Abstract
BACKGROUND
Intestinal ischemia has been described in case reports of patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease (coronavirus disease 19, COVID-19).
AIM
To define the clinical and histological, characteristics, as well as the outcome of ischemic gastrointestinal manifestations of SARS-CoV-2 infection.
METHODS
A structured retrospective collection was promoted among three tertiary referral centres during the first wave of the pandemic in northern Italy. Clinical, radiological, endoscopic and histological data of patients hospitalized for COVID-19 between March 1st and May 30th were reviewed. The diagnosis was established by consecutive analysis of all abdominal computed tomography (CT) scans performed.
RESULTS
Among 2929 patients, 21 (0.7%) showed gastrointestinal ischemic manifestations either as presenting symptom or during hospitalization. Abdominal CT showed bowel distention in 6 patients while signs of colitis/enteritis in 12. Three patients presented thrombosis of main abdominal veins. Endoscopy, when feasible, confirmed the diagnosis (6 patients). Surgical resection was necessary in 4/21 patients. Histological tissue examination showed distinctive features of endothelial inflammation in the small bowel and colon. Median hospital stay was 9 d with a mortality rate of 39%.
CONCLUSION
Gastrointestinal ischemia represents a rare manifestation of COVID-19. A high index of suspicion should lead to investigate this complication by CT scan, in the attempt to reduce its high mortality rate. Histology shows atypical feature of ischemia with important endotheliitis, probably linked to thrombotic microangiopathies.
Keywords: Coronavirus, COVID-19, Ischemic colitis, Small bowel ischemia, Endothelial inflammation
Core Tip: Ischemic manifestations have been described as possible presenting symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. These manifestations are frequent in the respiratory tract and bear a high fatality rate. Our retrospective observational trial aims to describe the prevalence, the characteristics and the evolution of patients presenting with intestinal ischemic manifestations of SARS-CoV-2 infection.
INTRODUCTION
The end of December 2019 marked the recent world history for the advent of a new zoonosis caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 rapidly spread around the world causing a pandemic responsible for a dramatic number of deaths due to a disease referred to as “coronavirus disease 19 (COVID-19)” (https://www.healthmap.org/covid-19/).
The Lombardy region (Italy) was the second epicentre of the pandemic[1]. A recent report of the Italian National Institute of Statistics revealed that in March 2020, there was an overall increase in the general population mortality rate of + 185% (+ 568% in the Bergamo province) compared to the same period of the previous 4 years (2015-2019) (https://www.istat.it/it/files//2020/05/Rapporto_Istat_ISS.pdf).
The most frequent clinical manifestation of COVID-19 is an atypical pneumonia, presenting with respiratory symptoms such as fever, cough, shortness of breath and hypoxia[2]. However, several cohort studies worldwide showed that in a minority of cases COVID-19 can present with isolated gastrointestinal symptoms (GI), including abdominal pain, nausea, vomiting and diarrhoea[3-6]. A metanalysis demonstrated a poorer prognosis of COVID-19 patients presenting with gastrointestinal symptoms, compared to other COVID-19 patterns of presentation[7]. This may be due to the severe intestinal ischemic lesions found in 3 of the first 12 autopsies performed in COVID-19 patients in Germany[8]. Furthermore, a preliminary report highlighted that, during the first wave of SARS-CoV-2 pandemic, there was a higher prevalence of gastrointestinal ischemic events compared with the same timeframe before the virus advent[9].
During the first wave of the pandemic the medical community was unaware of the multifaceted manifestations of this new infectious agent. Now that the world has faced the second wave of this pandemic, it is crucial to identify retrospectively all the possible life-threatening manifestations of SARS-CoV-2 for which appropriate management may influence the final outcome.
The aim of the present multicentre study, named ABDOCOVID, is to analyse retrospectively the main clinical, endoscopic, pathological and imaging findings in patients with intestinal ischemic manifestations of SARS-CoV-2, in three tertiary referral centres of the highly hit region of Lombardy.
MATERIALS AND METHODS
Patients and methods
All patients admitted from March 1st to May 30th to the emergency department (ED) of one of the three enrolling Hospitals (Papa Giovanni XXIII, Bergamo; Humanitas Clinical and Research Center, IRCCS, Rozzano, Milano; Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan) and with confirmed SARS-CoV-2 infection, were reviewed and analysed. All confirmed COVID-19 patients who underwent abdominal computed tomography (CT) scan or endoscopy were evaluated, and data on the diagnostic workup were retrospectively collected.
In order to calculate the prevalence of intestinal ischemic manifestations, aggregate data on all patients admitted for COVID-19 in the three enrolling Hospital were pooled.
The local Ethical Committee approved the study protocol (protocol 96/20). All patients’ data were anonymized and treated in accordance with the declaration of Helsinki.
Clinical data collection
The medical notes of enrolled patients were examined to gather information on their past medical history, symptoms at presentation to the ED, concomitant pulmonary findings and their evolution. In addition, clotting parameters of the patients, such as prothrombin time, partial thromboplastin time, platelet count and D-dimer level were collected.
