Abstract
Background
Chronic obstructive pulmonary disease (COPD) and bronchiectasis can overlap and share pathologic features, such as small airway disease (SAD). Whether the presence of SAD and emphysema in smokers with CT-derived bronchiectasis is associated with exacerbations is unknown.
Purpose
To assess whether SAD and emphysema in smokers with CT-derived bronchiectasis are associated with future exacerbations.
Materials and Methods
SAD and emphysema were quantified using the parametric response map method in former and current heavy smokers with and without bronchiectasis at CT from the COPDGene Study (from July 2009 to July 2018). Exacerbations were prospectively assessed through biannual follow-up. An exacerbation was defined as an increase in or new onset of respiratory symptoms treated with antibiotics and/or corticosteroids. Severe exacerbations were defined as those that required hospitalization. The association of a high burden of SAD (≥15.6%) and high burden of emphysema (≥5%) at CT with exacerbations was assessed with generalized linear mixed models.
Results
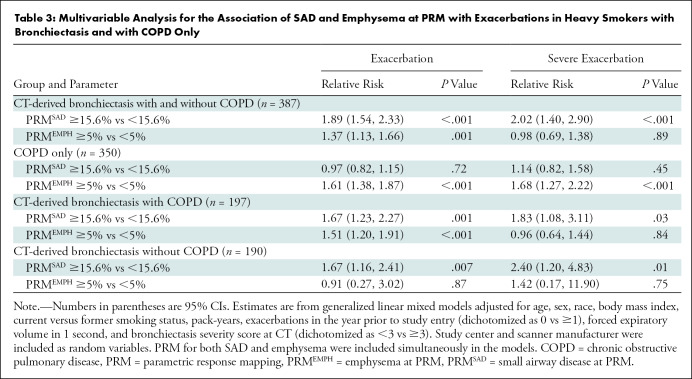
Of 737 participants, 387 (median age, 64 years [interquartile range, 58–71 years]; 223 women) had CT-derived bronchiectasis. During a 9-year follow-up, after adjustment for age, sex, race, body mass index, current smoking status, pack-years, exacerbations before study entry, forced expiratory volume in 1 second, or FEV1, and bronchiectasis severity CT score, high burden of SAD and high burden of emphysema were associated with a higher number of exacerbations per year (relative risk [RR], 1.89 [95% CI: 1.54, 2.33] and 1.37 [95% CI: 1.13, 1.66], respectively; P ≤ .001 for both). Results were comparable among participants with bronchiectasis meeting criteria for COPD (n = 197) (RR, 1.67 [95% CI: 1.23, 2.27] for high burden of SAD and 1.51 [95% CI: 1.20, 1.91] for high burden of emphysema; P ≤ .001 for both).
Conclusion
In smokers with CT-derived bronchiectasis and chronic obstructive pulmonary disease, structural damage to lung parenchyma and small airways was associated with a higher number of exacerbations per year.
Clinical trial registration no. NCT00608764
© RSNA, 2021
Summary
In current and former heavy smokers from the COPDGene Study cohort, increased emphysema and small airway disease at CT were each associated with increased risk of exacerbations in those with bronchiectasis and chronic obstructive pulmonary disease (COPD) and in those with bronchiectasis without COPD.
Key Results
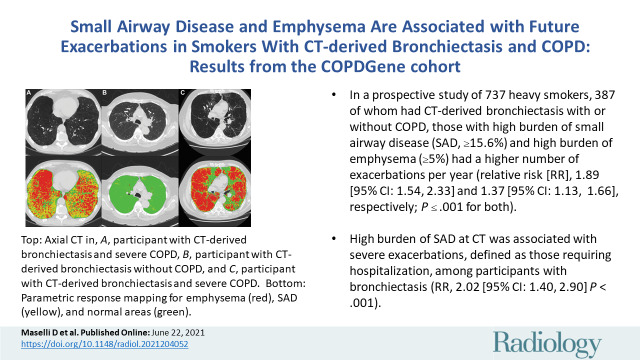
■ In a prospective study of 737 heavy smokers, 387 of whom had CT-derived bronchiectasis with or without chronic pulmonary obstructive disease, those with high burden of small airway disease (SAD) (≥15.6%) and high burden of emphysema (≥5%) had a higher number of exacerbations per year (relative risk [RR], 1.89 [95% CI: 1.54, 2.33] and 1.37 [95% CI: 1.13, 1.66], respectively; P ≤ .001 for both).
■ High burden of SAD at CT was associated with severe exacerbations, defined as those requiring hospitalization, among participants with bronchiectasis (RR, 2.02 [95% CI: 1.40, 2.90]; P < .001).
Introduction
Bronchiectasis is a disease characterized by repeated cycles of infection and inflammation, leading to progressive structural damage of the airways and lung parenchyma (1). CT examination is the standard method to confirm or rule out the disease in patients with a suggestive clinical feature. Furthermore, bronchiectasis at CT is also reported in smokers undergoing lung cancer screening or enrolled in chronic obstructive pulmonary disease (COPD) studies, with a prevalence of approximately 12% and 20%–30%, respectively (2–4). However, in these settings, the clinical meaning of bronchiectasis, including the occurrence of exacerbations, is incompletely understood. Bronchiectasis exacerbations are acute respiratory events associated with higher risk of hospitalization, health care use, and mortality (5–8). Although several risk factors for future exacerbations in patients with bronchiectasis have been identified, including high levels of markers of inflammation, Pseudomonas aeruginosa infection, and a history of previous exacerbations, there is a paucity of information in smokers (5,9,10). Identifying risk factors for future exacerbations in smokers with bronchiectasis at CT for this clinically relevant outcome may inform disease management.
