Abstract
INTRODUCTION:
Duodenal epithelial barrier impairment and immune activation may play a role in the pathogenesis of functional dyspepsia (FD). This study was aimed to evaluate the duodenal epithelium of patients with FD and healthy individuals for detectable microscopic structural abnormalities.
METHODS:
This is a prospective study using esophagogastroduodenoscopy enhanced with duodenal confocal laser endomicroscopy (CLE) and mucosal biopsies in patients with FD (n = 16) and healthy controls (n = 18). Blinded CLE images analysis evaluated the density of epithelial gaps (cell extrusion zones), a validated endoscopic measure of the intestinal barrier status. Analyses of the biopsied duodenal mucosa included standard histology, quantification of mucosal immune cells/cytokines, and immunohistochemistry for inflammatory epithelial cell death called pyroptosis. Transepithelial electrical resistance (TEER) was measured using Ussing chambers. Epithelial cell-to-cell adhesion proteins expression was assessed by real-time polymerase chain reaction.
RESULTS:
Patients with FD had significantly higher epithelial gap density on CLE in the distal duodenum than that of controls (P = 0.002). These mucosal abnormalities corresponded to significant changes in the duodenal biopsy samples of patients with FD, compared with controls, including impaired mucosal integrity by TEER (P = 0.009) and increased number of epithelial cells undergoing pyroptosis (P = 0.04). Reduced TEER inversely correlated with the severity of certain dyspeptic symptoms. Furthermore, patients with FD demonstrated altered duodenal expression of claudin-1 and interleukin-6. No differences in standard histology were found between the groups.
DISCUSSION:
This is the first report of duodenal CLE abnormalities in patients with FD, corroborated by biopsy findings of epithelial barrier impairment and increased cell death, implicating that duodenal barrier disruption is a pathogenesis factor in FD and introducing CLE a potential diagnostic biomarker in FD.
INTRODUCTION
The term “dyspepsia” encompasses a constellation of symptoms including epigastric pain or burning, early satiety, and postprandial fullness (1). Dyspeptic symptoms are reported in 10%–20% of the general population (2). Up to 70% of patients with dyspepsia who undergo endoscopy have unremarkable examination and are diagnosed with functional dyspepsia (FD) (3). The Rome diagnostic criteria divides FD into 2 main subgroups: postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) (1). FD is one of the most common reasons for primary care visits, negatively impacts productivity at the workplace, and has annual associated costs in the United States exceeding $18 billion (4,5).
The pathophysiology of FD is incompletely understood but likely complex with contributions from a number of different underlying mechanisms (6). Low-grade inflammation of the duodenal mucosa (7) and impaired duodenal epithelial integrity with increased permeability (8) have been suggested as a pathophysiologic mechanism in a subset of patients with FD. Indeed, observational studies using the standard histological evaluation report subtle duodenal immune cell infiltration, predominantly with eosinophils and/or mast cells, in up to 40% of patients with FD (9–12). However, the role of the impaired duodenal epithelial barrier structure in the pathogenesis of FD, including its relationship with the low-grade immune activation and individual dyspeptic symptoms, has been less well studied. Furthermore, the mechanisms leading to immune activation and/or impaired mucosal permeability in patients with FD are not well defined. Currently, the diagnosis and treatment of FD is almost entirely predicated on the patient’s symptoms because there is no diagnostic biomarker, based on underlying pathophysiology, for this common and bothersome condition.
Recently, a novel, immune-mediated inflammatory form of cell death called “pyroptosis” was described to account for the intestinal epithelial cells’ (IECs) extrusion process that breaches mucosal integrity in health and disease (13–15). Pyroptosis is an inflammatory form of cell death that epithelial cells undergo in response to environmental stimuli, which has been proposed to contribute to epithelial barrier dysfunction in patients with inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) (15,16). The role of pyroptosis in FD has not been investigated.
Confocal laser endomicroscopy (CLE) is a technology that can be performed during routine gastrointestinal (GI) endoscopy and provides “real-time,” exceptionally high magnification and resolution endoluminal gut imaging (17). CLE is a technique capable of dynamically visualizing altered mucosal barrier function, including visualization of the extrusion zones left in the mucosal surface after IECs have been shed (18,19). In recent studies using CLE, a subset of patients with IBS had mucosal barrier disruption (20,21). To our knowledge, studies using CLE to assess the duodenal mucosal architecture in patients with FD have not been performed.
We hypothesized that patients with FD, but not healthy individuals, have impaired duodenal epithelial barrier integrity, increased duodenal immune activation, and increased duodenal epithelial pyroptosis. The aim of this exploratory study was to assess whether these duodenal epithelial abnormalities might contribute to the pathogenesis of FD. We addressed this question using several experimental techniques, including CLE and histology with “ex vivo” measures of epithelial barrier integrity, immune activation, and pyroptosis, to compare the duodenal mucosal structure and function between patients with FD and healthy volunteers.
METHODS
This was a prospective, controlled, pilot study where symptomatic patients with FD and healthy individuals without significant GI symptoms were assessed with esophagogastroduodenoscopy (EGD) enhanced by probe-based CLE (pCLE) and duodenal mucosa biopsies for comprehensive histological evaluation of structural differences between the groups.
Patient population
Included patients had active upper GI symptoms that fulfilled the Rome IV criteria for PDS, with or without concomitant EPS, while consuming an unrestricted US diet (1). To reduce clinical heterogeneity, we chose to focus on patients with FD with postprandial symptoms. We also believed that the exclusion of patients with isolated EPS would reduce overlap with gastroesophageal reflux disease (GERD). All patients had a normal complete blood count and no evidence of celiac disease (negative serology or duodenal histology). Healthy individuals with no active or history of significant GI symptoms, consuming an unrestricted US diet, comprised the control group. In addition, patients referred for EGD to evaluate conditions unrelated to dyspepsia, including globus sensation or asymptomatic iron deficiency without overt anemia, were included in the control group for tissue-based analyses. These individuals were carefully evaluated at the time of recruitment to assure the absence of any GI symptoms or disease (except globus sensation) and the consumption of an unrestricted diet.
Exclusion criteria for patients with FD included pregnancy, age younger than 18 years, or presence of alarm clinical features including weight loss, nocturnal symptoms, vomiting, or dysphagia. Patients consuming nonsteroidal anti-inflammatory drugs, opioids, or having known allergy to fluorescein, midazolam, or fentanyl were also excluded. Patients previously diagnosed with structural GI disease (e.g., peptic ulcer disease, erosive esophagitis, and IBD), including Helicobacter pylori infection, and those with a comorbid medical condition known to affect the GI system (e.g., scleroderma, unstable thyroid disease, or diabetes mellitus) were excluded. Patients with previous surgery to the GI tract were not eligible except those who underwent appendectomy or cholecystectomy if performed >6 months before enrollment. The same exclusion criteria were applied to the control group.
Study protocol
Patients with FD.
Patients undergoing evaluation for dyspeptic symptoms in the Gastroenterology Clinics at the University of Michigan were invited to participate in the study. Interested patients were assessed for eligibility during an initial visit when baseline descriptive data were collected (age, sex, race, pertinent medical history, vital signs, weight, and use of medications). At this visit, the patients were also assessed for their past medical, surgical, and social history. Any reported comorbidity was confirmed through review of the patient’s medical record. Baseline symptoms were assessed according to the Rome 4 diagnostic criteria for FD (1), comprising 7 questions that include cardinal dyspeptic symptoms (early satiety, postprandial fullness, epigastric pain, epigastric burning, epigastric bloating, nausea, and belching). Patients were also evaluated for meal-related symptoms and excluded if following a specific restriction diet (e.g., gluten-free diet or low fermentable oligo-, di-, mono-saccharides and polyols diet).
Eligible patients received written instructions regarding a standard, 2,000 kcal/d, US diet to which they adhered for at least 2 weeks. During this time, patients consumed standard amounts of wheat and dairy for a typical US diet. Using a web-based survey, patients rated their daily dyspeptic symptoms using a 7-point numerical rating scale (NRS; 0, none to 7, very severe symptoms) over 2 weeks. Eligible patients had a mean daily score ≥3 for either “early satiety” and/or “postprandial fullness” and ≥2 d/wk experiencing the same symptom based on NRS responses. Patients who reported concomitant epigastric pain or burning were allowed to participate. Eligible patients were scheduled for EGD with pCLE.
Control group.
Asymptomatic healthy volunteers (N = 10) were recruited through paper and electronic advertisements posted throughout the University of Michigan campus. Interested individuals were scheduled for an initial visit, when baseline and descriptive data were obtained as described for patients with FD. These individuals were symptomatically assessed to specifically rule out any GI symptoms. Eligible volunteers received the same dietary instructions as FD patients to which they adhered for at least 2 weeks and were scheduled for EGD with pCLE.
Control group participants (N = 10) were also recruited from patients clinically scheduled to undergo EGD for globus sensation and/or laboratory-detected iron deficiency without overt anemia. These individuals were symptomatically assessed before their EGD to assure the absence of any GI symptoms or previously diagnosed GI disease (as described in exclusion criteria). Baseline descriptive data were obtained and a dietary assessment ensured patients were consuming an unrestricted diet. Eligible participants underwent EGD, and if no endoscopic abnormality was present, duodenal biopsies for research were obtained and processed as described below. This subgroup of controls did not undergo CLE, and their study participation was restricted to tissue-based analyses of the biopsied duodenal mucosa.
