Abstract
INTRODUCTION
In chronic obstructive pulmonary disease (COPD), macrophages play an indispensable role. In the lung tissues of COPD patients and smokers, macrophages can be observed to polarize towards M2 phenotype. The molecular mechanism of this process is unclear, and it has not been fully elucidated in COPD.
METHODS
We bought laboratory animals [C57BL/6 and miR-21–/– C57BL/6(F1)] from the Jackson Laboratory. The model of COPD mice was established by cigarette smoke (CS) exposure combined with intraperitoneal injection of cigarette smoke extract (CSE). RT-PCR detected the expression levels of inflammatory factors and markers associated with M1 and M2 macrophages. The ratio of M2 macrophages to M1 macrophages was detected by immunohistochemical staining.
RESULTS
The level of miR-21 was increased in RAW264.7 cells intervened by CSE and in lung tissue and bone marrow-derived macrophages (BMDMs) from COPD mice. CSE can gradually over time increase the level of miR-21. The proportion of M2 macrophages to M1 macrophages had a positive correlation with miR-21. Knockdowning miR-21 can reduce lung tissue damage. CSE also increased the levels of related inflammatory factors and markers associated with M2 macrophages, and an miR-21 inhibitor can reverse this conversion.
CONCLUSIONS
We confirmed that CSE can lead to macrophage transformation to the M2 phenotype and an increase in the expression level of miR-21. Knockdown of the miR-21 gene could inhibit the transformation of macrophages to the M2 phenotype in COPD.
Keywords: macrophage polarization, chronic obstructive pulmonary disease, miR-21, cigarette smoke extract
INTRODUCTION
COPD is one of the diseases with high economic burden and mortality in the world. It is estimated that more than 300 million people were affected by COPD in 2020 in the world, and 4.7 million of the 68 million deaths are caused by COPD1-3. The number of macrophages is increasing in the small airways of COPD patients4, and there is a close relationship between the number of macrophages and the severity of COPD4,5. Multiple studies have shown that the number of macrophages in the lung tissue of COPD patients is increased; in addition, there is a correlation between disease progression and the number of macrophages5,6. Alveolar macrophages (AM) play an indispensable role in the occurrence and development of COPD, and, at the same time, it can affect the structure of lung tissue through the initiation and degeneration of inflammation7.
Mutative exogenous stimuli and pathological processes can change the phenotypes and functional characteristics of alveolar macrophages8. Since a large number of M1-type macrophage-related cytokines, such as TNF-α and IL-8, are found in bronchoalveolar lavage fluid (BAL), pulmonary macrophages from patients with COPD have been recommended to display M1 phenotypes9. In addition, TNF-α has been shown to be closely related to cigarette smoking-induced emphysema in mice10. Research has shown that CS can induce macrophages to reprogram to the M2 phenotype in patients with COPD11. In vitro, using different concentrations of CSE to interfere with macrophages, the expression of M2 macrophagerelated markers (CD163, CD204, and CD206)12-14 and related factors (IL-10, TGF-β1, TGF-β2) was up-regulated15,16. Similarly, our previous studies showed that the proportion of M2 macrophages to M1 macrophages in BAL and lung tissue in a COPD mouse model were both significantly increased17,18. A number of etiology factors may contribute to changes in macrophage phenotypes including gram-negative anaerobe actinomycetes (AA), smoking and porphyromonas gingival (PG), and smoking is the most important factor19. However, the regulatory mechanism of macrophage polarization in COPD is unclear.
MicroRNAs (miRNAs) are small endogenous non-coding RNAs which bind in a sequence-specific manner to incomplete complementary sites in target messenger RNA (mRNA), thereby directly inhibiting protein translation or transcriptome degradation. In this way, miRNAs can simultaneously interact with hundreds of genes and regulate various developmental and physiological processes, including cell proliferation, differentiation, apoptosis, and innate and adaptive immune responses20. The research shows that miRNAs influence macrophage activation and polarization21. Our previous study confirmed that miR-21 was increased in lungs from a COPD mouse model and the peripheral blood of COPD patients, and its expression level correlated with lung function22. However, whether miR-21 is involved in COPD development by targeting macrophage polarization is unknown.
Therefore, in this study, we used CS exposure combined with intraperitoneal injection of CSE to construct miR-21–/– COPD animal models, to observe the effect of CSE on miR-21 and investigate the possible role of miR-21 in COPD-associated macrophage polarization.
