Abstract
Human papillomavirus (HPV) is a ubiquitous DNA virus that infects squamous epithelia. Though HPV only encodes 8 genes, it is capable of causing cellular transformation and ultimately cancer in host cells. In this article we review the classification of HPV viruses, their genetic structure and life cycle, viral gene biology, and provide an overview of the role of HPV in cancer. We explain how the viral life cycle can lead to integration of viral DNA into the host genome leading to increased cell cycle progression, decreased apoptosis, altered DNA repair, and chromosomal instability. We describe the multifaceted roles of the canonical oncogenes E6 and E7 in promoting tumorigenesis and the important role of other viral genes in regulating cancer development. We also review how the virus actively suppresses innate and adaptive immunity to evade immune detection and promote a pro-tumorigenic microenvironment. The biology presented here will serve as a foundation to the other chapters in this edition and we hope it will incite enthusiasm for continued research on this fascinating virus that causes significant morbidity and mortality worldwide.
HPV classification and genome
Human papillomavirus (HPV) is a small, non-enveloped, double-stranded DNA virus with a tropism for squamous epithelium. It is a member of the Papillomaviridae (PV) virus family, which, as of 2019, is divided into 53 genera, each denoted by a letter of the Greek alphabet (1). Only five genera of papillomaviruses can infect humans. Genera are based upon nucleotide sequence identities in the L1 major capsid protein open reading frame (ORF) and papillomaviruses that share less than 60% of their L1 ORFs are classified as separate genera. PVs within a genus that share 60–70% L1 sequence identity are known as a species, or clade, while those within a species and with a 71–89% L1 sequence identity are considered a type. Furthermore, PVs that share a 90–98% nucleotide sequence similarity are termed subtypes, while those with greater than 98% similarity are classified as variants (1).
There are 222 types of human papillomavirus that have been identified as of December, 2020 (1). These types fall into five main genera associated with different diseases: there are 65 Alphapapillomaviruses, 54 Betapapillomaviruses, 99 Gammapapillomaviruses, 3 Mupapillomaviruses, and a single Nupapillomavirus. These genera differ in their tropism, which are determined by viral entry driven by the interaction of the L1 capsid protein with the cell surface (2). HPVs in the beta, gamma, mu, and nu genera have a cutaneous tropism which typically result in warts on the hands or feet, while members of the alpha genus have a mucosal tropism and can be associated with more serious diseases including cancer.
Alpha papillomaviruses are known as either low-risk (LR) or high-risk (HR) based on their ability to transform cells and cause cancer. Infection by LR HPVs, such as HPV6 and HPV11 (α−10 clade), is non carcinogenic and typically causes anogenital warts (condylomata acuminata). In contrast, infection by HR HPV types (most commonly clades α −7 and α −9) may lead to the development of several cancers, such as cervical, vaginal, vulvar, anal, penile, and head and neck (3). HR HPVs include types 16, 18, 31, 33, 35, 39, 45, 52, 56, 58, 59, 66, and 68, among others, and are the causative agent of around 5% of all cancers globally (3).
The virus itself measures approximately 60 nM in diameter and is composed of an icosahedral capsid that contains a single circular double-stranded DNA of approximately 8,000 base pairs. Only one strand of the dsDNA is used as a template for transcription, and contains three genomic regions and 10 ORFs (4). The early (E) region of the genome occupies over 50% of the viral genome and encodes 6 ORFs: E1, E2, E4, E5, E6, and E7, each with differing functions and importance, which will be discussed in more detail below (and in Table 1). The late (L) region of the viral genome accounts for nearly 40% of the entire genome, lies downstream of the early genes, and encodes the major and minor viral capsid proteins, L1 and L2, respectively. The HPV icosahedral capsid contains 360 copies of L1 constructed by 72 pentamers with a single L2 protein in the center of each (5). This unique structure allowed for the development of the HPV vaccine, a groundbreaking development in cancer prevention that is discussed in the accompanying article by X and colleagues [CITATION]. Finally, the third region of the viral genome is the long control region (LCR), a segment of about 850 bp (10% of the genome). The LCR does not have any protein coding function but contains the origin of DNA replication as well as sequences involved in transcriptional regulation (6).
Table 1:
Summary of the functions of Human Papilloma Virus genes. Oncogenes E6 and E7 are summarized in Figure 2. E, early genes and L, late genes.