EGD With pCLE.
Eligibility was confirmed before EGD with pCLE. Procedures were performed with a standard upper endoscope (Olympus Medical, USA). CLE imaging was performed using a probe (Cellvizio, Mauna Kea Technologies, Paris, France) advanced through the endoscope’s accessory channel.
During the standard diagnostic EGD, the endoscope was guided into the third portion of the duodenum. The duodenal intubation was performed with particular care by a single, experienced endoscopist (B.N.) to avoid scope-induced mucosal damage that can confound interpretation of pCLE images. The pCLE was not performed if standard EGD revealed an endoscopic abnormality that could explain a patient’s dyspepsia (e.g., peptic ulcer disease and erosive esophagitis). In such patients, EGD was completed and study participation ended. In patients who had normal diagnostic EGD, pCLE was performed. Once the duodenum was intubated, 5 mL, 10% fluorescein was administered intravenously, and the laser was activated for CLE scanning. Frame-by-frame confocal images of the duodenum, starting from its third portion, were collected and digitally stored for analysis.A minimum of 3 different locations per site were imaged with pCLE for the third, second, and first portions (D3, D2, and D1) of the duodenum. Continuous recordings of the pCLE image videos were made for at least 10 minutes in all patients. Immediately after the pCLE completion to assess for gross histologic abnormalities, duodenal epithelial cell counts (eosinophils, mast cells, and lymphocytes), structural/functional epithelial barrier measures (described below), and/or epithelial pyroptosis, a minimum of 8 biopsies including each of the duodenal segments were obtained.
Detailed review and analysis of recorded pCLE images was performed in a post hoc manner as previously described (15,20). These images were independently analyzed in detail by 2 different investigators (R.D.D., J.J.L.), who were blinded to the status of participants to minimize bias (control vs FD patient). The primary CLE characteristic of duodenal epithelium that was assessed included the number of epithelial gaps, with or without fluorescein leaks into the lumen, counted per 1,000 IECs. Cell and gap counts were performed in each of the 3 duodenal segments (D3 through D1). Adequacy of imaged villi selected for the analysis were pre-defined such as the villi having more than 75% surface area visualized on the pCLE images, with a minimum of 3 consecutive views of the villi seen. Epithelial gaps were counted in those villi images, and the highest frequency of epithelial gaps for any individual patient was used to determine the gap density. The mean numbers of epithelial gaps per 1,000 epithelial cells were compared between patients with FD and healthy controls in each of the duodenal segments and overall. Figure 1 depicts representative pCLE images of duodenal villi in asymptomatic individual without epithelial gaps and the patient with FD with epithelial gaps.
Figure 1.
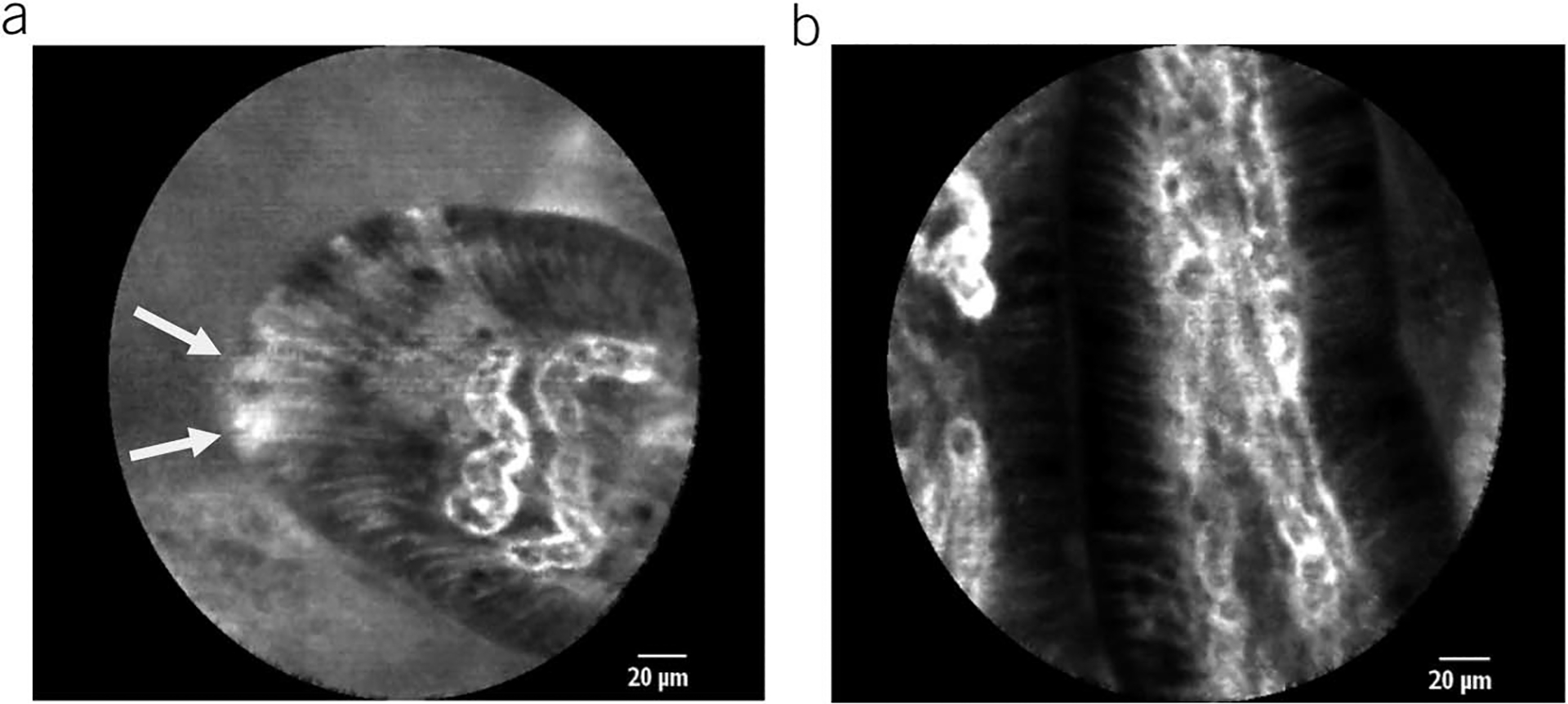
Representative probe-based CLE images of duodenal villi: (a) a patient with functional dyspepsia with several adjacent epithelial gaps (white arrowheads indicating epithelial gaps); (b) healthy individual without epithelial gaps.
Biopsy sample analyses.
Standard histology including specific stains for intraepithelial eosinophils, mast cells, and lymphocytes counts were assessed in each participant.
Intestinal epithelial barrier function was assessed by measuring the transepithelial electrical resistance (TEER) of ex vivo tissues as reported previously (22,23). TEER reflects paracellular resistance imparted by tight junctions and the lateral paracellular space and is a sensitive measure of barrier integrity (24). Duodenal mucosal biopsies were washed twice in sterilized PBS and transferred to Petri dishes containing Dulbecco’s modified eagle medium culture medium. After a 30-minute incubation at 37°C and pH stabilization, the TEER was measured using the micro-Snapwell system with an Endohm sural sensory nerve action potential (SNAP) electrode attached to an EVOM2 epithelial volt-ohm meter (World Precision Instruments) and expressed in ohms per square centimeter (Ω/cm2).
Quantitative or semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) for cell-to-cell adhesion proteins and inflammatory cytokines from the biopsied duodenal mucosa was performed. Total RNA was extracted from duodenal tissue samples using the TRIzol reagent (Life Technologies, Rockville, MD), according to the manufacturer’s instructions. Complementary DNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Quantitative polymerase chain reaction (PCR) for cell-to-cell adhesion proteins, inflammatory cytokines, and glyceraldehyde-3-phosphate dehydrogenase was performed with a CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories) using SYBR Green detection. Primers used for claudin (CLDN) 1–4 and 15, occludin (OCLN), zonula occludens (ZO) 1–3, junctional adhesion molecule 1, β-catenin, E-cadherin, interleukin (IL) 1β and 6, tumor necrosis factor (TNF) α, and interferon (IFN) γ were obtained from Qiagen. The PCR conditions were as follows: one cycle at 95°C for 10 minutes, followed by 40 two-temperature cycles at 95°C for 15 seconds and 60°C for 60 seconds. PCR amplifications were performed in a total volume of 25 μL, containing iQSYBR Green supermix (Bio-Rad Laboratories). Cytokine transcript levels were normalized to that of GADPH, and relative gene expression was expressed as the fold change (2−ΔΔCt) relative to expression in the control samples.
For evaluation of epithelial cell pyroptosis, slides were stained using the Maximus Biological Assay staining kits (Maximus Diagnostics LLC, Little Rock, AR). Samples with a minimum of 10 intact villi per patient were analyzed for quantitation of duodenal epithelial cell pyroptosis by a gastroenterologist blinded to the patient’s status. IECs and cells positive for activated caspase were manually counted in 10 villi for the derivation of duodenal pyroptosis because the number cells stained positive for activated caspase-1 were (green in Figure 2)/1,000 IECs. In addition to activated caspase-1, the slides were stained for anti-CD3 (red), a lymphocyte marker used to differentiate intraepithelial lymphocytes from IECs. The stained slides were imaged with a Zeiss LSM 880 confocal microscope equipped with Airyscan (Zeiss USA, Dublin, CA).
