Abstract
Objective:
Ovarian suppression is recommended to complement endocrine therapy in premenopausal women with breast cancer and high-risk features. It can be achieved by either medical ovarian suppression or therapeutic bilateral salpingo-oophorectomy. Our objective was to evaluate characteristics of patients with stage I-III hormone receptor-positive primary breast cancer who underwent bilateral salpingo-oophorectomy at our institution.
Materials and Methods:
Premenopausal women with stage I-III hormone receptor-positive primary breast cancer diagnosed between January 2010-December 2014 were identified from a database. Patients with confirmed BRCA1/2 mutations were excluded. Distribution of characteristics between treatment groups were assessed using Chi-square test and univariate logistic regression. A multivariate model was based on factors significant on univariate analysis.
Results:
Of 2,740 women identified, 2,018 (74%) received endocrine treatment without ovarian ablation; 516 (19%) endocrine treatment plus ovarian ablation; 206 (7.5%) received no endocrine treatment. Among patients undergoing ovarian ablation 282/516 (55%) received medical ovarian suppression, while 234 (45%) underwent bilateral salpingo-oophorectomy. By univariate logistic analyses, predictors for ovarian ablation were younger age (Odds ratio (OR) 0.97), histology (other vs. ductal: OR 0.23), lymph node involvement (OR 1.89), higher International Federation of Gynecology and Obstetrics (FIGO) stage (stage II vs. I: OR 1.48; stage III vs. I: OR 2.86), higher grade (grade 3 vs. 1: OR 3.41; grade 2 vs. 1: OR 2.99), chemotherapy (OR 1.52), more recent year of diagnosis (2014 vs. 2010; OR 1.713). Only year of diagnosis, stage, HER-2 treatment remained significant in the multivariate model. Within the cohort undergoing ovarian ablation, older age (OR 1.05) was associated with therapeutic bilateral salpingo-oophorectomy. Of 234 undergoing bilateral salpingo-oophorectomy, 12 (5%) mild-to-moderate adverse surgical events were recorded.
Conclusions:
Bilateral salpingo-oophorectomy is used frequently as endocrine ablation strategy. Older age was associated with bilateral salpingo-oophorectomy. Perioperative morbidity was acceptable. Evaluation of long-term effects and quality of life associated with endocrine ablation will help guide patient/provider decision-making.
Keywords: Gynecologic surgical procedures, Breast Neoplasms, Premenopause, Salpingo-oophorectomy
INTRODUCTION
In 2020, an estimated 276,480 women will be diagnosed with breast cancer in the United States1. Approximately 85% of newly diagnosed breast cancers are hormone receptor-positive (HR+)2, 92% being potentially curable stage I-III disease3. Among women with HR+ breast cancer 19–30% are below 50 years of age at time of diagnosis3. Traditionally, premenopausal women were treated with tamoxifen for 5 years 4, allowing a switch to an aromatase inhibitor if a postmenopausal state was reached. This changed following the SOFT and TEXT trial results5,6, published in 2014. Especially in premenopausal women who had undergone adjuvant chemotherapy due to high-risk features, adding an aromatase inhibitor with ovarian suppression resulted in significant improvement in disease-free survival (71.4% tamoxifen alone vs. 80.4% exemestane plus ovarian suppression)7. Bui et al performed a systematic Cochrane review and meta-analysis that included 15 earlier trials to evaluate the effects of ovarian ablation for the treatment of premenopausal women with hormone receptor-positive breast cancer8. The authors found evidence to support the addition of ovarian ablation in this patient population, with persisting benefit compared to observation, or when added to tamoxifen, or when added to chemotherapy and tamoxifen.
Bilateral salpingo-oophorectomy is an accepted alternative to medical ovarian suppression9 but is irreversible. Due to current recommendations10,11 premenopausal women with high-risk features commit to ovarian ablation and prolonged endocrine therapy. Ovarian function can be suppressed either with gonadotrophin-releasing hormone agonists (GnRHa) goserelin, leuprolide, or triptorelin administered subcutaneously monthly or 3-monthly, by ovarian irradiation, or surgical bilateral salpingo-oophorectomy12. Because of the resulting implications for fertility and family planning, some patients may choose definitive surgical ablation. However, there is a lack of data regarding use and timing of therapeutic bilateral salpingo-oophorectomy. We sought to evaluate the patient, disease, and treatment characteristics of premenopausal women undergoing bilateral salpingo-oophorectomy, compared with women receiving medical ovarian suppression, as part of adjuvant treatment of HR+ breast cancer. This information will provide an insight into the current use of bilateral salpingo-oophorectomy in this population, and may improve patient/provider decision-making.
