. Small numbers of studies for patients with PCA and MND+FTD are less surprising, given the low prevalence of these phenotypes and difficulty in gathering large samples.
Research question 1: What are the currently reported spoken language impairments in non‐language led dementias?
The scoping review revealed a wide range of language deficits across the dementia subtypes examined. Within the PDD/DLB spectrum, these often included increased pausing, longer latencies of utterances, and reduced phrase length in speech production. Such features appear consistent with the general mental slowing and bradyphrenia observed in parkinsonian syndromes. Poor ability to maintain topic and difficulties in narrative organization and comprehension were also commonly reported and linked with impaired executive function, a cognitive domain known to be impacted in PDD.96 Other impairments, such as in grammar, appeared purely linguistic, without associations with additional cognitive deficits.
As with previous reviews in PSP and CBS,94 language impairments consistent with non‐fluent aphasia were reported in these two syndromes. Although impairments in verbal fluency, naming, and syntactic processing were also commonly reported in PDD, bvFTD, PCA, and FTD+MND, a full nfvPPA profile was never described in these dementia subtypes. In PSP, additional difficulties with comprehension were reported in association with a loss of sustained attention.42
This review also found that people with bvFTD and those with MND+FTD share similar characteristics of language dysfunction with regard to perseverations, echolalia, and difficulties with grammar and comprehension.69 In addition, people with bvFTD may show poor organization of speech, phonological errors, and impairments in repetition and action naming. As the literature base for MND+FTD is currently small, it is still unclear whether these additional features will also be found in MND+FTD. Given the similarities in their dysexecutive features, one might predict overlapping language impairments. However, the additional life‐threatening features and complex care requirements that characterize MND+FTD may limit the perceived relevance of researching these language issues in the context of the broader clinical picture.
Poorer ability to process verbs and actions has been associated with damage of motor cortices, whereas difficulties with phonology and comprehension of quantifiers described in CBS are associated with disruption of posterior cortical regions.62 Interestingly, impairments in verb processing and action naming were observed in a number of dementia syndromes including bvFTD, PDD, PSP, and CBS. Evidence from both PD and FTD suggest that such deficits are associated with changes in the frontostriatal motor network.97, 98
We identified only four studies looking at the language profile of EOAD,79, 80, 81, 82 with mixed reports over the presence of language dysfunction. One study showed impaired comprehension for words, sentences, and commands,81 while another described a patient who developed full aphasia.82 The remaining two studies, however, did not identify any language impairments. Given both the paucity of studies and discrepancies found, further research into EOAD is warranted to determine the nature of language impairments and whether such difficulties significantly limit communication.
Atrophy encroaching the posterior portion of the left superior and middle temporal gyri and the inferior parietal lobe is an anatomical marker for logopenic aphasia1 but it is also present in PCA. This perhaps explains the logopenic language profile (albeit milder than that in logopenic variant PPA) exhibited in PCA.84, 85 In addition, the extended inferior parietal atrophy of PCA may play a pivotal role in the impairment of quantifiers and magnitude knowledge, similar to that described in people living with CBS.
Research question 2: What is the clinical significance of these language impairments?
Although the extent of the language dysfunction described in certain cases indicates substantial disability for some individuals (e.g., resulting in mutism or global aphasia), no studies directly measured the impact of language difficulties on activities of daily living or quality of life. In part, this may arise due to the lack of suitable tools that can effectively capture or monitor how language impairments affect everyday living and well‐being. The lack of development and validation of such questionnaires or inventories may reflect the limited attention received to date within the literature compared to other variables (e.g., descriptions of deficits, classification). Notably, a quality of life and life participation approach is becoming increasingly recognized as a recommended management approach for PPA.100, 101 This might accelerate the creation of new measures aimed to capture the impact of language impairment in daily living and quality of life in PPA. Such progress will likely have the potential to benefit the broader field of progressive language disorders.
Research question 3: What are the reported language‐based interventions?
No language‐based interventions were reported in any of the eight dementia syndromes examined, including in patients with PSP or CBS. This is surprising considering that not only is non‐fluent aphasia common in PSP, but numerous therapeutic studies have been conducted in nfvPPA with positive results.3, 99 Likewise, no investigations were identified to support the language difficulties of PCA, despite its overlap with the logogenic variant of PPA. People living with PCA, PSP, and CBS may therefore likely benefit from the language therapies currently available to treat PPA.3 It appears that both linguistic and other cognitive causes can be simultaneously involved in the language dysfunction of people with CBS, PSP, PDD, DLB, and bvFTD. If this is true, a close and comprehensive assessment of both cognitive and linguistic functions and their potential involvement in communication outcomes may be needed to develop effective interventions. For instance, it will be unhelpful administering a purely language‐based therapy to a patient whose language impairment is predominantly caused by a disruption in working memory.