Figure 2.
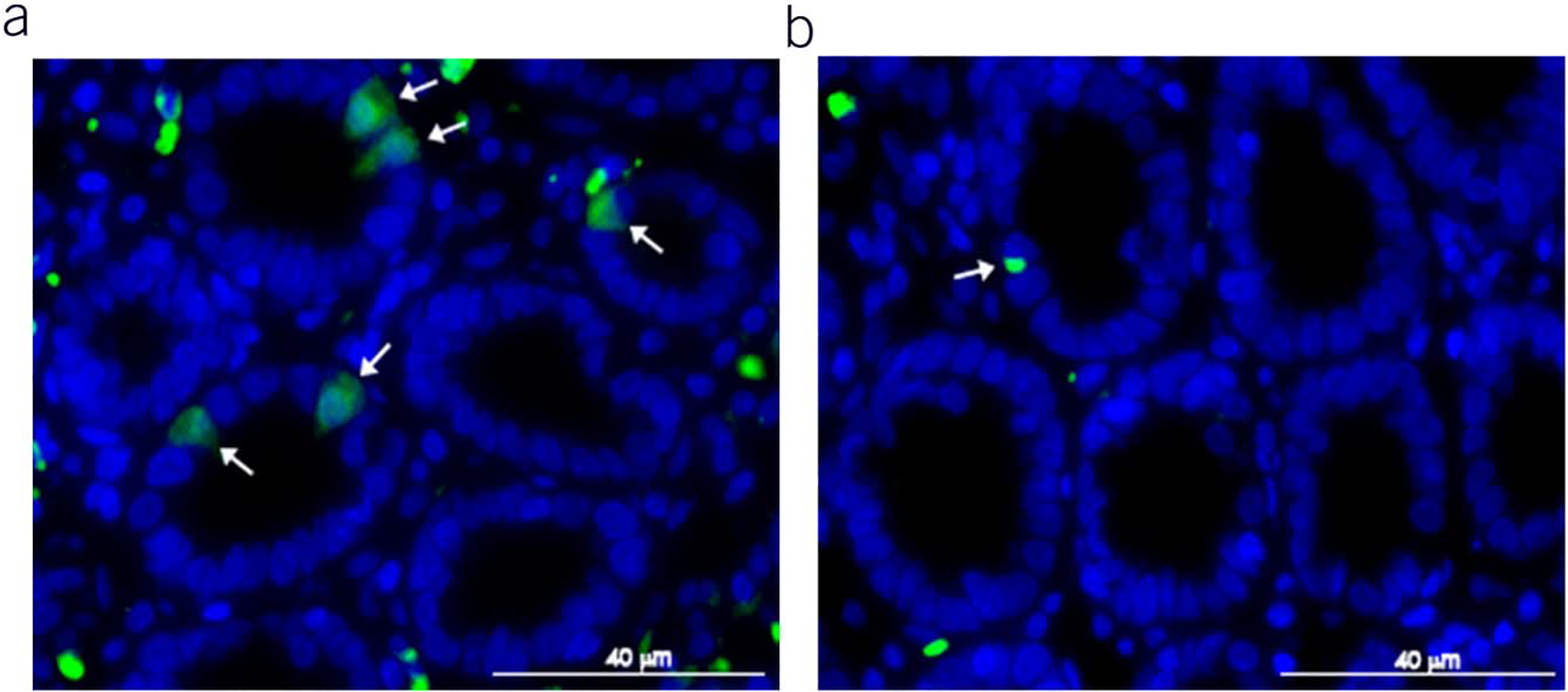
Representative image of biopsy immunohistochemistry stain for activated caspase from: (a) a patient with functional dyspepsia with positive stain (green) and (b) healthy control.
Statistical considerations.
The primary study outcome was the comparison of CLE findings (duodenal epithelial gap density) between patients with FD and healthy controls. Secondary outcomes included the comparison of measurements of duodenal epithelial barrier integrity (TEER; cell-to-cell adhesion proteins expression), immune activation (quantification of epithelial inflammatory cells and cytokines), and pyroptosis (immunohistochemistry staining for caspase-positive epithelial cells) from the biopsied mucosa between patients with FD and healthy controls. The relationship between markers of epithelial barrier integrity, mucosal inflammation, and pyroptosis with the severity of dyspeptic symptoms was also evaluated. The sample size calculation was performed based on the CLE epithelial gap density data of healthy control and patients with IBS from our previous study (20) because there are no available data on CLE duodenal epithelial gaps density in patients with FD. Assuming a difference in the mean gap density of 20 gaps/1,000 cells and a standard deviation of 10 gaps/1,000 cells, 6 patients per group would be required to achieve 80% power with type I error (α) of 0.05.
Bivariate analysis (including patient demographics and association with all study outcomes) were completed using the Student t test for all continuous variables and the Pearson χ2 and Fisher exact tests for categorical variables. Models were created to generate adjusted and unadjusted P values for differences between the groups in the primary outcome (CLE) and all secondary outcomes using the analysis of variance (general linear mixed model), which included interactions for possible confounders (body mass index [BMI], age, gender, IBS status, and proton pump inhibitor [PPI] use). R2 values were used to determine correlations when appropriate. All analyses were conducted by using SAS 9.4 data analysis and statistical software (Copyright (c) 2002–2012 by SAS Institute Inc., Cary, NC).
RESULTS
Patient population
A total of 38 participants (18 FD; 20 controls) were recruited and completed the study between January, 2017, and December, 2019. Two patients with FD and 2 controls were excluded from final analyses because of the presence of gross structural disease found on endoscopy or histology (1 patient with FD and 1 control with erosive esophagitis; 1 patient with FD with a large gastric bezoar; 1 control with increased duodenal intraepithelial lymphocytes). CLE was not completed in 2 additional patients with FD because of sedation difficulties or poor-quality CLE images. Patients with FD reported moderately severe early satiety (3.8 ± 1.7) and postprandial fullness (4.4 ± 1.6) over the 2-week screening period (Table 1). There were no statistically significant differences in the baseline demographic characteristics between patients with FD and controls, although patients with FD tended to be younger and to have lower BMI (Table 2). The majority were female participants in both the study groups. Anxiety/depression (5 patients), IBS (4 patients), and migraine headache (3 patients) were the most common comorbidities in patients with FD. Asthma, allergic rhinitis, constipation, and GERD (nonerosive) were each present in 2 patients with FD. The 2 patients with FD who had a history of GERD were on once daily PPI therapy with no active GERD symptoms. The 4 patients with FD with concomitant IBS included 2 patients with mixed bowel habits, 1 with constipation, and 1 with diarrhea predominance. All patients with FD were negative for Helicobacter pylori infection on histologic evaluation of gastric mucosal biopsies. Neuromodulators (5 patients), laxatives (5 patients), anticholinergics (3 patients), and PPIs (3 patients) were the most commonly used medications for patients with FD.
Table 1.
Average reported daily dyspepsia symptoms severity among functional dyspepsia patients
| Average daily scorea | |
|---|---|
| Epigastric pain | 2.96 ± 1.78 |
| Epigastric burning | 2.29 ± 1.65 |
| Early satiety | 3.84 ± 1.69 |
| Postprandial fullness | 4.43 ± 1.66 |
| Bloating | 4.25 ± 1.73 |
| Nausea | 2.51 ± 1.91 |
| Belching | 2.43 ± 1.91 |
0 = no symptom and 7 = worst symptom severity.
Table 2.
Baseline patient characteristics
| Functional dyspepsia (n = 16) | Control group (n = 18) | P value | |
|---|---|---|---|
| Average age—yr (range) | 31.7 ± 9.1 (21–48) | 40.3 ± 16.7 (21–73) | 0.07 |
| Sex—No of patients (%) | 0.54 | ||
| Female | 13 (81) | 13 (72) | |
| Male | 3 (19) | 5 (28) | |
| Average BMI, kg/m2 | 26.2 ± 6.8 | 31.7 ± 10.3 | 0.08 |
| Tobacco use—No of patients (ex-smokers) | 1 (4) | 0 (4) | 0.47 |
| Comorbidities (No of patients) | Anxiety/depression 5 | Globus sensation 6 | |
| IBS 4 | Anxiety/depression 5 | ||
| Constipation 3 | Iron-deficiency 3 (without anemia) | ||
| Migraine 3 | |||
| Asthma 2 | |||
| NERD* 2 | |||
| Allergic rhinitis 2 |
BMI, body mass index; IBS, irritable bowel syndrome; NERD, nonerosive reflux disease.
Globus sensation, stable anxiety/depression, and laboratory evidence of iron deficiency without overt anemia were the most common comorbidities in the control group. The 5 individuals diagnosed with anxiety/depression were each taking a stable dose of a neuromodulator. Four individuals with globus sensation were taking PPI once daily and 2 individuals used histamine-2 (H2) blocker on as-needed basis.