METHODS
Database and Patient Selection
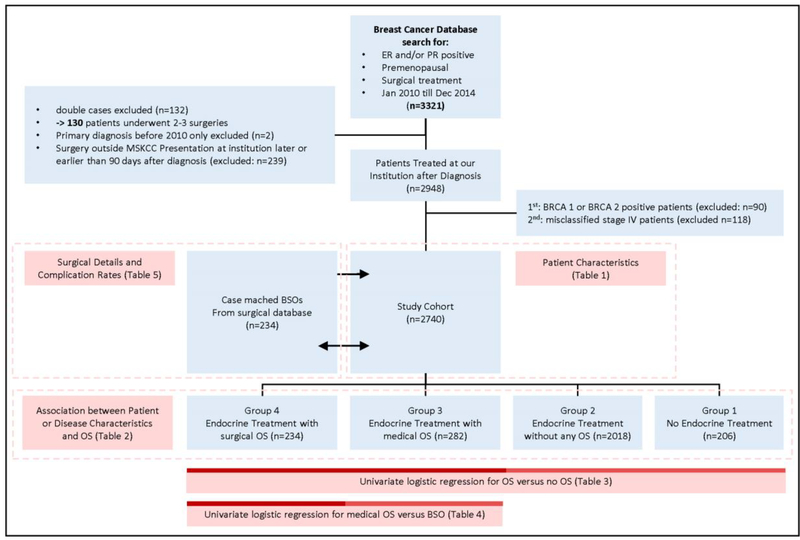
This study was approved by our Institutional Review Board. We performed a retrospective review of a prospective institutional breast cancer database, identifying all pre-menopausal women with HR+ (estrogen or progesterone receptor >1%) breast cancer diagnosed between January 2010-December 2014, who underwent mastectomy or breast conserving surgery and either neoadjuvant or adjuvant medical treatment. Premenopausal status was determined by the clinician at initial consult and was defined by regular menses without exogenous hormones before treatment initiation. This period was chosen to capture all patients undergoing bilateral salpingo-oophorectomy in the first 5 years of adjuvant treatment. Clinical, pathologic, and treatment variables were collected; 3321 women were identified (Figure 1). Any malignant histology was included and assigned to one of 5 categories: any ductal no lobular; any lobular no ductal; both lobular and ductal; inflammatory; other. Women who did not undergo breast surgery, presented >90 days from initial diagnosis, who had stage IV disease, or a known BRCA mutation, or insufficient documentation, were excluded. A total of 2740 premenopausal women with stage I-III were included in the final analysis and assigned to the following groups: Group 1, no endocrine therapy; Group 2, endocrine therapy without ovarian suppression; Group 3, endocrine therapy with medical ovarian suppression; Group 4, endocrine treatment with bilateral salpingo-oophorectomy at any time point. Patients receiving medical ovarian suppression (Leuprorelin or Goserelin) at any point during adjuvant treatment were assigned to Group 3, unless ovarian suppression was started after a recurrence or a bilateral salpingo-oophorectomy was performed. Women undergoing bilateral salpingo-oophorectomy after documented relapse, progression of disease, or for other reasons (adnexal mass, ovarian cancer, uterine cancer) were not classified in Group 4 for the primary diagnosis, instead these patients were assigned to Groups 1–3 irrespective of the endocrine treatment they had received prior to recurrence.
Figure 1.
Study Cohort Selection
For bilateral salpingo-oophorectomy gynecologic oncologists were consulted and minimally invasive surgery was the preferred method. Surgical details and complications were extracted from the gynecologic oncology surgical database. Complications were recorded and graded on a 1–5 scale according to a previously published classification system13.
Statistical Analysis
Association between the treatment groups and patient or disease characteristics was assessed using the χ2 test/Fisher’s Exact test for categorical variables and Kruskal-Wallis test for continuous variables. Two sets of univariate logistic regression analyses were performed to identify predictors among patient and tumor characteristics: first regression analysis compared women undergoing any type of ablation (medical ovarian suppression and bilateral salpingo-oophorectomy) with those who did not. A multivariate logistic model was created, based on all the variables with p<0.05 in univariate analysis. The second regression analysis was performed among all ovarian ablation patients, comparing those receiving medical ovarian suppression versus bilateral salpingo-oophorectomy. Microsoft Excel was used for data collection, SAS9.4 for statistical analyses.
RESULTS
Patient Demographics
The final cohort comprised 2740 premenopausal women with primary diagnosis of HR+ breast cancer during the study period, with median follow-up of 62.2 months (interquartile range (IQR): 47.7–81.1) (Table 1). Median age was 45 years (IQR: 40–48). Half (n=1,445; 54%) of the study cohort had a family history of breast cancer. Most (n=1,991; 73%) had children at time of diagnosis. Most cancers were ductal histology (n=2,188; 80%), poorly differentiated (tumor grade 3; n=1,694; 67%). One-third (n= 991; 36%) of women had lymph node involvement. The majority were diagnosed with International Federation of Gynecology and Obstetrics (FIGO) stage I (n=1,377; 54%) or II (n=846; 33%) disease; 307 (12%) with stage III. Most received adjuvant or neoadjuvant chemotherapy (n=1,720; 63%), adjuvant endocrine therapy (n=2527; 92%) and/or HER-2 targeted treatments (n=434; 16%). Of 516 receiving ovarian ablation, 234 (45%) had bilateral salpingo-oophorectomy, 282 (55%) underwent medical ovarian suppression.
Table 1.
Patient and Disease Characteristics (N=2740)
| N | % | |
|---|---|---|
| Age at Diagnosis | ||
| Median (Mean) | 45(43.8) | |
| Range | 20–60 | |
| IQR | 40–48 | |
| Year of Diagnosis | ||
| 2010 | 530 | 19.3% |
| 2011 | 537 | 19.6% |
| 2012 | 498 | 18.2% |
| 2013 | 597 | 21.8% |
| 2014 | 578 | 21.1% |
| Family History* | ||
| No | 1218 | 45.7% |
| Yes | 1445 | 54.3% |
| Unknown | 77 | |
| Live Children | ||
| No | 749 | 27.3% |
| Yes | 1991 | 72.7% |
| Histology | ||
| Any ductal no lobular | 2188 | 79.9% |
| Any lobular no ductal | 268 | 9.8% |
| Lobular and Ductal | 176 | 6.4% |
| Inflammatory | 12 | 0.4% |
| Other | 96 | 3.5% |
| Grade* | ||
| G1 | 196 | 7.8% |
| G2 | 631 | 25.0% |
| G3 | 1694 | 67.2% |
| Unknown | 219 | |
| LN Pos Exact* | ||
| No | 1746 | 63.8% |
| Yes | 991 | 36.2% |
| Unknown | 3 | |
| Stage* | ||
| I | 1377 | 54.4% |
| II | 846 | 33.4% |
| III | 307 | 12.2% |
| Unknown | 210 | |
| Chemotherapy | ||
| No | 1020 | 37.2% |
| Yes | 1720 | 62.8% |
| HER-2 Targeted Therapy | ||
| None | 2306 | 84.2% |
| Trastuzumab | 328 | 12.0% |
| Trastuzumab + Other | 106 | 3.9% |
| Any endocrine Treatment | ||
| No | 213 | 7.8% |
| Yes | 2527 | 92.2% |
| BSO | ||
| None | 2506 | 91.5% |
| Yes | 234 | 8.5% |
| medical OS | ||
| None | 2458 | 89.7% |
| Yes | 282 | 10.3% |
for these variables, the unknowns are not considered in the percentage reporting.