5. LIMITATIONS
Following the standard methodology of a scoping review, data were gathered from a wide range of studies and methods to create a map of current evidence. As we were also interested in the quality of available data to inform a future systematic review, we extended beyond a typical scoping review to also conduct a risk of bias assessment. While the ratings given to the majority of studies suggested satisfactory methodological rigor, it is important to note that approximately one third of studies identified fell below the accepted threshold. This indicates a need for more controlled studies in the future, to confirm and strengthen confidence in the current findings.
6. CONCLUSIONS
This scoping review describes the volume, extent, and nature of the research evidence regarding spoken language impairment in non–language‐led dementias. From this piece of work, three calls for action emerge: (1) research studies should seek to assess the clinical relevance of any spoken language deficits examined; (2) both linguistic and cognitive underlying mechanisms should be explored and sufficiently described (to assist in the design of effective language and behavioral interventions); and (3) trials of language therapies previously found useful in the PPA should be undertaken in patients with PSP, CBS, and PCA.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
Aida Suarez‐Gonzalez and Joshua Stott receive funding from a grant jointly funded by the Economic and Social Research Council (ESRC UK) and the National Institute for Health Research (NIHR UK) (ES/S010467/1). Joshua Stott also receives funding from the NIHR (R76G48), The Dunhill Medical Trust (RPGF1910/191), and Alzheimer's Society (AS‐PG‐18‐013 and AS‐CTF‐14‐005).
Suárez‐González A, Cassani A, Gopalan R, Stott J, Savage S. When it is not primary progressive aphasia: A scoping review of spoken language impairment in other neurodegenerative dementias. Alzheimer's Dement. 2021;7:e12205. 10.1002/trc2.12205
DATA AVAILABILITY STATEMENT
Data sharing in not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Gorno‐Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology. 2012;78(21):1670‐1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotelli M, Mannenti R, Ferrari C, Gobbi E, Macis A, Cappa SF. Effectiveness of language training and non‐invasive brain stimulation on oral and written naming performance in primary progressive aphasia: a meta‐analysis and systematic review. Neurosci Biobehav Rev. 2020(108):498‐525. [DOI] [PubMed] [Google Scholar]
- 4.Imamura T, Takatsuki Y, Fujimori M, et al. Age at onset and language disturbances in Alzheimer's disease. Neuropsychologia. 1998;36(9):945‐949. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Luo X‐G, Dy C‐L, et al. Characteristics of language impairment in Parkinson's disease and its influencing factors. Transl Neurodegener. 2015;4(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teichmann M, Migliaccio R, Kas A, Dubois B. Logopenic progressive aphasia beyond Alzheimer's–An evolution towards dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2013;84(1):113‐114. [DOI] [PubMed] [Google Scholar]
- 7.Crutch SJ, Lehmann M, Warren JD, Rohrer JD. The language profile of posterior cortical atrophy. J Neurol Neurosurg Psychiatry. 2013;84(4):460‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxon JA, Thompson JC, Jones M, et al. Examinig the language and behavioural profile in FTD and ALS‐FTD. J Neurol Neurosurg Psychiatry. 2017;88:675‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burrell JR, Ballard KJ, Halliday GM, Hodges JR. Aphasia in progressive supranuclear palsy: as severe as progressive non‐fluent aphasia. J Alzheimers Dis JAD. 2018;61(2):705‐715. [DOI] [PubMed] [Google Scholar]
- 10.McMonagle P, Blair M, Kertesz A. Corticobasal degeneration and progressive aphasia. Neurology. 2006;67(8):1444‐1451. [DOI] [PubMed] [Google Scholar]
- 11.Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(4):a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698‐1706. [DOI] [PubMed] [Google Scholar]
- 13.Savage SA, Suárez‐González A, Cassani A, Gopalan R, Stott J. Non‐primary progressive language impairment in neurodegenerative conditions: protocol for a scoping review. Syst Rev. 2021;10(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Stewart L, Shekelle P. Implementing PRISMA‐P: recommendations for prospective authors. Syst Rev. 2016;5(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. [DOI] [PubMed] [Google Scholar]