Imaging acquisition protocol and analysis
All abdominal CT scans performed at Papa Giovanni XXIII Hospital (Bergamo) were acquired with the patient in supine position, on a 64-detector scanner (Revolution EVO; GE Medical), in the ED. All CTs included at least a portal venous phase, acquired 80-90 s following the injection of a non-ionic contrast medium (iomeprol 350 mg/mL, Bracco Imaging, Italy), in an antecubital vein. The mean contrast dose was 1.3-1.5 mL per patient’s kg, with a flow rate of 3/3.5 mL/s. A rapid saline solution flush (about 30/40 mL, flow rate at least 3 mL/s) was injected thereafter. In specific cases (suspected bowel ischemia or bleeding), an unenhanced scan and an arterial phase were additionally performed. The CT scanning protocol and contrast medium characteristics were similar for Humanitas (64-detector scanner, Philips Brilliance) and Policlinico (Flash 64 and SOMATON 256, Siemens) cohorts of patients.
Image analysis was performed by expert radiologists: Papa Giovanni XXIII: 2 board-certified radiologists in consensus (Bonaffini PA, and Valle C, 12 and 6 years of experience in abdominal imaging, respectively); Humanitas: 1 radiologist (Bonifacio C, 17 years of experience in abdominal imaging); Ospedale Maggiore Policlinico: 1 radiologist (Forzenigo L, 20 years of experience in abdominal imaging).
On CT scans of enrolled patients we evaluated the following parameters: (1) Small bowel distension, with corresponding maximum calibre (mm) and extension (ileal, jejunal, both); (2) Large bowel distension, with corresponding maximum calibre (mm) and extension (cecum, ascending, transverse, descending, sigmoid colon, rectum, all); (3) Thrombosis in the inferior vena cava (IVC), superior mesenteric vein (SMV) and portal vein (PV) and/or in the superior mesenteric artery (main trunk and branches); (4) Intraluminal air in SMV and/or PV; (5) Bowel wall characteristics (preserved/ absent enhancement, layered enhancement, thickening, parietal pneumatosis); (6) Pneumoperitoneum and/or ascites; and (7) Other ischemic sites in the abdomen (either vessels or organs). At the same time, the concomitant presence of pulmonary embolism (PE) with binary score (yes/no) and lung disease stages at chest CT were also recorded, if present. Lung involvement was classified as early, progressive, peak or absorption, as previously reported by Pan et al[10].
Endoscopic screening and findings
Endoscopic procedures were performed only in patients with COVID-19 GI symptoms and concomitant clinical history or radiological findings compatible with suspected colitis. All procedures were performed by expert endoscopists for each centre (Indriolo A, Elli L, Furfaro F, experienced endoscopists for more than 10 years). All endoscopic procedures on COVID-19 positive patients were organized in a COVID-19 dedicated operating room or in intensive care unit, depending on patients’ clinical conditions. Appropriate protection equipment was worn to perform the endoscopic procedures, and the ventilators used to assist the patients were sanitized according to internal antiseptic protocols.
At GI endoscopy we recorded data on the aspects of ischemic colitis such as haemorrhagic purple nodules, indicating submucosal bleeding. Other common endoscopic findings in cases of ischemic colitis could be petechiae, oedema, easily friable mucosa, and development of pseudo membranes[11-13].
Pathology findings
When available, histological specimens from intestinal biopsies, surgical resection, autoptic samples were analysed. All biopsies were reviewed by the same pathologist with over 20 years of experience in intestinal biopsy interpretation. In addition to routine staining and fixation, CD34 immunostaining for endothelial cells identification was performed systematically on all specimens.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 21. Categorical variables were compared using Chi Square. Correlations were analysed by Pearson or Spearman test depending on parametric or non-parametric variables. Proportions are reported with 95% exact binomial confidence intervals (CI). To compare mortality rate between COVID-19 patients with and without intestinal ischemia we used risk ratios as an effect measure with 95%CI calculated by the Fisher's exact test.
RESULTS
Clinical characteristics upon admission
During the study period, 2929 patients with COVID-19 were admitted to the three enrolling centres. GI presenting symptoms were found in 16% (95%CI: 0.14-0.17) of patients. We identified 21 patients (66% male, median age 69 years, range 62-79), who met the aforementioned inclusion criteria. The prevalence of intestinal ischemia in COVID-19 hospitalized patients was 0.7% (95%CI: 0.04-0.10). Baseline characteristics of the patients are presented in Table 1.
Table 1.
