Abstract
Background
Obtaining ventricular volumetry and mass is key to most cardiac MRI but challenged by long multibreath-hold acquisitions.
Purpose
To assess the image quality and performance of a highly accelerated, free-breathing, two-dimensional cine cardiac MRI sequence incorporating deep learning (DL) reconstruction compared with reference standard balanced steady-state free precession (bSSFP).
Materials and Methods
A DL algorithm was developed to reconstruct custom 12-fold accelerated bSSFP cardiac MRI cine images from coil sensitivity maps using 15 iterations of separable three-dimensional convolutions and data consistency steps. The model was trained, validated, and internally tested in 10, two, and 10 adult human volunteers, respectively, based on vendor partner-supplied fully sampled bSSFP acquisitions. For prospective external clinical validation, consecutive children and young adults undergoing cardiac MRI from September through December 2019 at a single children's hospital underwent both conventional and highly accelerated short-axis bSSFP cine acquisitions in one MRI examination. Two radiologists scored overall and volumetric three-dimensional mesh image quality of all short-axis stacks on a five-point Likert scale and manually segmented endocardial and epicardial contours. Scan times and image quality were compared using the Wilcoxon rank sum test. Measurement agreement was assessed with intraclass correlation coefficient and Bland-Altman analysis.
Results
Fifty participants (mean age, 16 years ± 4 [standard deviation]; range, 5–30 years; 29 men) were evaluated. The mean prescribed acquisition times of accelerated scans (non–breath-held) and bSSFP (excluding breath-hold time) were 0.9 minute ± 0.3 versus 3.0 minutes ± 1.9 (P < .001). Overall and three-dimensional mesh image quality scores were, respectively, 3.8 ± 0.6 versus 4.3 ± 0.6 (P < .001) and 4.0 ± 1.0 versus 4.4 ± 0.8 (P < .001). Raters had strong agreement between all bSSFP and DL measurements, with intraclass correlation coefficients of 0.76 to 0.97, near-zero mean differences, and narrow limits of agreement.
Conclusion
With slightly lower image quality yet much faster speed, deep learning reconstruction may allow substantially shorter acquisition times of cardiac MRI compared with conventional balanced steady-state free precession MRI performed for ventricular volumetry.
© RSNA, 2021
Summary
Free-breathing highly accelerated two-dimensional cine cardiac MRI with deep learning reconstruction is promising for reducing scan time while maintaining similar image quality and accuracy of ventricular volumetry compared with conventional balanced steady-state free precession MRI.
Key Results
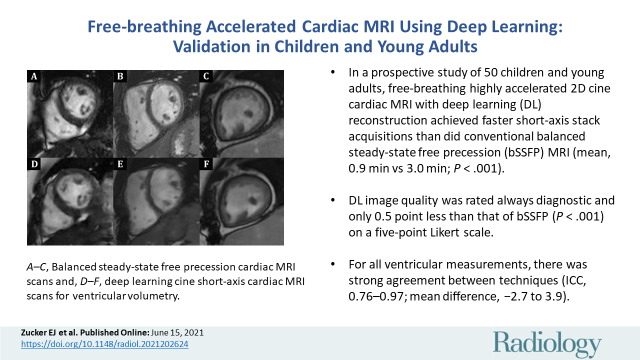
■ In a prospective study of 50 children and young adults, free-breathing highly accelerated two-dimensional cine cardiac MRI with deep learning (DL) reconstruction achieved faster short-axis stack acquisitions than did conventional balanced steady-state free precession (bSSFP) MRI (mean, 0.9 minute vs 3.0 minutes; P < .001).
■ The DL image quality was rated always diagnostic and only 0.5 points less than that of bSSFP (P < .001) on a five-point Likert scale.
■ For all ventricular measurements, there was strong agreement between techniques (intraclass correlation coefficients, 0.76–0.97; mean difference, −2.7 to 3.9).
Introduction
Cardiac MRI is considered the reference standard for the noninvasive assessment of ventricular volumes and function. Typically, a multisection two-dimensional cine balanced steady-state free precession (bSSFP) short-axis acquisition through the ventricles is obtained over multiple breath holds to mitigate respiratory motion (1–4). But the multibreath-hold approach is generally lengthy (on the order of 5 minutes for complete short-axis coverage) and can prove difficult in patients unable to comply with breath-holding instructions (1,4). Even patients who are able to comply can struggle to maintain consistent breath-hold amplitudes, leading to section-misalignment errors (1). These challenges can be partially overcome with free-breathing bSSFP using multiple signal averaging or respiratory triggering, but usually at the expense of degraded image quality or increased scan time (1,5).
Artificial intelligence approaches are increasingly promising for accomplishing a variety of medical imaging tasks, including rapid reconstruction of undersampled MRI k-space data to maintain or improve clinically diagnostic image quality while reducing scan time (6–12). Recently, a deep learning (DL)–based superresolution technique was proposed to estimate high-spatial-resolution cardiac MRI bSSFP images from low-resolution short-axis scans with reduced matrix size although no increased acceleration factors. In a small cohort of adults, there were no statistically significant differences in left ventricular (LV) volumetry results obtained from the DL-resolved low-resolution images and full-spatial-resolution images (13). Our group recently described an alternative approach using a highly accelerated two-dimensional cine cardiac MRI sequence with model-based DL reconstruction to iteratively estimate cardiac MRI bSSFP images directly from sparsely sampled k-space data (14). In comparison with DL superresolution approaches, this model-based DL reconstruction method directly leverages the raw k-space data and coil sensitivity information to improve cine image reconstruction quality. As a result, the approach demonstrated better image quality and LV volumetry than that of state-of-the-art cardiac MRI with parallel imaging and compressed sensing in healthy adult volunteers (14). However, this approach has not been optimized for or prospectively evaluated in the clinical setting, including assessment of right ventricular volumetry and inclusion of pediatric patients who might most benefit from it.
The purpose of this study was to further optimize and then prospectively validate the clinical performance of our highly accelerated DL cine sequence in children and young adults undergoing free-breathing cardiac MRI for structural or congenital heart disease, using conventional bSSFP as the reference standard. We hypothesized that the DL cine sequence could achieve similar overall image quality and quantitative ventricular volume and mass data at reduced scan times.