- 16.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19‐32. [Google Scholar]
- 17.Mateen FJ, Oh J, Tergas AI, Bhayani NH, Kamdar BB. Titles versus titles and abstracts for initial screening of articles for systematic reviews. Clin Epidemiol. 2013;5:89‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kmet L, Lee R. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. Heritage Foundation for Medical Research; 2004. [Google Scholar]
- 19.Lee L, Packer TL, Tang SH, Girdler S. Self‐management education programs for age‐related macular degeneration: a systematic review. Australas J Ageing. 2008;27(4):170‐176. [DOI] [PubMed] [Google Scholar]
- 20.Cummings JL, Darkins A, Mendez M, Hill MA, Benson DF. Alzheimer's disease and Parkinson's disease: comparison of speech and language alterations. Neurology. 1988;38(5):680‐684. [DOI] [PubMed] [Google Scholar]
- 21.Frank EM, McDade HL, Scott WK. Naming in dementia secondary to Parkinson's Huntington's and Alzheimer's diseases. J Commun Disord. 1996;29(3):183‐197. [DOI] [PubMed] [Google Scholar]
- 22.Lewis FM, LaPointe LL, Murdoch BE, Chenery HJ. Lewis language impairment in Parkinson's disease. Aphasiology. 1998;12(3):193‐206. [Google Scholar]
- 23.Magdalinou NK, Golden HL, Nicholas JM, et al. Verbal Adynamia in Parkinsonian syndromes: behavioral correlates and neuroanatomical substrate. Neurocase. 2018;24(4):204‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azuma T, Bayles KA, Cruz RF, et al. Comparing the difficulty of letter, semantic, and name fluency tasks for normal elderly and patients with Parkinson's disease. Neuropsychology. 1997;11(4):488‐497. [DOI] [PubMed] [Google Scholar]
- 25.Bayles KA, Trosset MW, Tomoeda CK, Montgomery EB, Wilson J. Generative naming in Parkinson disease patients. J Clin Exp Neuropsychol. 1993;15(4):547‐562. [DOI] [PubMed] [Google Scholar]
- 26.Suhr JA, Jones RD. Letter and semantic fluency in Alzheimer's, Huntington's and Parkinson's dementias. Arch Clin Neuropsychol. 1998;13(5):447‐454. [PubMed] [Google Scholar]
- 27.Piatt AL, Fields JA, Paolo AM, Koller WC, Troster AI. Lexical, semantic, and action verbal fluency in Parkinson's disease with and without dementia. J Clin Exp Neuropsychol. 1999;21(4):435‐443. [DOI] [PubMed] [Google Scholar]
- 28.Ash S, Jester C, York C, et al. Longitudinal decline in speech production in Parkinson's disease spectrum disorders. Brain Lang. 2017;171:42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ash S, McMillan C, Gross RG, et al. Impairments of speech fluency in Lewy body spectrum disorder. Brain Lang. 2012;120(3):290‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ash S, McMillan C, Gross RG, et al. The organization of narrative discourse in Lewy body spectrum disorder. Brain Lang. 2011;119(1):30‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossman M, Irwin DJ, Jester C, et al. Narrative oraganization deficit in Lewy body disorders is related to Alzheimer's pathology. Front Neurosci. 2017;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ash S, Xie SX, Gross RG, et al. The organisation and anatomy of narrative comprehension and expression in Lewy body spectrum disorders. Neuropsychology. 2012;26(3):368‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross RG, Camp E, McMillan CT, et al. Impairment of script comprehension in Lewy body spectrum disorders. Brain Lang. 2013;125(3):330‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grossman M, Gross RG, Moore P, et al. Difficulty processing syntactic ambiguities in Lewy body spectrum disorder. Brain Lang. 2012;120(1):52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross RG, McMillan CT, Chandrasekaran K, et al. Sentence processing in Lewy body spectrum disorder, the role of working memory. Brain Cogn. 2012;78(2):85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson G, Shallice T, Cipolotti L. Dynamic aphasia in progressive supranuclear palsy: a deficit in generating a fluent sequence of novel thought. Neuropsychologia. 2006;44(8):1344‐1360. [DOI] [PubMed] [Google Scholar]