IQR, interquartile range; LN, lymph node; BSO, bilateral salpingo-oophorectomy; OS, ovarian suppression
Distribution of Treatment Groups
Patients were assigned to four groups (Table 2): Group 1 (n=206; 11%), no endocrine treatment; Group 2 (n=2,018; 74%), any endocrine treatment without ovarian ablation; Group 3 (n=282; 10%), any endocrine treatment with medical ovarian suppression; Group 4 (n=234; 9%), therapeutic bilateral salpingo-oophorectomy at any time during primary treatment. Median follow-up for women receiving endocrine treatment without or with ovarian suppression (Groups 2 and 3) was 64.1 (IQR: 48.9–82.7) and 60.1 (IQR: 49.2–77.7) months, respectively. Median follow-up for women undergoing bilateral salpingo-oophorectomy was 67.3 months (IQR: 51.9–84.4).
Table 2:
Distribution of Patients and Disease Characteristics between Treatment Groups. Percentages are calculated by row.
| Group 1 | Group 2 | Group 3 | Group 4 | ||
|---|---|---|---|---|---|
| No Endocrine Treatment (n=206) | Endocrine Treatment without OS (n=2018) | Endocrine Treatment with medical OS (n=282) | Endocrine Treatment with BSO (n=234) | ||
|
|
|||||
| N (%) | N (%) | N (%) | N (%) | p-value** | |
| Follow-Up | |||||
| Median | 37.1 | 64.1 | 60.1 | 67.3 | |
| Range | 0.6–105.9 | 0.5–115.1 | 3.1–108.3 | 6.2–108.4 | |
| IQR | 7.5–58.2 | 48.9–82.7 | 49.2–77.7 | 51.9–84.4 | |
| Age at Diagnosis | |||||
| Median (Mean) | 45(43.9) | 45(44) | 43(42.2) | 45(44.1) | <0.001‡ |
| Range | 20–56 | 20–60 | 24–59 | 26–55 | |
| IQR | 41–48 | 40–49 | 37–48 | 40–48 | |
| Year of Diagnosis | |||||
| 2010 | 40(7.5%) | 404(76.2%) | 41(7.7%) | 45(8.5%) | <0.001 |
| 2011 | 31(5.8%) | 421(78.4%) | 39(7.3%) | 46(8.6%) | |
| 2012 | 37(7.4%) | 373(74.9%) | 46(9.2%) | 42(8.4%) | |
| 2013 | 42(7%) | 442(74%) | 69(11.6%) | 44(7.4%) | |
| 2014 | 56(9.7%) | 378(65.4%) | 87(15.1%) | 57(9.9%) | |
| Family History* | |||||
| No | 85(7%) | 922(75.7%) | 116(9.5%) | 95(7.8%) | 0.202 |
| Yes | 114(7.9%) | 1041(72%) | 157(10.9%) | 133(9.2%) | |
| Unknown | 7 | 55 | 9 | 6 | |
| Live Children | |||||
| No | 62(8.3%) | 556(74.2%) | 79(10.5%) | 52(6.9%) | 0.263 |
| Yes | 144(7.2%) | 1462(73.4%) | 203(10.2%) | 182(9.1%) | |
| Histology | |||||
| Any ductal no lobular | 166(7.6%) | 1606(73.4%) | 232(10.6%) | 184(8.4%) | 0.008 |
| Any lobular no ductal | 19(7.1%) | 190(70.9%) | 34(12.7%) | 25(9.3%) | |
| Lobular and Ductal | 8(4.5%) | 135(76.7%) | 11(6.3%) | 22(12.5%) | |
| Inflammatory | 0(0%) | 9(75%) | 2(16.7%) | 1(8.3%) | |
| Other | 13(13.5%) | 78(81.3%) | 3(3.1%) | 2(2.1%) | |
| Grade* | |||||
| G1 | 27(13.8%) | 155(79.1%) | 7(3.6%) | 7(3.6%) | <0.001 |
| G2 | 35(5.5%) | 478(75.8%) | 72(11.4%) | 46(7.3%) | |
| G3 | 113(6.7%) | 1229(72.6%) | 186(11%) | 166(9.8%) | |
| Unknown | 31 | 156 | 17 | 15 | |
| LN Pos Exact* | |||||
| No | 163(9.3%) | 1319(75.5%) | 141(8.1%) | 123(7%) | <0.001 |
| Yes | 43(4.3%) | 698(70.4%) | 140(14.1%) | 110(11.1%) | |
| Unknown | 1 | 1 | 1 | ||
| Stage (unknown removed) | |||||
| I | 135(9.8%) | 1048(76.1%) | 107(7.8%) | 87(6.3%) | <0.001 |
| II | 47(5.6%) | 634(74.9%) | 86(10.2%) | 79(9.3%) | |
| III | 16(5.2%) | 193(62.9%) | 55(17.9%) | 43(14%) | |
| Chemotherapy | |||||
| No | 138(13.5%) | 729(71.5%) | 88(8.6%) | 65(6.4%) | <0.001 |
| Yes | 68(4%) | 1289(74.9%) | 194(11.3%) | 169(9.8%) | |
| HER-2 Targeted | |||||
| None | 183(7.9%) | 1698(73.6%) | 242(10.5%) | 183(7.9%) | <0.001 |
| Trastuzumab | 17(5.2%) | 256(78%) | 25(7.6%) | 30(9.1%) | |
| Trastuzumab+other | 6(5.7%) | 64(60.4%) | 15(14.2%) | 21(19.8%) | |
for these variables the unknowns are not considered in the percentage reporting and the test for p-value
p-values for continuous variables are calculated using Kruskal-Wallis test
if not otherwise labeled:
p-values.