Bronchiectasis is associated with a myriad of conditions, including COPD (8–13). Individuals with coexistent COPD and bronchiectasis have a higher number of exacerbations than those with COPD alone (11,12). Furthermore, various studies have given insight into distinct pathologic features of COPD and bronchiectasis, including emphysema and small airway disease (SAD) (14,15). We and others have demonstrated that heavy smokers with COPD and bronchiectasis at CT have a higher burden of emphysema than those with COPD alone and that in bronchiectasis, a greater burden of emphysema is associated with hospitalizations (14–17). SAD has been previously described in lung tissue in patients with bronchiectasis and recently in those with both COPD and bronchiectasis (18,19). Based on observations in a small number of participants (n = 60), small airway abnormalities were associated with the sputum volume of bronchiectasis exacerbations, but not with the frequency of those events (20). New techniques, such as parametric response mapping (PRM), use matched inspiratory and expiratory images that allow objective assessment of SAD on clinical CT scans. Using such a technique in patients with bronchiectasis, the burden of SAD has been linked to reduced lung function and exercise capacity (14). In patients with COPD, SAD at PRM has been associated with future exacerbations (21). The purpose of this study was to examine the association of SAD and emphysema with future exacerbations in participants with bronchiectasis at CT.
Materials and Methods
COPDGene Study
We used baseline data from the COPDGene Study (ClinicalTrials.gov: NCT00608764; www.COPDGene.org) (22). Briefly, current and former heavy smokers (10 pack-years or more) aged 45–80 years were recruited in the COPDGene Study. Participants with an active pulmonary disease other than COPD or asthma and those who had an exacerbation in the 4 weeks prior to study entry were excluded. All participants completed questionnaires and underwent spirometry and standardized CT scanning. All participants provided written informed consent to participate. The institutional review board at each participating clinical center approved the protocol. COPDGene sites obtained Health Insurance Portability and Accountability Act authorization as part of the consent form.
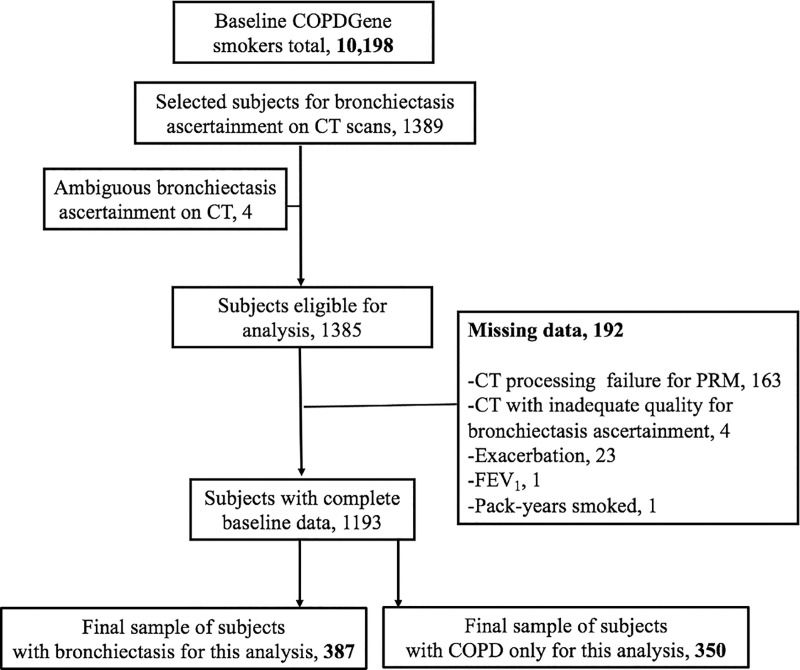
We used a convenience sample of COPDGene smokers in whom bronchiectasis assessments from CT scans were available. Because the COPDGene participants, by nature of the study design, did not have a clinical diagnosis of bronchiectasis, we believe that the term CT-derived bronchiectasis is more appropriate in this context and is used hereafter. Cross-sectional studies comparing emphysema, SAD, and pulmonary vasculature between COPDGene participants with and without CT-derived bronchiectasis were previously reported (2,14), and 737 participants from those analyses are included in the present study. The current study expands on this by evaluating the rate of exacerbation prospectively regarding the presence of emphysema and SAD in patients with bronchiectasis. To test our hypothesis, this study focused on current and former smokers with CT-derived bronchiectasis, including those with COPD and those without COPD, and examined exacerbations longitudinally. A subset of participants with COPD without bronchiectasis (referred to as “COPD only”) was also used (Fig 1).
Figure 1:
Flowchart shows participant selection. COPD = chronic obstructive pulmonary disease, FEV1 = forced expiratory volume in 1 second, PRM = parametric response mapping.
Exacerbation Ascertainment
Participants reported the occurrence of exacerbations at baseline and every 6 months afterward by telephone contact and web-based surveys from July 2009 to July 2018. An exacerbation was defined as an increase in or new onset of respiratory symptoms (cough, phlegm, and/or dyspnea) treated with antibiotics and/or corticosteroids (23). When an episode required hospitalization, it was counted as a severe exacerbation. This definition aligns with that of a recent expert consensus on bronchiectasis exacerbation (6).