- 37.Robinson GA, Spooner D, Harrinson WJ. Frontal dynamic aphasia in progressive supranuclear palsy: distinguishing between generation and fluent sequencing of novel thoughts. Neuropsychologia. 2015;77:62‐75. [DOI] [PubMed] [Google Scholar]
- 38.Esmonde T, Giles E, Xuereb J, Hodges J. Progressive supranuclear palsy presenting with dynamic aphasia. J Neurol Neurosurg Psychiatry. 1996;60(4):403‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohrer JD, Paviour D, Bronstein AM, O'Sullivan SS, Lees A, Warren JD. Progressive supranuclear palsy syndrome presenting as progressive nonfluent aphasia: a neuropsychological and neuroimaging analysis. Mov Disord. 2010;25(2):179‐188. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Josephs KA, Boeve BF, Duffy JR, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11(4):283‐296. [DOI] [PubMed] [Google Scholar]
- 41.Burrell JR, Ballard KJ, Halliday GM, Hodges JR. Aphasia in progressive supranuclear palsy: as severe as progressive non‐fluent aphasia. J Alzheimers Dis. 2018;61(2):705‐715. [DOI] [PubMed] [Google Scholar]
- 42.Barker MS, Nelson NL, O'Sullivan JD, Adam R, Robinson GA. Energization and spoken language production: evidence from progressive T supranuclear palsy. Neuropsychologia. 2018;119:349‐362. [DOI] [PubMed] [Google Scholar]
- 43.Catricala E, Boschi V, Cuoco S, et al. The language profile of PSP. Cortex. 2019;115:294‐308. [DOI] [PubMed] [Google Scholar]
- 44.Rosser A, Hodges JR. Initial letter and semantic category fluency in Alzheimer's disease, Huntington's disease, and progressive supranuclear palsy. Neurosurg Psychiatry. 1994;57(11):1389‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lebrun Y, Devreux F, Rousseau JJ. Language and speech in a patient with a clinical diagnosis of progressive supranuclear palsy. Brain Lang. 1986;27(2):247‐256. [DOI] [PubMed] [Google Scholar]
- 46.Boeve B, Dickson D, Duffy D, et al. Progressive nonfluent aphasia and subsequent aphasic dementia associated with atypical progressive supranuclear palsy pathology. Eur Neurol. 2003;49(2):72‐78. [DOI] [PubMed] [Google Scholar]
- 47.Daniele A, Barbier A, Di Giuda D, et al. Selective impairment of action‐verb naming and comprehension in progressive supranuclear palsy. Cortex. 2013;49(4):948‐960. [DOI] [PubMed] [Google Scholar]
- 48.Podoll K, Schwarz M, Noth J. Language functions in progressive supranuclear palsy. Brain. 1991:1457‐1472. Pt 3. [DOI] [PubMed] [Google Scholar]
- 49.Cotelli M,Borroni B, Manenti R, et al. Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology. 2006;20(5):558‐565. [DOI] [PubMed] [Google Scholar]
- 50.Cotelli M, Borroni B, Manenti R, et al. Universal grammar in the frontotemporal dementia spectrum: evidence of a selective disorder in the corticobasal degeneration syndrome. Neuropsychologia. 2007;45(13):3015‐3023. [DOI] [PubMed] [Google Scholar]
- 51.Levin J, Bak TH, Rominger A, et al. The association of aphasia and right‐sided motor impairment in corticobasal syndrome. J Neurol. 2015;262(10):2241‐2246. [DOI] [PubMed] [Google Scholar]
- 52.Donovan NJ, Kendall DL, Moore AB. Why consider impaired social language usage in a case of corticobasal degeneration? Clin Neuropsychol. 2007;21(1):190‐203. [DOI] [PubMed] [Google Scholar]
- 53.Kertesz A, Martinez‐Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55(9):1368‐1375. [DOI] [PubMed] [Google Scholar]
- 54.Takao M, Tsuchiya K, Mimura M, et al. Corticobasal degeneration as cause of progressive non‐fluent aphasia: clinical, radiological and pathological study of an autopsy case. Neuropathology. 2006;26(6):569‐578. [DOI] [PubMed] [Google Scholar]
- 55.Tree JJ, Kay J. Longitudinal assessment of language and memory impairments in pathologically confirmed cortico‐basal ganglionic degeneration. Cortex. 2008;44(9):1234‐1247. [DOI] [PubMed] [Google Scholar]
- 56.Ferrer I, Hernandez I, Boada M, et al. Primary progressive aphasia as the initial manifestation of corticobasal degeneration and unusual tauopathie. Acta Neuropathol. 2003;106(5):419‐435. [DOI] [PubMed] [Google Scholar]
- 57.Grossman M, McMillan C, Moore P, et al. What's in a name: voxel‐based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628‐649. Pt 3. [DOI] [PubMed] [Google Scholar]