METHODS
Animals, preparation of CSE and animal model
C57BL/6 and miR-21–/– C57BL/6(F1) mice were randomly divided into a control group and a COPD model group, all of which were purchased from the Jackson Laboratory. All animals were kept in clean rooms with a temperature of 23–25°C, a humidity of 50–60% and a 12-hour light/dark cycle. The study followed the guidelines for animal and human research23. The preparation of CSE followed previous procedure24. The COPD mice model was established by CS exposure combined with intraperitoneal injection of CSE. The whole experiment lasted 28 days. The modeling box was made as previously described25. First, five cigarettes were lit simultaneously for 15 minutes. Second, the box is opened and the animals allowed to rest for five minutes. Then, the first step is repeat. The entire process is called CS exposure cycle, with exposure twice a day during the entire experimental cycle, except for the 1st, the 12th, and 23rd day; the control group was kept in the experimental animal center of the Third Xiangya Hospital of Central South University. The intraperitoneal injection of CSE was carried out according to the previous method. On days 1, 12, and 23, the control animals were intraperitoneally injected with 0.3 mL/20 g PBS, while the model animals were intraperitoneally injected with 0.3 mL/20 g CSEPBS. On day 29, the lung function of the mice was measured, and lung tissue and BMDMs were collected. This research was approved by the Institutional Review Board of Central-South University and followed the guidelines for animal and human research23.
Cell culture and production of BMDMs
RAW264.7 and L929 cell lines were purchased from the American Type Culture Collection and were cultured using DMEM (Life Technologies, Carlsbad, CA, USA) containing 10% (v/v) heat-inactivated FBS. According to our previous experiments, CSE interferes with RAW264.7 cells at a concentration of 5%. BMDMs are established as previously described26.
Histomorphology of lung tissue
The left lower lung was lavaged with 4% paraformaldehyde, and then fixed with 4% paraformaldehyde overnight27. The lung tissue was embedded in paraffin (Sigma, MO, USA) , cut it into 4 μm sections after embedding, and stained with hematoxylin and eosin (H & E) (Sigma). The three indicators of mean linear intercept (MLI), mean alveolar interval thickness (MAST) and destruction index (DI) were used to quantify the pathological severity of emphysema. The measurement methods have been previously described25.
Immunohistochemical staining
The dewaxing and descaling sections were incubated with 1% H2O2 at room temperature for 30 minutes to eliminate endogenous peroxidase activity, and then anti-CD68, anti-CD206 and anti-CD86 antibodies (ProInTech, Chicago, IL, USA) were added and incubated overnight at 4°C. The sections were washed thoroughly on the second day and incubated with the secondary antibody for 1 hour at room temperature. The secondary antibody needs to be coupled with appropriate horseradish peroxidase. The unreacted secondary antibody was removed and the section was incubated with 3,39-diaminobiphenyl-4HCl (DAB; Sigma, MO, USA) -H2O2 solution to observe the immunolabeling, and then HE was used to counterstain the section using a fixed glass cover. The presence of CD68, CD206 and CD86 can be indicated by varying degrees of tan or brown particles or flake deposits. Four or five CD68, CD206 and CD86 positive images were randomly selected to calculate the integrated photometric value (IOD) and average optical density (AOD). Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) software was used for high magnification of each slice to visualize high-definition color pathological images and analyze the results.
Transient miRNA transfections
The miRNA-21 inhibitor was purchased from Qiagen, and the all-star negative mimic (Qiagen) was used as a control. According to the transfection reagent instructions, the cells were transiently transfected with Hiperfect Transfection Reagent (Qiagen) at a final concentration of 50 nM. Red siGLO oligos (Thermo Fisher Scientific, Waltham, MA, USA) was used to confirm the transfection efficiency of the cells. The transfection efficiency was greater than 90% and the transfection was considered successful28.
Quantitative real-time PCR
According to the instructions, TRIzol reagent (Takara, Dalian, Liaoning, China) is used to isolate total RNA, and SYBR Green PCR Master Mix (Bio-Rad, CA, USA) used to detect mRNA levels. The mRNA expression was detected using a two-step quantitative real-time polymerase chain reaction (Applied Biosystems, Carlsbad, CA, USA). As endogenous control β-actin was used, and the DD cycle threshold method was used to determine the relevant expression level. Table 1 identifies the primer sequences.
Table 1.