Materials and Methods
The institutional review board at Stanford University approved this study. Written informed consent was obtained from patients aged 18 years and older or from a parent/guardian for patients younger than 18 years. Written informed assent was obtained for participants aged 7 to 17 years.
DL Reconstruction and Accelerated Cine Sequence
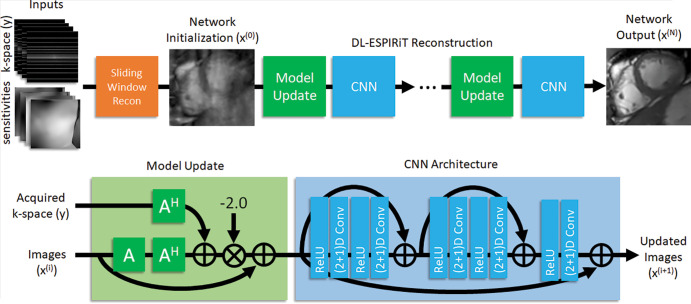
We enhanced our previously published (2+1)D deep learning–eigenvalue iterative self-consistent parallel imaging reconstruction, or DL-ESPIRiT, technique using 15 (previously 10) unrolled iterations and 128 (previously 96) feature maps (Fig 1) (14). Using a pixelwise L1 loss function, this physics-based algorithm leverages DL to optimize parameters associated with the eigenvalue autocalibrating parallel MRI approach based on initial coil sensitivity maps (15,16). This method was developed in our previous study with a cohort of 22 healthy adult volunteers who underwent fully sampled two-dimensional cine bSSFP acquisitions (data supplied by GE Healthcare) (14). Ten participants were used for model training, corresponding to a total of 183 image sections (including short-axis and long-axis views); two for validation; and 10 for internal testing. Cloud-based DL reconstructions were performed on a high-performance server with NVIDIA Titan X and Titan Xp video cards. The trained algorithm will be made available to any researcher who signs a research agreement with our vendor partner (GE Healthcare).
Figure 1:
Flowchart illustrates deep learning–eigenvalue iterative self-consistent parallel imaging reconstruction (DL-ESPIRiT) MRI network that is trained to reconstruct highly accelerated two-dimensional cine cardiac MRI section by section. First, sliding window reconstruction of undersampled k-space data is performed. These images are then coil-combined and used to initialize network, which iteratively applies model updates followed by three-dimensional (3D) convolutional neural networks (CNNs). Network assumes signal model, denoted “A,” comprised of coil sensitivity maps and Fourier encoding operations to simulate data acquisition. During model updates, A is used to transform intermediate images back into k-space to enforce consistency with acquired k-space samples. During CNN updates, complex-valued data are separated into real and imaginary channels and de-aliased by using separable 3D convolutions, whose weights are learned during training phase. A^H = complex conjugate of “A” (transform from k-space to image domain), Conv = convolution, Recon = reconstruction, ReLU = rectified linear unit.
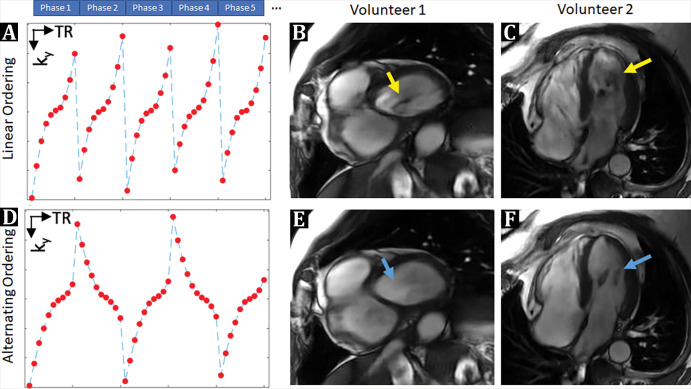
A custom 12-fold accelerated bSSFP cine sequence, reconstructed through the DL network, was then developed based on our previously published prototype (14). This was modified to minimize phase encoding variations between adjacent repetition times, reducing dark flow artifacts otherwise induced by high undersampling factors (Fig 2) (17,18).
Figure 2:
A, D, Schematic representation of k-space sample ordering strategy, designed to mitigate, B, C, E, F, dark flow artifacts. Although readout gradient waveforms are flow-compensated in balanced steady-state free precession acquisitions, phase-encoding (PE) gradients are not. As a result, PE gradients encode additional phase in areas with rapid blood flow, which can perturb signal steady state. This effect is exaggerated in highly accelerated cine acquisitions because PE gradients need to be rapidly switched every repetition time (TR) to efficiently sample k-space. Consequently, B, C, examples derived from testing in human volunteers, which show dark flow artifacts (yellow arrows) in left ventricle blood pool of 12-fold accelerated deep learning (DL) cine acquisition with linear ordering. Thus, linear ordering was modified to reduce PE gradient variations between adjacent TRs using an alternating k-space ordering scheme. In, E, F, a 12-fold accelerated DL cine acquisition using proposed alternating ordering scheme shows that flow-related artifacts are eliminated (blue arrow).
Study Participants
To externally prospectively validate the DL cine technique, children and young adults undergoing cardiac MRI from September through December 2019 at a single tertiary care children's hospital were consecutively recruited to undergo short-axis stacks using both the novel sequence and a routine clinically indicated (non–fully sampled) bSSFP acquisition. The only exclusion criteria were inability to obtain consent and/or assent or absence of a complete bSSFP short-axis stack, neither of which occurred. Routine demographic data and prescribed acquisition times were abstracted from the medical record and Digital Imaging and Communications in Medicine elements, respectively. Total sequence duration times inclusive of participant coaching and breath-hold recovery were estimated as the time between cine sequence start (bSSFP or DL) and next sequence start.