- 58.Santos‐Santos MA, Mandelli ML, Binney RJ, et al. Cross‐sectional and longitudinal features of non‐fluent/agrammatic primary progressive aphasia with underlying corticobasal degeneration or progressive supranuclear palsy pathology. JAMA Neurol. 2016;73(6):733‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brito‐Marques PR, Vieira‐Mello RJ, Montenegro L, et al. Clinicopathologic analysis of progressive non‐fluent aphasia and corticobasal degeneration. Dement Neuropsychol. 2011;5(2):135‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graham NL, Bak T, Patterson K, Hodges JR. Language function and dysfunction in corticobasal degeneration. Neurology. 2003;61(4):493‐499. [DOI] [PubMed] [Google Scholar]
- 61.Gross RG, Ash S, McMillan CT, et al. Impaired information integration contributes to communication difficulty in corticobasal syndrome. Cogn Behav Neurol. 2010;23(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Troiani V, Clark R, Grossman M. Impaired verbal comprehension of quantifiers in corticobasal syndrome. Neuropsychology. 2011;25(2):159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McMillan CT, Clark R, Moore P, Grossman M. Quantifier comprehension in corticobasal degeneration. Brain Cogn. 2006;62(3):250‐260. [DOI] [PubMed] [Google Scholar]
- 64.Morgan B, Gross R, Clark R, et al. Some is not enough: quantifier comprehension in corticobasal syndrome and bahevioral variant frontotemproal dementia. Neuropsychologia. 2011;49(13):3532‐3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMonagle P, Blair M, Kertesz A. Corticobasal degeneration and progressive aphasia. Neurology. 2006;67(8):1444‐1451. [DOI] [PubMed] [Google Scholar]
- 66.Hardy CJD, Buckley AH, Downey L, et al. The language profile of behavioural variant frontotemporal dementia. J Alzheimers Dis. 2015;50(2):359‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D'Honincthun P, Pillon A. Verb comprehension and naming in frontotemporal degeneration—The role of the static depiction of actions. Cortex. 2008;44(7):834‐847. [DOI] [PubMed] [Google Scholar]
- 68.Snowden JS, Harris JM, Saxon JA, et al. Naming and conceptual understanding in frontotemporal dementia. Cortex. 2019;120:22‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saxon JA, Thompson JC, Jones M, et al. Examining the language and behavioural profile in FTD and ALS‐FTD. J Neurol Neurosurg Psychiatry. 2017;88(8):675‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pakhomov SVS, Smith GE, Chacon D, et al. Computerized analysis of speech and language to identify psycholinguistic correlates of frontotemporal lobar degeneration. Cogn Behav Neurol. 2010;23(3):165‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis C, Heidler‐Gary J, Gottesman RF, et al. Action versus animal naming fluency in subcortical dementia, frontal dementias and Alzheimer disease. Neurocase. 2010;16(3):259‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silveri MC, lvigni BL, Cappa A, et al. Impairment of verb processing in frontal variant frontotemporal dementia: a dysexecutive symptom. Dement Geriatr Cogn Disord. 2003;16(4):296‐300. [DOI] [PubMed] [Google Scholar]
- 73.Ash S, Moore P, Antani S. Trying to tell a tale: discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66(9):1405‐1413. [DOI] [PubMed] [Google Scholar]
- 74.Ash S, Naomi Nevler N, Jeffrey Phillips J, et al. A longitudinal study of speech production in primary progressive aphasia and behavioral variant frontotemporal dementia. Brain Lang. 2019;194:46‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peelle JE, Troiani V, Gee J, et al. Sentence comprehension and voxel‐based morphometry in progressive nonfluent aphasia, semantic dementia. J Neurolinguistics. 2008;21(5):418‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peelle JE, Cooke A, Moore P, Vesely L, Grossman M. Syntactic and thematic components of sentence processing in progressive nonfluent aphasia and frontotemporal dementia. J Neurolinguistics. 2007;20(6):482‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grossman M, Rhee J, Moore P. Sentence processing in frontotemporal dementia. Cortex. 2005;41(6):764‐777. [DOI] [PubMed] [Google Scholar]