Primer sequences of the genes investigated in RT-PCR analysis
| Gene | Primer sequences |
|---|---|
| Β-Actin | F 5'-AACGGCTCCGGCATGTGCAA-3' |
| R 5'-CTTCTGACCCATGCCCACCA-3' | |
| TNF-a | F 5'-CAGCCTCTTCTCCTTCCTGAT-3' |
| R 5'-GCCAGAGGGCTGATTAGAGA-3' | |
| IL-10 | F5'-ACCAAGACCCAGACATCA-3' |
| R 5'-TTCACAGGGAAGAAATCG-3' | |
| IL-6 | F 5'-GGAACTCTACCAGAAATATAGC-3' |
| R 5'-CCTGTATTCCGTCTCCTTG-3' | |
| CD206 | F 5'-GTGGAGTGATGGAACCCCAG-3' |
| R 5'-CTGTCCGCCCAGTATCCATC-3' | |
| Ym1 | F 5'-GAAGGAGCCACTGAGGTCTG-3' |
| R 5'-TGTTGTCCTTGAGCCACTGA-3' | |
| Fizz1 | F 5'-CGTGGAGAATAAGGTCAAGGA-3' |
| R 5'-CAGTAGCAGTCATCCCAGCA-3' | |
| ARG1 | F 5'-GCATATCTGCCAAAGACATCG-3' |
| R 5'-CCATCACCTTGCCAATCCC-3' | |
| iNOS | F 5'-AGGGAATCTTGGAGCGAGTT-3' |
| R 5'- GCAGCCTCTTGTCTTTGACC-3' |
Statistical analysis
The results were described using mean ± standard deviation, and GraphPad Prism Software 6.0 (GraphPad Software, La Jolla, CA, USA) was used for data analysis. One-way analysis of variance was used for statistical evaluation, and the correlation between groups was analyzed using the Pearson correlation coefficient. A p<0.05 was considered statistically significant.
RESULTS
The expression of miR-21 is up-regulated in RAW264.7 cells intervened by CSE and lung tissue and BMDMs from COPD mouse model.
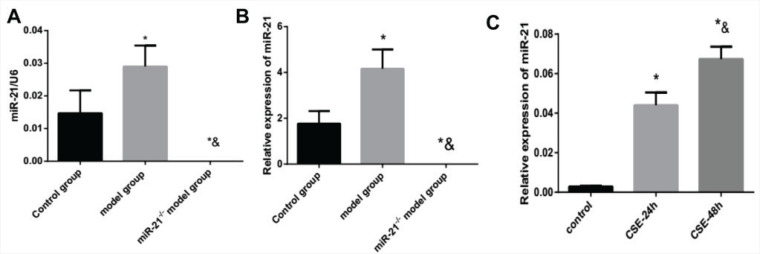
Our experiment detected the expression of miR-21 in RAW264.7 cells intervened by CSE and lung tissue and BMDMs from COPD mouse model and miR-21-/- mice using RT-PCR. The RAW264.7 cells were intervened by 5% CSE for 24 h and 48 h in vitro. Compared with the control group, the expression of miR-21 in lung tissues and BMDMs of COPD mice was significantly increased (p<0.05, Figures 1A and 1B). Moreover, CSE stimulation increased the expression of miR-21, which gradually rose over time (p<0.05, Figure 1C).
Figure 1.
The expression of miR-21 is up-regulated by cigarette smoke extracts. A: the expression level of miR-21 in lung tissue. Control group: normal control group. Model group: C57BL/6 mice were intervened by CS exposure combined with CSE intraperitoneal injection. The miR-21–/– model group: miR-21–/– C57BL/6 mice were intervened by CS exposure combined with CSE intraperitoneal injection. *p<0.05, versus the control group; *&p<0.05, versus COPD model group. B: the expression level of miR-21 in BMDMs.*p<0.05, versus the control group; *&p<0.05, versus COPD mouse model. C: the expression level of miR-21 in RAW264.7 cells treated with CSE. *p<0.05, versus the control group. *&p<0.05, versus the CSE-24h group.