| Viral gene | Function |
|---|---|
| E1 | • ATP-dependent helicase essential for viral genome replication • Recruits host cell DNA replication proteins |
| E2 | • Activates or represses viral gene transcription • Initiates viral DNA replication by interacting with E1 • Tethers viral genomes to host chromosomes in mitosis |
| E4 | Expressed during later stages of infection • Amplifies viral genome • Role in viral synthesis • Promotes and contributes to virion release |
| E5 | • Activates mitogenic signaling pathways • Inhibits MHC I expression • suppression of IFN-κ • modulates transit of signaling proteins through ER • Alters activity of EGFR • Enhances transforming abilities of E6 and E7 • Prevents keratinocyte differentiation |
| L1 | • Major viral capsid protein |
| L2 | • Minor viral capsid protein, involved in encapsidation of viral DNA • Associates with viral DNA to assist with trafficking to host nucleus |
HPV life cycle
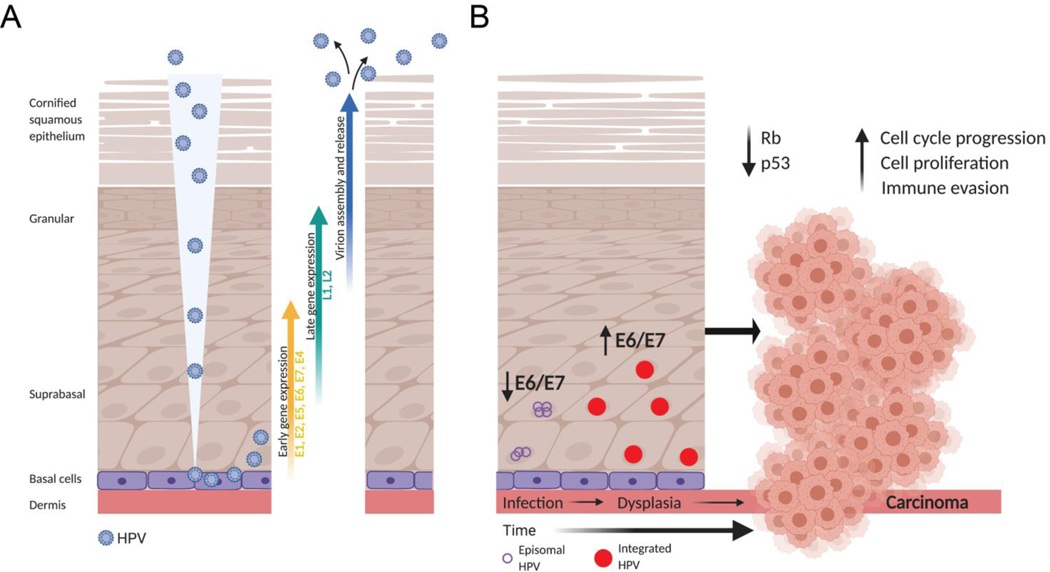
HPV targets cutaneous or mucosal squamous epithelia which are composed of a basal layer of undifferentiated cells along the basement membrane followed by suprabasal, granular, and cornified squamous cells as the outermost layer, which become increasingly differentiated in this outward direction (Figure 1A). HPV can only establish an infection within actively dividing cells because it relies on host cells for genome replication given the virus itself does not encode for any enzymes or polymerases that would facilitate self-replication. Therefore only the basal cells are infected because the more differentiated epithelial cells do not undergo mitosis. Infections typically occurs through microabrasions in the tissue (7). In the cervix, basal cells are more freely exposed at the transformation zone, the region where the epithelium transitions from the columnar epithelial cells of the endocervix to the stratified squamous epithelium of the ectocervix, and may not require any tears or cuts to establish infection. This region also contains specialized stem cells known as reserve cells, which themselves are commonly the target for HPV infection (8). A multistep process coordinates cell binding and viral entry via endocytosis (reviewed in (9,10)). Following endocytosis, HPVs are delivered to endosomes and/or lysosomes where a low pH environment is required for the uncoating of the HPV capsid. L2 remains associated with the viral DNA and mediates DNA trafficking through the trans-Golgi network (TGN) eventually leading to access to the host nucleus during prophase upon breakdown of the nuclear envelope (11). In order for HPV to evade host cell innate immune recognition, the viral DNA remains in the luminal component of the TGN and there is evidence that it resides in a transport vesicle throughout mitosis (12).
Figure 1:
The Human Papillomavirus life cycle and path to transformation and carcinogenesis. (A) HPV gains entry to the mitotically active basal cells in squamous epithelia often through microwounds in the tissue. Following infection of basal cells, HPV initiates its own viral genome replication using the E1, E2, and E4 proteins, while E6 and E7 function to promote host cell proliferation and prevent apoptosis. As the keratinocytes differentiate (upward direction in the figure), L1 and L2 are transcribed in order to package the many copies of newly replicated viral DNA into capsids. Complete virions are then shed from the surface of the squamous epithelium in a non-lytic manner. (B) Though viral replication is HPV’s primary goal, the viral genome can also be amplified and remain episomal (purple circles) in the host cells for long periods of time, though viral E6 and E7 gene expression is kept low in this state. After many rounds of mitosis, the viral genome can become integrated into the host genome (red circles), leading to upregulation of the oncoproteins E6 and E7, increased cell proliferation with relaxed cell cycle checkpoints, and increased chromosomal instability leading to aneuploidy, dysplasia and ultimately carcinoma. Created with BioRender.com
The productive life cycle of HPV can be loosely grouped into three phases: establishment, maintenance, and vegetative/productive amplification. HPV replicates and assembles exclusively in the nucleus of the host cell and utilizes the host machinery to do so (13). The establishment phase involves viral transcription and genome amplification following nuclear entry. The viral proteins E1 and E2 are involved in viral genome amplification (11,14) and viral DNA is initially maintained as episomes where viral gene expression is tightly regulated (15). E2 is a DNA binding protein and plays an important role in the initiation of viral DNA replication by binding to the origin of replication (ori) on the LCR. It recruits E1, a helicase and the only HPV encoded enzyme, to the site and forms a sequence-specific complex. E2 dissociates from the E1/E2/ori complex in an ATP-dependent fashion and the resulting E1 complex is able to unwind the viral DNA and recruits host DNA replication factors to replicate the viral DNA (16). Ultimately, each host cell will contain approximately 50–100 viral episomes (17) though more have been detected in some cell culture model systems.
After the initial establishment phase, the maintenance phase is initiated to uphold a constant number of viral genomes and to establish a persistent infection. Replication of the viral genome occurs once per S phase of the cell cycle (18). E2 is critical to this phase as it tethers the viral genome to host chromosomes, supporting viral genome partitioning into daughter cells during mitosis (19). In this phase HPV genomes can be maintained in basal epithelial cells for years to several decades after initial infection allowing for production of premalignant precursor lesions with increasing cellular dysplasia that either spontaneously revert or advance to invasive cancer in the minority of cases (20). Thankfully, less than 10% of new infections lead to persistent infections, dysplasia and cancer implying a role for immune mediated clearance of virally infected cells (21).
At some point, HPVs switch from stable maintenance of the viral genome to vegetative replication which occurs in the differentiating epithelial cells (4). Little is known about the regulation or mechanisms of this switch. The infected cells differentiate and move towards the surface of the epithelium. Normally, differentiating cells would not be capable of supporting viral DNA synthesis given they are no longer mitotically active, however, the viral proteins E6 and E7 activate host cell DNA replication machinery allowing for continued viral DNA synthesis. The viral proteins L1 and L2 are expressed later, leading to viral assembly, maturation and release from the outermost epithelial cell layer in a non-lytic manner (Figure 1A).