OS, ovarian suppression; BSO, bilateral salpingo-oophorectomy; IQR, interquartile range; LN, lymph node
Neither family history of breast cancer nor history of giving birth to one or more children showed association with any treatment group. All other characteristics—histologic subtype, lymph node status, tumor grade, chemotherapy, HER-2 targeted treatment, age at diagnosis—were unevenly distributed between the groups.
Factors Associated with Ovarian Suppression
Univariate logistic regression was performed (Table 3). Women who did not receive ovarian ablation (Group 1–no endocrine, Group 2–endocrine without ovarian suppression) were pooled and compared with those undergoing either medical ovarian suppression or bilateral salpingo-oophorectomy (Group 3–ovarian suppression, Group 4– bilateral salpingo-oophorectomy). Younger age (OR 0.98; 95% CI: 0.96–0.99; p=0.001), more recent diagnosis (2014 vs. 2010; OR 1.71; 95% CI: 1.27–2.31; p< 0.001), higher-grade tumors (grade 3 vs. 1: OR 3.41; 95% CI: 1.95–5.95; grade 2 vs. 1: OR 2.99; 95% CI: 1.67–5.33; p<0.001), lymph node involvement (OR 1.89; 95% CI: 1.56–2.30; p<0.001), higher FIGO stage (stage II vs. I: OR 1.48; 95% CI: 1.18–1.86; stage III vs. I: OR 2.86; 95% CI: 2.15–3.80), uncommon histology (other vs. ductal: OR 0.23; 95% CI: 0.10–0.58; p=0.019), or chemotherapy (OR 1.52; 95% CI: 1.23–1.87; p<0.001) were associated with likelihood of either medical ovarian suppression or bilateral salpingo-oophorectomy. Positive family history, and children, were not associated with ovarian ablation.
Table 3:
Univariate Logistic Regression for Patients Undergoing Endocrine Treatment Without Ovarian Ablation (Group 1 and Group 2: n=2224) versus With Ovarian Ablation (Group 3 and Group 4: n=516)
| Variables | Levels | OR | 95%CI Lower Bounds | 95%CI Upper Bounds | p-value |
|---|---|---|---|---|---|
|
| |||||
| Age at Diagnosis | as 1 yr increase | 0.975 | 0.961 | 0.990 | 0.001 |
| Year of Diagnosis | 2011 vs 2010 | 0.971 | 0.700 | 1.347 | <0.001 |
| 2012 vs 2010 | 1.108 | 0.800 | 1.535 | ||
| 2013 vs 2010 | 1.205 | 0.885 | 1.641 | ||
| 2014 vs 2010 | 1.713 | 1.271 | 2.308 | ||
| Family History (77 unk.) | Yes vs No | 1.198 | 0.985 | 1.458 | 0.071 |
| Live Children | Yes vs No | 1.131 | 0.909 | 1.408 | 0.271 |
| Histology | |||||
| Lobular no ductal vs Ductal no lobular | 1.202 | 0.884 | 1.636 | 0.019 | |
| Lobular and ductal vs Ductal no lobular | 0.983 | 0.663 | 1.457 | ||
| Inflammatory vs Ductal no lobular | 1.420 | 0.383 | 5.268 | ||
| Other vs Ductal no lobular | 0.234 | 0.095 | 0.579 | ||
| Grade (219 unk.) | G2 vs G1 | 2.988 | 1.674 | 5.331 | <0.001 |
| G3 vs G1 | 3.407 | 1.954 | 5.940 | ||
| LN Pos Exact (3 unk.) | Yes vs. No | 1.894 | 1.560 | 2.300 | <0.001 |
| Stage (210 unk.) | II vs. I | 1.477 | 1.176 | 1.856 | <0.001 |
| III vs. I | 2.859 | 2.153 | 3.798 | ||
| Chemotherapy | Yes vs. No | 1.516 | 1.232 | 1.865 | <0.001 |
| HER-2 Targeted | Trastuzumab vs. None | 0.892 | 0.655 | 1.214 | <0.001 |
| Trastuzumab+Other vs. None | 2.276 | 1.502 | 3.449 | ||
|
| |||||
The Odds Ratio (OR) is modeled for ovarian suppression (OS) = yes. OR>1 means more likely to get OS; OR<1 less likely to get OS. unk., unknown: number of patients in analysis who did not have documentation for this variable. LN, lymph node
A multivariate model using all patient and disease characteristics, showed significance on univariate analyses (Table S1). With existence of other covariates in the same model, only more recent year of diagnosis (2014 vs. 2010; OR 1.557; 95%CI 1.11–2.24), higher stage (stage III vs. I: OR 2.26; 95%CI: 1.37–3.72), and HER-2 treatment (trastuzumab plus other vs. no HER-2 targeting; OR 2.39; 95%CI 1.342–4.231) were significantly associated with medical ovarian suppression or bilateral salpingo-oophorectomy.