The change in M2/M1 macrophages and its correlation with miR-21
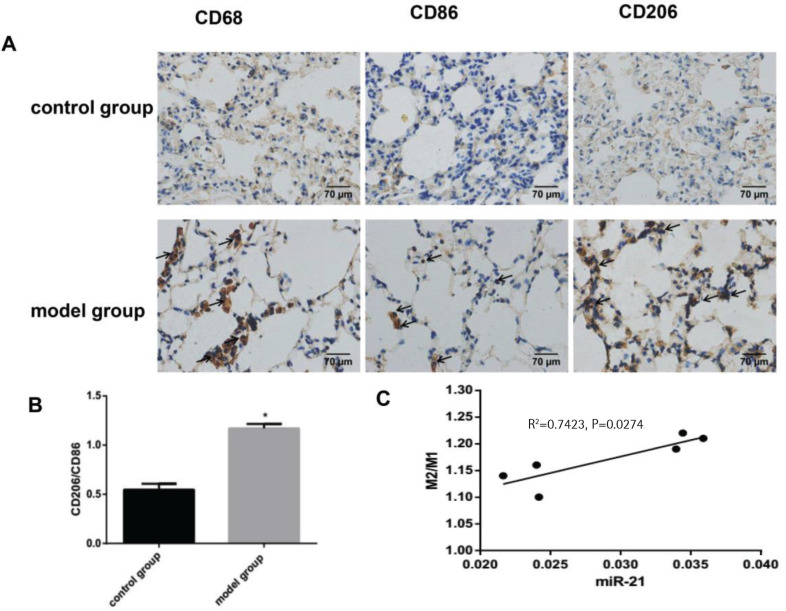
In this experiment, immunohistochemical staining showed a rising proportion of M2 macrophages to M1 macrophages, which indicated the macrophages in the lung tissue of COPD mice had a tendency to polarize towards M2 macrophages (p<0.05, Figures 2A and 2B). Furthermore, the proportion of M2 macrophages to M1 macrophages was positively correlated with the expression of miR-21 (p<0.05, Figure 2C). In our COPD mice induced by the combination of CS exposure and intraperitoneal injection of CSE, macrophages were more likely to have the M2 phenotype.
Figure 2.
The change in M2/M1 macrophages and its correlation with miR-21. Control group: normal control group. Model group: C57BL/6 mice were intervened by CS exposure combined with CSE intraperitoneal injection. A: the protein expression levels of CD68, CD86 and CD206 as well as immunohistochemical staining for CD206 expression in lung tissue from COPD mouse model showing increased deposition of brown granules in cell membranes (black arrows, 400×magnification). B: ratio of M2/M1 macrophages. *p<0.05, versus the control group. C: ratio of M2 /M1 macrophages and its correlation with miR-21.
The pathological morphological changes in lung tissue after knocking out miR-21
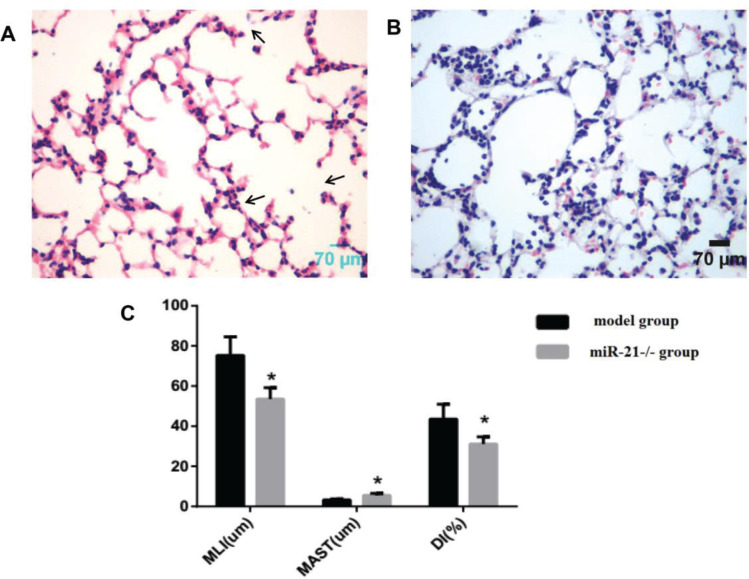
Using the same way to build the COPD mouse model, the alveolar septum thickness was increased compared to wild-type mice, and the alveolar size and destruction of the alveolar wall were reduced in miR-21 knockout mice (Figures 3A and 3B). MLI and DI were reduced, and MAST was increased in miR-21 knockout mice (p<0.05, Figure 3C).
Figure 3.