HPV DNA can integrate into the host DNA over the long maintenance phase and this is commonly found in both pre-malignant and malignant lesions. This is not part of the viral life cycle, signifying a “dead-end” for the virus, but unfortunately can have severe consequences for the host. The HPV genome can be disrupted upon integration or can remain intact with multiple copies present flanked by partial copies (22). While the mechanism of integration is not completely understood, a looping model has been proposed where viral and host DNA concatemers are formed from looped structures during regular DNA replication. This same study suggests that HPV integration directly promotes genomic instability, a common characteristic of cancer (23). HPV genome integration into the host does not appear to occur in a defined set of genomic hotspots, but HPV appears to integrate in common fragile sites and transcriptionally active regions (24,25). HPV can also insert its genome near oncogenes but this is not a major mechanism of oncogenesis. Integration usually disrupts the E1 and E2 genes, which terminates E2 driven transcriptional repression of E6 and E7 leading to increased oncogene expression (26). These fusion transcripts are more stable than their viral counterparts (22), which further contributes to enhanced oncogene expression.
HPV-associated cancers can contain viral DNA as isolated episomes, integrated into the host genome, or a combination of both. 83% of HPV-positive cervical cancers have integrated HPV genomes, including 76% of HPV16-positive cancers and 100% of HPV18-positive cancers (27). Viral integration increases with lesion grade and is highest in invasive carcinoma (28,29), but other studies have found that HPV integration was present in low-grade lesions as well (30). Viral integration is associated with patient outcome in many HPV-associated cancers. Cervical tumors with higher levels of HPV integration had a lower survival rate post-treatment compared to tumors with episomal genomes (31). Because viral integration is inversely correlated with circulating viral load (integrated virus cannot replicate and release new virions), a low initial viral load measured in cervical cancer tumors has come to be associated with a higher relapse rate following radiotherapy (32). Viral integration has been less studied in HPV-associated head and neck cancer but the TCGA analysis of head and neck cancer revealed that 25/36 (69.4%) of HPV+ tumors had viral integration without a definite predilection for integration sites (27). Similar to cervical cancer, HPV+ head and neck cancer patients with no viral integration had increased survival compared to patients with integrated viral genomes and this was associated with decreased immune cell infiltration in the latter case (33). Along these lines, head and neck tumors lacking E2 mRNA had worse 3-year progression free survival compared to tumors with higher levels of E2 likely given the inverse relationship between E2 and E6/E7 expression (34). Interestingly, 45% of HPV+ anal cancers maintain HPV episomally without integration, and similar to cervical cancer, higher viral load is associated with improved overall survival (35). Overall, integration of viral DNA is most common in cervical cancer and is associated with increased E6 and E7 expression, a lower viral load, and worse prognosis in many HPV-associated cancers.
Cellular transformation and tumor initiation
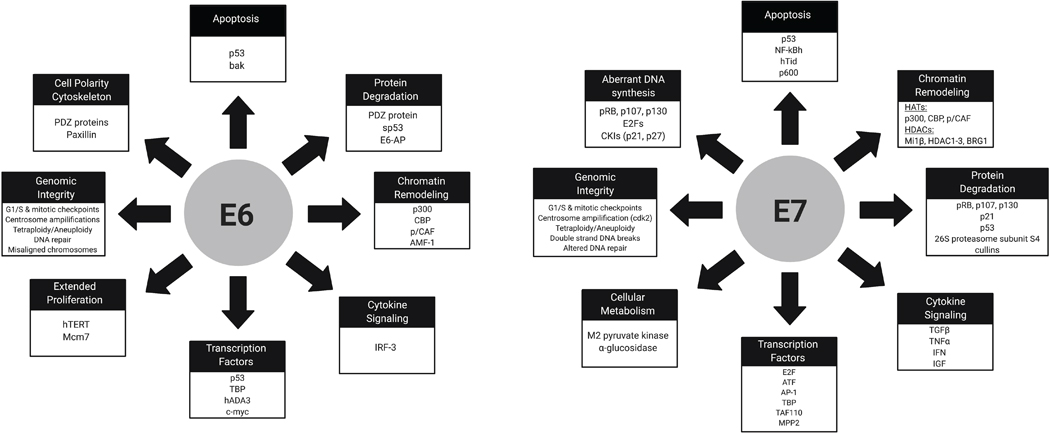
The HPV proteins E6 and E7 are critical for initially conferring a growth advantage, and are necessary for transformation and maintenance of a transformed phenotype (36). Both E6 and E7 are localized in the nucleus but there is some evidence that they may also have a role in the cytoplasm. E7 has more immortalizing and transformative properties than E6 alone, however these two proteins are cooperative and together promote tumorigenesis. The most well characterized functions of these oncoproteins is their degradation of critical tumor suppressor proteins, leading to uncontrolled cell growth. HR HPV E7 binds retinoblastoma protein (Rb), a cell cycle regulator, and other retinoblastoma pocket proteins, p107 and p130 (37,38), leading to inhibition or proteasomal degradation of Rb. Rb is then unable to inhibit E2F, a family of transcription factors, leading to active E2F-dependent transcription which promotes transition to the S phase of the cell cycle leading to increased cell proliferation and coincidentally increased viral gene transcription (39,40). E7 also controls cell proliferation by binding p21 and p27 which leads to increased activity of CDK2, further promoting G1-S phase entry of the cell cycle (41,42). One cellular consequence to targeting of Rb-E2F and other cell cycle regulators is the upregulation of another tumor suppressor, p53, which would normally counteract these effects by inhibiting cell growth and inducing apoptosis (43). To prevent this from halting cell proliferation and hence viral genome replication, HPV E6 targets p53 for proteasomal degradation by forming a complex with the E3 ubiquitin-protein ligase E6-associated protein, E6AP (44). E6 can also bind p53 and block transcription of tumor-suppressive genes, a property that is unique to certain HPV types (45). It is important to note that despite a significant decrease in functional p53, HPV+ cells continue to have some p53 activity which may partially explain their enhanced radiosensitivity (46). Though E6 mediated p53 degradation aids in productive viral replication, it also allows for genetic mutations to accumulate, which ultimately can lead to transformation, dysplasia, and cancer (Figure 1B). E6 also has many p53-independent targets that play essential roles in its transformative properties. These include the PDZ proteins that control cell signaling and attachment, the p300/CBP transcriptional activators involved in regulating differentiation and the cell cycle, and proteins involved in apoptosis (47). HR E6 also activates transcription of telomerase reverse transcriptase (TERT) which plays an essential role in cellular immortalization (48,49). The host cell targets of E6 and E7 are reviewed in Figure 2.
