Abstract
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor in China. Preoperative diagnosis of HCC is challenging because of atypical imaging manifestations and the diversity of focal liver lesions. Artificial intelligence (AI), such as machine learning (ML) and deep learning, has recently gained attention for its capability to reveal quantitative information on images. Currently, AI is used throughout the entire radiomics process and plays a critical role in multiple fields of medicine. This review summarizes the applications of AI in various aspects of preoperative imaging of HCC, including segmentation, differential diagnosis, prediction of histopathology, early detection of recurrence after curative treatment, and evaluation of treatment response. We also review the limitations of previous studies and discuss future directions for diagnostic imaging of HCC.
Keywords: Hepatocellular carcinoma, Radiomics, Artificial intelligence, Diagnosis, Treatment, Recurrence
Core Tip: Hepatocellular carcinoma (HCC) threatens human health because of its high morbidity and recurrence rates. Patients with HCC may benefit from early diagnosis, timely treatment, and appropriate follow-up strategies. In the era of big data, artificial intelligence (AI) provides critical information regarding the diagnosis, treatment, and prognosis of HCC. We herein discuss the role of AI in the following aspects of preoperative imaging: Segmentation, differential diagnosis, prediction of histopathology, early detection of recurrence after curative treatment, and evaluation of treatment response.
INTRODUCTION
Hepatocellular carcinoma (HCC), which most often arises from chronic hepatitis B virus infection, is one of the main causes of cancer-related deaths worldwide, particularly in China[1]. HCC presents with characteristic radiological features and can be diagnosed without biopsy. Imaging is therefore crucial for diagnosis and management. Computed tomography (CT) is the most widely used method for HCC diagnosis, although magnetic resonance imaging (MRI) is the optimal diagnostic modality, owing to its multi-parameter imaging techniques. However, even with the application of dynamic contrast-enhanced MRI (DCE-MRI), the imaging diagnosis of HCC is challenging because of atypical imaging manifestations and liver tumor diversity.
A single clinical image contains a large amount of quantitative information that can provide crucial data for diagnostic and treatment purposes. This information can be processed using innovative methods. Radiomics has recently gained attention for its potential to further analyze images. It allows for the extraction of a large amount of quantitative objective data included in radiological images that could be explored for determining underlying biological processes[2]. The workflow of a radiomics study generally includes five stages: Image acquisition, segmentation, feature extraction, exploratory analysis, and modeling[3] (Figure 1). Every stage is closely related to artificial intelligence (AI). The concept of AI was first advocated in 1955 by McCarthy et al[4], who described AI as a computer program that attempts to simulate human cognitive functions. AI can learn and solve problems to improve itself.
Figure 1.
The five stages of a radiomics study where artificial intelligence can play a role.
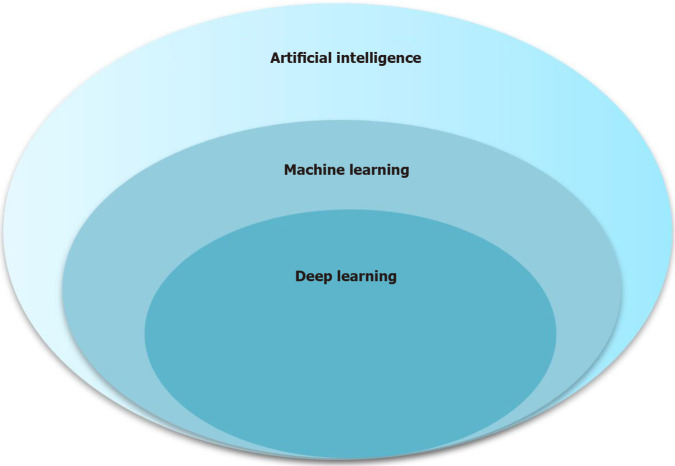
Machine learning (ML) is at the core of AI and involves various techniques, such as artificial neural networks, support vector machines (SVM), and random forest (RF). Deep learning (DL) is an important branch of ML, and convolutional neural networks (CNNs) are a type of DL algorithm. The relationships among AI, ML, and DL are described in Figure 2. With technological advances and the use of AI, radiomics has rapidly developed in recent years, and all radiomics techniques have now been utilized in studies within the medical field. This review focuses on the application of AI for HCC imaging, including segmentation, differential diagnosis, prediction of histopathology, early detection of recurrence after curative treatment, and evaluation of treatment response.
Figure 2.
Relationship of artificial intelligence, machine learning, and deep learning.