The pathological morphological changes of lung tissue after knocking out miR-21 while using the same method to build the COPD model. Model group: C57BL/6 mice were intervened by CS exposure combined with CSE intraperitoneal injection. The miR-21–/– group: miR-21–/– C57BL/6 mice were intervened by CS exposure combined with CSE intraperitoneal injection. A: HE staining of lung tissue from model mice (400×magnification) showing an enlarged alveolar space, a thinner alveolar septum and a destroyed alveolar wall (black arrows). B: HE staining of lung tissue from miR-21-/- mice (400×magnification) showing increased alveolar septum thickness, reduced alveolar size and destruction of the alveolar wall. C: comparison of lung histomorphology in the model group and the miR-21-/- group. MLI (μm): mean linear intercept. MAST (μm): mean alveolar septal thickness. DI (%): destruction index. *p<0.05, versus the model group.
The effect of CSE and miR-21 on macrophage polarization in BMDMs and RAW264.7 cells
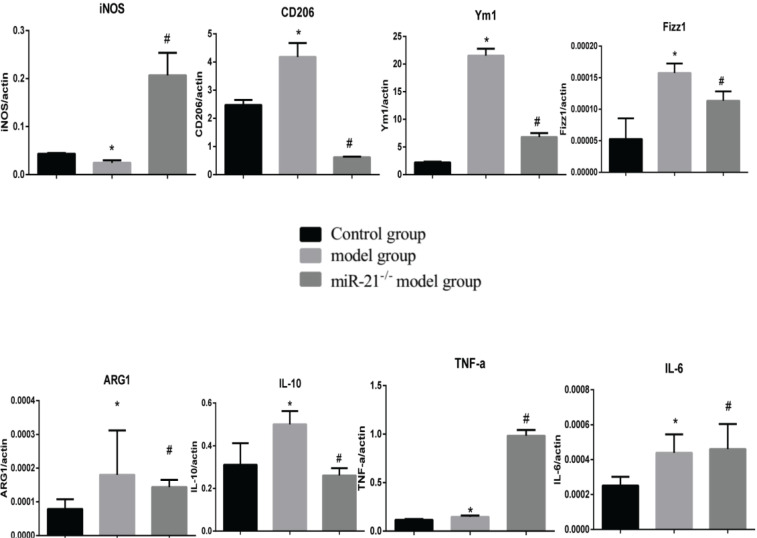
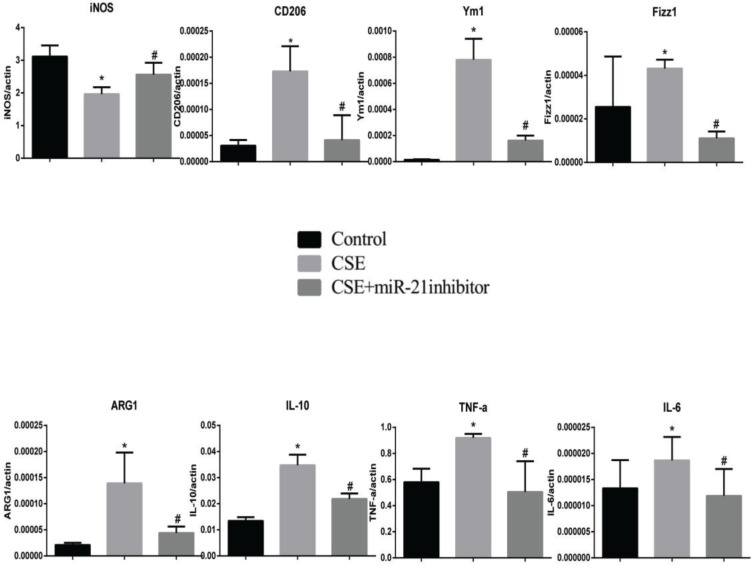
To clarify the effects of CSE and miR-21 on the polarization of macrophages, we used RT-PCR to detect the expression levels of inflammatory factors and markers associated with M1 and M2 macrophages (Table 1). Inducible nitric oxide synthase (iNOS) , IL-6 and TNF-a were representative markers for M1 macrophages, and CD206, arginase 1 (ARG1), chitinase-like secretory lectin Ym1, resistin-like-a (Fizz1) and IL-10 were used as representative markers of M2 macrophages. Results suggested, compared with the control group, in BMDMs of COPD mice and RAW264.7 cells intervened by CSE, the mRNA expression levels of inflammatory factors and markers associated with M2 macrophages (CD206, Arg1, YM1, FizZ1, and IL-10) were increased (p<0.05, Figures 4 and 5), and the expression of iNOS was decreased (p<0.05, Figures 4 and 5). In the BMDMs from the miR-21-/- model group, the mRNA expression levels of inflammatory factors and markers associated with M2 macrophages (CD206, Arg1, YM1, FizZ1, and IL-10) decreased (p<0.05, Figure 4), whereas the RNA expression level of inflammatory factors and markers associated with M1 macrophages (iNOS and TNF-a) increased (p<0.05, Figure 4B). However, there is no change in expression level of IL-6 (p>0.05, Figure 4). Adding miR-21 inhibitor to the RAW264.7 cells intervened by CSE reduced the changes of RNA expression levels of CD206, Arg1, YM1, FizZ1 and IL-10 (p<0.05, Figure 5), while the RNA expression levels of iNOS, IL-10 and IL-6 were increased (p<0.05, Figure 5).