Figure 2:
Diverse functions of high-risk HPV E6 and E7 proteins. Collectively, these functions result in transformation of the host cell, increased cell cycle progression, decreased apoptosis, immune evasion, and chromosomal instability, which can lead to carcinogenesis and tumor formation.
The ability of E6 and E7 to bind the aforementioned cellular targets differentiates between the LR and HR HPV types. For example, LR HPV E6 proteins do not contain a PDZ binding motif, partially explaining the ability of HR HPVs to cause cancer (50). Furthermore, it is known that both HR and LR E6 proteins are able to bind to p53, but LR E6 cannot induce its degradation (51). Similarly, LR HPV E7 proteins are still able to target Rb but with a lower affinity compared to HR HPV E7 proteins, possibly contributing to their difference in progression to cancer (52).
A consequence of E7 mediated degradation of Rb and subsequent increased E2F activity is a feedback loop that results in increased expression of p16INK4A (53). p16INK4A (CDKN2A) is a tumor suppressor and inhibits CDK4/6 thereby preventing phosphorylation of Rb (and subsequently transcription of E2F target genes), slowing progression from G1 to S phase in the cell cycle. Thus p16 is upregulated in cells without functional Rb in order to attempt to put a brake on the system and halt cell cycle progression. Increased levels of p16INK4A does not seem advantageous to tumor growth, however, high levels of p16INK4A do not lead to growth inhibition in cells with non-functional Rb (54), allowing HPV+ cells to continue to rapidly proliferate. In fact, p16 has been found to be necessary for survival in some cervical cancer cell lines (55). Because of this HPV E7 specific upregulation of p16, it is used as a surrogate marker in clinical samples for HPV positivity and has been shown to be prognostic in patients with HNSCC treated with definitive chemoradiation (56,57). Immunohistochemistry (IHC) for p16 is the most commonly used diagnostic tool to determine HPV status, however mRNA expression of E6 and E7 remains the gold standard for diagnosis as this indicates active viral gene transcription. There is excellent correlation between HPV E6 and E7 mRNA expression and p16 IHC in oropharyngeal tumors with 97% of p16+ tumors also expressing E6/E7 mRNA (58). However, 5% of these tumors expressed HPV mRNA but were p16 negative indicating a low false-negative rate using p16 IHC alone. Interestingly, the correlation between p16 and HPV mRNA is not as strong in abnormal cervical cytology specimens as 188/300 samples were positive for HPV E6/E7 mRNA but only 96/300 of these were positive for p16 by IHC (59). However, the correlation increased with increasing grade of cervical intraepithelial neoplasia (CIN), implying better p16 sensitivity for HPV gene expression with higher lesion grade.
Genome instability and DNA repair
Although E6 and E7 are sufficient in transforming primary human keratinocytes (36), they are not able to directly induce carcinogenesis and malignant progression. This step requires additional oncogenic events such as chromosomal instability, which can lead to gains or losses of oncogenes or tumor suppressors, respectively. Expression of the oncogenes ras or fos in addition to HPV18 leads to malignant progression and invasive squamous cell carcinoma in vivo, while (unintegrated) HPV18 alone does not (60). HR HPV is known to cause chromosomal instability with E6 and E7 causing gene amplifications, rearrangements, deletions, inversions, and chromosomal translocations (23,61). Chromosomal instability only occurs upon integration of HPV, and E7 levels have been shown to correlate with extent of numerical and structural aberrations (62). HR E6 and E7 also induce multiple mitotic defects including centrosome amplification, anaphase bridges and multipolar mitosis, which ultimately lead to chromosome missegregation and aneuploidy (63,64). Normally, cells harboring these mitotic errors would be targeted for cell death, however E6 and E7 act together to relax cell cycle checkpoints and prevent apoptosis, allowing these genetically abnormal cells to continue to proliferate.
HR HPVs also hijack the host DNA damage response in an effort to promote viral genome replication, which increases replication stress and contributes to the genomic instability discussed above (65). E6 and E7 activate the ataxia telangiectasia-mutated (ATM) and ATM and Rad3-related (ATR) DNA damage repair pathways which are induced in the presence of double-strand or single-strand DNA breaks respectively (66). Activation of these DNA damage repair pathways would usually result in cell cycle arrest, however, E7 prevents this by inducing the degradation of claspin, a protein essential for DNA damage checkpoint recovery (67). Host cells are thus tricked into thinking that DNA damage repair has occurred and the cell proceeds through mitosis in the presence of DNA damage further contributing to genomic instability and malignant progression. Expression of the DNA repair factors phospo-CHK1, pCHK2, FANCD2, and BRCA1 increase with increasing grade of cervical squamous intraepithelial lesions suggesting a role for these pathways in malignant progression (68). Manipulation of DNA damage repair has also been shown to play a role in head and neck cancer as HPV16 E7 promotes DNA damage in an Rb-dependent manner causing tumor development in the presence of other DNA repair defects (69).
Ionizing radiation induces double-stranded DNA breaks which can be repaired to allow cell survival. However, defects in DNA damage repair lead to increased cell death and hence increased radiosensitivity, which is a mechanism thought to explain the enhanced radiosensitivity of HPV-associated cancers. DNA repair typically occurs by non-homologous end joining (NHEJ), or homologous recombination (HR) but there are other noncanonical pathways such as alternative end-joining (Alt-EJ), which includes microhomology-mediated end-joining (MMEJ). HPV+ cells tend to have a delay in DNA damage repair as dictated by resolution of γH2AX foci following exposure to ionizing radiation and also have been shown to have defects in both NHEJ and HR (70). In a non-squamous cell model system, HPV16 E7 induced both MMEJ activity and HR and repressed NHEJ while the other HPV proteins also upregulated HR (71). This group also found that E7 suppresses NHEJ following radiation-induced damage. Thus not only does HPV hijack host DNA repair pathways, but it also seems to alter them in order to promote viral DNA replication. This can lead to more error-prone DNA repair in the host resulting in genomic instability.