Bronchiectasis Assessment on CT Scans
CT scan protocols have been previously described (22). Briefly, nonenhanced inspiratory and expiratory CT were used. Image reconstruction parameters included section thicknesses of 0.625–0.9 mm, with 0.45–0.625-mm intervals. The detection and scoring of bronchiectasis and the related interreader agreement assessment, which was calculated with use of κ and concordance correlation coefficient tests, were performed as previously described (2,24). Briefly, each CT scan was read independently twice by trained primary readers for this ascertainment (one chest radiologist, Y.O., with 16 years of experience; two pulmonologists, D.M. and A.D., with >3 and 10 years of lung imaging experience; and one physician, W.D., with >1 year of lung imaging experience). Bronchiectasis was coded as present, absent, or equivocal. Then, another trained reader (A.Y., a chest radiologist with 12 years of experience) assigned the final code when there was disagreement among the primary readers. All readers were blinded to the participants’ clinical information. CT-derived bronchiectasis was considered present if one or more of the following criteria were present in one or more lung lobes: (a) airway dilatation (airway lumen diameter greater than adjacent pulmonary vessel diameter), (b) abnormal airway tapering of any extent (no change in airway lumen diameter moving from proximal to distal airways), or (c) a bronchus within 1 cm of the pleura. The severity of CT-derived bronchiectasis was based on the degree of bronchial dilatation, the type of bronchiectasis, the number of bronchopulmonary segments affected, and the presence of wall thickness and mucus plugging in the affected bronchi (24). A higher score (range, 1–40) indicates greater severity (24).
SAD and Emphysema Assessment on CT Scans
SAD and emphysema were quantified with use of the PRM method, which has been validated in lung tissue. PRM was applied on paired expiratory and inspiratory CT scans to identify and quantify the extent of SAD and emphysema at PRM (LungQ, Thirona) (25,26). Briefly, the PRM method involves a deformable registration to spatially align the expiratory scan with the inspiratory scan, allowing them to share the same spatial geometry. Classification of voxel-by-voxel attenuation maps into discrete zones allows for quantification of measures of normal parenchyma, emphysema, and SAD (ie, nonemphysematous air trapping) by using two thresholds of lung attenuation: –950 HU on inspiratory scans and –856 HU on expiratory scans. The extent of PRM measurements is expressed as the percentage of the whole lung area.
Clinical and Physiologic Assessments
Demographic and clinical data were obtained with standardized questionnaires and procedures (www.COPDGene.org). Spirometric measures of lung function were performed per American Thoracic Society recommendations (27). Postbronchodilator forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) are expressed as a percentage of predicted values (28). COPD was defined as a postbronchodilator FEV1/FVC ratio less than 0.7.
Statistical Analysis
A generalized linear mixed analysis was used to test the PRM measurements in relationship to the number of exacerbations per year during the follow-up period. The Poisson distribution (which accounts for the dispersion of the outcome), log link, and an unstructured correlation matrix were used in the multivariable models. We used the follow-up time in years as the offset. Model fitting was assessed and was adequate. PRM measurements were dichotomized as high and low burden of SAD and emphysema based on median values and a previously used cutoff point (SAD at PRM ≥15.6% vs <15.6%; emphysema at PRM ≥5% vs <5%) (29). Both binary PRM variables were used simultaneously in the models. Fixed effect covariates for multivariable models were chosen a priori and included age, sex, race, body mass index, current smoking status, pack-years smoked, exacerbation in the year prior to study entry (dichotomized as 0 vs ≥1), FEV1, and bronchiectasis severity score at CT (dichotomized as <3 vs ≥3). CT scanner manufacturer and clinical center were used as random effects. These models provide relative risks (RRs) for exacerbation episodes per year over the follow-up time. Analysis is presented by group: all participants with CT-derived bronchiectasis, those with COPD only, those with bronchiectasis with COPD, and those with bronchiectasis without COPD. The two latter groups are derived from the first. P < .05 was considered indicative of a statistically significant difference. SAS software (version 9.4, SAS Institute) was used for the analysis. All analyses were performed by a statistician (W.W., with 20 years of experience). Data generated or analyzed during the study are available from the corresponding author by request.