Figure 4.
RNA expression levels of M1/M2 macrophages-related cytokines and markers in BMDMs from the control, COPD and miR-21–/– COPD groups. Control group: normal control group. Model group: C57BL/6 mice were intervened by CS exposure combined with CSE intraperitoneal injection. The miR-21–/– model group: miR-21–/– C57BL/6 mice were intervened by CS exposure combined with CSE intraperitoneal injection. *p<0.05, versus the control group. #p<0.05, versus the model group.
Figure 5.
RNA expression levels of M1/M2 macrophages-related cytokines and markers in RAW264.7 cells from the control, cigarette smoke extract (CSE)-treated and miR-21 inhibitor-treated groups. CSE: 5% CSE interfering with RAW264.7 cells. CSE+ miR-21 inhibitor: miR-21 inhibitor interfering with CSE-treated RAW264.7 cells. *p<0.05, versus the control group. #p<0.05, versus the CSE group.
DISCUSSION
We discovered the miR-21 expression was increased in RAW264.7 cells intervened by CSE and gradually increased with the CSE-intervention duration. At the same time, compared with the control group, the expression of miR-21 in BDMDs cells from COPD mice was significantly increased, which was basically consistent with our previous experimental results22. As an important regulator, miRNAs exert a fine regulatory function on specific cellular events in the development of various organs, including the lungs. Our previous study also confirmed the differential expression of miRNAs in lung tissues of COPD rats and may have an important impact on the occurrence and development of COPD22,29. At the same time, our experiments suggested miR-21 is closely related to pathological changes in lung tissue. When our miR-21- / - mice were modelled using the same method as the method for wild-type mice, we found that compared with wild-type mice, miR-21- / - mice have significantly reduced alveolar damage, which suggests miR-21 may have an important impact on the formation of emphysema and knockout miR-21 can prevent the occurrence of emphysema as well as provide a new targeted therapy for the treatment of COPD.
Research has suggested CD68+ macrophages were increased in bronchial mucosa from COPD patients with mild/moderate stability compared with a control group30. Previously, the expression of CD68+ cells in the small airways was inaccurate, but now studies have shown that compared with smokers with normal lung function, the number of CD68+ macrophages in small airways increases with the severity of COPD31. Our experiments also verified that the protein expression of CD68 expression was increased in the COPD mouse model. It is currently unclear whether M1 or M2 macrophages are dominant in COPD, and it is possible that an intermediate phenotype exists32. Our previous study showed that macrophage polarization occurred in bronchoalveolar lavage fluid in COPD mice and that there was a tendency towards M2 macrophages, which were closely associated with lung function and lung tissue destruction17. Our experiment also confirmed that compared with the control group, the ratio of CD206/CD86 is significantly increased, which was consistent with our previous results18; by adopting the two continuous section-contrasted staining method we confirmed that the proportion of M2 macrophages to M1 macrophages increased in the lung tissue from COPD mouse model18.
MiRNAs may have an important influence and effect on the maturation of monocytes/macrophages. Our experiments verified that miR-21 inhibitors could decrease the expression of related inflammatory factors and markers associated with M2 macrophages in RAW264.7 cells with a CSE intervention. We found that M2 macrophage-related markers and factor expression were reduced in miR-21 knockout mice BDMDs cells compared with wild-type model mice, which indicated that miR-21 can mediate macrophage phenotype changes. Consistent with our observations, miR-21 is the central site for the mediation of macrophage phenotype changes from pro-inflammatory (M1) to pro-apoptotic (M2) via phagocytosis (phagocytotic apoptotic cells)33.