MRI Protocol
MRI examinations were performed at either 1.5 T (SIGNA Artist, GE Healthcare) or 3.0 T (Discovery MR750 or SIGNA Architect, GE Healthcare) using a 32-channel cardiac coil (Invivo), nonsedated unless clinically necessary. Conventional two-dimensional multisection short-axis cine bSSFP (FIESTA, GE Healthcare) images were obtained breath held when feasible (one slice per breath hold) and otherwise when free breathing with multiple signal averages. This cardiac cine imaging was performed with the following parameters: slice thickness, 6–8 mm; matrix size, 160–204 × 192–224; repetition time msec/echo time msec, 3.0–4.2/1.2–1.9; flip angle, 45–60°; views per segment, 8–18 depending on heart rate; 2–3 signal averages with retrospective gating; and standard acceleration. All DL cine acquisitions were performed free-breathing and respiratory triggered, with the following parameters: slice thickness, 6–8 mm depending on participant size; matrix size, 200 × 180; 3.0–3.8/1.1–1.4; and flip angle, 45°–60°. Both short-axis cine acquisitions were performed without contrast material except when intravenous ferumoxytol (Feraheme, AMAG Pharmaceuticals) was previously given per clinical protocol (19–21).
Image Assessment
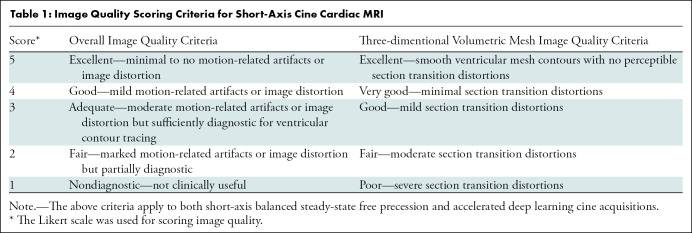
Two pediatric cardiovascular radiologists (E.J.Z. and A.K., with 11 years and 15 years of experience, respectively), blinded to participant demographics, sequence type, and scan parameters, independently scored overall image quality of the DL cine and bSSFP reconstructions on a five-point Likert scale (Table 1). They also assessed for LV regional wall motion abnormalities by using the echocardiographic wall motion score index (22) and segmented biventricular endocardial and LV epicardial contours in Medis Suite MR (version 3.1.16.24; QMass module, Medis Medical Imaging). A commercial artificial intelligence algorithm (Arterys) was then used to auto-segment and create three-dimensional volumetric mesh representations of the ventricles. From the contours, biventricular volume and LV mass data were derived. The two radiologists also scored mesh map image quality in LV end-diastole on a five-point Likert scale (Table 1).
Table 1:
Image Quality Scoring Criteria for Short-Axis Cine Cardiac MRI
Statistical Analysis
Descriptive statistics were calculated in Excel (version 2016; Microsoft Corporation). Using Stata (version 14; Stata-Corp), differences in scan times and image quality scores were compared with the Wilcoxon rank-sum test, and intra- and interobserver agreement were assessed by using the intraclass correlation coefficient, correlated to agreement as poor (< 0.50), fair (0.50–0.75), good (>0.75–0.90), and excellent (>0.90) (23). Bland-Altman plots were created in Matlab (version R2014; MathWorks). Statistical significance was set at P < .05. Using a two-sample t test, a sample size of 44 was calculated in R (version 4.02; the R Project for Statistical Computing) to provide greater than 90% power to detect an absolute difference of 2.5% in LV ejection fraction (LVEF) measurements derived from conventional bSSFP and DL cine sequences (LVEF mean ± standard deviation, 66% ± 5 in male participants aged 8–17 years), corresponding to a medium effect size (Cohen d) of 0.5 (24).
Results
Participant and MRI Characteristics
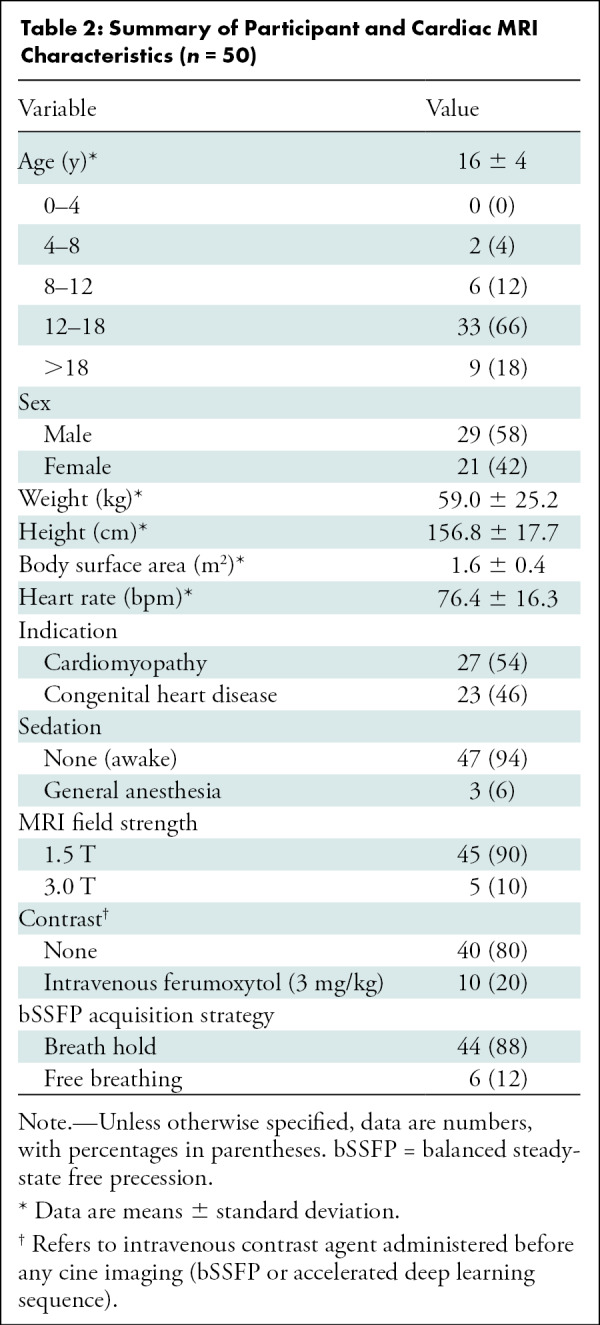
Demographics and MRI characteristics of the final clinical validation sample are summarized in Table 2. Among the 50 participants recruited, 29 (58%) were male (mean age, 16 years ± 4; range, 5–26 years). Most (44 of 50, 88%) bSSFP acquisitions were performed nonsedated and breath held; the remaining six were performed non–breath held, of which three patients were awake and three were under anesthesia. Slightly more than half of participants (27 of 50, 54%) underwent MRI examinations for cardiomyopathy evaluation, and the remainder were for congenital heart disease assessment.