A subgroup analysis was performed for all women receiving ovarian ablation, comparing Group 3—ovarian suppression versus Group 4— bilateral salpingo-oophorectomy) (Table 4). Older age at diagnosis (1.05; 95% CI: 1.02–1.08; p<0.001) was associated with higher likelihood of bilateral salpingo-oophorectomy. For all other patient and disease characteristics there were no significant differences in distribution between bilateral salpingo-oophorectomy and medical ovarian suppression.
Table 4:
Univariate Logistic Regression for Patients Undergoing Bilateral Salpingo-oophorectomy (n=234) versus Ovarian Suppression (n=282) for Ovarian Ablation
| Variables | Levels | OR | 95%CI Lower Bounds | 95%CI Upper Bounds | p-value |
|---|---|---|---|---|---|
|
| |||||
| Age at Diagnosis | as 1 yr increase | 1.051 | 1.021 | 1.082 | <0.001 |
| Year of Diagnosis | 2011 vs 2010 | 1.075 | 0.589 | 1.960 | 0.082 |
| 2012 vs 2010 | 0.832 | 0.459 | 1.508 | ||
| 2013 vs 2010 | 0.581 | 0.329 | 1.025 | ||
| 2014 vs 2010 | 0.597 | 0.348 | 1.023 | ||
| Family History (15 unk.) | Yes vs No | 1.034 | 0.724 | 1.477 | 0.852 |
| Live Children | Yes vs No | 1.362 | 0.910 | 2.038 | 0.133 |
| Histology | 0.176 | ||||
| Lobular no ductal vs Ductal no lobular | 0.927 | 0.534 | 1.609 | ||
| Lobular and ductal vs Ductal no lobular | 2.522 | 1.192 | 5.334 | ||
| Inflammatory vs Ductal no lobular | 0.630 | 0.057 | 7.007 | ||
| Other vs Ductal no lobular | 0.841 | 0.139 | 5.083 | ||
| Grade (32 unk.) | G2 vs G1 | 0.639 | 0.210 | 1.941 | 0.286 |
| G3 vs G1 | 0.892 | 0.307 | 2.598 | ||
| LN Pos Exact (2 unk.) | Yes vs. No | 0.901 | 0.636 | 1.275 | 0.556 |
| Stage (59 unk.) | II vs. I | 1.130 | 0.745 | 1.713 | 0.777 |
| III vs. I | 0.962 | 0.590 | 1.568 | ||
| Chemotherapy | Yes vs. No | 1.179 | 0.806 | 1.727 | 0.396 |
| HER-2 Targeted | Trastuzumab vs. None | 1.587 | 0.902 | 2.790 | 0.076 |
| Trastuzumab+Other vs. None | 1.851 | 0.929 | 3.691 | ||
|
| |||||
The Odds Ratio (OR) is modeled for BSO yes, as OR>1 means more likely to get BSO, OR<1 less likely to get BSO. unk., unknown: number of patients in analysis who did not have documentation for this variable.
BSO, bilateral salpingo-oophorectomy; OS, ovarian suppression; LN, lymph node
In total, 335 women started medical ovarian suppression; 53 of these underwent bilateral salpingo-oophorectomy later. When comparing these 53 with the 282 who received only medical ovarian suppression, older age was the only factor associated with bilateral salpingo-oophorectomy (p=0.035).
Timing of Ovarian Ablation
Endocrine treatment began after a median 6.7 months (IQR: 4.3–8.6) in all three treatment groups (Group 2: 6.8, IQR: 4.4–8.7; Group 3: 6.3 months, IDR: 3.7–8.3; Group 4: 6.6 months, IQR: 3.8–8.4). Median time from diagnosis to any type of ovarian suppression was 12.4 months (IQR: 64–28.4) (Table S2).
In Group 3, 119 women started endocrine therapy and medical ovarian suppression at the same time; 135 had medical ovarian suppression after a median endocrine treatment time of 13.6 months (IQR: 4–34). Median time from diagnosis to bilateral salpingo-oophorectomy was 22.9 months (IQR: 13.5–37.7). Most women undergoing bilateral salpingo-oophorectomy, (n=193, 93%) received endocrine therapy without medical ovarian suppression for a median 18.3 months (IQR: 9.4–34.7) before bilateral salpingo-oophorectomy. Of 234 patients undergoing bilateral salpingo-oophorectomy, 53 (22%) started medical ovarian suppression and had bilateral salpingo-oophorectomy later. For women crossing over to bilateral salpingo-oophorectomy, median time from first administration of medical ovarian suppression to bilateral salpingo-oophorectomy was 11.7 months (IQR: 5.95–22.8).
Complications of Surgical Ovarian Ablation
The majority (n=192, 85%) underwent outpatient surgery (Table 5); 33 (15%) had inpatient surgery. Median length of hospitalization was 0 (range 0–7). Most surgeries were laparoscopically (n=152, 67%) or robotically assisted (n=62, 27%); 14 (6%) were laparotomies. All laparotomies included additional abdominal procedures at time of bilateral salpingo-oophorectomy. Eighty-nine women (39%) undergoing bilateral salpingo-oophorectomy had concomitant surgical procedures, including breast reconstruction (46, 20%), hysterectomy (35, 15%), hernia repair, vulvar surgery, additional intraabdominal resections (24, 11%). Twelve (5%) had postoperative complications with 4 readmissions. Among those undergoing bilateral salpingo-oophorectomy without concomitant surgery, 2 (1.5%) had complications with 1 readmission. Grade 1 complications were documented in 2 patients: 1 urinary tract infection after laparoscopic bilateral salpingo-oophorectomy, 1 postoperative wound infection and seroma after laparotomy for total abdominal hysterectomy/bilateral salpingo-oophorectomy in a patient with diabetes. Grade 2 complications included a wound infection requiring readmission for intravenous antibiotics after laparoscopic bilateral salpingo-oophorectomy with concomitant breast surgery, and symptomatic anemia requiring transfusion after robotic-assisted total laparoscopic hysterectomy/bilateral salpingo-oophorectomy. One patient was readmitted for a grade 3 pelvic hematoma requiring drainage; she had a history of peritonitis with adhesions, requiring conversion to laparotomy with enterolysis for bilateral salpingo-oophorectomy.