Results
Participant Characteristics
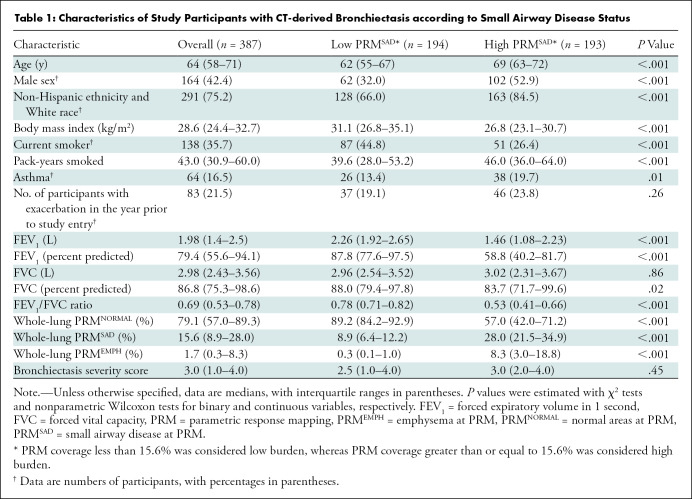
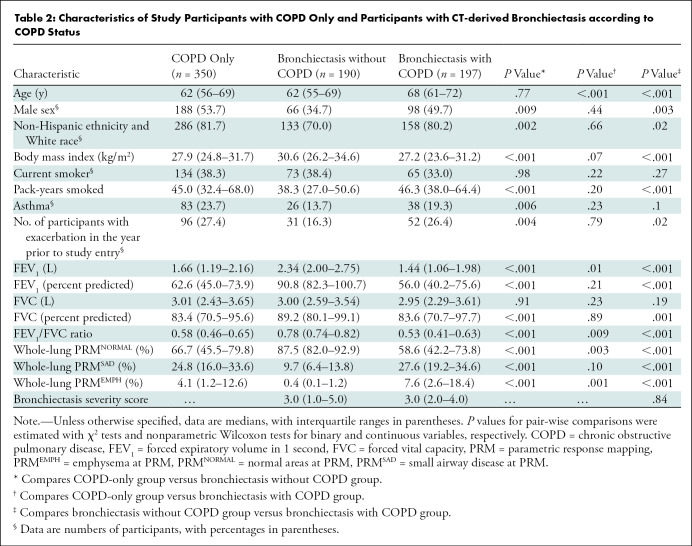
A total of 387 participants (median age, 64 years [interquartile range, 58–71 years]; 223 women) with CT-derived bronchiectasis and 350 with COPD only (median age, 62 years [interquartile range, 56–69 years]; 162 women) who had complete data on PRM, exacerbations, and other covariates were included in the analysis (Fig 1). Participant characteristics are shown in Table 1 according to status of SAD at PRM. Our results showed that compared with participants with low burden of SAD at PRM (n = 194), those with high burden of SAD at PRM (n = 193) were older, non-Hispanic White men. Most of these participants were former smokers, with higher pack-years than those with low burden of SAD at PRM. A higher proportion of participants with high versus low burden of SAD at PRM had one or more exacerbations before study entry. Participants with high burden of SAD at PRM had lower FEV1. Finally, participants in the high burden of SAD at PRM group had a greater burden of emphysema at PRM and bronchiectasis severity score at CT (Table 1). Table 2 shows the characteristics of the participants with COPD only and CT-derived bronchiectasis according to COPD status. Compared with those participants with COPD only, those with CT-derived bronchiectasis and COPD were older. These participants also had lower FEV1/FVC ratio. The participants with CT-derived bronchiectasis and COPD had higher burden of emphysema at PRM than their counterparts with COPD only. We did not find evidence that any other characteristics were different between these two groups.
Table 1:
Characteristics of Study Participants with CT-derived Bronchiectasis according to Small Airway Disease Status
Table 2:
Characteristics of Study Participants with COPD Only and Participants with CT-derived Bronchiectasis according to COPD Status
Association of SAD and Emphysema at PRM with Exacerbations
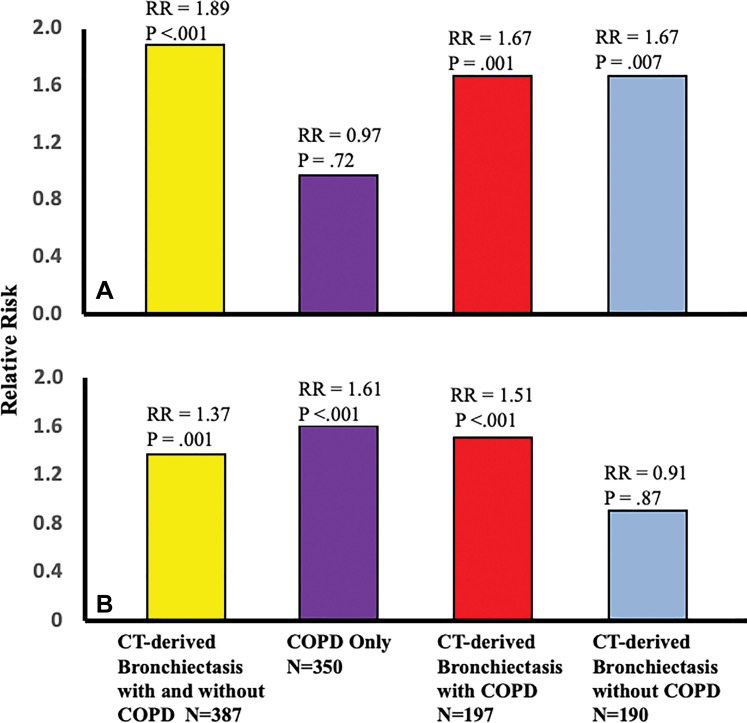
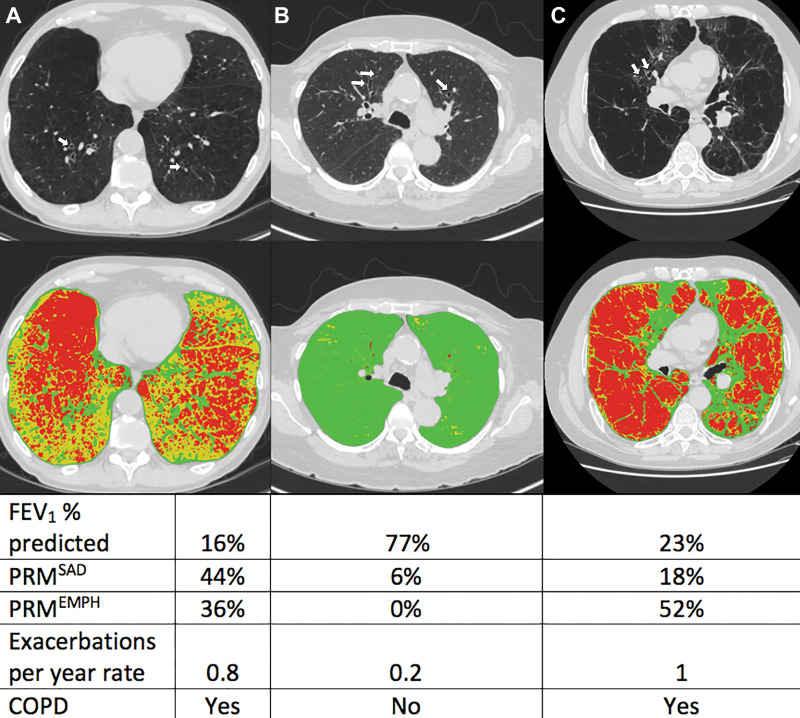
During a median follow-up time of 9 years (interquartile range, 8.3–9.5 years), 211 of the 387 participants with CT-derived bronchiectasis (54.5%) had one or more future exacerbations, yielding a median of 0.3 exacerbations per year. The multivariable analyses showed high burden of SAD and emphysema at PRM and were each related to increased risk of exacerbations (RR, 1.89 [95% CI: 1.54, 2.33] and 1.37 [95% CI: 1.13, 1.66], respectively; P ≤ .001) (Table 3, Fig 2). When participants with bronchiectasis and physician-diagnosed asthma were excluded, corresponding RRs were 2.26 (95% CI: 1.76, 90) for high burden of SAD and 1.61 (95% CI: 1.29, 2.03) for high burden of emphysema at PRM (P < .001 for both). The RRs for exacerbations among all participants with COPD only (n = 350) were 0.97 (95% CI: 0.82, 1.15; P = .72) for high burden of SAD and 1.61 (95% CI: 1.38, 1.87; P < .001) for high burden of emphysema at PRM (Table 3, Fig 2). Illustrative cases of CT-derived bronchiectasis with corresponding PRM measurements are shown in Figure 3.
Table 3:
Multivariable Analysis for the Association of SAD and Emphysema at PRM with Exacerbations in Heavy Smokers with Bronchiectasis and with COPD Only