The standard EGD with routine histological evaluation of the duodenal mucosa biopsies revealed no abnormalities in patients with FD or healthy controls. No histological abnormalities were identified in gastric biopsies from the FD cohort and biopsied controls.
CLE revealed epithelial barrier disruption in the distal duodenum of patients with FD
Representative pCLE images of the duodenal epithelium from a healthy volunteer and a patient with FD with increased epithelial gaps are shown in Figure 1. Patients with FD had significantly higher mean epithelial gap density on pCLE in the third portion of the duodenum (D3) when compared with healthy controls (P = 0.002; Table 3 and Figure 3). The mean D3 gap density in patients with FD was 26.9 ± 9.4/1,000 epithelial cells vs 10.05 ± 6.5/1,000 epithelial cells for controls. The estimated mean difference in duodenal gap density between FD and controls was 17 (95% CI 5.3–25.8) gaps/1,000 cells. The difference in D3 epithelial gap density on CLE between patients with FD and controls remained statistically significant (P = 0.002) after controlling for potential confounders including: age, sex, BMI, comorbid IBS, and PPI use. There was no significant difference in the mean duodenal epithelial gap densities on pCLE in the first and second portions of the duodenum. When duodenal gap density was averaged including first, second, and third portions of the duodenum, there was a numerical difference between FD and healthy, which did not reach statistical significance (13.7 ± 6.4 vs 8.2 ± 5.4, P = 0.18).
Table 3.
Differences in mean epithelial gap density per 1,000 cells between healthy patients and patients with functional dyspepsia (mean ± SD)
| Functional dyspepsia (n = 14) | Healthy control (n = 8) | P value | |
|---|---|---|---|
| First portion of the duodenum (D1) | 6.2 ± 7 | 5.2 ± 9.5 | 0.75 |
| Second portion of the duodenum (D2) | 8.4 ± 11.2 | 9.5 ± 7.4 | 0.64 |
| Third portion of the duodenum (D3) | 26.9 ± 9.4 | 10.05 ± 6.5 | 0.002 |
| Mean of all sections ([D1 + D2 + D3]/3) | 13.7 ± 6.4 | 8.2 ± 5.4 | 0.18 |
Figure 3.
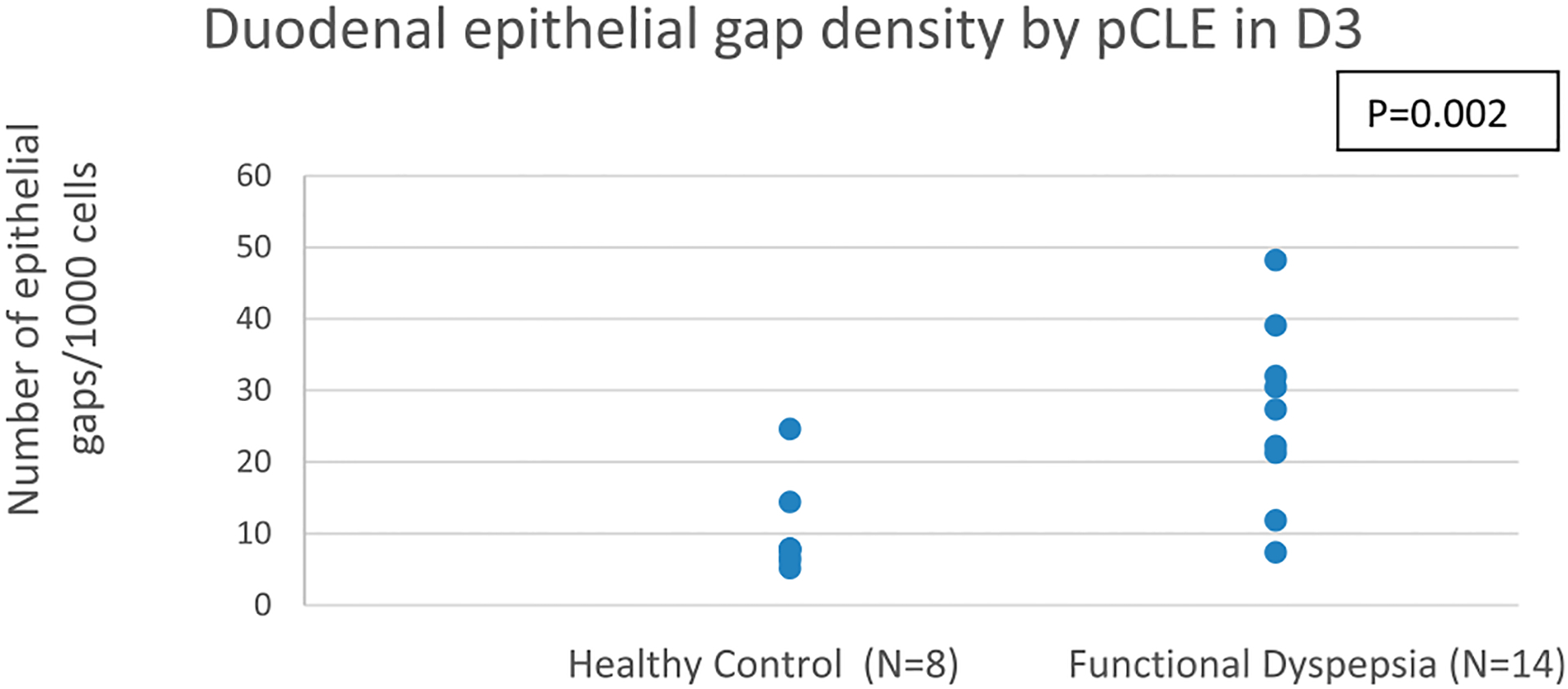
Epithelial gap density assessed by pCLE in the third portion of the duodenum (D3) in healthy controls and functional dyspepsia (FD) patients. pCLE, probe-based CLE.
An exploratory analysis to estimate the diagnostic accuracy of the duodenal gap density on pCLE in D3, as a diagnostic marker for FD, is shown in Table 4. Using 15 gaps/1,000 cells as the cutoff for an abnormal gap density, the estimated diagnostic sensitivity of gap density for FD is 78.6% and the specificity is 87.5%, with a positive predictive value of 92% and a negative predictive value of 70%.
Table 4.
Diagnostic accuracy of gap density for diagnosing FD
| FD (n = 14) | Healthy control (n = 8) | Total | |
|---|---|---|---|
| Elevated gap density | 11 | 1 | 12 |
| Normal gap density | 3 | 7 | 10 |
| Total | 14 | 8 |
Diagnostic accuracy of pCLE duodenal gap density assessment for FD (15 gaps/1,000 cells are used for cutoff of an abnormal gap density).
FD, functional dyspepsia; pCLE, probe-based CLE.
“Ex vivo” analysis of the biopsied mucosa revealed impaired duodenal epithelial barrier integrity
The integrity of duodenal mucosa from biopsied samples was evaluated by the measurement of TEER in a Ussing chambers, performed in subgroup of patients with FD and controls. Patients with FD showed significantly lower TEER in comparison to the control group (15.2 ± 5.7 vs 23.48 ± 6.6, P = 0.009; Figure 4), indicating impaired duodenal barrier integrity. When TEER measurements were correlated with severity scores of individual dyspeptic symptoms in patients with FD, there was a significant inverse correlation with abdominal pain (r = −0.751; P = 0.01) and bloating (r = −0.856; P = 0.001). There was a borderline significant inverse correlation of TEER with early satiety (r = −0.619; P = 0.05) and postprandial fullness (r = −0.601; P = 0.06) severity scores. These findings indicate that patients with impaired duodenal barrier integrity have more severe dyspeptic symptoms. There was no statistically significant correlation between TEER measurements and the epithelial gap density score assessed by CLE in patients with FD.
Figure 4.