Patients clinical characteristics upon admission
|
Patients
|
Age
|
Sex
|
Comorbidities
|
Ongoing ACT/APT
|
Entry symptoms
|
D-dimer
|
| 1 | 85 | M | HT, CVD | Yes | Low GI bleeding | Not done |
| 2 | 71 | F | HT | Yes | Loss of appetite, vomiting, low GI bleeding | 10 N |
| 3 | 69 | M | DM, liver cirrhosis | No | Diarrhoea, fever and dyspnea | 8 N |
| 4 | 79 | F | HT, CVD, HCV | Yes | Abdominal pain | 8 N |
| 5 | 63 | M | No | No | Lower limb pain | > 70 N |
| 6 | 62 | M | Obesity, DM, HT, liver cirrhosis | No | Abdominal pain and vomiting | > 70 N |
| 7 | 83 | F | HT | Yes | Abdominal pain and dyspnoea | 4 N |
| 8 | 65 | M | No | No | Abdominal pain | Not done |
| 9 | 88 | M | CVD | Yes | Abdominal pain | N |
| 10 | 79 | M | CVD | No | Cardiac failure | N |
| 11 | 56 | M | HCV, liver cirrhosis | No | Ascites | 5 N |
| 12 | 61 | M | DM, HT | No | Dyspnoea | 3 N |
| 13 | 78 | F | HT | Yes | Lower limb oedema | 8 N |
| 14 | 41 | M | No | No | Fever and mental slowdown | 6 N |
| 15 | 64 | F | HT | Yes | Fever and caught | 2 N |
| 16 | 89 | F | Alzheimer disease | Yes | Caught and diarrhoea | N |
| 17 | 59 | M | Obesity, HT | No | Shortness of breathing | 2 N |
| 18 | 74 | M | CVD, pharynx carcinoma | No | Loss of conscious | 4 N |
| 19 | 63 | M | DM, HT | No | Fever, caught and dyspnoea | 2 N |
| 20 | 62 | F | HCV, COPD | No | Melena and anaemia | Not done |
| 21 | 72 | M | Parkinson disease and dementia | No | Fever and dysuria | > 70 N |
N: Normal value; ACT: Anticoagulant therapy; APT: Antiplatelets therapy; HT: Hypertension; CVD: Cardiovascular disorders; HCV: Hepatitis C virus; DM: Diabetes mellitus; COPD: Chronic obstructive pulmonary disease; GI: Gastrointestinal.
All but one patient presented with positive SARS-CoV-2 nasopharyngeal swab. SARS-CoV-2 diagnosis in one patient was made through SARS-CoV-2 RNA detection in the intestinal mucosa[14]. All patients were admitted to the Hospital after an ED access. In 11/21 (52%) cases, the clinical picture was characterized by gastrointestinal symptoms, being abdominal pain the most represented (5/11, 45%). Two patients did not present any comorbidity, while the most prevalent in the remainders was hypertension (in 10/21, 48%).
Eight of 21 patients (38%) were under anticoagulants or antiplatelets therapies at the time of ED admission. D-dimer was elevated in 17/21 (81%) of patients, while the rest of coagulation parameters were compatible with their treatment.
Radiological findings
Bowel distension was evident in 45% of cases. In 6/20 (30%) patients there was small bowel distension (mean maximum diameter 41.2 mm; range 30-56), generally involving the ileum (4/6 cases) and diffuse in 1 case. Large bowel was distended (mean diameter 94 mm; range 91-97) in 3/20 (15%) patients (2 with associated ileal involvement): In 2 cases the dilatation extended from transverse to cecum, while in 1 it involved the whole colon (Figure 1).
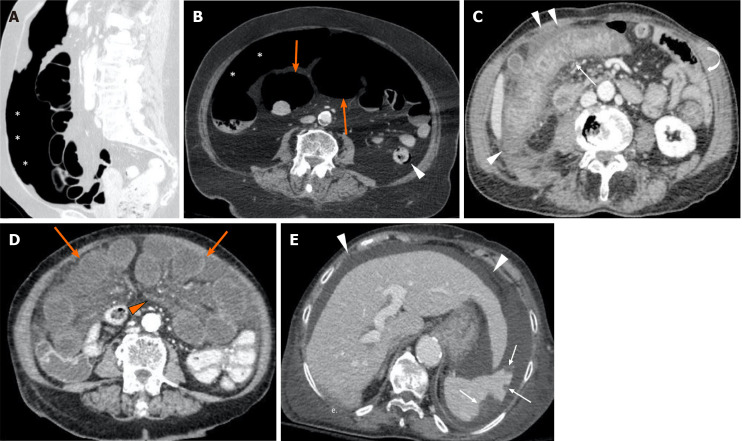
Figure 1.
Computed tomography images from positive coronavirus disease 19 patients with abdominal signs and symptoms. A and B: The computed tomography (CT) scan revealed pneumoperitoneum (asterisks, A and B), dilated bowel loops with thin non-enhancing walls (orange arrows, B) and focal areas of pneumatosis in the descending colon (arrowhead, B), in keeping with intestinal ischemia. The main mesenteric vessels were patent (not shown); C: CT evidence of ischemic colitis involving the transverse and right portions of the colon: Markedly thickened and layered walls (thin arrow), compared to other normal appearing small and large bowel loops (curved arrow), with associated free fluid and oedematous stranding of the adjacent fat tissue (arrowheads); D and E: Axial CT scan shows multiple small bowel loops moderately dilated, with reduced thickness and poorly enhancing walls (orange arrows, D); concomitant diffuse oedema of the mesentery (orange arrowhead, D). In the same patient there were associated multiple peripheral splenic infarcts (withe arrows, E) with areas of mottled increased attenuation; the main splenic vessels were patent (not shown). Diffuse ascites (white arrowheads, E) is noted.
Six patients (30%) had CT scan evidence of small or large bowel ischemia, with reduced wall thickness and enhancement. In four of these cases there was parietal pneumatosis with associated loop over distension (3 small, 2 large bowel). One patient had pneumoperitoneum. In four cases (3 with bowel ischemia) there were associated splenic (3) or renal (1) parenchymal infarcts.