Figure 2:
Bar graphs show relative risk (RR) of exacerbations in participants with high burden of small airway disease (SAD) and emphysema at parametric response mapping (PRM) according to group: CT-derived bronchiectasis with and without chronic obstructive pulmonary disease (COPD) (yellow), COPD only (purple), CT-derived bronchiectasis with COPD (red), and CT-derived bronchiectasis without COPD (blue). A, RR of exacerbation in participants with high versus low burden of SAD at PRM (≥15.6% vs <15.6%) according to group. B, RR of exacerbation in participants with high versus low burden of emphysema at PRM (≥5% vs <5%) according to group.
Figure 3:
Axial noncontrast CT scans (top row) and corresponding images from parametric response mapping (PRM) (bottom row) for small airway disease (SAD) and emphysema illustrate bronchiectasis. CT scans show bronchiectasis (arrows), and color-coded images show emphysema in red, SAD in yellow, and normal areas in green. Values of PRM measures, predicted percentage for forced expiratory volume in 1 second (FEV1), and the rates of exacerbation per year are shown in the table. A, Images in a participant with CT-derived bronchiectasis and severe chronic obstructive pulmonary disease (COPD). B, Images in a participant with CT-derived bronchiectasis without COPD. C, Images in a participant with CT-derived bronchiectasis and severe COPD. PRMEMPH = emphysema at PRM, PRMSAD = small airway disease at PRM.
In a stratified analysis, the RRs for the association of SAD and emphysema at PRM with exacerbations in participants with CT-derived bronchiectasis and COPD (n = 197) were 1.67 (95% CI: 1.23, 2.27) and 1.51 (95% CI: 1.20, 1.91), respectively (P ≤ .001 for both). Corresponding RRs for those with CT-derived bronchiectasis without COPD (n = 190) were 1.67 (95% CI: 1.16, 2.41; P = .007) for high burden of SAD and 0.91 (95% CI: 0.27, 3.02; P = .87) for high burden of emphysema at PRM (Table 3, Fig 2). Similarly, when participants with physician-diagnosed asthma were excluded, the RRs for those with CT-derived bronchiectasis and COPD (n = 159) were 2.01 (95% CI: 1.42, 2.83) for high burden of SAD and 1.61 (95% CI: 1.24, 2.08) for high burden of emphysema at PRM (P < .001 for both). Among those with CT-derived bronchiectasis only without asthma (n = 164), corresponding RRs were 1.52 (95% CI: 0.88, 2.61; P = .13) for high burden of SAD and 1.45 (95% CI: 0.41, 5.09; P = .56) for high burden of emphysema at PRM.
The RRs for severe exacerbations in those with high burden of SAD at PRM were 2.02 (95% CI: 1.40, 2.90; P < .001) for all participants with CT-derived bronchiectasis, 1.14 (95% CI: 0.82, 1.58; P = .45) for those with COPD only, 1.83 (95% CI: 1.08, 3.11; P = .03) for those with bronchiectasis with COPD, and 2.40 (95% CI: 1.20, 4.83; P = .01) for those with bronchiectasis without COPD. The corresponding RRs in those with high burden of emphysema at PRM were 0.98 (95% CI: 0.69, 1.38; P = .89) for all participants with CT-derived bronchiectasis, 1.68 (95% CI: 1.27, 2.22; P < .001) for those with COPD only, 0.96 (95% CI: 0.64, 1.44; P = .84) for those with bronchiectasis with COPD, and 1.42 (95% CI: 0.17, 11.90; P = .75) for those with bronchiectasis without COPD (Table 3).