SEGMENTATION OF HCC LESIONS
Although HCC segmentation is challenging, it is crucial for various medical imaging analyses. Manual and semi-manual segmentation are time-consuming and susceptible to interobserver variability, which might in turn lead to the biased results. In contrast, automatic segmentation based on an AI algorithm is more repeatable and efficient. CNNs have been successfully applied for the automatic segmentation of hepatic lesions in both CT and MRI. Chlebus et al[5] figured out a CNN model for the automatic segmentation of liver tumors on CT; however, with respect to tumor detection, the model performed poorly compared with human performance. Bousabarah et al[6] established a DL algorithm to automatically delineate the liver and HCC lesions on MRI. The model was a combination of a deep CNN, RF, and thresholding. The mean dice similarity coefficient between the DL model and manual segmentation was 0.64/0.68 (validation/test) for tumor segmentation and 0.91/0.91 for liver segmentations. Current methods do not achieve a comparably high level of performance in liver imaging for automatic tumor segmentation. This may be due to the heterogeneity of the liver parenchyma and the tumor itself. Larger datasets will be required to improve the accuracy of DL algorithms and will ensure optimal sensitivity and specificity.
DIFFERENTIAL DIAGNOSIS
Imaging-based differential diagnosis improves the accuracy of diagnosis. For HCC, the primary diagnostic modalities are CT and MRI. Radiomics with AI has been gradually applied in clinical research, as it can provide quantitative information, including additional differential characteristics not yet recognized in current radiological diagnosis. This could, in turn, help less-experienced radiologists diagnose focal liver lesions more accurately. Table 1 summarizes the studies on the differential diagnosis of HCC with AI.
Table 1.
Summary of studies on differential diagnosis of hepatocellular carcinoma
|
Ref.
|
Aim of the study
|
Modality
|
Patients
|
Method
|
AUC of the final model
|
| Nie et al[7] | Differentiation between HCC and HCA | CT | 131 | ML | T: 0.96, V: 0.94 |
| Nie et al[8] | Differentiation between HCC and FNH | CT | 156 | ML | T: 0.979, V: 0.917 |
| Mokrane et al[9] | Differentiation between HCC and non-HCC nodules | CT | 178 | ML | T: 0.70, V: 0.66 |
| Ponnoprat et al[10] | Differentiation between HCC and ICC | CT | 257 | ML | NA |
| Shi et al[11] | Differentiation of HCC from other FLLs | CT | 342 | DL | 0.925 |
| Yasaka et al[12] | Liver mass classification | CT | 560 | DL | 0.92 |
| Cao et al[13] | FLL classification | CT | 375 | DL | V: 0.88-0.99 |
| Jiang et al[14] | Comparing the diagnostic accuracies of EASL (v2018), LI-RADS criteria, and radiomics models for HCC | MRI | 211 | ML | T: 0.861, V: 0.810 |
| Zhen et al[15] | Classification of liver tumors | MRI | 1411 | DL | 0.963-0.998 |
| Liu et al[16] | Differentiation of cHCC-CC from CC and HCC | MRI | 85 | ML | 0.77 |
| Huang et al[17] | Diagnosis of DPHCC | MRI | 100 | ML | 0.784 |
| Jian et al[18] | Characterization of HCC | MRI | 112 | DL | NA |
| Wu et al[19] | Classification of HCC and hepatic hemangioma | MRI | 369 | ML | T: 0.86, V: 0.89 |
T: Training cohort; V: Validation cohort; NA: Not available; AUC: The area under the receiver operating characteristic curve; ML: Machine learning; DL: Deep learning; HCC: Hepatocellular carcinoma; HCA: Hepatocellular adenoma; FNH: Focal nodular hyperplasia; ICC: Intrahepatic cholangiocarcinoma; cHCC-CC: Combined hepatocellular cholangiocarcinoma; CC: Cholangiocarcinoma; DPHCC: Dual-phenotype hepatocellular carcinoma; FLL: Focal liver lesion.
CT
Some studies have applied conventional ML algorithms for the differentiation of liver masses. In the non-cirrhotic liver, Nie et al[7,8] reported CT-based radiomics nomograms for the preoperative differentiation of HCC from hepatocellular adenoma (HCA) and focal nodular hyperplasia (FNH). It showed good discrimination capability, with an area under the curve (AUC) of 0.96/0.94 and 0.979/0.917 (training/validation) for differentiating HCC from HCA and FNH, respectively. In patients with cirrhosis, ML techniques could classify indeterminate liver nodules as HCC or non-HCC based on triphasic CT scans, with an AUC of 0.70/0.66 (training/validation)[9]. Ponnoprat et al[10] developed an ML method to differentiate between HCC and intrahepatic cholangiocarcinoma (ICC), the two most common malignant liver tumors, based on multi-phase CT. They reported an 88% accuracy in HCC-ICC classification. DL with CNN for the differential diagnosis of liver lesions also showed relatively high diagnostic capability, reaching an AUC of 0.86-0.99[11-13].