Twelve patients (60%) had CT evidence of colitis/enteritis, with layered enhancement (mucosal enhancing, submucosal oedema) and wall thickening, (mean thickness 10.9 mm; range 7-17). The most commonly involved sites were left/sigmoid colon and/or terminal ileum; one patient had pancolitis. One patient demonstrated the same features in the oesophagus.
In the majority of cases (13/20, 65%) there was no evidence of venous or arterial acute thrombosis, including almost all patients with CT scan signs of bowel ischemia or colitis/enteritis. Thrombosis of a main abdominal vein was present only in three patients: IVC with SMV (one case with SB ischemia), PV with SMV (1) and PV alone (1). Three patients had iliac-femoral veins thrombosis, without involvement of any other vessel. In one case, we observed an acute splenic artery thrombosis, with associated parenchymal infarcts. None of these patients had evidence of intraluminal arterial or venous air, hematomas or signs of bleeding in the abdomen. In eight cases ascites was present.
Five patients had simultaneous evidence of PE but in 15/21 cases the assessment of pulmonary artery was not feasible. Pulmonary stage evaluation from chest CT was feasible in 11/21 (52%) patients: The most common one was early (5/11), followed by progressive (3/11), peak (2/11) and absorption (1/11).
Endoscopic findings
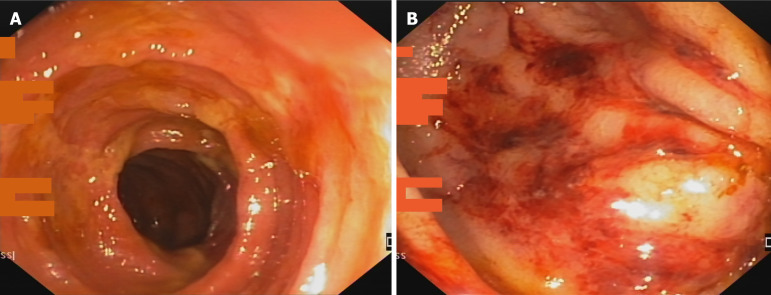
Endoscopy was performed in 8/21 (38 %) patients. Five (63%) demonstrated typical features of ischemic colitis with active bleeding from an oedematous mucosa, with petechiae in three of them and mucosal oedema and ischemic ulceration in the remaining 2 (Figure 2). Neither patchy lesions nor stenotic complication of ischemia were found in any of the performed endoscopies.
Figure 2.
Endoscopic image of ischemic colitis. A: Oedematous mucosa with ischemic ulcerations; B: Presence of petechiae in an oedematous colic mucosa.
Histopathology findings
In three colic biopsies collected from four patients’ mucosal lamina propria oedema, blood congestion, coagulative epithelial necrosis and ulcerations, intramucosal haemorrhagic foci were observed and interpreted as suggestive of acute ischemic injury. One biopsy showed normal histology; however, endoscopy was performed 2 mo after admission. One specimen from small bowel resection and one from autopsy were analysed. Both showed extensive haemorrhagic necrosis of mucosal layer, extended to the underlying wall. Necrosis was invariably accompanied by severe acute inflammation with diffuse micro-abscesses.
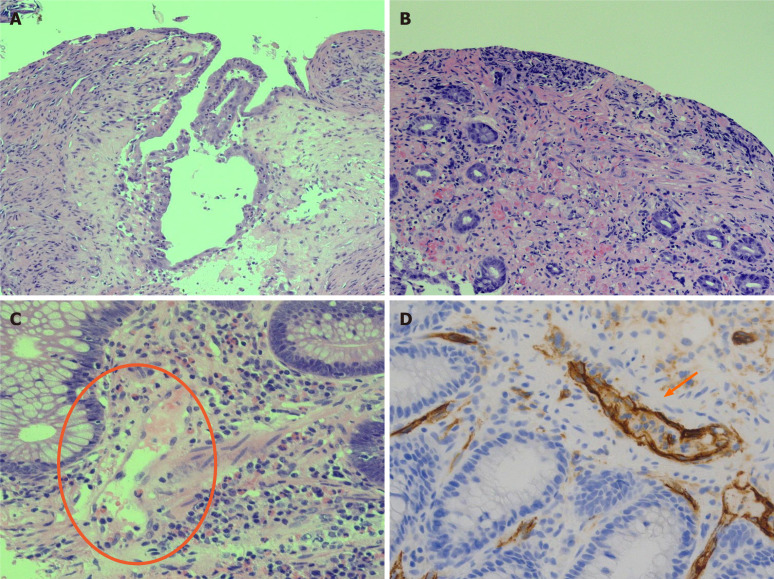
Findings of microvascular damage (endothelial shrinkage, leucocytes adhesion, vascular wall partial destruction) were reported in colonic (n. 3) and small bowel (n. 2) specimens. These features were studied in depth with CD34 staining for endothelial cells, and considered highly suggestive of SARS-CoV-2 infection related disease (Figure 3).
Figure 3.