Table 2:
Summary of Participant and Cardiac MRI Characteristics (n = 50)
Scan Times and Image Quality
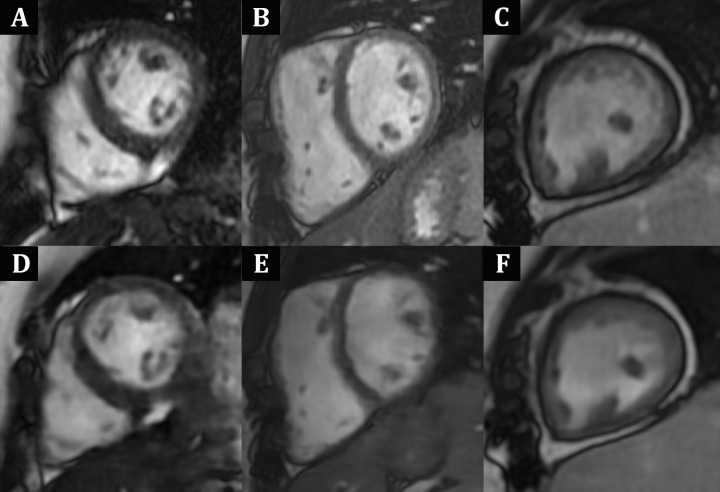
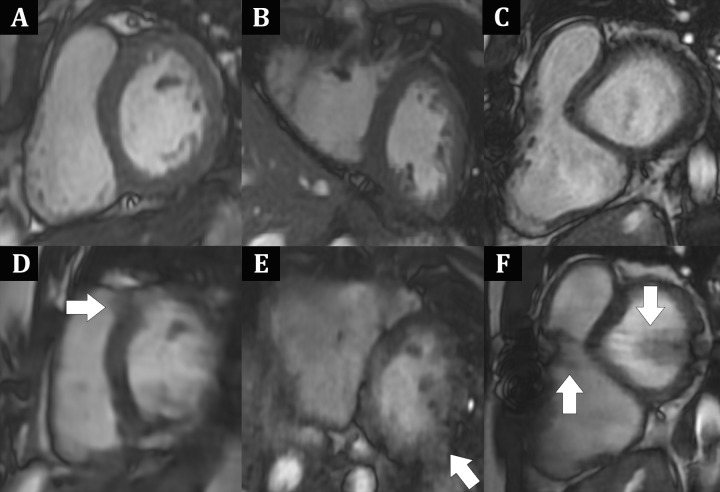
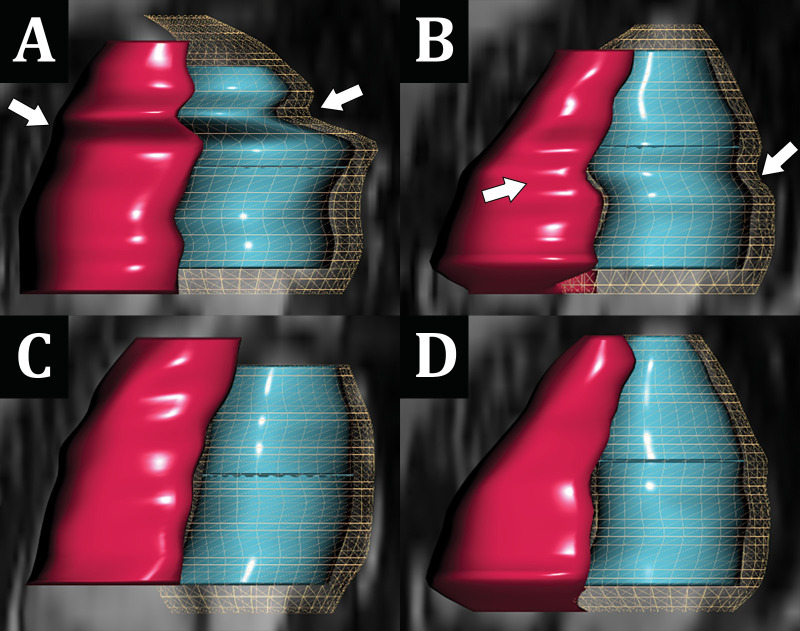
For complete short-axis coverage, the DL cine sequence, reconstructed on average in 1.8 minutes ± 0.4 for the multiple independently processed sections, was faster to acquire than bSSFP. Mean scan time and total sequence duration were 0.9 minute ± 0.3 and 1.7 minutes ± 1.1, respectively, for DL cine compared with 3.0 minutes ± 1.9 and 10.9 minutes ± 3.7 for bSSFP (both P < .001). Mean radiologist overall image quality scores were only minimally lower for DL cine (3.8 ± 0.6) than for bSSFP (4.3 ± 0.6; P < .001) (Fig 3). In addition, both raters' overall image quality scores for all acquisitions (ie, DL cine and bSSFP) were 3 or greater, indicating at least diagnostic quality. In six of 50 (12%) cases, DL cine was rated more than one point lower in image quality than bSSFP by at least one radiologist. Examples of DL cine images that were visibly degraded due to artifacts are shown in Figure 4. The mean three-dimensional mesh image quality scores were only slightly lower for DL cine (4.0 ± 1.0) than for bSSFP (4.4 ± 0.8; P < .001). In seven of 50 cases (14%), DL mesh image quality was rated higher than that of bSSFP by at least one radiologist (Fig 5).
Figure 3:
Case examples show similar diagnostic image quality between, A–C, balanced steady-state free precession cardiac MRI acquisitions and, D–F, deep-learning (DL) cine short-axis cardiac MRI acquisitions for ventricular volumetry, with only subtle contrast differences. All images shown are in end-diastole; C and F are obtained after intravenous ferumoxytol administration, whereas remainder are without contrast material. A, D, Images in a10-year-old boy with Duchenne muscular dystrophy. B, E, Images in a 10-year-old girl with repaired tetralogy of Fallot. C, F, Images in an 18-year-old woman with complex single-ventricle congenital heart disease after Fontan palliation.