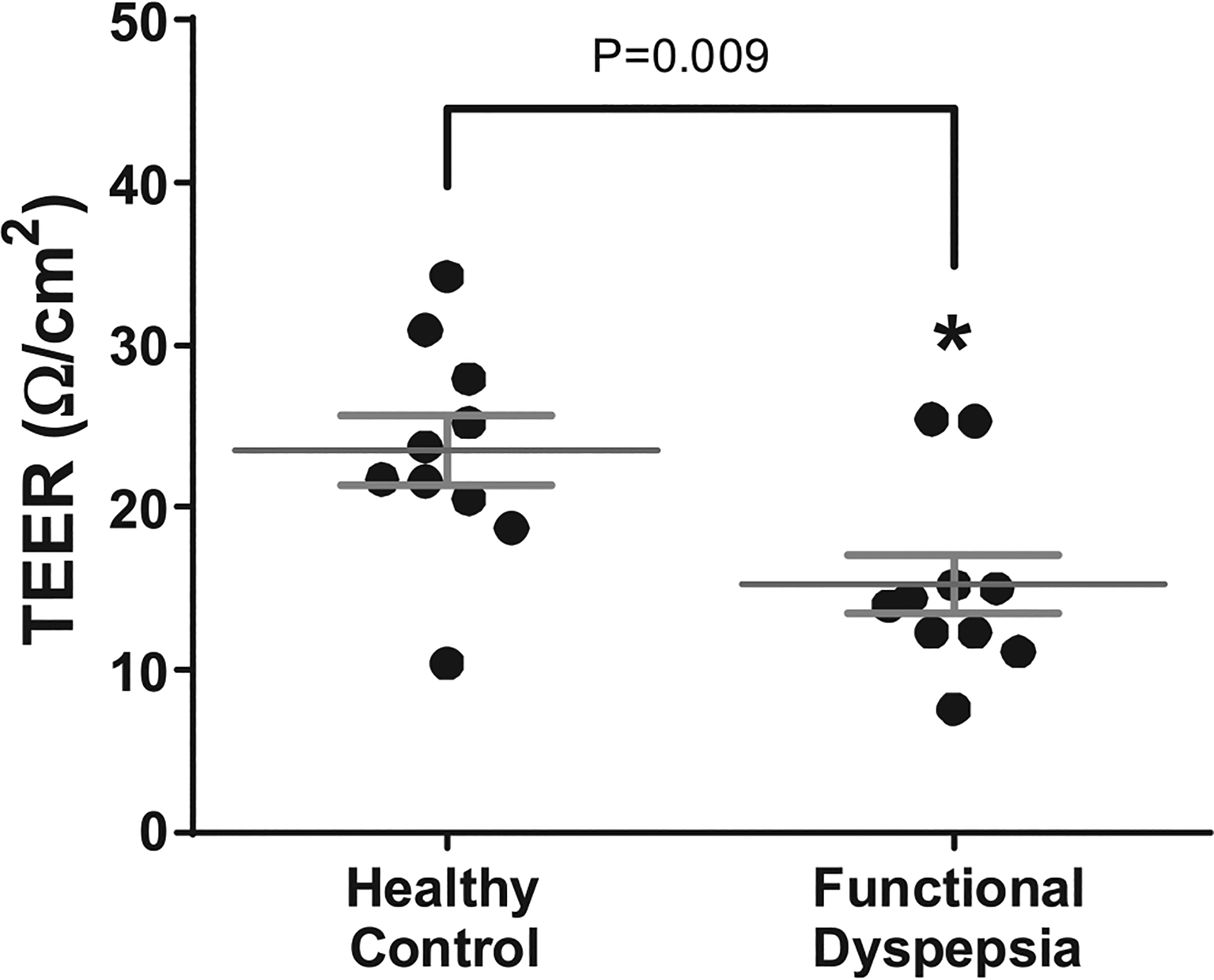
Assessment of duodenal epithelial integrity by TEER measurements in biopsied duodenal mucosa of healthy controls (n = 10) and FD patients (n = 10). TEER, transepithelial electrical resistance.
To further evaluate the duodenal epithelial barrier integrity, we assessed the gene expression by RT-PCR of several cell-to-cell adhesion proteins in duodenal biopsy samples from a subset of patients with FD and controls (Figure 5). The expression of CLDN 1 at the tight junction was significantly lower in patients with FD than in controls (1.0 ± 1.17 vs 0.51 ± 0.1, P = 0.017). There was no statistically significant difference in the gene expression of other tested tight junction proteins (CLDN 2–4 and 15, OCLN, ZO 1–3, junctional adhesion molecule 1) or adherence junction proteins (β-catenin and E-cadherin) between the 2 groups. There was no statistically significant correlation of cell-to-cell adhesion proteins expression with TEER measurements or epithelial gap density score by pCLE in patients with FD.
Figure 5.
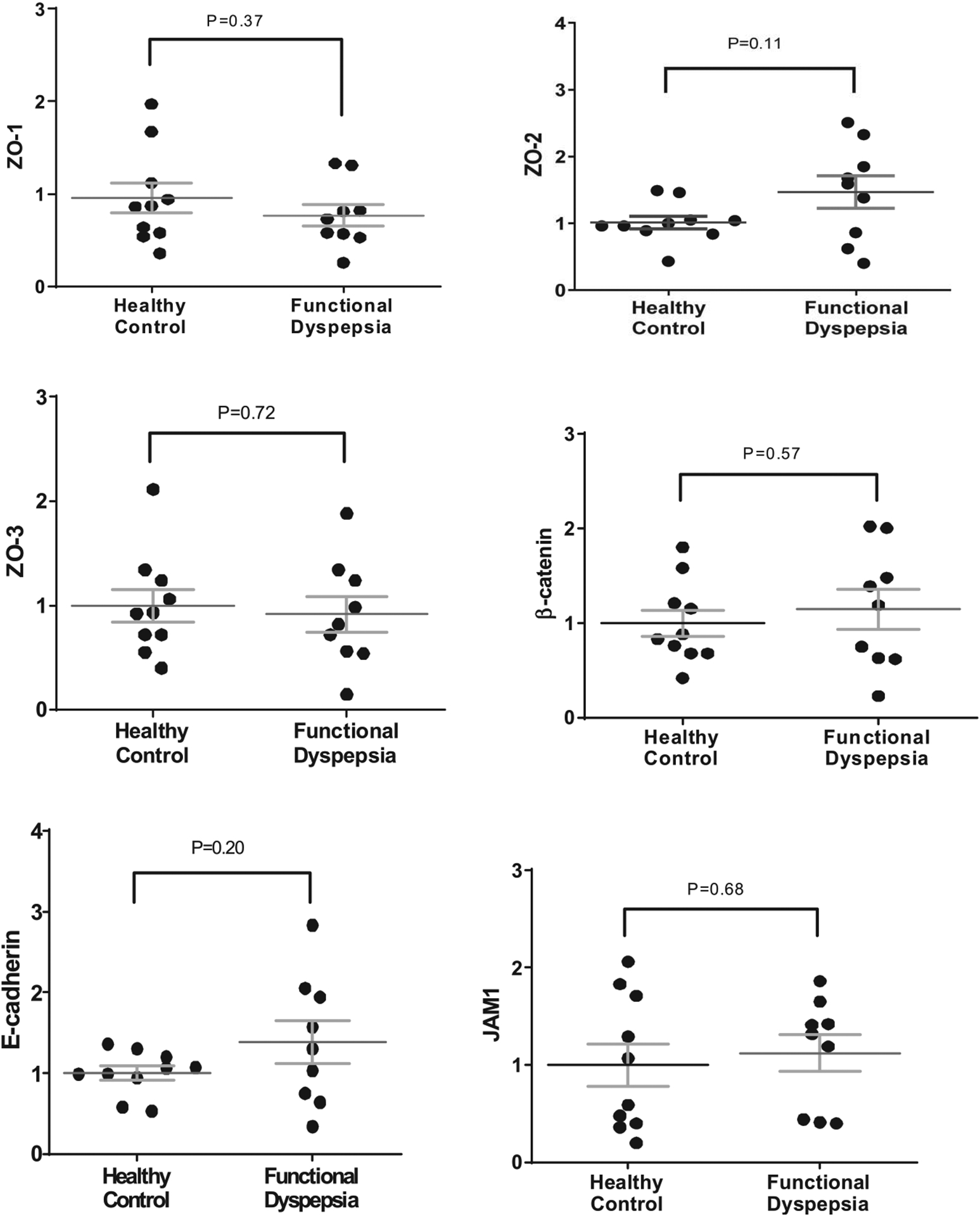
Gene extpression of tight junction and adherens junction proteins evaluated by real-time reverse transcriptase PCR in the biopsied duodenal mucosa from healthy controls and patients with FD. CLDN, claudin; OCLN, occludin; ZO 1–3, zonula occludens; and JAM1, junctional adhesion molecule 1.
The difference in TEER and CLD-1 expression between patients with FD and controls remained statistically significant after adjusting for possible confounders including: age, sex, BMI, comorbid IBS, and PPI use.
Assessment of duodenal “low-grade” inflammation
Histological analysis (including specific staining for intramucosal eosinophils, mast cells, and lymphocytes) of the duodenal mucosal biopsies from patients with FD and controls revealed no significant differences in the mean numbers (per high power field) of eosinophils (51.1 ± 29 vs 64.6 ± 48), mast cells (83.2 ± 17.2 vs 89.2 ± 30.2), or lymphocytes (101.9 ± 48.5 vs 95.1 ± 36.9) (P = NS for all comparisons; Table 5). To further assess for differences in duodenal mucosal immune activation, gene expression of certain cytokines including IL-1β, IL-6, TNF-α, and IFN-γ were quantified by RT-PCR in subset of patients with FD and controls. There was significantly higher expression of IL-6 in patients with FD compared with controls (1.7 ± 0.54 vs 1.0 ± 0.67, P = 0.02; Figure 6). There were no significant differences between groups in the quantitative assessment of other tissue cytokines. Interestingly, when individual cytokine expression was correlated with TEER measurements, IFN-γ expression inversely correlated with TEER (r = −0.695; P = 0.03), but there were no significant correlations for other cytokines. There was a significant inverse correlation between IL-6 expression and CLDN 2 (r = −0.666; P = 0.05), CLDN 4 (r = −0.716; P = 0.03), and borderline significance with β-catenin (r = −0.616; P = 0.07). Duodenal mucosa cytokine expression did not correlate with the epithelial gap density on CLE, nor with individual FD symptoms severity scores.
Table 5.