Histological features of ischemic damage of small and large bowel characterized by mucosal ulceration and fibrosis and vascular congestion of lamina propria associated with moderate mixed inflammatory infiltrate. A: Histological features of ischemic damage of small bowel; B: Histological features of ischemic damage of large bowel; C: Distinctive severe acute respiratory syndrome coronavirus 2 blood vessels inflammatory pattern with endothelial shrinkage and layering of granulocytes and lymphocytes on damaged vascular wall; D: Endothelial injury is emphasized by CD34 immunostaining of endothelial cells showing marked infiltration of inflammatory cells.
Outcomes
Surgery was necessary in four (19%) patients. Three had small bowel perforations repaired, and one small bowel resection. None of the surgeries was performed on the colon.
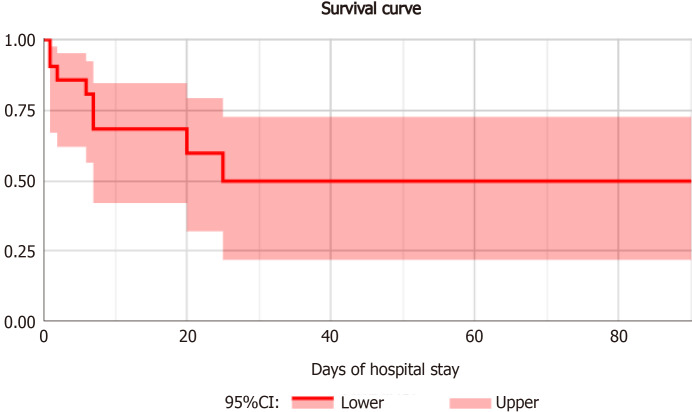
Median hospital stay was 9 d (interquartile range: 6-25). Mortality rate was 38% (95%CI: 0.18-0.62) as specified in Figure 4. No significant difference was found in univariate analysis when comparing patients stratified by outcome (Table 2).
Figure 4.
Survival curve of patients with intestinal ischemia.
Table 2.
No significant difference was found in univariate analysis when comparing patients stratified by outcome
|
Variables
|
Recovered, n = 13
|
Dead, n = 9
|
P
value
|
| Age in yr1 | 65 (61-78) | 71 (62-82) | 0.63 |
| Sex (F)2 | 6 (46%) | 1 (11%) | 0.17 |
| ACT/APT2 | 5 (38%) | 3 (33%) | 0.99 |
| D-dimer1 | 5 N (2 N-9 N) | 5 N (3 N-54 N) | 0.92 |
| PT (INR)1 | 1 (0.9-1.3) | 1.1 (1-1.5) | 0.48 |
| aPTT (INR)1 | 0.9 (0.8-1.1) | 1 (0.9-1.3) | 0.4 |
| Platelets (× 109/L)1 | 271 (226-325) | 232 (68-278) | 0.11 |
Express in median (Interquartile range).
Express in number of subjects (percentage).
F: Female; ACT: Anticoagulant therapy; APT: Antiplatelets therapy; PT: Prothrombin time; INR: International normalized ratio; aPPT: Activated partial thromboplastin time; N: Normal value.
Mortality among all the COVID-19 admitted patients was 23% (95%CI: 0.21-0.25). Mortality in COVID-19 patients with ischemic GI involvement had a relative risk for of 1.47 (95%CI: 0.007-0.031; P = 0.31), if compared to all COVID-19 hospitalized patients.
DISCUSSION
After the publication of several case reports or series of patients with COVID-19 gastrointestinal ischemia[15-18], this multicentre retrospective study demonstrated that intestinal ischemic manifestations of SARS-CoV-2 were diagnosed in 0.7% of patients hospitalized with COVID-19 during the first pandemic wave. Ischemic signs must be suspected in all patients presenting to the ED with important abdominal symptoms and a positive SARS-CoV-2 swab, but they may also appear later as complications of SARS-CoV-2 pneumonia. Previous anticoagulant treatment does not seem to protect from the development of this complication. Contrast enhanced CT scan appears to be the best tool to promptly diagnose these manifestations and their associated complications. Endoscopy is useful in case of suspected ischemic colitis. Atypical features on GI histology could guide the diagnosis even in patients with a SARS-CoV-2 negative nasopharyngeal swab. Nonetheless, a high index of suspicion must be kept, since mortality in these cases is as high as one in three patients.
Northern Italy and especially the Lombardy region were the most SARS-CoV-2 affected regions worldwide, with a seroprevalence after the first wave ranging from 24% to 38.5%[19-21], and an hospitalisation rate < 1%; thus, intestinal ischemic manifestations seem to be a very rare complication of SARS-Cov-2 infection.
Although the median hospital stay of the studied patients is similar to that of other larger cohorts of COVID-19 patients[22,23], the mortality is confirmed to be higher[22,23]. Furthermore, if we consider the latest results from Imperial College on Infection Fatality Rate (2.3% in SARS-CoV-2 infected patients in Italy)[24], intestinal ischemic manifestations impact with an over 15 times higher lethality.