Table 5:
Surgical Details and Complication Rates of Bilateral Salpingo-oophorectomy in the Study Cohort. Missing variables are not included in the percentage denominators.
| N | % | |
|---|---|---|
|
|
||
| All BSO | 234 | |
| Admission | ||
| Inpatient | 33 | 14.7 |
| Outpatient | 192 | 85.3 |
| Surgery Type | ||
| laparoscopy | 152 | 66.7 |
| laparotomy | 14 | 6.1 |
| robotic | 62 | 27.2 |
| Concomitant Surgery | 89 | 38.9 |
| Breast Surgery | 46 | 20.1 |
| Hysterectomy | 35 | 15.3 |
| Other Surgery | 24 | 10.5 |
| BSO with and without Concomitant Surgery (n=234) | ||
| Readmission | 4 | 1.8 |
| Complications | 12 | 5.4 |
| Surgery Duration [min] | ||
| Median (Mean) | 95(112.1) | |
| Range | 18–647 | |
| IQR | 53.2–145.8 | |
| EBL [ml] | ||
| Median (Mean) | 20(41.5) | |
| Range | 0–1000 | |
| IQR | ||
| Hospitalization [days] | ||
| Median (Mean) | 0(0.5) | |
| Range | 0–7 | |
| IQR | 0–0 | |
| Comorbidity* | 52 | 23.3 |
|
| ||
| BSO without Concomitant Surgery (n=145) | ||
| Readmission | 1 | 0.7 |
| Complications | 2 | 1.5 |
| Surgery Duration [min] | ||
| Median (Mean) | 62 (71.4) | |
| Range | 18–185 | |
| IQR | 40–92 | |
| EBL [ml] | ||
| Median (Mean) | 20 (24.8) | |
| Range | 0–300 | |
| IQR | ||
| Hospitalization [days] | ||
| Median (Mean) | 0(0.1) | |
| Range | 0–1 | |
| IQR | 0–0 | |
| Comorbidity* | 32 | 23.7 |
|
| ||
Comobidity includes at least one incidence of “hypothyroidism”, “arterial hypertension”, “heart disease”, “pulmonary embolism or DVT” or “diabetes”.
BSO, bilateral salpingo-oophorectomy; IQR, interquartile range; EBL, estimated blood loss
Median duration of surgery was 95 minutes (IQR: 53.2–145.8 minutes). Eighty-four (38%) patients had operative time >120 minutes. Twenty-five (11%) had operative time >180 minutes; in 24 of these 25, combined surgical procedures were performed. Median blood loss was 20 ml, (range, 0–1000). Among women undergoing bilateral salpingo-oophorectomy only, median surgical duration was 62 minutes (IQR: 40–92); 16 (12%) exceeded 120 minutes.
DISCUSSION
In this study, we assessed the characteristics of premenopausal women undergoing surgical ovarian ablation compared with women receiving medical ovarian suppression for HR+ breast cancer at our institution. Of 2,740 women identified, 516 (19%) were treated with medical or surgical ovarian ablation. Those selected for ovarian ablation presented with high-risk tumor features (higher tumor grade or stage, lymph node involvement), or were younger at time of diagnosis. Among those selected for ovarian ablation, older age was associated with bilateral salpingo-oophorectomy. We detected a delayed induction of ovarian suppression therapy in a large proportion of women in both the medical ablation (13.6 months) and the bilateral salpingo-oophorectomy (22.9 months) treatment groups. Surgical complications were few, even with combined surgical procedures.
The landscape of adjuvant endocrine therapy in premenopausal women has changed significantly since the joint analysis of the SOFT and TEXT trials7. This data had a median follow-up of 8 years, with findings suggesting an overall survival benefit of 1.8% for women receiving tamoxifen plus ovarian suppression versus tamoxifen alone (HR 0.59; 95% CI 0.42–0.84). This group was characterized by high-risk clinicopathological features and younger age (median, 40 years). The absolute benefits of ovarian suppression were prominent in women who remained premenopausal after chemotherapy. Among those patients the rate of disease-free survival observed with tamoxifen plus ovarian suppression was 5.3% higher than tamoxifen alone and 9% higher with exemestane plus ovarian suppression. Our dataset presents similar distribution of high-risk features among women undergoing ovarian suppression. This may be related to the clinical conduct adopted by the specialists after the results of the SOFT and TEXT trial. Within the cohort undergoing ovarian ablation, the association of older age (OR 1.05) and bilateral salpingo-oophorectomy was significant. We hypothesize that women of older age are more inclined to consider surgical ovarian suppression.
In the combined analysis of TEXT and SOFT trials addition of ovarian suppression was associated with a substantial increase in grade 3 adverse events: 24.6% in tamoxifen versus 31.0% tamoxifen plus ovarian suppression versus 32.3% exemestane plus ovarian suppression. Similar increase was recorded for musculoskeletal symptoms (6.7% vs. 5.7% vs. 11.4%) and osteoporosis (3.9% vs. 7.2 vs. 14.8%), respectively. Vaginal dryness and dyspareunia were most frequent in the ovarian suppression plus exemestane group. Adverse events regarding specifically patients who opted for bilateral salpingo-oophorectomy or ovarian irradiation were not presented.