We also found that in different intervention environments, the expression of TNF-a, IL-6 and IL-10 may be different, which indicates that CSE-induced macrophage transformation may exist between M1 and M2 dynamic changes. Additionally, inflammatory cytokines and immunosuppressive factors coexist, which is a condition that results in a low but persistent inflammation and leads to COPD, and regulation of macrophage polarization can be used as a treatment for COPD patients34. Our experiment also confirmed this paradox, which indicates that miR-21 may exert a different effect at different stages of COPD and may lead to the coexistence of a variety of macrophage types. This requires further study to discover the role of this dynamic change in COPD. Studies have shown that pri-miR-21 may have an effect in the early stages of inflammation, while mature miR-21 has an effect on inflammation repair35. At an early stage of inflammation, pri-miR-21 is predominant and exhibits proinflammatory effects. In contrast, during the inflammatory repair stage, mature miR-21 exerts an anti-inflammatory effect and also causes macrophages to transform into M2 macrophages, which exhibits a low inflammation level and an immunosuppressive state and leads to the continuation of inflammation. Whether this phenomenon exists in COPD requires further study. Moreover, our previous study establishing a dynamic COPD rat model showed that miR-21 expression was a dynamic process.
Limitations
Our experiment has some limitations. First, the mechanism of miR-21 on macrophage polarization was not studied in depth. Second, we have not studied the specific role of miR-21 in COPD, for example, epithelial-mesenchymal transition, endothelial-mesenchymal transition, and small airway remodeling etc. We thus need to further study the mechanism and the function of miR-21 and M2 macrophages in COPD.
CONCLUSIONS
CSE can lead to macrophage transformation to the M2 phenotype and a rise in the expression of miR-21. Knockout of the miR-21 gene may reduce lung injury in COPD mouse model and could inhibit the transformation of macrophages to the M2 phenotype in COPD.
CONFLICTS OF INTEREST
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none was reported.
FUNDING
This study was supported by grants from the National Natural Science Foundation of China (No. 81800044).
ETHICAL APPROVAL AND INFORMED CONSENT
This research was approved by the Institutional Review Board of Central-South University (No. 81800044, 2019.01-2021.12) and followed the guidelines for animal and human research.
DATA AVAILABILITY
The data supporting this research are available from the authors on reasonable request.
AUTHORS’ CONTRIBUTIONS
JJL conceived the study, performed the experiment, data analysis, and drafted the manuscript. SHS coordinated the study. LHX guided the experiment. All authors read and approved the final manuscript.
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer reviewed.
REFERENCES
- 1.Vogelmeier CF, Criner GJ, Martínez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. In Spanish. Arch Bronconeumol. 2017;53(3):128–149. doi: 10.1016/j.arbres.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Negewo NA, Gibson PG, Wark PA, Simpson JL, McDonald VM. Treatment burden, clinical outcomes, and comorbidities in COPD: an examination of the utility of medication regimen complexity index in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2929–2942. doi: 10.2147/COPD.S136256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/s0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Stefano A, Capelli A, Lusuardi M, et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med. 1998;158(4):1277–1285. doi: 10.1164/ajrccm.158.4.9802078. [DOI] [PubMed] [Google Scholar]
- 5.Hogg JC, Paré PD, Hackett TL. The Contribution of Small Airway Obstruction to the Pathogenesis of Chronic Obstructive Pulmonary Disease. Physiol Rev. 2017;97(2):529–552. doi: 10.1152/physrev.00025.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortaz E, Folkerts G, Redegeld F. Mast cells and COPD. Pulm Pharmacol Ther. 2011;24(4):367–372. doi: 10.1016/j.pupt.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 2014;5:435. doi: 10.3389/fimmu.2014.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S, Plüddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262(1):36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangedal S, Aanerud M, Persson LJ, Brokstad KA, Bakke PS, Eagan TM. Comparison of inflammatory markers in induced and spontaneous sputum in a cohort of COPD patients. Respir Res. 2014;15(1):138. doi: 10.1186/s12931-014-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med. 2004;170(5):492–498. doi: 10.1164/rccm.200404-511OC. [DOI] [PubMed] [Google Scholar]