As already highlighted by Bhayana and colleagues, the radiologists play a central role in the diagnosis of these complications, with intestinal wall abnormalities described in nearly a third of abdominal CT scans performed in COVID-19 patients admitted to the intensive care unit (ICU)[25]. The main relevant finding in our cohort of patients is that in almost all patients with CT signs of bowel ischemia or colitis/enteritis there was no evidence of acute venous or arterial vessels thrombosis. Only one of six patients with bowel ischemia showed concomitant acute IVC and SMV thrombosis. This is in line with the report from Bhayana[25]. Interestingly, in 4/20 patients CT scan showed splenic (3) or renal (1) parenchymal infarcts, but only in one case we identified splenic artery thrombosis. The rate of complications related to bowel involvement was relatively low, accounting for CT scan evidence of perforation in one case and loops over distension in 6/20 patients. Bowel over distension, with no evidence of obstructing aetiologies, was therefore most likely functional.
Endoscopy may help confirming the diagnosis of patients with ischemic colitis, as outlined by the early published reports[26,27], although caution is mandatory when performing endoscopy in unstable patients admitted to ICU[28]. This is in agreement with a multicentre study on endoscopic findings of SARS-CoV-2 positive patients during the first wave in northern Italy, which showed histologically confirmed colon ischemia as one of the most prevalent findings[29].
In our series the histological analysis of colon biopsies together with small bowel resected segments showed very distinctive aspects of endothelium inflammation and endotheliitis, which were firstly described in the pulmonary, renal and gastrointestinal tract of SARS-CoV-2 infected patients[30]. These findings may give an explanation to the previously mentioned radiological evidence of ischemic damage without main venous or arterial thrombosis. Furthermore, in our cohort they may explain the absence of topographical segmental and patchy distribution of endoscopic ischemic lesions, which are usually expression of occluded vasculature[13].
The strong tropism of SARS-CoV-2 for the gastrointestinal tract is probably mediated by the abundance of Angiotensin Converting Enzyme 2 receptors in the intestinal mucosa[31]. A possible explanation for the virus induced endothelial damage in the gastrointestinal tract could lie in the lectin pathway, which is supposed to be responsible for SARS-CoV-2 mediated thrombotic microangiopathy in lung tissues[32]. The central role of Lectin and mannan-binding lectin-associated serine protease-2 in the gastrointestinal ischemic reperfusion damage has already been described in murine models in the pre-COVID-19 era[33,34]. These findings, if confirmed, could open new interesting therapeutic fields of research on SARS-CoV-2 ischemic manifestations.
CONCLUSION
Despite several limitations, mainly due the retrospective design on an infrequent and previously unknown manifestation of SARS-CoV-2, this multicentre study was able to confirm early isolated observations of gastrointestinal ischemia. Furthermore, new findings on epidemiology, clinical presentation, distinctive diagnostics features and outcomes were illustrated and discussed. This preliminary results in a small series of patients might raise the awareness on an infrequent but potentially lethal manifestation of COVID-19, aiding clinicians in proper management and prognostic stratification of complicated cases.
ARTICLE HIGHLIGHTS
Research background
Clinical manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus are heterogenous and can affect different organs, including the gastrointestinal tract. Angiotensin converting enzyme 2 receptor, which mediates SARS-CoV-2 infection, is abundantly present in the intestinal mucosa.
Research motivation
Since the cytokine storm mediated by SARS-CoV-2 in coronavirus disease 19 (COVID-19) seems to determine a vascular damage which could explain hypercoagulability and pulmonary embolism, intestinal ischemic events in SARS-CoV-2 positive patients may be linked to the same pathogenic mechanism.
Research objectives
The aim of the present study is to collect and analyse the intestinal ischemic events in SARS-CoV-2 positive patients in order to calculate the incidence and determine the prognosis of affected subjects.
Research methods
The study was designed as a retrospective observational multicentre collection involving three among the largest COVID hospitals in Lombardy.
Research results
Intestinal ischemia is a rare but fatal manifestation of SARS-CoV-2 infection. The condition should be suspected in case of severe abdominal pain. In order to define localization and extent of intestinal ischemia, abdominal computed tomography scan and possibly endoscopy should be carried out. Intestinal biopsies main finding is endotheliitis.
Research conclusions
Severe endotheliitis in the intestinal mucosa could mediate intestinal ischemic manifestations in the gastrointestinal tract. Such manifestations are rare but frequently fatal, thus they should be ruled out in SARS-CoV-2 positive patients with gastrointestinal symptoms.
Research perspectives
One of the possible mechanisms of ischemic intestinal manifestations of SARS-CoV-2 could be mediated by mannan-binding lectin-associated serine protease-2 which is also a possible target for future therapy of COVID-19.
Footnotes
Institutional review board statement: The study was reviewed and approved by the ASST Papa Giovanni XXIII Institutional Review Board, No. 2020-0096.
Informed consent statement: Informed consent from patients was waived because of the retrospective nature of the study.
Conflict-of-interest statement: The authors declare no conflict of interest and no financial support for this study.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Manuscript source: Invited manuscript
Peer-review started: March 26, 2021
First decision: April 29, 2021
Article in press: July 23, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sivanand N S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Lorenzo Norsa, Department of Pediatric Gastroenterology Hepatology and Transplantation, ASST Papa Giovanni XXIII, Bergamo 24127, Italy. lnorsa@asst-pg23.it.
Pietro Andrea Bonaffini, Department of Radiology Papa Giovanni XXIII Bergamo, University of Milano-Bicocca, Milan 20126, Italy.