While optimal duration of ovarian suppression is not known, a postmenopausal state in young women comes with significant morbidity. In the Nurses’ Health Study, in the cohort undergoing hysterectomy between ages 35–50 without estrogen replacement therapy, the addition of bilateral salpingo-oophorectomy resulted in a significant increase in all-cause mortality14. Long-term morbidity data is not available for medical ovarian suppression; however, it can be assumed that women treated with medical ovarian suppression and aromatase inhibitor would encounter long-term effects similar to those of premenopausal women undergoing bilateral salpingo-oophorectomy. Applying both the benefits of ovarian suppression for breast cancer prognosis and the resulting morbidity to a Markov Monte Carlo simulation model, Kwon et al. estimated 577 and 787 additional deaths in the medical ovarian suppression and bilateral salpingo-oophorectomy groups, respectively15. When considering deaths from breast cancer and treatment-related adverse events, this model makes tamoxifen the optimal choice in endocrine therapy for premenopausal breast cancer; it is preferred for low-risk disease.
It is essential to identify candidates for ovarian suppression whose high risk of recurrence outweighs the risk of long-term morbidity. Regan et al. incorporated clinicopathological features in a continuous score termed “composite risk”16,17. The absolute improvement of freedom from distant metastases for women with high composite risk was 10–15%. Although the composite risk score was not applied at our institution, women with high-risk features such as younger age, high tumor grade, stage III, or lymph node involvement were more likely to undergo medical ovarian suppression or bilateral salpingo-oophorectomy.
This retrospective study has limitations. As a single-institution study at a specialty center, the findings may reflect multidisciplinary care delivered by a relatively small number of clinicians; therefore, some findings may not be generalizable to other institutions. Menopausal status was extracted from physicians’ charts at initial consult, not by objective hormone level measurements; thus, we were unable to differentiate between pre- and perimenopausal status. It is unclear how many women were perimenopausal at time of diagnosis, or how many transitioned into menopause after chemotherapy. For this reason, we analyzed the distribution of women older than 50 years between the four treatment groups. The distributions were even, ranging from 8.3–12.7% in each group (data not shown). Our observed rate of bilateral salpingo-oophorectomy versus medical ovarian suppression is higher than those cited in the SOFT and TEXT trials (16–18% of patients assigned to ovarian suppression opted to undergo bilateral oophorectomy or bilateral ovarian irradiation7). In our cohort, the majority of women seeking bilateral salpingo-oophorectomy did so before initiating medical ovarian suppression. Many (n=193, 93%) began tamoxifen for a median duration of 18 months before crossing over to bilateral salpingo-oophorectomy. Women enrolled in the SOFT trial were offered a choice of medical ovarian suppression, bilateral salpingo-oophorectomy, or ovarian irradiation. Medical ovarian suppression was preferred (91%). In the TEXT trial, bilateral salpingo-oophorectomy or ovarian irradiation was allowed after 6 months of medical ovarian suppression. The rate of early cessation of medical ovarian suppression without substitution of ovarian ablation was 19% in the combined population of SOFT and TEXT. The prognostic impact of discontinuing medical ovarian suppression is unclear. In our study, only 53 women (22%) who had bilateral salpingo-oophorectomy started with medical ovarian suppression. The higher rate of bilateral salpingo-oophorectomy may be a result of the time period, during which medical ovarian suppression for premenopausal women was not yet fully established. Another limitation is the lack of specific reasons cited for different forms of ovarian ablation; detailed information regarding the decision-making processes about bilateral salpingo-oophorectomy, or discussions about alternatives, were often not specified in physicians’ notes. Future research should examine whether adverse events associated with endocrine therapy, and/or quality of life concerns (i.e. time commitment, mood disturbance) associated with medical ovarian suppression impact choice. There is a paucity of data regarding postoperative satisfaction and/or regret in women choosing bilateral salpingo-oophorectomy. This information would be crucial in guiding discussions between patients and providers regarding treatment options.
The only direct comparison of bilateral salpingo-oophorectomy versus treatment with luteinizing hormone-releasing hormone agonists was performed in women with metastatic disease. The authors show similar progression-free and overall survival in both groups18. Bilateral salpingo-oophorectomy in the adjuvant setting was tested prior to that study and was shown to be equivalent to cyclophosphamide, methotrexate and fluorouracil when combined with tamoxifen19; a second analysis 10 years later yielded similar results20. The E-3193 study randomized 337 women to tamoxifen with and without ovarian suppression, with most choosing to undergo bilateral salpingo-oophorectomy (n=74; 42%). Neither quality of life nor complication rates differed between the groups21. The adequacy of maintaining estrogen level suppression was examined in the SORT-EST Substudy; at 3, 6 and 12 months, 34.2% of 79 treated with ovarian suppression and exemestane demonstrated at least one E2 level >2.72 pg/mL22. It is unclear whether these small transient increases of estradiol levels are also present, but less likely, in women undergoing bilateral salpingo-oophorectomy. In a recent study, Ferrandina et al. analyzed the cost-effectiveness of laparoscopic bilateral salpingo-oophorectomy and GnRHa administration in patients aged 40–49 years with hormone-sensitive breast cancer through a probabilistic decision tree model23. The authors concluded that bilateral salpingo-oophorectomy is more cost-effective than GnRHa in the adjuvant setting.
Conclusion
Ovarian ablation is known to improve survival in premenopausal women with HR+ breast cancer with high-risk features. Therapeutic bilateral salpingo-oophorectomy is associated with low morbidity and is a reasonable alternative to medical ovarian suppression. However, many questions remain. Future prospective studies addressing the decision-making process, patients’ treatment preferences, and long-term effects of endocrine ablation are needed. Patient-reported outcomes, health-related quality of life, investigation of provider factors, treatment considerations and choice--including postoperative satisfaction or regret—will help guide future discussions between patients and providers, facilitating more informed decisions about treatment.