Figure 4:
Case examples of noncontrast MRI images obtained midcardiac cycle, which shows better image quality of, A–C, balanced steady-state free precession compared with, D–F, deep learning (DL) cine short-axis cardiac MRI acquisitions. A, D, Images in a 14-year-old adolescent boy with suspected left ventricular (LV) noncompaction. Portions of LV myocardium appear “smeared” and ill-defined in, D, DL cine image, most notable at anteroseptum (arrow). B, E, Images in 19-year-old woman after atrial and ventricular septal defect as well as aortic coarctation repair. E, DL cine image is notable for prominent motionlike artifact with blurring most notable at LV inferolateral wall (arrow). C, F, Images in 20-year-old man with repaired tetralogy of Fallot. In F, DL cine image, bandlike dark signal artifact (arrows), aligned with region of median sternotomy wires, obscures portions of both ventricles. DL cine image degradation is attributed likely to undersampling and inherent variability in DL reconstruction process.
Figure 5:
Comparison of three-dimensional volumetric mesh contours auto-generated between, A, B, breath-held balanced steady-state free precession (bSSFP) and, C, D, corresponding free-breathing deep learning (DL) cine short-axis acquisitions. Note the greater distortion (arrows) in, A, B, the bSSFP mesh contours, which is related to inconsistent breath holding with consequent section misregistration errors. A, C, Images in a 17-year-old adolescent girl undergoing cardiomyopathy evaluation and, B, D, images in a 14-year-old adolescent boy with suspected left ventricular noncompaction.
In participants with high heart rates (≥80 beats per minute) compared with those with lower heart rates (<80 beats per minute), we did not find evidence of statistical differences in mean image quality for either bSSFP (4.2 ± 0.6 vs 4.4 ± 0.6; P = .10) or DL cine (3.8 ± 0.5 vs 3.9 ± 0.6; P = .41). In unenhanced compared with ferumoxytol-enhanced examinations, mean overall image quality scores were rated higher for bSSFP (4.4 ± 0.5 vs 3.9 ± 0.7; P = .003), but we did not find evidence of a difference for DL cine (3.9 ± 0.6 vs 3.7 ± 0.6; P = .12).
We also did not find evidence of differences in mean mesh image quality scores for either unenhanced bSSFP (4.5 ± 0.7 vs 4.1 ± 1.2; P = .18) or DL cine (4.0 ± 1.0 vs 3.9 ± 1.1; P = .98) or in mean combined radiologist wall motion score index scores between DL cine and bSSFP reconstructions (1.1 ± 0.2 vs 1.1 ± 0.2, respectively; P = .99). However, most MRI (46 of 50, 92%), irrespective of acquisition strategy, were deemed to have normal wall motion by both radiologists, limiting granular comparison.
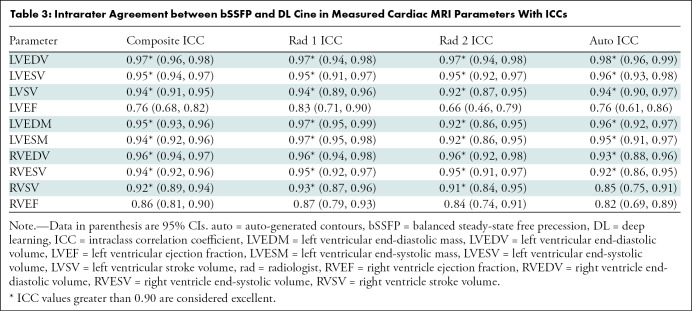
Intra- and Interobserver Agreement
Intraclass correlation coefficient intra- and interobserver agreement analysis according to each rater (two radiologists plus auto-generated contours) is summarized in Table 3 and Table E1 (online), respectively. For all three raters, there was excellent agreement between all biventricular volume and LV mass measurements derived from bSSFP and DL cine images, with the exception of good agreement for right ventricular stroke volume using auto-generated contours. Ejection fraction agreement was lower, in the good range for right ventricular ejection fraction in all three raters and for LVEF in two of three raters. For the second radiologist rater (A.K.), LVEF agreement was only moderate. For the two radiologists, there was good interobserver agreement in ejection fractions and excellent interobserver agreement in all other cardiac MRI parameters both for DL cine and bSSFP images. For auto-generated both bSSFP and DL cine contours, the pattern of interobserver agreement was similar regardless of technique, with good to excellent agreement for most parameters but moderate agreement for LV stroke volume and LVEF and poor to moderate agreement for right ventricular stroke volume and right ventricular ejection fraction.
Table 3:
Intrarater Agreement between bSSFP and DL Cine in Measured Cardiac MRI Parameters With ICCs
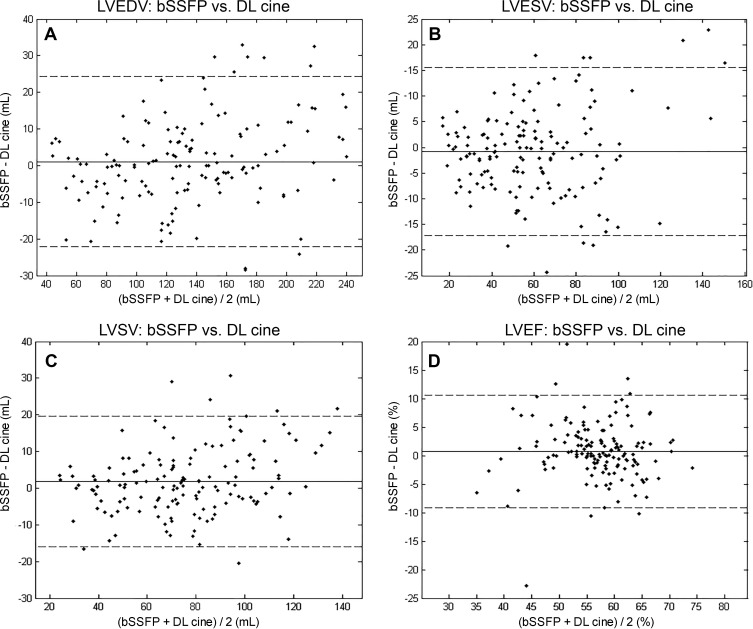
Composite-reader Bland-Altman plots for left and right ventricular volumetry and LV mass are shown in Figure 6 and Figures E1 and E2 (online), respectively. For combined readers, there were minimally greater than zero mean differences (95% CIs) in LV stroke volume, LV end-systolic mass, right ventricular end-diastolic volume, and right ventricular stroke volume of 1.9 mL (0.4–3.3 mL), −2.7 g (−4.7 to −0.6 g), 3.9 mL (1.3–6.5 mL), and 2.3 mL (0.3–4.2 mL), respectively. For all other measurements obtained with bSSFP versus DL cine, we did not find evidence for a difference (supporting concordance) with narrow 95% CIs overlapping with 0 (volumes, −2.1 to 3.6 mL; LV end-diastolic mass, −3.1 to 0.8 g; and ejection fractions, −0.5% to −4.6%). Corresponding limits of agreement are specified in Figure 6 and Figures E1 and E2 (online).