HPV and immune evasion
HPV infection is nearly ubiquitous, yet only a small number of people infected with an oncogenic form of HPV ultimately develop cancer. In most cases, HPV is cleared by the immune system and low-grade dysplastic lesions can also spontaneously revert. However, in order for HPV to complete its life cycle and replicate, it has evolved mechanisms to escape host immune detection, which increases the chances of long-term infection and cellular transformation. First, viral DNA and proteins remain in the host cell nucleus thereby evading detection. As discussed above, early HPV viral gene expression is initially low due to the transcriptional repression activity of E2 thereby minimizing potential presentation to the host immune system. Upon viral genome integration, E2 disruption results in increased E6 and E7 expression (26). This increase in viral oncogene expression occurs in the upper epithelial layers which have a lower density of antigen presenting cells (APC), further allowing HPV escape from immune recognition (72). Newly packaged virions are shed in sloughed epithelium rather than through cell lysis so there is little opportunity for APCs to engulf virions and present viral antigens to the immune system.
In addition to physical avoidance of immune cells, HPV also has direct ways of inhibiting both innate and adaptive immune cell function. As a first line of defense, mammalian cells have several mechanisms to detect foreign viral or bacterial DNA in order to activate rapid clearance by the immune system. Cyclic GMP-AMP synthase (cGAS) is one of the major cytosolic DNA sensors that recognizes double-stranded DNA in a sequence independent manner (73), which activates STING (stimulator of interferon genes) leading to transcription of interferons, chemokines and cytokines to mount an antiviral response (74). In order to stay undetected by the host immune system, HPV18 E7 binds STING and prevents upregulation of interferons in the presence of cytosolic DNA (75) while HPV16 E7 evades STING-induced IFN activation via an intermediary protein, NLRX1 (76). In addition to this mechanism of innate immune evasion, HR HPV oncoproteins also repress transcription of many IFN inducible genes including IFN-α, IFN-β, and STAT1 (77–79). HPV16 E7 and HPV18 E7 can also bind to the transactivation domain of IRF1 (77,80), whilst HPV16 E6 can bind IRF3 (81), blocking IFNβ transcription and an innate immune response.
Another important pathway regulating inflammatory cytokines that is manipulated by HPV oncoproteins is the NF-κB pathway. Several studies have noted that high-risk HPV E6 and E7 are capable of binding to coactivators of NF-κB in the nucleus, leading to the downregulation of the NF-κB pathway which results in impaired antiviral activity, promoting persistence of HPV infection and preventing HPV from immune recognition (82–84). Additionally, both HPV16 E5 and E6 have been shown to suppress the cytokine IFN-κ in keratinocytes which blunts IFN-stimulated gene expression (85,86). HPV16 E5 has also been shown to decrease MHC I expression which inhibits the adaptive immune response (87). Additional immune evasion strategies include deregulation of DNA methylation and histone modification, intracellular sequestration of MHC molecules, and alteration of HPV dinucleotides to avoid APOBEC3 antiviral editing (reviewed in (88)). Thus, HPV has multiple mechanisms to prevent immune signaling to allow for continued viral replication in the host and consequently has the potential to maintain a long-term infection leading to viral genome integration and cancer.
As discussed above, HPV tends to promote an anti-inflammatory environment while suppressing pro-inflammatory cytokines. This has been noted in HPV+ squamous cervical epithelium compared to normal epithelium which contributes to blunted antigen presentation by Langerhans cells (LC), specialized APCs that reside in the epidermis (89). CCL20, a chemokine that attracts LCs to the site of inflammation, is downregulated by the expression of HPV16 E6 and E7 oncogenes (90,91). Similarly, CCR7 expression on dendritic cells is downregulated in HPV-positive tumors (92), resulting in low infiltration of DCs in secondary lymphoid tissues. These, and other, factors collectively impair trafficking of innate immune cells in HPV-associated cancers.
Host T-cell responses such as CD4+ T-helper cells (Th) and CD8+ cytotoxic T cells are critical in HPV clearance and response to chemoradiotherapy. Tumor infiltrating lymphocytes, specifically CD8+ T-cells, are a known positive prognostic factor in many cancer types including cervical and head and neck (93,94). These cytotoxic T-cells have been shown to be inactive, as evidenced by a lack of granzyme, in a subset of HPV16+ cervical cancers (95), which other studies have shown is associated with a poor prognosis (96) implying that not only T-cell presence, but activity, is important in patient outcomes. Similarly, patients with cervical tumors with low CD8+ T-cells and Granzyme-A and Perforin expression during chemoradiotherapy were more likely to fail treatment and die of their disease (97). These tumors also had higher HPV E6 and E7 expression and it has been shown that HPV16 E7 can repress Granzyme-B expression and alter mechanisms of cytotoxic T cell killing (98,99). These studies provide evidence that HPV can directly inhibit T-cell infiltration and activation in tumors, which contributes to immune evasion and treatment resistance. More research is needed in this exciting area to determine how HPV affects the immune response prior to, and during, chemoradiotherapy such that treatment outcomes can be improved, particularly in cervical cancer.