Discussion
To examine the association of small airway disease (SAD) and emphysema with exacerbations, we followed up 737 heavy smokers with and without CT-derived bronchiectasis from the COPDGene Study over a median of 9 years. Our results showed that among participants with bronchiectasis, a higher burden of SAD and emphysema is associated with a higher number of exacerbations per year (relative risk [RR], 1.89 and 1.37, respectively; P ≤ .001 for both). In participants with CT-derived bronchiectasis, SAD was associated with exacerbations regardless of chronic obstructive pulmonary disease (COPD) status (RR, 1.67; P ≤ .007 for both groups), whereas emphysema was associated with exacerbations in those with bronchiectasis and COPD (RR, 1.51; P < .001) but not in those with CT-derived bronchiectasis without COPD (RR, 0.91; P = .87). In participants with COPD only, emphysema at CT was also associated with a higher number of exacerbations per year (RR, 1.61; P < .001), but SAD was not (RR, 0.97; P = .72).
Patients with coexistent COPD and bronchiectasis (versus COPD only) have a greater frequency of exacerbations, particularly those with emphysema (11–13). CT studies have also helped identify another pathologic feature in bronchiectasis—SAD (14,15). However, it has been unclear whether SAD in patients with bronchiectasis is associated with clinically relevant outcomes, such as exacerbations. Our study adds evidence that a greater burden of emphysema and SAD in heavy smokers with CT-derived bronchiectasis is linked to exacerbation episodes. The association was observed in participants with bronchiectasis and COPD and in those with bronchiectasis and without COPD. Increased SAD was also associated with severe exacerbations among participants with bronchiectasis at CT (RR, 2.02; P < .001), further strengthening the association when excluding participants with asthma (RR, 2.26; P < .001). Emphysema was associated with a higher number of exacerbations in those with bronchiectasis and COPD but not among those with CT-derived bronchiectasis without COPD, suggesting that the effects of emphysema on exacerbation events are somewhat synergistic in participants with the bronchiectasis-COPD overlap compared with bronchiectasis alone. In contrast, among participants with CT-derived bronchiectasis, we found no evidence for an association between emphysema and exacerbations necessitating hospitalization (RR, 0.98; P = .89). This finding differs from a prior study demonstrating such an association (17). Differences in the number of severe exacerbations per year between the studies may, in part, explain this discrepancy.
Our results are relevant, as CT is widely used in various clinical settings. It often leads to the detection of abnormalities, including bronchiectasis, in participants with minimal or without clinical manifestations (4). For example, chest CT for lung cancer screening, performed in patients already at risk for pulmonary parenchymal abnormalities, has been suggested as potentially useful for the evaluation of other diseases, including incidental bronchiectasis (3,30,31). Furthermore, patients undergoing lung cancer screening may have a higher prevalence of incidental bronchiectasis than the general population (31,32). Thus, an understanding about the potential clinical impact of such an incidental finding in smokers is needed. Herein, we have expanded these findings by showing that those with parenchymal and small airway structural abnormalities and bronchiectasis at CT are linked to future exacerbations only in the presence of COPD. This supports the possibility of considering referral to a pulmonologist and clinical follow-up of smokers with “incidental” bronchiectasis at CT, particularly in those with known or suspected COPD.
Although this study was not intended to elucidate the mechanisms of the associations we observed, there are several possible explanations. First, bronchiectasis is characterized by repeated cycles of an exaggerated inflammatory response, including elevated neutrophil elastase activity. In adults with bronchiectasis, elevated neutrophil elastase activity was associated with more exacerbations, and uninhibited neutrophil elastase leads to emphysema (9,33). Additionally, the gene expression signature of interleukin-17A, which is involved in neutrophilic inflammation and corticosteroid resistance, was directly associated with SAD, supporting a potential link between inflammation and exacerbations (34). Second, it is conceivable that the extent of SAD might be related to changes in defense mechanisms of the lung, such as the mucociliary system. For example, a study showed that altered mucus properties, such as increased mucus consistency, are associated with higher SAD (35). Notably, more hyper-concentrated mucus and mucus plugging in the airway lumen may also contribute to exacerbation by impeding airway clearance and serving as a nidus for pathogen growth (36,37).
Our study has limitations. First, the cohort included heavy smokers only, and the overall severity of CT-derived bronchiectasis was mild on average. Thus, caution should be exercised in extrapolating our findings to other cohorts, including those with more severe bronchiectasis. Despite this, participants in the COPDGene Study were well-characterized. State-of-the-art lung imaging allowed us to explore the association between parenchymal abnormalities and future exacerbations. Second, the PRM method algorithm attributes a specific pathologic feature to every voxel (25). Although it is likely that one process may be predominant, mixed pathologic abnormalities might be present in a given voxel. Third, while COPDGene has no information on other risk factors for future exacerbations, such as data on pathogens, we were able to include the majority of covariates used in other studies (5). Finally, although the method of measuring the bronchial lumen–to–vessel diameter ratio to define bronchiectasis may be imperfect, it is still widely used in clinical studies (2,5,9,24). Objective measurements of bronchiectasis under development might improve the ability to assess this disease in the future (38).
In summary, in heavy smokers with bronchiectasis, we have demonstrated that greater burden of small airway disease and emphysema at CT are associated with a higher number of exacerbations per year. The findings warrant further investigation in other cohorts.
The COPDGene Study was supported by National Institutes of Health grants R01HL089897 and R01HL089856 and the COPD Foundation through contributions made to an industry advisory committee comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Sunovion. A.A.D. is supported by National Heart, Lung, and Blood Institute grants R01HL133137 and R01HL149861 and the Brigham and Women’s Hospital Minority Faculty Career Development Award.