Intramucosal eosinophils, lymphocytes and mast cells in FD and healthy controls—histological cell counts from duodenal biopsy samples
| Mean number (range) | FD (n = 16) | Healthy control (n = 13) | P value |
|---|---|---|---|
| Eosinophils per 10 HPF | 51.1 ± 29 (21–132) | 64.6 ± 48 (17–167) | 0.356 |
| Lymphocytes per 2 HPF | 101.9 ± 48.5 (14–212) | 95.1 ± 36.9 (38–185) | 0.684 |
| Mast cells per 2 HPF | 83.2 ± 17.2 (42–115) | 89.2 ± 30.2 (34–131) | 0.504 |
FD, functional dyspepsia; HPF, high power field.
Figure 6.
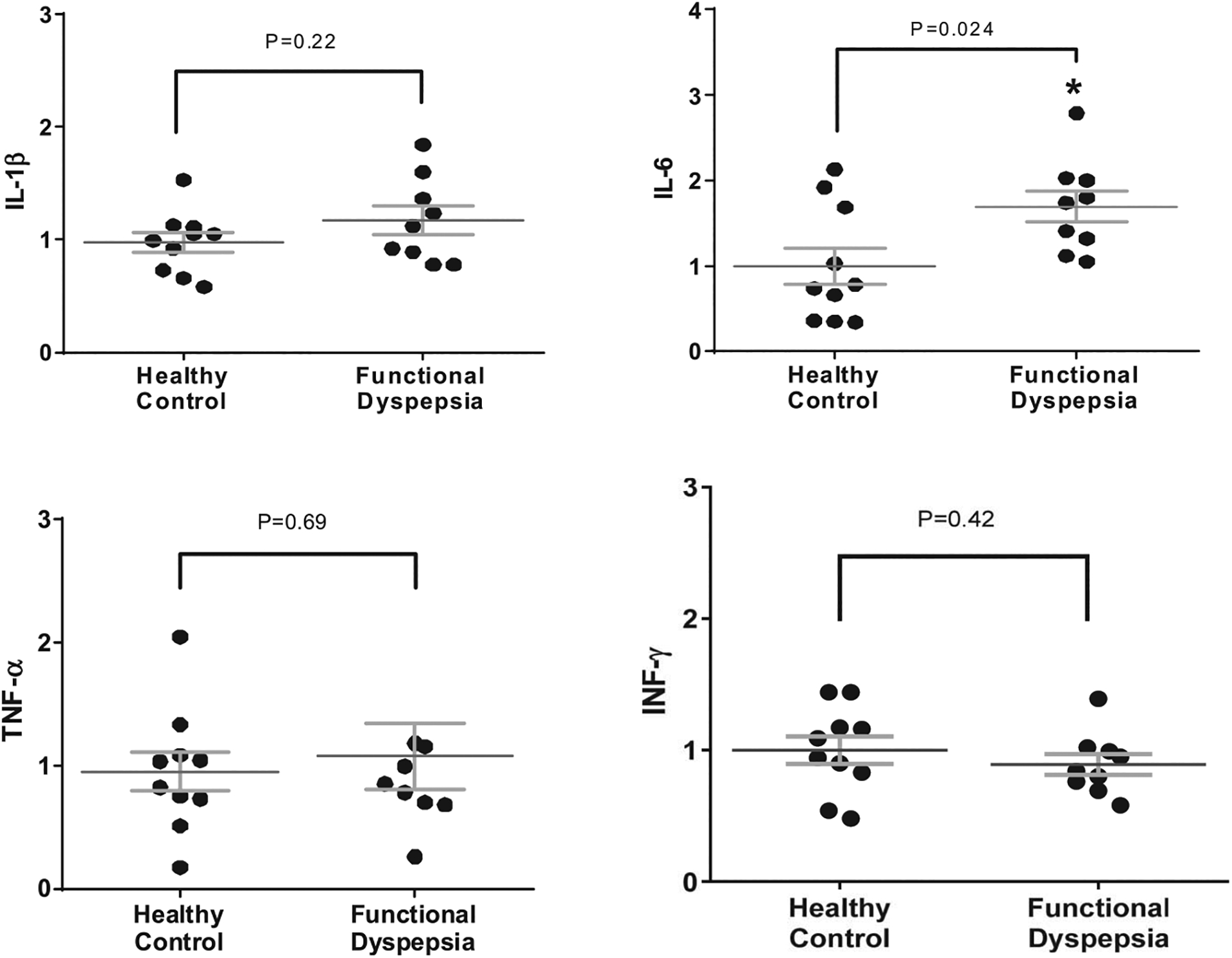
Gene expression of tissue cytokines evaluated by reverse transcriptase PCR in the biopsied duodenal mucosa from healthy controls and patients with FD. IL, interleukin; TNF, tumor necrosis factor; INF, interferon.
Increased duodenal pyroptosis in patients with FD
There was a quantitative increase in inflammatory cell death, or pyroptosis, on analysis of duodenal mucosal biopsies (number of cells/1,000 IECs staining positive for activated caspase-1) in patients with FD compared with controls. The average duodenal pyroptosis in patients with FD was 20.8 ± 6.4 vs 13.8 ± 7.2 positive cells/1,000 epithelial cells in healthy controls (P = 0.04). Figure 7 depicts the quantitative assessment of caspase-positive epithelial cells in the 2 groups. There was no significant difference in pyroptosis quantification in FD patients dependent on IBS presence (P = 0.64) and PPI use (P = 0.15). The difference between patients with FD and controls remained statistically significant after adjusting for age, sex, and BMI.
Figure 7.
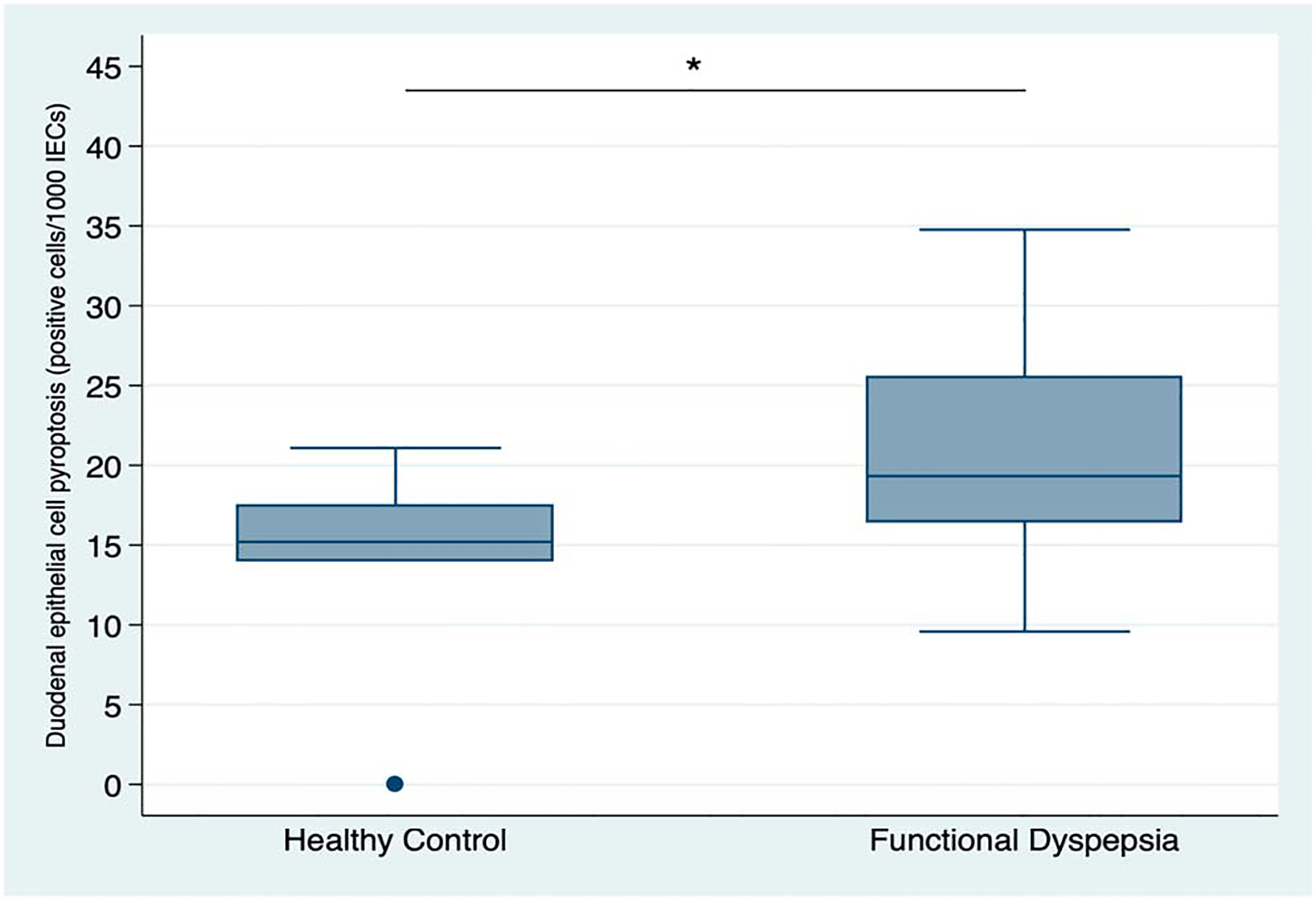
Quantitative assessment of duodenal epithelial cells staining positive for activated caspase-1 (immunohistochemistry) on biopsy samples from healthy controls (n = 6) and patients with FD(n = 14). *P = 0.04.