Conclusions
HPV remains a significant cause of cancer and cancer deaths worldwide despite the advent of the HPV vaccine. Though HPV encodes only 8 genes, they affect hundreds of cellular pathways in the host target cells. HPVs have evolved to replicate viral DNA efficiently, suppress host responses to viral DNA, and promote an immune privileged microenvironment in order to ensure viral replication and production of viral particles. Unfortunately, prioritizing viral survival can have detrimental consequences for the host: viral genomes become integrated into the host DNA leading to cell cycle dysregulation, uncontrolled cell proliferation, increased genomic instability and aneuploidy, and ultimately carcinogenesis. Though this phenomenon has been studied for over 65 years and we have gained a deeper understanding of HPV-mediated carcinogenesis, there remain many questions regarding virus and host interactions. This is especially true with regard to how HPV affects anti-tumoral immunity in the context of radiotherapy and immune checkpoint blockade. Improved understanding of how HPV and its viral proteins promote cancer and affect the anti-tumoral immune response is critical for the design of improved therapies for patients with HPV associated cancers.
Acknowledgments
Financial support and disclosures: Supported in part by an ASCO Young Investigator Award (PFC), RSNA Fellow Research Grant (PFC), the Wisconsin Head and Neck Cancer SPORE grant P50DE026787 (RJK) and the UW Comprehensive Cancer Center Support Grant P30 CA014520 (RJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.International Human Papillomavirus (HPV) Reference Center [Internet]. Available from: https://www.hpvcenter.se/human_reference_clones/
- 2.Mistry N, Wibom C, Evander M. Cutaneous and mucosal human papillomaviruses differ in net surface charge, potential impact on tropism. Virol J. 2008;5(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. Global Cancer Observatory [Internet]. Available from: https://gco.iarc.fr/
- 4.Harden ME, Munger K. Human papillomavirus molecular biology. Mutat Res Rev Mutat Res. 2017June;772:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnen RL, Erickson KD, Chen XS, Garcea RL. Interactions between Papillomavirus L1 and L2 Capsid Proteins. JVI. 2003April15;77(8):4818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harden ME, Munger K. Human papillomavirus molecular biology. Mutation Research/Reviews in Mutation Research. 2017April;772:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003January;16(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doorbar J, Griffin H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Research. 2019June;7:176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aksoy P, Gottschalk EY, Meneses PI. HPV entry into cells. Mutat Res Rev Mutat Res. 2017June;772:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raff AB, Woodham AW, Raff LM, Skeate JG, Yan L, Da Silva DM, et al. The evolving field of human papillomavirus receptor research: a review of binding and entry. J Virol. 2013June;87(11):6062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pyeon D, Pearce SM, Lank SM, Ahlquist P, Lambert PF. Establishment of Human Papillomavirus Infection Requires Cell Cycle Progression. Münger K, editor. PLoS Pathog. 2009February27;5(2):e1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiGiuseppe S, Luszczek W, Keiffer TR, Bienkowska-Haba M, Guion LGM, Sapp MJ. Incoming human papillomavirus type 16 genome resides in a vesicular compartment throughout mitosis. Proc Natl Acad Sci U S A. 2016May31;113(22):6289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Z-M, Baker CC. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front Biosci. 2006September1;11:2286–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozbun MA. Human Papillomavirus Type 31b Infection of Human Keratinocytes and the Onset of Early Transcription. JVI. 2002November15;76(22):11291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedell MA, Hudson JB, Golub TR, Turyk ME, Hosken M, Wilbanks GD, et al. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J Virol. 1991May;65(5):2254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergvall M, Melendy T, Archambault J. The E1 proteins. Virology. 2013October;445(1–2):35–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011June5;414(2):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinson T, Henno L, Toots M, Ustav M, Ustav M. The Cell Cycle Timing of Human Papillomavirus DNA Replication. Zheng Z-M, editor. PLoS ONE. 2015July1;10(7):e0131675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekhar V, Reed SC, McBride AA. Interaction of the Betapapillomavirus E2 Tethering Protein with Mitotic Chromosomes. JVI. 2010January1;84(1):543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 201601;2:16086. [DOI] [PubMed] [Google Scholar]
- 21.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007September8;370(9590):890–907. [DOI] [PubMed] [Google Scholar]
- 22.Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A. 1995February28;92(5):1654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akagi K, Li J, Broutian TR, Padilla-Nash H, Xiao W, Jiang B, et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Research. 2014February1;24(2):185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorland EC, Myers SL, Gostout BS, Smith DI. Common fragile sites are preferential targets for HPV16 integrations in cervical tumors. Oncogene. 2003February;22(8):1225–37. [DOI] [PubMed] [Google Scholar]
- 25.Christiansen IK, Sandve GK, Schmitz M, Dürst M, Hovig E. Transcriptionally active regions are the preferred targets for chromosomal HPV integration in cervical carcinogenesis. PLoS One. 2015;10(3):e0119566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thierry F, Yaniv M. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987November;6(11):3391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017March;543(7645):378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudelist G, Manavi M, Pischinger KID, Watkins-Riedel T, Singer CF, Kubista E, et al. Physical state and expression of HPV DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecologic Oncology. 2004March;92(3):873–80. [DOI] [PubMed] [Google Scholar]
- 29.Hu Z, Zhu D, Wang W, Li W, Jia W, Zeng X, et al. Genome-wide profiling of HPV integration in cervical cancer identifies clustered genomic hot spots and a potential microhomology-mediated integration mechanism. Nat Genet. 2015February;47(2):158–63. [DOI] [PubMed] [Google Scholar]