Figure 6:
Composite-rater (two radiologists plus auto-contour) Bland-Altman plots compare measurement differences in cardiac MRI left ventricular (LV) volume and function parameters between balanced steady-state free precession (bSSFP) and accelerated deep learning (DL) cine techniques. Solid lines correspond to mean differences, and dashed lines indicate limits of agreement (± 1.96 standard deviations from mean difference), with volumes in milliliters and ejection fractions in percentages. For, A, LV end-diastolic volume, B, LV end-systolic volume, C, LV stroke volume, and, D, LV ejection fraction, mean differences (95% CIs) were effectively zero at 1.1 (−0.8, 3.0), −0.8 (−2.1, 0.5), 1.9 (0.4, 3.3), and 0.7 (−0.1, 1.6), respectively. Corresponding limits of agreement were −22.7 to 24.8, −17.4 to 15.9, −16.3 to 20.0, and −9.3 to 10.8, respectively.
Individual Bland-Altman intra- and interobserver statistics are detailed in Figures E3–E11 (online). Intraobserver (bSSFP vs DL cine) comparisons were similar to composite-reader metrics, with the exception of wider right ventricular ejection fraction limits of agreement for auto-generated contours compared with those of the radiologists. For inter-radiologist comparisons, mean measurement differences for both bSSFP and DL cine techniques were at most minimally greater than zero (suggesting concordance), with narrow CIs, and wider limits of agreement for right ventricular parameters compared with those of LV parameters. There was less measurement concordance for radiologists versus auto-contours (ie, greater mean differences and wider limits of agreement), with overall better performance for LV parameters.
Select subgroup analyses of composite intraobserver agreement for bSSFP versus DL cine measurements were performed (Table E2 and Figs E11–E16 [online]). The congenital heart disease subgroup (23 of 50 participants, 46%) was notable for slightly lower LVEF intraclass correlation coefficient and slightly higher nonzero mean differences for right ventricular end-diastolic volume and right ventricular stroke volume compared with those of other subgroups and the total study sample, although with overlapping CIs. Other analyzed subgroups were similar to the composite study sample.
Discussion
In this study, we sought to develop and prospectively validate a highly accelerated cardiac MRI cine sequence with deep learning (DL) reconstruction to enable more rapid short-axis scans for volumetry assessment. We found in a cohort of 50 children, adolescents, and young adults that our accelerated DL sequence was significantly faster than reference standard balanced steady-state free precession (bSSFP; mean acquisition time, 0.9 minute vs 3.0 minutes; P < .001) with only slightly lower overall image quality (mean score, 3.8 vs 4.3; P < .001). Moreover, there was good or better agreement in DL cine-derived biventricular volume and left ventricular mass measurements and at least moderate agreement in ejection fraction estimates compared with those obtained from bSSFP images, as verified by two radiologists and auto-contouring software (composite rater intraclass correlation coefficients, 0.76–0.97 with near-zero mean differences).
In recent years, there has been marked progress in DL methods to accomplish a variety of radiology tasks, focusing primarily on lesion detection, diagnosis, or classification, risk prediction, and image segmentation or quantification (25–27). However, comparatively less attention has been paid to the tremendous potential of DL to fundamentally transform existing image reconstruction paradigms, with ready applications to MRI (7–10,12–14,28,29). The current work thus contributes to this growing field, including its promise for rapid, direct clinical translation and deployment.
A recent report (30) urged caution about the possible instabilities of DL-based image reconstruction methods, with small model perturbations causing unique artifacts. As shown in case examples in our study, image contrast differences and artifacts were observed with the DL technique, likely related to undersampling and the image reconstruction process and contributing to slightly lower image quality scores compared to the reference standard. A prudent approach might thus be to review the DL cine images in real time to determine whether follow-up conventional scanning is still required.
In our study, intra- and interrater agreement in ejection fraction measurements was lower compared with that of other parameters, likely related to compounding of measurement errors in end-diastolic volume and end-systolic volume, from which ejection fraction is derived (20,31). The level of variation in DL cine versus bSSFP volume and function estimates as interrogated by Bland-Altman limits of agreement, while nonzero, is within a range at least as good as or better than that reported in similar studies comparing bSSFP to nonconventional short-axis techniques (20,32,33). Lesser interobserver agreement in general for right ventricular parameters and machine-derived versus radiologist measurements are attributed to intrinsically more complex right ventricular geometry and inherent challenges in contour automation, respectively (20,28,34,35). At our institution, clinical use of the commercial artificial intelligence algorithm, with often imperfect initial traces, is currently at the imager's discretion, and auto-generated contours are manually reviewed and edited as deemed necessary to ensure accuracy.