Maja Caldato, Fondazione IRCCS Ca’ Granda, Department of Emergency Medicine, Ospedale Maggiore Policlinico, Milano 20122, Italy.
Cristiana Bonifacio, Department of Radiology, Humanitas Clinical and Research Center, IRCCS, Rozzano 20089, Italy.
Aurelio Sonzogni, Department of Pathology, ASST Papa Giovanni XXIII, Bergamo 24127, Italy.
Amedeo Indriolo, Department of Gastroenterology and Endoscopy, ASST Papa Giovanni XXIII, Bergamo 24127, Italy.
Clarissa Valle, Department of Radiology Papa Giovanni XXIII Bergamo, University of Milano-Bicocca, Milan 20126, Italy.
Federica Furfaro, IBD Center, Humanitas Clinical and Research Center IRCCS, Humanitas University, Rozzano 20089, Italy.
Alice Bonanomi, Post-Graduate School of Diagnostic Radiology, University of Milano-Bicocca, Milano 20126, Italy.
Paolo Niccolò Franco, Post-Graduate School of Diagnostic Radiology, University of Milano-Bicocca, Milano 20126, Italy.
Mauro Gori, Cardiovascular Department, ASST Papa Giovanni XXIII, Bergamo 24127, Italy.
Veronica Smania, Department of Gastroenterology and Endoscopy, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano 20122, Italy; Department of Pathophysiology and Transplantation, University of Milan, Milano 20122, Italy.
Lucia Scaramella, Department of Gastroenterology and Endoscopy, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano 20122, Italy; Department of Pathophysiology and Transplantation, University of Milan, Milano 20122, Italy.
Laura Forzenigo, Department of Radiology, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano 20122, Italy.
Maurizio Vecchi, Department of Gastroenterology and Endoscopy, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano 20122, Italy; Department of Pathophysiology and Transplantation, University of Milan, Milano 20122, Italy.
Monica Solbiati, Fondazione IRCCS Ca’ Granda, Department of Emergency Medicine, Ospedale Maggiore Policlinico, Department of Clinical Sciences and Community Health, University of Milan, Milano 20122, Italy.
Giorgio Costantino, Fondazione IRCCS Ca’ Granda, Department of Emergency Medicine, Ospedale Maggiore Policlinico, Department of Clinical Sciences and Community Health, University of Milan, Milano 20122, Italy.
Silvio Danese, IBD Center, Humanitas Clinical and Research Center IRCCS, Humanitas University, Rozzano 20089, Italy.
Lorenzo D'Antiga, Department of Pediatric Gastroenterology Hepatology and Transplantation, ASST Papa Giovanni XXIII, Bergamo 24127, Italy.
Sandro Sironi, Department of Radiology Papa Giovanni XXIII Bergamo, University of Milano-Bicocca, Milan 20126, Italy; Post-Graduate School of Diagnostic Radiology, University of Milano-Bicocca, Milano 20126, Italy.
Luca Elli, Department of Gastroenterology and Endoscopy, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano 20122, Italy; Department of Pathophysiology and Transplantation, University of Milan, Milano 20122, Italy.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at lnorsa@asst-pg23.it.
References
- 1.Alicandro G, Remuzzi G, La Vecchia C. COVID-19 pandemic and total mortality in the first six months of 2020 in Italy. Med Lav. 2020;111:351–353. doi: 10.23749/mdl.v111i5.10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut . 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology. 2020;159:373–375.e2. doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao R, Qiu Y, He J-S, Tan J-Y, Li X-H, Liang J, Shen J, Zhu L-R, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen M-H. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol . 2020 doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norsa L, Bonaffini PA, Indriolo A, Valle C, Sonzogni A, Sironi S. Poor Outcome of Intestinal Ischemic Manifestations of COVID-19. Gastroenterology. 2020;159:1595–1597.e1. doi: 10.1053/j.gastro.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moszkowicz D, Mariani A, Trésallet C, Menegaux F. Ischemic colitis: the ABCs of diagnosis and surgical management. J Visc Surg. 2013;150:19–28. doi: 10.1016/j.jviscsurg.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Theodoropoulou A, Koutroubakis IE. Ischemic colitis: clinical practice in diagnosis and treatment. World J Gastroenterol. 2008;14:7302–7308. doi: 10.3748/wjg.14.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt LJ, Feuerstadt P, Longstreth GF, Boley SJ American College of Gastroenterology. ACG clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI) Am J Gastroenterol. 2015;110:18–44; quiz 45. doi: 10.1038/ajg.2014.395. [DOI] [PubMed] [Google Scholar]