- 30.Kulmala S-MA. Early integration of high copy HPV16 detectable in women with normal and low grade cervical cytology and histology. Journal of Clinical Pathology. 2006February16;59(5):513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin H-J, Joo J, Yoon JH, Yoo CW, Kim J-Y. Physical Status of Human Papillomavirus Integration in Cervical Cancer Is Associated with Treatment Outcome of the Patients Treated with Radiotherapy. Lo AWI, editor. PLoS ONE. 2014January10;9(1):e78995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J-Y, Park S, Nam B-H, Roh J-W, Lee CH, Kim Y-H, et al. Low Initial Human Papilloma Viral Load Implicates Worse Prognosis in Patients With Uterine Cervical Cancer Treated With Radiotherapy. JCO. 2009October20;27(30):5088–93. [DOI] [PubMed] [Google Scholar]
- 33.Koneva LA, Zhang Y, Virani S, Hall PB, McHugh JB, Chepeha DB, et al. HPV Integration in HNSCC Correlates with Survival Outcomes, Immune Response Signatures, and Candidate Drivers. Mol Cancer Res. 2018January;16(1):90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramqvist T, Mints M, Tertipis N, Näsman A, Romanitan M, Dalianis T. Studies on human papillomavirus (HPV) 16 E2, E5 and E7 mRNA in HPV-positive tonsillar and base of tongue cancer in relation to clinical outcome and immunological parameters. Oral Oncology. 2015December;51(12):1126–31. [DOI] [PubMed] [Google Scholar]
- 35.Morel A, Neuzillet C, Wack M, Lameiras S, Vacher S, Deloger M, et al. Mechanistic Signatures of Human Papillomavirus Insertions in Anal Squamous Cell Carcinomas. Cancers (Basel). 2019November22;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. Journal of Virology. 1989;63(10):4417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Münger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Research. 2002November;89(2):213–28. [DOI] [PubMed] [Google Scholar]
- 38.Gheit T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front Oncol. 2019May8;9:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helin K, Harlow E, Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993October;13(10):6501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chellappan S, Kraus VB, Kroger B, Munger K, Howley PM, Phelps WC, et al. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proceedings of the National Academy of Sciences. 1992May15;89(10):4549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones DL, Alani RM, Münger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997August15;11(16):2101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz JW, Jansen-Dürr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996December5;13(11):2323–30. [PubMed] [Google Scholar]
- 43.Demers GW, Halbert CL, Galloway DA. Elevated Wild-Type p53 Protein Levels in Human Epithelial Cell Lines Immortalized by the Human Papillomavirus Type 16 E7 Gene. Virology. 1994January;198(1):169–74. [DOI] [PubMed] [Google Scholar]
- 44.Scheffner M, Whitaker NJ. Human papillomavirus-induced carcinogenesis and the ubiquitin–proteasome system. Seminars in Cancer Biology. 2003February;13(1):59–67. [DOI] [PubMed] [Google Scholar]
- 45.Lechner MS, Laimins LA. Inhibition of p53 DNA binding by human papillomavirus E6 proteins. J Virol. 1994July;68(7):4262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013August1;73(15):4791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantovani F, Banks L. The Human Papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001November;20(54):7874–87. [DOI] [PubMed] [Google Scholar]
- 48.Katzenellenbogen RA . Activation of telomerase by HPVs. Virus Res. 2017March2;231:50–5. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Dakic A, Zhang Y, Dai Y, Chen R, Schlegel R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc Natl Acad Sci U S A. 2009November3;106(44):18780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas M, Narayan N, Pim D, Tomaić V, Massimi P, Nagasaka K, et al. Human papillomaviruses, cervical cancer and cell polarity. Oncogene. 2008November;27(55):7018–30. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Coffino P. High-risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol. 1996July;70(7):4509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989December20;8(13):4099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khleif SN, DeGregori J, Yee CL, Otterson GA, Kaye FJ, Nevins JR, et al. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci U S A. 1996April30;93(9):4350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Medema RH, Herrera RE, Lam F, Weinberg RA. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci U S A. 1995July3;92(14):6289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaughlin-Drubin ME, Park D, Munger K. Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 2013October1;110(40):16175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010July1;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006February10;24(5):736–47. [DOI] [PubMed] [Google Scholar]
- 58.Gao G, Chernock RD, Gay HA, Thorstad WL, Zhang TR, Wang H, et al. A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer. 2013February15;132(4):882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Y, Ren C, Yang L, Zhang X, Liu L, Wang Z. Performance of p16/Ki67 immunostaining, HPV E6/E7 mRNA testing, and HPV DNA assay to detect high-grade cervical dysplasia in women with ASCUS. BMC Cancer. 2019March27;19(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pei XF, Meck JM, Greenhalgh D, Schlegel R. Cotransfection of HPV-18 and v-fos DNA Induces Tumorigenicity of Primary Human Keratinocytes. Virology. 1993October;196(2):855–60. [DOI] [PubMed] [Google Scholar]
- 61.White AE, Livanos EM, Tlsty TD. Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev. 1994March15;8(6):666–77. [DOI] [PubMed] [Google Scholar]
- 62.Pett MR, Alazawi WOF, Roberts I, Dowen S, Smith DI, Stanley MA, et al. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 2004February15;64(4):1359–68. [DOI] [PubMed] [Google Scholar]
- 63.Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A. 2000August29;97(18):10002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duensing S, Münger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 2002December1;62(23):7075–82. [PubMed] [Google Scholar]
- 65.Sitz J, Blanchet SA, Gameiro SF, Biquand E, Morgan TM, Galloy M, et al. Human papillomavirus E7 oncoprotein targets RNF168 to hijack the host DNA damage response. Proc Natl Acad Sci U S A. 2019September24;116(39):19552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spriggs CC, Laimins LA. Human Papillomavirus and the DNA Damage Response: Exploiting Host Repair Pathways for Viral Replication. Viruses. 2017August18;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spardy N, Covella K, Cha E, Hoskins EE, Wells SI, Duensing A, et al. Human papillomavirus 16 E7 oncoprotein attenuates DNA damage checkpoint control by increasing the proteolytic turnover of claspin. Cancer Res. 2009September1;69(17):7022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spriggs CC, Blanco LZ, Maniar KP, Laimins LA. Expression of HPV-induced DNA Damage Repair Factors Correlates With CIN Progression. Int J Gynecol Pathol. 