DISCUSSION
In this exploratory study, we found that patients with FD have significantly greater epithelial gap density in the distal duodenum by CLE compared with healthy controls. We further found that impaired duodenal epithelial barrier integrity by tissue-based TEER analyses was more common in patients with FD than controls and correlated with severity of symptoms. We also found evidence of increased cellular pyroptosis in the duodenal mucosa of patients with FD. In aggregate, these findings suggest that abnormalities in the duodenal mucosal barrier structure and function are present in a subset of patients with FD and may contribute to the pathogenesis and illness experience of this common and bothersome condition.
To our knowledge, this is the first study from North America investigating duodenal barrier integrity in patients with FD. Multiple factors have been suggested to play a role in the pathogenesis of FD (25). More recently, investigators have suggested that abnormalities in duodenal mucosal permeability and immune activation may play a role in the pathogenesis of FD (6–12). Indeed, we found impaired barrier integrity demonstrated in vivo on CLE and ex vivo on tissue analyses from endoscopic duodenal biopsies obtained from patients with FD vs controls. Our study provides novel insights regarding the use of pCLE to detect duodenal epithelial barrier impairment in patients with FD. We also found that altered epithelial integrity, measured by TEER, is associated with higher severity of certain dyspeptic symptoms (e.g., pain, bloating, and early satiety) suggesting that duodenal barrier impairment may play a role in selected FD symptoms. These results support other recent reports from Europe and Asia that also reported lower duodenal TEER measurements and altered expression of certain epithelial junctional proteins in patients with FD (8,26–28). However, ours is the first study to find direct association between impaired duodenal integrity on TEER and severity of dyspeptic symptoms.
Measuring the mucosal permeability with a clinically applicable in vivo tool has been difficult to achieve. A recent study by Ishigami et al. (26) used mucosal admittance (inverse of impedance), a novel catheter-based technique applied during EGD, to evaluate duodenal epithelial permeability. These authors found a negative correlation between mucosal admittance and TEER values in the duodenum and demonstrated higher mean mucosal admittance values suggesting increased duodenal epithelial permeability in patients with FD compared with matched healthy individuals. However, unlike mucosal admittance that requires further validation and is not widely available, pCLE technology is commercially available and in use in a number of centers for indications unrelated to functional GI disorders. Thus, if our results are validated in larger trials, CLE can potentially be introduced into clinical practice as an early diagnostic tool capable to identify patients with FD with impaired duodenal epithelial structure and function. It is exciting to envision subsequent opportunities to use CLE in the development of mechanism-guided therapies for FD based on the identification of specific physiologic abnormalities identified by a validated biomarker.
Preliminary studies of CLE in patients with functional GI disorders are scarce but have yielded intriguing data (20,21,29). There is only one other study of CLE in patients with FD, a report from China that assessed the gastric epithelium in H. pylori infected and noninfected patients with FD vs healthy controls (29). In this CLE study, the authors reported increased gastric paracellular permeability score in patients with FD compared with controls, but no difference in the gastric cell shedding score. Ours is the first study to perform CLE in the duodenum, which has increasingly been implicated in the pathogenesis of FD (30) and is less exposed to luminal factors, such as gastric acid and H. pylori infection, which could complicate the interpretation of CLE imaging. We focused our primary CLE analysis on epithelial gap density score that has been validated as a marker of the epithelial cell extrusion and established as a reliable method to assess epithelial barrier structure and function (16,18–21,31,32). An early study by Kiesslich et al. (18) showed that CLE is capable of identifying cell shedding events and epithelial gaps by using acriflavine, applied topically to the mucosal surface. These results were validated in parallel studies of anesthetized mice by using rigid confocal probe microscopy, 2-photon confocal microscopy, and electron microscopy (18). A subsequent study by these authors showed that CLE can identify cell shedding by using intravenous fluorescein as a contrast agent (19). Furthermore, experiments in mice demonstrated that both efflux of intravenous dye and influx of luminal dye occurs at the sites of epithelial shedding (gaps), which was influenced by the osmotic gradient across the epithelium (33). Several successive clinical studies with CLE have successfully used the intestinal epithelial gap density score in patients with IBD and IBS, further supporting CLE as an objective measurement tool of the epithelial barrier structure (16,20,31,32).
We did not find a statistically significant correlation between the epithelial gap density score on CLE and tissue-based measures of duodenal epithelial integrity (TEER), suggesting the pathophysiological mechanisms for these 2 processes may be different. We also did not find statistically significant difference in the epithelial gap density on CLE between patients with FD and controls when images from the more proximal duodenum (D1 and D2) were analyzed individually. Additional studies are needed to validate and better understand this finding. Interestingly, in a study of patients with IBD undergoing CLE in an endoscopically normal duodenum, there were comparatively higher numbers of epithelial gaps in the distal vs proximal duodenum (D2/D3 vs D1) (31). Previous studies of epithelial barrier integrity in FD did not compare the permeability in different duodenal segments and most studies evaluated tissue samples from the second portion of the duodenum (8,26,28).
This study found no significant difference in numbers of duodenal mucosal eosinophils, mast cells, or lymphocytes on duodenal mucosa histology in patients with FD vs controls. This differs from a recent meta-analysis, which reported an overall increased number of duodenal eosinophils and mast cells in patients with FD (12). It is important to note that there was significant heterogeneity between included studies and evidence of publication bias in this analysis. In the current study, we also assessed the duodenal gene expression of selected mucosal cytokines and found increased expression of IL-6 in patients with FD, but no difference in expression of IL-1β, TNF-α, and IFN-γ. Previous studies report increased peripheral blood cytokines in FD compared with controls, including TNF-α, IL-1β, and IL-10 (34), but there is minimal literature on duodenal tissue cytokines in FD. A recent study from Japan reported increased expression of IL-1β in the duodenum of patients with FD, compared with non-FD patients with GI symptoms (27). To our knowledge, no other study evaluated duodenal mucosa cytokines in patients with FD. IL-6 is a pleiotropic cytokine generated in response to environmental stress factors that plays an important role in inflammatory diseases, including IBD (35). Interestingly, a recent animal study with an FD rat model found stress-induced gastric hyperalgesia to be modulated through the type-2 corticotropin releasing factor receptor, including the IL-6 pathway (36).
This study is also the first to explore the role of pyroptosis in patients with FD, measured through quantification of activated caspase-1 on immunohistochemistry. Pyroptosis is an inflammatory form of cell death resulting from the mucosal innate immune activation by the inflammasome/caspase-1 complex in response to sensation of microbial/danger signals (37). The significance of pyroptosis in IECs is that it can mechanistically explain cell extrusion breaches of the mucosal integrity (15,16), making it possible for luminal microbes to trespass the mucosal barrier. Although we only analyzed a small sample of patients with FD patients and controls, these preliminary results are intriguing and hypothesis generating. It is tempting to postulate that in a predisposed person with altered intestinal microbial content, an increase in the duodenal epithelial cell pyroptosis levels may cause increased epithelial cell shedding and accentuate mucosal barrier defects, permitting microbial trespass into the host tissue and stimulating subtle duodenal inflammation. Consequently, inflamed duodenum may be hypersensitive to luminal contents (e.g., acid and bile) and/or induce reflex responses or cytokines release that alter gastroduodenal function and result in FD symptoms. Further research to understand the role of pyroptosis in the pathogenesis of FD is warranted.
We also found that expression of the tight junction protein, CLDN-1 was significantly reduced in patients with FD compared with controls, whereas expression of other cell-to-cell adhesion proteins were not affected. CLDNs regulate the high-capacity, size and charge-selective, pore pathway ions transport route (38). Lower duodenal CLDN-1 expression was also reported in a study of patients with FD from China (39), whereas other small studies have failed to identify differences in CLDNs expression in patients with FD (8,27). Impaired duodenal expressions of other junctional proteins (e.g., ZO-1, p-OCLN) have been reported with variable frequency in other studies of patients with FD (8,27,28). This variability may be due to small sample sizes in the available studies, failure to differentiate between FD subtypes (PDS vs EPS), and differences in dietary intake. The role of CLDNs and the pore pathway route on increased duodenal permeability will require further study in well-characterized cohorts of patients with FD.
Our pilot study has a number of limitations. First, we enrolled a relatively small number of patients from a single tertiary care center. This could have led to underestimation or overestimation of the outcomes assessed and may influence the generalizability of our findings to patients from primary or secondary care settings. Although we tried to control for potential environmental confounders such as medications and diet, it is possible that differences in environmental exposures could have affected our results. Second, not every participant completed every aspect of the study. Performance of EGD with CLE, followed by acquisition of multiple duodenal biopsies and immediate tissue processing created practical challenges that made it impossible to conduct all aspects of the study in every subject. Our study was powered to detect a difference in CLE; therefore, secondary measurements in this exploratory study may not reach 80% power and require further investigation. In addition, our study was purely observational and cannot discern whether the duodenal epithelial barrier disruption is a cause or effect of FD. Furthermore, we cannot say whether the increased gap density by pCLE and/or abnormalities on tissue-based analyses can predict response to specific treatments for FD. Clearly, our preliminary findings require confirmation in larger, well-controlled clinical trials.