- 14.Norsa L, Valle C, Morotti D, Bonaffini PA, Indriolo A, Sonzogni A. Intestinal ischemia in the COVID-19 era. Dig Liver Dis. 2020;52:1090–1091. doi: 10.1016/j.dld.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianco F, Ranieri AJ, Paterniti G, Pata F, Gallo G. Acute intestinal ischemia in a patient with COVID-19. Tech Coloproctol. 2020;24:1217–1218. doi: 10.1007/s10151-020-02255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan W, Ramadan HK. COVID-19 and pneumatosis intestinalis: An early sign of intestinal ischemia. Dig Liver Dis . 2021;53:289–290. doi: 10.1016/j.dld.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh B, Mechineni A, Kaur P, Ajdir N, Maroules M, Shamoon F, Bikkina M. Acute Intestinal Ischemia in a Patient with COVID-19 Infection. Korean J Gastroenterol. 2020;76:164–166. doi: 10.4166/kjg.2020.76.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung S, Quiwa JC, Pillai A, Onwu C, Tharayil ZJ, Gupta R. Superior Mesenteric Artery Thrombosis and Acute Intestinal Ischemia as a Consequence of COVID-19 Infection. Am J Case Rep. 2020;21:e925753. doi: 10.12659/AJCR.925753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagani G, Conti F, Giacomelli A, Bernacchia D, Rondanin R, Prina A, Scolari V, Gandolfi CE, Castaldi S, Marano G, Ottomano C, Boracchi P, Biganzoli E, Galli M. Seroprevalence of SARS-CoV-2 significantly varies with age: Preliminary results from a mass population screening. J Infect. 2020;81:e10–e12. doi: 10.1016/j.jinf.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perico L, Tomasoni S, Peracchi T, Perna A, Pezzotta A, Remuzzi G, Benigni A. COVID-19 and lombardy: TESTing the impact of the first wave of the pandemic. EBioMedicine. 2020;61:103069. doi: 10.1016/j.ebiom.2020.103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norsa L, Cosimo P, Indriolo A, Sansotta N, D'Antiga L, Callegaro A. Asymptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Patients With Inflammatory Bowel Disease Under Biologic Treatment. Gastroenterology . 2020;159:2229–2231.e2. doi: 10.1053/j.gastro.2020.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, Klauber J, Janssens U, Marx G, Weber-Carstens S, Kluge S, Pfeifer M, Grabenhenrich L, Welte T, Busse R. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imperial College London. Report 34 - COVID-19 Infection Fatality Ratio Estimates from Seroprevalence. 29 October 2020. [cited 26 March 2021]. Available from: http://www.imperial.ac.uk/medicine/departments/school-public-health/infectious-disease-epidemiology/mrc-global-infectious-disease-analysis/covid-19/report-34-ifr/
- 25.Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA, Catalano O, Gee MS, Hahn PF, Harisinghani M, Kilcoyne A, Lee SI, Mojtahed A, Pandharipande PV, Pierce TT, Rosman DA, Saini S, Samir AE, Simeone JF, Gervais DA, Velmahos G, Misdraji J, Kambadakone A. Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology. 2020;297:E207–E215. doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida Vargas A, Valentí V, Sánchez Justicia C, Martínez Regueira F, Martí Cruchaga P, Luján Colás J, Aliseda Jover D, Esteban Gordillo S, Cienfuegos JA, Rotellar Sastre F. Severe colon ischemia in patients with severe coronavirus-19 (COVID-19) Rev Esp Enferm Dig. 2020;112:784–787. doi: 10.17235/reed.2020.7329/2020. [DOI] [PubMed] [Google Scholar]
- 27.Paul T, Joy AR, Alsoub HARS, Parambil JV. Case Report: Ischemic Colitis in Severe COVID-19 Pneumonia: An Unforeseen Gastrointestinal Complication. Am J Trop Med Hyg . 2021;104:63–65. doi: 10.4269/ajtmh.20-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan KH, Lim SL, Damati A, Maruboyina SP, Bondili L, Abu Hanoud A, Slim J. Coronavirus disease 2019 (COVID-19) and ischemic colitis: An under-recognized complication. Am J Emerg Med. 2020;38:2758.e1–2758.e4. doi: 10.1016/j.ajem.2020.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massironi S, Viganò C, Dioscoridi L, Filippi E, Pagliarulo M, Manfredi G, Conti CB, Signorelli C, Redaelli AE, Bonato G, Iiritano E, Frego R, Zucchini N, Ungari M, Pedaci M, Bono F, Di Bella C, Buscarini E, Mutignani M, Penagini R, Dinelli ME, Invernizzi P. Endoscopic Findings in Patients Infected With 2019 Novel Coronavirus in Lombardy, Italy. Clin Gastroenterol Hepatol. 2020;18:2375–2377. doi: 10.1016/j.cgh.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rambaldi A, Gritti G, Micò MC, Frigeni M, Borleri G, Salvi A, Landi F, Pavoni C, Sonzogni A, Gianatti A, Binda F, Fagiuoli S, Di Marco F, Lorini L, Remuzzi G, Whitaker S, Demopulos G. Endothelial injury and thrombotic microangiopathy in COVID-19: Treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology. 2020;225:152001. doi: 10.1016/j.imbio.2020.152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, Dudler T, Parent B, Lhotta K, Wallis R, Farrar CA, Sacks S, Lee H, Zhang M, Iwaki D, Takahashi M, Fujita T, Tedford CE, Stover CM. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincenti M, Behrends M, Dang K, Park YH, Hirose R, Blasi-Ibanez A, Liu T, Serkova NJ, Niemann CU. Induction of intestinal ischemia reperfusion injury by portal vein outflow occlusion in rats. J Gastroenterol. 2010;45:1103–1110. doi: 10.1007/s00535-010-0262-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at lnorsa@asst-pg23.it.