This study had limitations. First, the DL model training data set size was relatively small. However, we note that it is larger than data sets used in previously published studies of cardiac cine DL reconstruction (36–40). Moreover, the use of physics-based modeling reduces the propensity of the model to overfit to specific patient anatomy. Second, our participant sample, although powered for pediatric LVEF and similar in size to that of other studies comparing bSSFP to nontraditional short-axis acquisitions, was still modest in size and both clinically and demographically heterogeneous, reducing its potential generalizability in all patient subgroups (20,31–34). Third, additional quantitative parameters (eg, circumferential and radial strain) and specific morphologic features (eg, valves and trabeculations) were not evaluated and beyond the scope of our study. Fourth, the DL cine was only tested in the short-axis plane, and translation to other planes has not been confirmed. Finally, the incremental benefit of the DL-based acceleration employed over the many existing non-DL based acceleration methods, including the non-DL eigenvalue autocalibrating parallel MRI approach, could not be assessed, because such techniques are not routinely used in our clinical cardiac MRI practice (15,16).
In conclusion, our highly accelerated cine cardiac MRI sequence with deep learning (DL) reconstruction achieved similar overall short-axis image quality and biventricular volume and function and left ventricular mass estimates when compared with those of reference standard balanced steady-state free precession MRI, despite markedly shorter acquisition and sequence duration times. This performance persisted even in the face of a clinically diverse study sample, with robust validation both from two expert radiologists and automated contouring software. With continued optimization, including mitigation of potential image contrast differences and artifacts, this DL cine method shows substantial promise for facilitating faster short-axis acquisitions for quantitative measurement, with less onerous examinations for patients in need.
Study supported by National Institutes of Health (grant nos. R01EB009690 and R01EB019241), NSF Graduate Research Fellowship, GE Healthcare, Google Cloud, Tashia and John Morgridge Faculty Scholar Fund, and Stanford Maternal & Child Health Research Institute.
Disclosures of Conflicts of Interest: E.J.Z. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Genetech. Other relationships: disclosed no relevant relationships. C.M.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has grants with General Electric Healthcare. Other relationships: disclosed no relevant relationships. A.K. disclosed no relevant relationships. P.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by General Electric Healthcare. Other relationships: disclosed no relevant relationships. S.S.V. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has grants/grants pending with General Electric Healthcare; holds stock in Arterys and InkSpace Imaging and is an advistory board member of HeartVista. Other relationships: disclosed no relevant relationships.
Abbreviations:
- bSSFP
- balanced steady-state free precession
- DL
- deep learning
- LV
- left ventricle
- LVEF
- LV ejection fraction
References
- 1. Atweh LA , Dodd NA , Krishnamurthy R , Pednekar A , Chu ZD , Krishnamurthy R . Comparison of two single-breath-held 3-D acquisitions with multi-breath-held 2-D cine steady-state free precession MRI acquisition in children with single ventricles . Pediatr Radiol 2016. ; 46 ( 5 ): 637 – 645 . [DOI] [PubMed] [Google Scholar]
- 2. Delgado JA , Abad P , Rascovsky S , et al . Assessment of cardiac volumes using an isotropic whole-heart dual cardiac phase sequence in pediatric patients . J Magn Reson Imaging 2014. ; 39 ( 3 ): 708 – 716 . [DOI] [PubMed] [Google Scholar]
- 3. Wech T , Pickl W , Tran-Gia J , et al . Whole-heart cine MRI in a single breath-hold--a compressed sensing accelerated 3D acquisition technique for assessment of cardiac function . Rofo 2014. ; 186 ( 1 ): 37 – 41 . 10.1055/s-0033-1350521 . [DOI] [PubMed] [Google Scholar]
- 4. Sievers B , Schrader S , Rehwald W , Hunold P , Barkhausen J , Erbel R . Left ventricular function assessment using a fast 3D gradient echo pulse sequence: comparison to standard multi-breath hold 2D steady state free precession imaging and accounting for papillary muscles and trabeculations . Acta Cardiol 2011. ; 66 ( 3 ): 349 – 357 . [DOI] [PubMed] [Google Scholar]
- 5. Krishnamurthy R , Pednekar A , Atweh LA , et al . Clinical validation of free breathing respiratory triggered retrospectively cardiac gated cine balanced steady-state free precession cardiovascular magnetic resonance in sedated children . J Cardiovasc Magn Reson 2015. ; 17 ( 1 ): 1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choy G , Khalilzadeh O , Michalski M , et al . Current applications and future impact of machine learning in radiology . Radiology 2018. ; 288 ( 2 ): 318 – 328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen F , Taviani V , Malkiel I , et al . Variable-density single-shot fast spin-echo MRI with deep learning reconstruction by using variational networks . Radiology 2018. ; 289 ( 2 ): 366 – 373 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeng DY , Shaikh J , Holmes S , et al . Deep residual network for off-resonance artifact correction with application to pediatric body MRA with 3D cones . Magn Reson Med 2019. ; 82 ( 4 ): 1398 – 1411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen F , Cheng JY , Taviani V , et al . Data-driven self-calibration and reconstruction for non-cartesian wave-encoded single-shot fast spin echo using deep learning . J Magn Reson Imaging 2020. ; 51 ( 3 ): 841 – 853 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hammernik K , Klatzer T , Kobler E , et al . Learning a variational network for reconstruction of accelerated MRI data . Magn Reson Med 2018. ; 79 ( 6 ): 3055 – 3071 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leng S , Yang X , Zhao X , et al . Computational platform based on deep learning for segmenting ventricular endocardium in long-axis cardiac MR imaging . Annu Int Conf IEEE Eng Med Biol Soc 2018. ; 2018 : 4500 – 4503 . [DOI] [PubMed] [Google Scholar]