Supplementary Material
Table S1. Multivariate Logistic Model for Patients Undergoing Endocrine Treatment Without Ovarian Ablation (Group 1 and Group 2: n=2224) versus With Ovarian Ablation (Group 3 and Group 4: n=516)
Table S2. Timing of Endocrine Therapy, Medical Ovarian Suppression and Bilateral Salpingo-oophorectomy Among the Three Endocrine Treatment Groups
PRECIS:
Ovarian ablation may be required for the treatment of high-risk breast cancer in premenopausal women. Therapeutic bilateral salpingo-oophorectomy is a safe alternative to medical ovarian suppression, with low complication rates.
HIGHLIGHTS:
Ovarian ablation for adjuvant breast cancer treatment was offered to women with high-risk features
A total of 45% of patients undergoing ovarian ablation had therapeutic bilateral salpingo-oophorectomy
Complication (5.4%) and readmission (1.8%) rates after bilateral salpingo-oophorectomy were low
FUNDING:
This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
DISCLOSURES: NAR reports grants from Stryker/Novadaq, grants from Olympus, grants from GRAIL, outside the submitted work. JC reports grants from Fidia grants from Sprout, outside the submitted work. DSC reports personal fees from Bovie Medical Co., personal fees from Verthermia Inc. (now Apyx Medical Corp.), personal fees from C Surgeries, personal fees from Biom ‘Up, other from Intuitive Surgical Inc., other from TransEnterix Inc., outside the submitted work. AI reports personal fees from Mylan, outside the submitted work. MML is a consultant for Intuitive Surgical Inc., outside the submitted work. KLR reports other from Intuitive Surgical Inc., outside the submitted work.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7–30, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Kohler BA, Sherman RL, Howlader N, et al. : Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst 107:djv048, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Altekruse SF, Li CI, et al. : US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies C, Godwin J, Gray R, et al. : Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis PA, Regan MM, Fleming GF, et al. : Adjuvant Ovarian Suppression in Premenopausal Breast Cancer. New England Journal of Medicine 372:436–446, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagani O, Regan MM, Walley BA, et al. : Adjuvant Exemestane with Ovarian Suppression in Premenopausal Breast Cancer. New England Journal of Medicine 371:107–118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis PA, Pagani O, Fleming GF, et al. : Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. New England Journal of Medicine 379:122–137, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bui KT, Willson ML, Goel S, et al. : Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer. Cochrane Database Syst Rev 3:Cd013538, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(NCCN©) NCCN: Breast Cancer - Version 6.2020. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®):MS-33, 2020 [Google Scholar]
- 10.Burstein HJ, Lacchetti C, Anderson H, et al. : Adjuvant Endocrine Therapy for Women With Hormone Receptor–Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. Journal of Clinical Oncology 34:1689–1701, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Paluch-Shimon S, Cardoso F, Partridge AH, et al. : ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann Oncol, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Burstein HJ, Lacchetti C, Anderson H, et al. : Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol 34:1689–701, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA: Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–13, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker WH, Feskanich D, Broder MS, et al. : Long-Term Mortality Associated With Oophorectomy Compared With Ovarian Conservation in the Nurses’ Health Study. Obstetrics & Gynecology 121:709–716, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon JS, Pansegrau G, Nourmoussavi M, et al. : Long-term consequences of ovarian ablation for premenopausal breast cancer. Breast Cancer Research and Treatment 157:565–573, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Regan MM, Francis PA, Pagani O, et al. : Absolute improvements in freedom from distant recurrence with adjuvant endocrine therapies for premenopausal women with hormone receptor-positive (HR+) HER2-negative breast cancer (BC): Results from TEXT and SOFT. Journal of Clinical Oncology 36:503–503, 2018 [Google Scholar]
- 17.Regan MM, Francis PA, Pagani O, et al. : Absolute Benefit of Adjuvant Endocrine Therapies for Premenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer: TEXT and SOFT Trials. J Clin Oncol 34:2221–31, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor CW, Green S, Dalton WS, et al. : Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol 16:994–9, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Nomura Y, Tashiro H, Hisamatsu K, et al. : A randomized trial of adjuvant endocrine therapy, chemotherapy, and chemoendocrine therapy for operable breast cancer stratified by estrogen receptors. Cancer 61:2168–2175, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Nomura Y, Shirouzu M, Takayama T: Direct comparisons of adjuvant endocrine therapy, chemotherapy, and chemoendocrine therapy for operable breast cancer patients stratified by estrogen receptor and menopausal status. Breast Cancer Res Treat 49:51–60, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Tevaarwerk AJ, Wang M, Zhao F, et al. : Phase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in premenopausal women with node-negative, hormone receptor-positive breast cancer (E-3193, INT-0142): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 32:3948–58, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellet M, Gray KP, Francis PA, et al. : Twelve-Month Estrogen Levels in Premenopausal Women With Hormone Receptor-Positive Breast Cancer Receiving Adjuvant Triptorelin Plus Exemestane or Tamoxifen in the Suppression of Ovarian Function Trial (SOFT): The SOFT-EST Substudy. J Clin Oncol 34:1584–93, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrandina G, Amadio G, Marcellusi A, et al. : Bilateral Salpingo-Oophorectomy Versus GnRH Analogue in the Adjuvant Treatment of Premenopausal Breast Cancer Patients: Cost-Effectiveness Evaluation of Breast Cancer Outcome, Ovarian Cancer Prevention and Treatment. Clin Drug Investig 37:1093–1102, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multivariate Logistic Model for Patients Undergoing Endocrine Treatment Without Ovarian Ablation (Group 1 and Group 2: n=2224) versus With Ovarian Ablation (Group 3 and Group 4: n=516)
Table S2. Timing of Endocrine Therapy, Medical Ovarian Suppression and Bilateral Salpingo-oophorectomy Among the Three Endocrine Treatment Groups