In conclusion, for the first time in patients with FD, we report distal duodenal structural changes detectable on pCLE, confirm the presence of duodenal epithelial barrier impairment using an ex-vivo model, and report evidence of increased duodenal pyroptosis. These results introduce pCLE as a potential diagnostic biomarker, capable of identifying patients with FD with impaired duodenal epithelial structure and function. Additional larger studies are warranted to further define the pCLE characteristics of the duodenal mucosa in patients with FD. Based on our findings, it is tempting to speculate that in a subset of patients with FD, environmental or luminal factors may cause junctional protein damage, leading to impaired barrier integrity and entry of luminal irritants/chemicals into the mucosa. The resulting epithelial immune activation and cell death could lead to increased epithelial gaps, enabling mucosal microbial trespass and ultimately, alternations in gut function and sensation. Further studies will be needed to validate this hypothetical conceptual model.
Study Highlights.
WHAT IS KNOWN
Duodenal epithelial immune activation and barrier impairment have been implicated in the pathogenesis of FD.
Mechanisms leading to duodenal immune activation and/or impaired mucosal permeability in FD are not well defined.
CLE is a clinically applicable endoscopic tool capable of detecting gut epithelial barrier impairments but its role in FD has not been studied.
WHAT IS NEW HERE
CLE displays structural epithelial changes in the distal duodenum of patients with FD compared with healthy controls.
Patients with FD have impaired duodenal epithelial barrier integrity by tissue-based analysis that correlates with the severity of certain dyspeptic symptoms.
There is evidence of increased inflammatory epithelial cell death called pyroptosis in the duodenal mucosa of patients with FD.
These results indicate that duodenal barrier impairment is a pathogenesis factor in FD and introduce CLE as a potential diagnostic biomarker in FD capable to identify the patients with impaired duodenal epithelial structure and function.
Financial support:
This work was supported by Michigan Institute for Clinical and Health Research (MICHR) T3 pilot project grant (grant number UL1TR000433) awarded to B.N.
Footnotes
Potential competing interests: None to report.
REFERENCES
- 1.Stanghellini V, Chan FD, Hasler WL et al. Gastroduodenal disorders. Gastroenterology 2016;150(6):1380–92. [DOI] [PubMed] [Google Scholar]
- 2.Ford AC, Marwaha A, Sood R, et al. Global prevalence of, and risk factors for, uninvestigated dyspepsia: A meta-analysis. Gut 2015;64:1049–57. [DOI] [PubMed] [Google Scholar]
- 3.Ford AC, Marwaha A, Lim A, et al. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2010;8:830–7. [DOI] [PubMed] [Google Scholar]
- 4.Sander GB, Mazzoleni LE, Francesconi CF, et al. Influence of organic and functional dyspepsia on work productivity: The HEROES-DIP study. Value Health 2011;14(Suppl 1):S126–9. [DOI] [PubMed] [Google Scholar]
- 5.Lacy BE, Weiser KT, Kennedy AT et al. Functional dyspepsia: The economic impact to patients. Aliment Pharmacol Ther 2013;38:170. [DOI] [PubMed] [Google Scholar]
- 6.Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med 2015;373(19): 1853–63. [DOI] [PubMed] [Google Scholar]
- 7.Kindt S, Tertychnyy A, de Hertogh G, et al. Intestinal immune activation in presumed post-infectious functional dyspepsia. Neurogastroenterol Motil 2009;21:832–e56. [DOI] [PubMed] [Google Scholar]
- 8.Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014; 63:262–71. [DOI] [PubMed] [Google Scholar]
- 9.Walker MM, Aggarwal KR, Shim LS, et al. Duodenal eosinophilia and early satiety in functional dyspepsia: Confirmation of a positive association in an Australian cohort. J Gastroenterol Hepatol 2014;29: 474–9. [DOI] [PubMed] [Google Scholar]
- 10.Futagami S, Shindo T, Kawagoe T, et al. Migration of eosinophils and CCR2-/CD68-double positive cells into the duodenal mucosa of patients with postinfectious functional dyspepsia. Am J Gastroenterol 2010;105; 1835–42. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: An adult endoscopic population based case-control study. Clin Gastroenterol Hepatol 2007;5:1175–83. [DOI] [PubMed] [Google Scholar]
- 12.Du L, Chen B, Kim JJ et al. Micro-inflammation in functional dyspepsia: A systematic review and meta-analysis. Neurogastroenterol Motil 2018. [DOI] [PubMed] [Google Scholar]
- 13.Miao EA, Leaf IA, Treuting PM, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 2010;11(12):1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knodler LA, Vallance BA, Celli J, et al. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci USA 2010;107(41):17733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JJ, Davis EM, Wine E, et al. Epithelial cell extrusion leads to breaches in the intestinal epithelium. Inflamm Bowel Dis 2013;19(5):912–21. [DOI] [PubMed] [Google Scholar]
- 16.Liu JJ, Kay TM, Davis EM et al. Epithelial cell extrusion zones observed on confocal laser endomicroscopy correlates with immunohistochemical staining of mucosal biopsy samples. Dig Dis Sci 2016;61(7):1895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology 2009;136:1509–25. [DOI] [PubMed] [Google Scholar]
- 18.Kiesslich R, Goetz M, Angus EM, et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 2007;133(6):1769–78. [DOI] [PubMed] [Google Scholar]
- 19.Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012;61:1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turcotte JF, Kao D, Mah SJ, et al. Breaks in the wall: Increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest Endosc 2013; 77(4):624–30. [DOI] [PubMed] [Google Scholar]
- 21.Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014;147:1012–20 e4. [DOI] [PubMed] [Google Scholar]
- 22.El Asmar R, Panigrahi P, Bamford P, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 2002;123(5):1607–15. [DOI] [PubMed] [Google Scholar]
- 23.Wallon C, Braaf Y, Wolving M, et al. Endoscopic biopsies in Ussing chambers evaluated for studies of macromolecular permeability in the human colon. Scand J Gastroenterol 2005;40:586–95. [DOI] [PubMed] [Google Scholar]
- 24.Buzza MS, Netzel-Arnett S, Shea-Donohue T, et al. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci USA 2010;107(9): 4200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbone F, Tack J. Gastroduodenal mechanisms underlying functional gastric disorders. Dig Dis 2014;32;222–9. [DOI] [PubMed] [Google Scholar]
- 26.Ishigami H, Matsumura T, Kasamatsu S, et al. Endoscopy guided evaluation of duodenal mucosal permeability in functional dyspepsia. Clin Transl Gastroenterol 2017;8(4):e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komori K, Ihara E, Minoda Y, et al. The altered mucosal barrier function in the duodenum plays a role in the pathogenesis of functional dyspepsia. Dig Dis Sci 2019;64:3228–39. [DOI] [PubMed] [Google Scholar]
- 28.Taki M, Oshima T, Li M, et al. Duodenal low-grade inflammation and expression of tight junction proteins in functional dyspepsia. Neurogastroenterol Motil 2019;31(10):e13576. [DOI] [PubMed] [Google Scholar]
- 29.Rui J, Wang P, Kou G, et al. Impaired gastric mucosal integrity identified by confocal endomicroscopy in Helicobacter pylori-negative functional dyspepsia. Neurogastroenterol Motil 2019:e13719. [DOI] [PubMed] [Google Scholar]
- 30.Wauters L, Talley NJ, Walker MM, et al. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut 2020;69(3): 591–600. [DOI] [PubMed] [Google Scholar]
- 31.Lim LG, Neumann J, Hansen T, et al. Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2014;20:892–900. [DOI] [PubMed] [Google Scholar]
- 32.Liu JJ, Wong K, Thiesen AL, et al. Increased epithelial gaps in the small intestine of patients with inflammatory bowel disease: Density matters. Gastrointest Endosc 2011;73:6. [DOI] [PubMed] [Google Scholar]
- 33.Buchner AM, Wallace MB. In vivo microscopy in the diagnosis of intestinal neoplasia and inflammatory conditions. Histopathology 2015; 66:137–46. [DOI] [PubMed] [Google Scholar]
- 34.Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 2011;106(6):1089–98. [DOI] [PubMed] [Google Scholar]
- 35.Nikolaus S, Waetzig GH, ButzinInt S, et al. Evaluation of interleukin-6 and its soluble receptor components sIL-6R and sgp130 as markers of inflammation in inflammatory bowel diseases. J Colorectal Dis 2018; 33(7):927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozakai Y, Hori K, Aye-Mon A, et al. The role of peripheral corticotropin-releasing factor signaling in a rat model of stress-induced gastric hyperalgesia. Biochem Biophys Res Commun 2019;519(4):797–802. [DOI] [PubMed] [Google Scholar]
- 37.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–22. [DOI] [PubMed] [Google Scholar]
- 38.Krause G, Winkler L, Mueller SL et al. Structure and function of claudins. Biochim Biophys Acta 2008;1778(3):631–45. [DOI] [PubMed] [Google Scholar]
- 39.Du L, Shen J, Kim JJ, et al. Impact of gluten consumption in patients with functional dyspepsia: A case-control study. J Gastroenterol Hepatol 2018; 33(1):128–33. [DOI] [PubMed] [Google Scholar]