2019January;38(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park JW, Shin M-K, Pitot HC, Lambert PF. High incidence of HPV-associated head and neck cancers in FA deficient mice is associated with E7’s induction of DNA damage through its inactivation of pocket proteins. PLoS One. 2013;8(9):e75056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weaver AN, Cooper TS, Rodriguez M, Trummell HQ, Bonner JA, Rosenthal EL, et al. DNA double strand break repair defect and sensitivity to poly ADP-ribose polymerase (PARP) inhibition in human papillomavirus 16-positive head and neck squamous cell carcinoma. Oncotarget. 2015September29;6(29):26995–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leeman JE, Li Y, Bell A, Hussain SS, Majumdar R, Rong-Mullins X, et al. Human papillomavirus 16 promotes microhomology-mediated end-joining. Proc Natl Acad Sci U S A. 2019October22;116(43):21573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanley MA. Epithelial Cell Responses to Infection with Human Papillomavirus. Clinical Microbiology Reviews. 2012April1;25(2):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013February15;339(6121):786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016September20;17(10):1142–9. [DOI] [PubMed] [Google Scholar]
- 75.Lau L, Gray EE, Brunette RL, Stetson DB. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. 2015October30;350(6260):568–71. [DOI] [PubMed] [Google Scholar]
- 76.Luo X, Donnelly CR, Gong W, Heath BR, Hao Y, Donnelly LA, et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J Clin Invest. 2020April1;130(4):1635–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park JS, Kim EJ, Kwon HJ, Hwang ES, Namkoong SE, Um SJ. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000March10;275(10):6764–9. [DOI] [PubMed] [Google Scholar]
- 78.Barnard P, Payne E, McMillan NA. The human papillomavirus E7 protein is able to inhibit the antiviral and antigrowth functions of interferon-alpha. Virology. 2000November25;277(2):411–9. [DOI] [PubMed] [Google Scholar]
- 79.Hong S, Mehta KP, Laimins LA. Suppression of STAT-1 expression by human papillomaviruses is necessary for differentiation-dependent genome amplification and plasmid maintenance. J Virol. 2011September;85(18):9486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Um S-J, Rhyu J-W, Kim E-J, Jeon K-C, Hwang E-S, Park J-S. Abrogation of IRF-1 response by high-risk HPV E7 protein in vivo. Cancer Letters. 2002May28;179(2):205–12. [DOI] [PubMed] [Google Scholar]
- 81.Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998July1;12(13):2061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang S-M, McCance DJ. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J Virol. 2002September;76(17):8710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel D, Huang S-M, Baglia LA, McCance DJ. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. The EMBO Journal. 1999September15;18(18):5061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zimmermann H, Degenkolbe R, Bernard H-U, O’Connor MJ. The Human Papillomavirus Type 16 E6 Oncoprotein Can Down-Regulate p53 Activity by Targeting the Transcriptional Coactivator CBP/p300. J Virol. 1999August;73(8):6209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scott ML, Woodby BL, Ulicny J, Raikhy G, Orr AW, Songock WK, et al. Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance. Banks L, editor. J Virol. 2019October30;94(2):e01582–19, /jvi/94/2/JVI.01582–19.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reiser J, Hurst J, Voges M, Krauss P, Münch P, Iftner T, et al. High-risk human papillomaviruses repress constitutive kappa interferon transcription via E6 to prevent pathogen recognition receptor and antiviral-gene expression. J Virol. 2011November;85(21):11372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyauchi S, Sanders PD, Guram K, Kim SS, Paolini F, Venuti A, et al. HPV16 E5 Mediates Resistance to PD-L1 Blockade and Can Be Targeted with Rimantadine in Head and Neck Cancer. Cancer Res. 2020February15;80(4):732–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Westrich JA, Warren CJ, Pyeon D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017March2;231:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mota F, Rayment N, Chong S, Singer A, Chain B. The antigen-presenting environment in normal and human papillomavirus (HPV)-related premalignant cervical epithelium. Clin Exp Immunol. 1999April;116(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caberg J-H, Hubert P, Herman L, Herfs M, Roncarati P, Boniver J, et al. Increased migration of Langerhans cells in response to HPV16 E6 and E7 oncogene silencing: role of CCL20. Cancer Immunol Immunother. 2009January;58(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borgne ML, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, et al. Dendritic Cells Rapidly Recruited into Epithelial Tissues via CCR6/CCL20 Are Responsible for CD8+ T Cell Crosspriming In Vivo. Immunity. 2006February1;24(2):191–201. [DOI] [PubMed] [Google Scholar]
- 92.Pahne-Zeppenfeld J, Schröer N, Walch-Rückheim B, Oldak M, Gorter A, Hegde S, et al. Cervical cancer cell-derived interleukin-6 impairs CCR7-dependent migration of MMP-9-expressing dendritic cells. International Journal of Cancer. 2014;134(9):2061–73. [DOI] [PubMed] [Google Scholar]
- 93.Balermpas P, Rödel F, Rödel C, Krause M, Linge A, Lohaus F, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int J Cancer. 2016January1;138(1):171–81. [DOI] [PubMed] [Google Scholar]
- 94.Piersma SJ, Jordanova ES, van Poelgeest MIE, Kwappenberg KMC, van der Hulst JM, Drijfhout JW, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007January1;67(1):354–61. [DOI] [PubMed] [Google Scholar]
- 95.Cromme FV, Walboomers JMM, Van Oostveen JW, Stukart MJ, De Gruijl TD, Kummer JA, et al. Lack of granzyme expression in T lymphocytes indicates poor cytotoxic T lymphocyte activation in human papillomavirus-associated cervical carcinomas. Int J Gynecol Cancer. 1995September;5(5):366–73. [DOI] [PubMed] [Google Scholar]
- 96.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015January15;160(1–2):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cosper PF, McNair C, González I, Wong N, Knudsen KE, Chen JJ, et al. Decreased local immune response and retained HPV gene expression during chemoradiotherapy are associated with treatment resistance and death from cervical cancer. Int J Cancer. 2020April1;146(7):2047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Munguía-Moreno JA, Díaz-Chavéz J, García-Villa E, Albino-Sanchez ME, Mendoza-Villanueva D, Ocadiz-Delgado R, et al. Early synergistic interactions between the HPV16-E7 oncoprotein and 17β-oestradiol for repressing the expression of Granzyme B in a cervical cancer model. Int J Oncol. 2018August;53(2):579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhat P, Bergot A-S, Waterhouse N, Frazer IH. Human papillomavirus E7 oncoprotein expression by keratinocytes alters the cytotoxic mechanisms used by CD8 T cells. Oncotarget. 2018January19;9(5):6015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]