- 78.Yunusova Y, Graham NL, Shellikeri S, et al. Profiling speech and pausing in amyotrophic lateral sclerosis (ALS) and Frontotemporal Dementia (FTD). PLoS One. 2016;11(1):e0147573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sa F, Pinto P, Cunha C, et al. Differences between early and late onset Alzheimer's disease in neuropsychological tests. Front Neurol. 2012;3:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Selnes OA, Carson K, Rovner B, Gordon B. Language dysfunction in early—and late—onset possible Alzheimer's disease. Neurology. 1988;38(7):1053‐1056. [DOI] [PubMed] [Google Scholar]
- 81.Imamura T, Takatsuki Y, Fujimori M, et al. Age at onset and language disturbances in Alzheimer's disease. Neuropsychologia. 1998;36(9):945‐949. [DOI] [PubMed] [Google Scholar]
- 82.Borges MK, Lopes TN, Biella MM, Siquieira A, Mauer S, Aprahamian I. Early‐onset Alzheimer's disease (EOAD) with Aphasia A case report. Front Psychiatry. 2018;9:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fitzpatrick D, Blanco‐Campal A, Kyne L. A case of overlap posterior cortical atrophy and logopenic variant primary progressive aphasia. Neurologist. 2019;24(2):62‐65. [DOI] [PubMed] [Google Scholar]
- 84.Crutch SJ, Lehmann M, Warren JD, Rohrer JD. The language profile of posterior cortical atrophy. J Neurol Neurosurg Psychiatry. 2013;84(4):460‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magnin E, Sylvestre G, Lenoir F, et al. Logopenic syndrome in posterior cortical atrophy. J Neurol. 2013;260(2):528‐533. [DOI] [PubMed] [Google Scholar]
- 86.Steeb B, Garcia‐Cordero I, Huizing M, et al. Progressive compromise of nouns and verbs in posterior cortical atrophy. Front Psychol. 2018;9:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shebani Z, Patterson K, Nestor PJ, et al. Semantic word category processing in semantic dementia and posterior cortical atrophy. Cortex. 2017;93:92‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suárez‐González A, Hoffman P, Crutch SJ. Where words meet numbers: comprehension of measurement unit terms in posterior cortical atrophy. Neuropsychologia. 2019;131:216‐222. [DOI] [PubMed] [Google Scholar]
- 89.Rakowic W, Hodges J. Dementia and aphasia in motor neuron disease: an underrecognised association?. J Neurol Neurosurg Psychiatry. 1998;65(6):881‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamminga J, Leslie FVC, Hsieh S, et al. Syntactic comprehension deficits across the FTD‐ALS continuum. Neurobiol Aging. 2016;41:11‐18. [DOI] [PubMed] [Google Scholar]
- 91.Bak TH, Hodges JR. The effects of motor neurone disease on language: further evidence. Brain Lang. 2004;89(2):354‐361. [DOI] [PubMed] [Google Scholar]
- 92.Bak TH, O'Donovan DG, Huereb JH, Boniface S, Hodges JR. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45. Brain. 2001;124:103‐120. Pt 1. [DOI] [PubMed] [Google Scholar]
- 93.Smith KM, Caplan DN. Communication impairment in Parkinson's disease: impact of motor and cognitive symptoms on speech and language. Brain Lang. 2018;185:38‐46. [DOI] [PubMed] [Google Scholar]
- 94.Peterson KA, Patterson K, Rowe JB. Language impairment in progressive supranuclear palsy and corticobasal syndrome. J Neurol. 2019;268(3):796‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32(6):853‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schapira A, Chaudhuri K, Jenner P. Non‐motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:435‐450. [DOI] [PubMed] [Google Scholar]
- 97.Birba A, Garcia‐Cordero I, Kozono G. Losing ground: frontostriatal atrophy disrupts language embodiment in Parkinson's and Huntington's disease. Neurosci Biobehav Rev. 2017;80:673‐687. [DOI] [PubMed] [Google Scholar]
- 98.Gardini S, Venneri A, McGeown WJ, et al. Brain activation patterns characterizing different phases of motor action: execution, choice and ideation. Brain Topogr. 2006;29(5):679‐692. [DOI] [PubMed] [Google Scholar]
- 99.Henry LH, Hubbard HI, Grasso SM, et al. Retraining speech production and fluency in non‐fluent/agrammatic primary progressive aphasia. Brain. 2018;141(6):1799‐1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rogalski EJ, Khayum B. A life participation approach to primary progressive aphasia intervention. Semin Speech Lang. 2018;39(3):284‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruggero L, Nickels L, Croot K. Quality of life in primary progressive aphasia: what do we know and what can we do next?. Aphasiology. 2019;33(5):498‐519. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing in not applicable to this article as no new data were created or analyzed in this study.