- 12. Sandino CM , Cheng JY , Chen F , Mardani M , Pauly JM , Vasanawala SS . Compressed Sensing: From Research to Clinical Practice with Deep Neural Networks . IEEE Signal Process Mag 2020. ; 37 ( 1 ): 111 – 127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masutani EM , Bahrami N , Hsiao A . Deep learning single-frame and multiframe super-resolution for cardiac MRI . Radiology 2020. ; 295 ( 3 ): 552 – 561 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandino CM , Lai P , Vasanawala SS , Cheng JY . Accelerating cardiac cine MRI using a deep learning-based ESPIRiT reconstruction . Magn Reson Med 2021. ; 85 ( 1 ): 152 – 167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lustig M , Pauly JM . SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space . Magn Reson Med 2010. ; 64 ( 2 ): 457 – 471 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uecker M , Lai P , Murphy MJ , et al . ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA . Magn Reson Med 2014. ; 71 ( 3 ): 990 – 1001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Storey P , Li W , Chen Q , Edelman RR . Flow artifacts in steady-state free precession cine imaging . Magn Reson Med 2004. ; 51 ( 1 ): 115 – 122 . [DOI] [PubMed] [Google Scholar]
- 18. Lai P , Sandino CM , Vasanawala SS , Janich M . Time-alternative data sampling for reducing flow artifacts in bSSFP cine in a single heart beat using deep learning reconstruction [Poster session] . SCMR 23rd Annual Scientific Sessions , Orlando, FL , February 12–15, 2020 . [Google Scholar]
- 19. Vasanawala SS , Nguyen KL , Hope MD , et al . Safety and technique of ferumoxytol administration for MRI . Magn Reson Med 2016. ; 75 ( 5 ): 2107 – 2111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanneman K , Kino A , Cheng JY , Alley MT , Vasanawala SS . Assessment of the precision and reproducibility of ventricular volume, function, and mass measurements with ferumoxytol-enhanced 4D flow MRI . J Magn Reson Imaging 2016. ; 44 ( 2 ): 383 – 392 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zucker EJ , Cheng JY , Haldipur A , Carl M , Vasanawala SS . Free-breathing pediatric chest MRI: Performance of self-navigated golden-angle ordered conical ultrashort echo time acquisition . J Magn Reson Imaging 2018. ; 47 ( 1 ): 200 – 209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lang RM , Badano LP , Mor-Avi V , et al . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging . J Am Soc Echocardiogr 2015. ; 28 ( 1 ): 1 – 39.e14 . [DOI] [PubMed] [Google Scholar]
- 23. Portney LG . Foundations of clinical research: applications to evidence-based practice . 4th ed. Philadelphia, Pa: : F.A. Davis; , 2020. . [Google Scholar]
- 24. Kawel-Boehm N , Maceira A , Valsangiacomo-Buechel ER , et al . Normal values for cardiovascular magnetic resonance in adults and children . J Cardiovasc Magn Reson 2015. ; 17 ( 1 ): 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McBee MP , Awan OA , Colucci AT , et al . Deep learning in radiology . Acad Radiol 2018. ; 25 ( 11 ): 1472 – 1480 . [DOI] [PubMed] [Google Scholar]
- 26. Lu MT , Ivanov A , Mayrhofer T , Hosny A , Aerts HJWL , Hoffmann U . Deep learning to assess long-term mortality from chest radiographs . JAMA Netw Open 2019. ; 2 ( 7 ): e197416 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banerjee I , Sofela M , Yang J , et al . Development and performance of the pulmonary embolism result forecast model (PERFORM) for computed tomography clinical decision support . JAMA Netw Open 2019. ; 2 ( 8 ): e198719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lundervold AS , Lundervold A . An overview of deep learning in medical imaging focusing on MRI . Z Med Phys 2019. ; 29 ( 2 ): 102 – 127 . [DOI] [PubMed] [Google Scholar]
- 29. Mardani M , Gong E , Cheng JY , et al . Deep generative adversarial neural networks for compressive sensing MRI . IEEE Trans Med Imaging 2019. ; 38 ( 1 ): 167 – 179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Antun V , Renna F , Poon C , Adcock B , Hansen AC . On instabilities of deep learning in image reconstruction and the potential costs of AI . Proc Natl Acad Sci U S A 2020. ; 117 ( 48 ): 30088 – 30095 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mooij CF , de Wit CJ , Graham DA , Powell AJ , Geva T . Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles . J Magn Reson Imaging 2008. ; 28 ( 1 ): 67 – 73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JP , Deng J , Robinson JD , Seiberlich N , Rigsby CK . A comparison of real-time radial GRAPPA and standard cine imaging for the evaluation of cardiac function in children and young adults . J Cardiovasc Magn Reson 2016. ; 18 ( Supplement 1 ): O74 . [Google Scholar]
- 33. Pednekar AS , Wang H , Flamm S , Cheong BY , Muthupillai R . Two-center clinical validation and quantitative assessment of respiratory triggered retrospectively cardiac gated balanced-SSFP cine cardiovascular magnetic resonance imaging in adults . J Cardiovasc Magn Reson 2018. ; 20 ( 1 ): 44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luijnenburg SE , Robbers-Visser D , Moelker A , Vliegen HW , Mulder BJ , Helbing WA . Intra-observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging . Int J Cardiovasc Imaging 2010. ; 26 ( 1 ): 57 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bai W , Sinclair M , Tarroni G , et al . Automated cardiovascular magnetic resonance image analysis with fully convolutional networks . J Cardiovasc Magn Reson 2018. ; 20 ( 1 ): 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schlemper J , Caballero J , Hajnal JV , Price AN , Rueckert D . A deep cascade of convolutional neural networks for dynamic MR image reconstruction . IEEE Trans Med Imaging 2018. ; 37 ( 2 ): 491 – 503 . [DOI] [PubMed] [Google Scholar]
- 37. Qin C , Schlemper J , Caballero J , Price AN , Hajnal JV , Rueckert D . Convolutional recurrent neural networks for dynamic MR image reconstruction . IEEE Trans Med Imaging 2019. ; 38 ( 1 ): 280 – 290 . [DOI] [PubMed] [Google Scholar]
- 38. Biswas S , Aggarwal HK , Jacob M . Dynamic MRI using model-based deep learning and SToRM priors: MoDL-SToRM . Magn Reson Med 2019. ; 82 ( 1 ): 485 – 494 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Küstner T , Fuin N , Hammernik K , et al . CINENet: deep learning-based 3D cardiac CINE MRI reconstruction with multi-coil complex-valued 4D spatio-temporal convolutions . Sci Rep 2020. ; 10 ( 1 ): 13710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hauptmann A , Arridge S , Lucka F , Muthurangu V , Steeden JA . Real-time cardiovascular MR with spatio-temporal artifact suppression using deep learning-proof of concept in congenital heart disease . Magn Reson Med 2019. ; 81 ( 2 ): 1143 – 1156 . [DOI] [PMC free article] [PubMed] [Google Scholar]