Disclosures of Conflicts of Interest: D.J.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has received compensation from GlaxoSmithKline, AstraZeneca, and Sanofi Regeneron for advisory board membership; received speaker fees from GlaxoSmithKline, AstraZeneca, and Sanofi Regeneron. Other relationships: disclosed no relevant relationships. A.Y. Activities related to the present article: received salary support from the National Institutes of Health (grant 2U01HL089897). Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. W.W. disclosed no relevant relationships. Y.O. disclosed no relevant relationships. W.R.D. disclosed no relevant relationships. C.M. disclosed no relevant relationships. A.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has received consulting fees from GlaxoSmithKline, Teva, Boehringer Ingelheim, AstraZeneca, and Abbott. Other relationships: disclosed no relevant relationships. M.I.R. disclosed no relevant relationships. T.R.A. disclosed no relevant relationships. A.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has received consulting fees from Hillrom, Insmed, and Zambon; received payment from Hillrom as part of a clinical trial. Other relationships: disclosed no relevant relationships. S.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has received consulting fees from Insmed, Chiesi, CSL Behring, Bayer, Grifols, GlaxoSmithKline, and Zambon; received or will receive grants from Insmed and Fisher & Paykel; received speaker fees from Insmed; receives royalties from McGraw Hill; received compensation from Insmed for the development of educational presentations. Other relationships: disclosed no relevant relationships. K.A.Y. disclosed no relevant relationships. G.L.K. disclosed no relevant relationships. J.M.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has received consulting fees from GlaxoSmithKline, AstraZeneca, and Takeda as an advisory board member and/or adjudication committee member; received or will receive grants from Bayer, Grifols, Vertex, Mereo BioPharma, and Verona to conduct translational research and clinical trials. Other relationships: disclosed no relevant relationships. R.S.J.E. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has received consulting fees from Leuko Labs; received or will receive grants from Insmed and Boehringer Ingelheim; received speaker fees from Chiesi; is the founder of Quantitative Imaging Solutions and an equity holder. Other relationships: disclosed no relevant relationships. D.A.L. disclosed no relevant relationships. A.A.D. disclosed no relevant relationships.
Abbreviations:
- COPD
- chronic obstructive pulmonary disease
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- PRM
- parametric response mapping
- RR
- relative risk
- SAD
- small airway disease
References
- 1. Maselli DJ , Amalakuhan B , Keyt H , Diaz AA . Suspecting non-cystic fibrosis bronchiectasis: What the busy primary care clinician needs to know . Int J Clin Pract 2017. ; 71 ( 2 ): e12924 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diaz AA , Maselli DJ , Rahaghi F , et al . Pulmonary vascular pruning in smokers with bronchiectasis . ERJ Open Res 2018. ; 4 ( 4 ): 00044 – 2018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanchez-Carpintero Abad M , Sanchez-Salcedo P , de-Torres JP , et al . Prevalence and burden of bronchiectasis in a lung cancer screening program . PLoS One 2020. ; 15 ( 4 ): e0231204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan WC , Hague CJ , Leipsic J , et al . Findings on thoracic computed tomography scans and respiratory outcomes in persons with and without chronic obstructive pulmonary disease: a population-based cohort study . PLoS One 2016. ; 11 ( 11 ): e0166745 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chalmers JD , Aliberti S , Filonenko A , et al . Characterization of the “frequent exacerbator phenotype” in bronchiectasis . Am J Respir Crit Care Med 2018. ; 197 ( 11 ): 1410 – 1420 . [DOI] [PubMed] [Google Scholar]
- 6. Hill AT , Haworth CS , Aliberti S , et al . Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research . Eur Respir J 2017. ; 49 ( 6 ): 1700051 . [DOI] [PubMed] [Google Scholar]
- 7. Goeminne PC , Hernandez F , Diel R , et al . The economic burden of bronchiectasis – known and unknown: a systematic review . BMC Pulm Med 2019. ; 19 ( 1 ): 54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martinez-Garcia MA , Athanazio R , Gramblicka G , et al . Prognostic value of frequent exacerbations in bronchiectasis: the relationship with disease severity . Arch Bronconeumol 2019. ; 55 ( 2 ): 81 – 87 . [DOI] [PubMed] [Google Scholar]
- 9. Chalmers JD , Moffitt KL , Suarez-Cuartin G , et al . Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis . Am J Respir Crit Care Med 2017. ; 195 ( 10 ): 1384 – 1393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shoemark A , Cant E , Carreto L , et al . A point-of-care neutrophil elastase activity assay identifies bronchiectasis severity, airway infection and risk of exacerbation . Eur Respir J 2019. ; 53 ( 6 ): 1900303 . [DOI] [PubMed] [Google Scholar]
- 11. De la Rosa D , Martínez-Garcia MA , Giron RM , et al . Clinical impact of chronic obstructive pulmonary disease on non-cystic fibrosis bronchiectasis. A study on 1,790 patients from the Spanish Bronchiectasis Historical Registry . PLoS One 2017. ; 12 ( 5 ): e0177931 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung WS , Lin CL . Acute respiratory events in patients with bronchiectasis-COPD overlap syndrome: a population-based cohort study . Respir Med 2018. ; 140 ( 6 ): 10 . [DOI] [PubMed] [Google Scholar]