- 11.Shaykhiev R, Krause A, Salit J, et al. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J Immunol. 2009;183(4):2867–2883. doi: 10.4049/jimmunol.0900473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunz LI, Lapperre TS, Snoeck-Stroband JB, et al . Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respir Res. 2011;12(1) doi: 10.1186/1465-9921-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaku Y, Imaoka H, Morimatsu Y, et al. Overexpression of CD163, CD204 and CD206 on alveolar macrophages in the lungs of patients with severe chronic obstructive pulmonary disease. PLoS One. 2014;9(1):e87400. doi: 10.1371/journal.pone.0087400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazzan E, Turato G, Tinè M, et al. Dual polarization of human alveolar macrophages progressively increases with smoking and COPD severity. Respir Res. 2017;18(1):40. doi: 10.1186/s12931-017-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan F, Fu X, Shi H, Chen G, Dong P, Zhang W. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS One. 2014;9(9):e107063. doi: 10.1371/journal.pone.0107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng H, Yin Y, Ren Y, et al. Effect of CSE on M1/M2 polarization in alveolar and peritoneal macrophages at different concentrations and exposure in vitro. In Vitro Cell Dev Biol Anim. 2020;56(2):154–164. doi: 10.1007/s11626-019-00426-4. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Xie L, Liu C, Zhang Q, Sun S. PTEN/PI3k/AKT Regulates Macrophage Polarization in Emphysematous mice. Scand J Immunol. 2017;85(6):395–405. doi: 10.1111/sji.12545. [DOI] [PubMed] [Google Scholar]
- 18.He S, Xie L, Lu J, Sun S. Characteristics and potential role of M2 macrophages in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3029–3039. doi: 10.2147/COPD.S147144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonetti MS. Cigarette smoking and periodontal diseases: etiology and management of disease. Ann Periodontol. 1998;3(1):88–101. doi: 10.1902/annals.1998.3.1.88. [DOI] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 21.Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61(1):91–103. doi: 10.1002/glia.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie L, Wu M, Lin H, et al. An increased ratio of serum miR-21 to miR-181a levels is associated with the early pathogenic process of chronic obstructive pulmonary disease in asymptomatic heavy smokers. Mol Biosyst. 2014;10(5):1072–1081. doi: 10.1039/c3mb70564a. [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association, American Physiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283(2):R281–R283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Hanaoka M, Droma Y, Chen P, Voelkel NF, Kubo K. Endothelin-1 receptor antagonists prevent the development of pulmonary emphysema in rats. Eur Respir J. 2010;35(4):904–912. doi: 10.1183/09031936.00003909. [DOI] [PubMed] [Google Scholar]
- 25.He ZH, Chen P, Chen Y, et al. Comparison between cigarette smoke-induced emphysema and cigarette smoke extract-induced emphysema. Tob Induc Dis. 2015;13(March) doi: 10.1186/s12971-015-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185(11):1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao H, Chung S, Hwang JW, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest. 2012;122(6):2032–2045. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fordham JB, Naqvi AR, Nares S. Regulation of miR-24, miR-30b, and miR-142-3p during macrophage and dendritic cell differentiation potentiates innate immunity. J Leukoc Biol. 2015;98(2):195–207. doi: 10.1189/jlb.1A1014-519RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R, Sun S, Ji F, et al. CNTN-1 Enhances Chemoresistance in Human Lung Adenocarcinoma Through Induction of Epithelial-Mesenchymal Transition by Targeting the PI3K/Akt Pathway. Cell Physiol Biochem. 2017;43(2):465–480. doi: 10.1159/000480473. [DOI] [PubMed] [Google Scholar]
- 30.Postma DS, Reddel HK, ten Hacken NH, van den Berge M. Asthma and chronic obstructive pulmonary disease: similarities and differences. Clin Chest Med. 2014;35(1):143–156. doi: 10.1016/j.ccm.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 32.Belchamber KBR, Donnelly LE. Macrophage Dysfunction in Respiratory Disease. Results Probl Cell Differ. 2017;62:299–313. doi: 10.1007/978-3-319-54090-0_12. [DOI] [PubMed] [Google Scholar]
- 33.Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol. 2014;192(3):1120–1129. doi: 10.4049/jimmunol.1300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Liu Y, Feng X, Li C, Li S, Zhao Z. The key role of macrophage depolarization in the treatment of COPD with ergosterol both in vitro and in vivo. Int Immunopharmacol. 2020;79:106086. doi: 10.1016/j.intimp.2019.106086. [DOI] [PubMed] [Google Scholar]
- 35.Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8(5):706–713. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this research are available from the authors on reasonable request.