- 13. Martínez-García MA , de la Rosa Carrillo D , Soler-Cataluña JJ , et al . Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease . Am J Respir Crit Care Med 2013. ; 187 ( 8 ): 823 – 831 . [DOI] [PubMed] [Google Scholar]
- 14. Martinez CH , Okajima Y , Yen A , et al . Paired CT measures of emphysema and small airways disease and lung function and exercise capacity in smokers with radiographic bronchiectasis . Acad Radiol 2021. ; 28 ( 3 ): 370 – 378 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts HR , Wells AU , Milne DG , et al . Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests . Thorax 2000. ; 55 ( 3 ): 198 – 204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dou S , Zheng C , Cui L , et al . High prevalence of bronchiectasis in emphysema-predominant COPD patients . Int J Chron Obstruct Pulmon Dis 2018. ; 13 ( 2041 ): 2047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bedi P , Chalmers JD , Goeminne PC , et al . The BRICS (Bronchiectasis Radiologically Indexed CT Score): a multicenter study score for use in idiopathic and postinfective bronchiectasis . Chest 2018. ; 153 ( 5 ): 1177 – 1186 . [DOI] [PubMed] [Google Scholar]
- 18. Everaerts S , McDonough JE , Verleden SE , et al . Airway morphometry in COPD with bronchiectasis: a view on all airway generations . Eur Respir J 2019. ; 54 ( 5 ): 1802166 . [DOI] [PubMed] [Google Scholar]
- 19. Whitwell F . A study of the pathology and pathogenesis of bronchiectasis . Thorax 1952. ; 7 ( 3 ): 213 – 239 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ooi GC , Khong PL , Chan-Yeung M , et al . High-resolution CT quantification of bronchiectasis: clinical and functional correlation . Radiology 2002. ; 225 ( 3 ): 663 – 672 . [DOI] [PubMed] [Google Scholar]
- 21. Han MK , Quibrera PM , Carretta EE , et al . Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort . Lancet Respir Med 2017. ; 5 ( 8 ): 619 – 626 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Regan EA , Hokanson JE , Murphy JR , et al . Genetic epidemiology of COPD (COPDGene) study design . COPD 2010. ; 7 ( 1 ): 32 – 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bowler RP , Kim V , Regan E , et al . Prediction of acute respiratory disease in current and former smokers with and without COPD . Chest 2014. ; 146 ( 4 ): 941 – 950 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diaz AA , Young TP , Maselli DJ , et al . Quantitative CT measures of bronchiectasis in smokers . Chest 2017. ; 151 ( 6 ): 1255 – 1262 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galbán CJ , Han MK , Boes JL , et al . Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression . Nat Med 2012. ; 18 ( 11 ): 1711 – 1715 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vasilescu DM , Martinez FJ , Marchetti N , et al . Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease . Am J Respir Crit Care Med 2019. ; 200 ( 5 ): 575 – 581 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Standardization of spirometry, 1994 update . American Thoracic Society. Am J Respir Crit Care Med 1995. ; 152 ( 3 ): 1107 – 1136 . [DOI] [PubMed] [Google Scholar]
- 28. Hankinson JL , Odencrantz JR , Fedan KB . Spirometric reference values from a sample of the general U.S. population . Am J Respir Crit Care Med 1999. ; 159 ( 1 ): 179 – 187 . [DOI] [PubMed] [Google Scholar]
- 29. Han MK , Tayob N , Murray S , et al . Association between emphysema and chronic obstructive pulmonary disease outcomes in the COPDGene and SPIROMICS Cohorts: a post hoc analysis of two clinical trials . Am J Respir Crit Care Med 2018. ; 198 ( 2 ): 265 - 267 10.1164/rccm.201801-0051LE . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mets OM , de Jong PA , Prokop M . Computed tomographic screening for lung cancer: an opportunity to evaluate other diseases . JAMA 2012. ; 308 ( 14 ): 1433 – 1434 . [DOI] [PubMed] [Google Scholar]
- 31. Singhvi D , Bon J . CT imaging and comorbidities in COPD: beyond lung cancer screening . Chest 2021. ; 159 ( 1 ): 147 – 153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MacRedmond R , Logan PM , Lee M , Kenny D , Foley C , Costello RW . Screening for lung cancer using low dose CT scanning . Thorax 2004. ; 59 ( 3 ): 237 – 241 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Campos MA , Diaz AA . The role of computed tomography for the evaluation of lung disease in alpha-1 antitrypsin deficiency . Chest 2018. ; 153 ( 5 ): 1240 – 1248 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christenson SA , van den Berge M , Faiz A , et al . An airway epithelial IL-17A response signature identifies a steroid-unresponsive COPD patient subgroup . J Clin Invest 2019. ; 129 ( 1 ): 169 – 181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kesimer M , Smith BM , Ceppe A , et al . Mucin concentrations and peripheral airway obstruction in chronic obstructive pulmonary disease . Am J Respir Crit Care Med 2018. ; 198 ( 11 ): 1453 – 1456 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramsey KA , Chen ACH , Radicioni G , et al . Airway mucus hyperconcentration in non-cystic fibrosis bronchiectasis . Am J Respir Crit Care Med 2020. ; 201 ( 6 ): 661 – 670 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Okajima Y , Come CE , Nardelli P , et al . Luminal plugging on chest CT scan: association with lung function, quality of life, and COPD clinical phenotypes . Chest 2020. ; 158 ( 1 ): 121 – 130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martínez-García MÁ , de la Rosa-Carrillo D , Soler-Cataluña JJ , et al . Bronchial infection and temporal evolution of bronchiectasis in patients with chronic obstructive pulmonary disease . Clin Infect Dis 2021. ; 72 ( 3 ): 403 – 410 . [DOI] [PubMed] [Google Scholar]