MRI
MRI can provide more comprehensive information for differential diagnosis than CT because of its multi-parameter techniques and various tissue contrast mechanisms. Accurate diagnosis of HCC remains a challenge because of liver tumor diversity and relies on the experience of radiologists. Jiang et al[14] established a radiomics signature from multi-sequence MRI using the least absolute shrinkage and selection operator (LASSO) model and multivariate logistic regression analysis. The AUC of the radiomics signature was 0.810. It showed a comparable accuracy with the LI-RADS (0.841) and EASL criteria (0.811) for HCC diagnosis. Zhen et al[15] used CNNs to develop a DL model to classify liver tumors based on MR images. The CNN model combined with clinical data performed well in identifying HCC (AUC, 0.985; 95%CI: 0.960-1.000), metastatic tumors (AUC, 0.998; 95%CI: 0.989-1.000), and other primary malignancies (AUC, 0.963; 95%CI: 0.896-1.000). Some researchers have also applied ML algorithms to the differential diagnosis of primary liver cancer subtypes. Liu et al[16] reported an ML analysis of MRI radiomics features for the differentiation of combined hepatocellular cholangiocarcinoma (cHCC-CC) from HCC and cholangiocarcinoma. The model showed acceptable performance, with an AUC of 0.77. Huang et al[17] created a radiomics signature from Gd-EOB-DTPA-enhanced MRI for the diagnosis of dual-phenotype HCC (DPHCC). They used LASSO and four classifiers: Multi-layer perceptron, SVM, logistic regression, and K-nearest neighbor. The combination of different phases and classifiers achieved the best performance in the preoperative diagnosis of DPHCC. ML and DL algorithms can also be used in non-contrast MRI to increase the diagnostic accuracy of HCC[18,19]. This is beneficial for patients who cannot receive contrast injections.
CT- or MRI-based ML and DL models have demonstrated promising predictive performance and have reached a high level of accuracy similar to that of experienced radiologists. Accurate preoperative diagnosis of focal liver lesions can help clinicians make proper decisions, optimize patient management, and improve patient prognosis.
PREOPERATIVE PREDICTION OF HISTOPATHOLOGY
Grade
Histological grade represents the biological behavior and aggressiveness of tumors. The high recurrence rate of HCC is associated with its pathological grade. The pathological grade of HCC determined using imaging may provide valuable prognostic information. Mao et al[20] developed an ML model based on contrast-enhanced CT for preoperative prediction of the pathological grade of HCC. The model achieved an AUC of 0.8014 when combined with clinical factors. The radiomics signature based on T1WI, T2WI, and DCE-MRI was also found to be significantly related to the histopathological grade of HCC[21,22]. Yang et al[22] proposed a DL model with a multichannel fusion three-dimensional CNN (3D-CNN) based on DCE-MR to differentiate among pathological grades. The model reached an average accuracy of 0.7396 ± 0.0104. Thus, further improvements are needed to achieve better diagnostic performance of radiomics models for histological grade.
Microvascular invasion
In addition to predicting the HCC grade, the prediction of microvascular invasion (MVI) has also become a common topic of study. MVI status is a crucial factor influencing treatment selection and follow-up planning; thus, it must be determined preoperatively[23,24]. Some studies have tried to predict MVI before surgery using radiological images and clinical factors. They found that MVI was closely related to several factors. Some studies used LASSO to construct radiomics signatures from CT and MRI and developed models with various conventional ML algorithms for the preoperative prediction of MVI[25-27]. The results showed favorable predictive accuracy for MVI status in HCC patients, especially when combined with clinical factors[28,29]. Jiang et al[30] figured out a CT-based model using eXtreme Gradient Boosting and DL algorithm with 3D-CNN to predict MVI preoperatively. The 3D-CNN model demonstrated high diagnostic capability, with an AUC of 0.980/0.906 (training/validation). However, different dimensionality reduction and modeling methods affect the diagnostic performance of the final models. Ni et al[31] retrospectively analyzed 206 HCC cases to explore the best radiomic-based diagnostic model. The LASSO + GBDT method showed better performance than the other methods, when the threshold probability was more than 0.22.
MVI usually occurs in the peritumoral region, but it is unclear whether including the features of the peritumoral region could improve predictive capability. Nebbia et al[32] developed an ML model for preoperative prediction of MVI status using multiparametric MRI. They found that radiomics features extracted from the tumor region had good diagnostic performance, with an AUC of 0.867. Radiomics features from the peritumoral region also showed an association with MVI; however, the AUC was slightly lower than that of intratumoral radiomics features. Although peritumoral enhancement pattern is reported to be a good predictor of MVI status[33], the usefulness of the features extracted from peritumoral regions for predicting MVI status needs further evaluation in larger populations.
Molecular profiling
HCC originates from hepatocytes and/or hepatic progenitor cells and can express various molecular phenotypes[34]. As such, prognosis varies even among patients with the same pathological grade. Molecular profiling is an important modality that may reflect the biological behavior and invasiveness of tumors. Several studies have used ML methods to identify the molecular phenotypes of HCC preoperatively. CK19 is a biliary-specific marker, and CK19 positivity is associated with a poor postoperative prognosis in HCC[35,36]. In a recent study, the radiomics signature derived from gadoxetic acid–enhanced MRI was used to predict CK19 status in HCC. The final model combined the radiomics signature and clinical factors and achieved a sensitivity of 0.818/0.769 (training/validation) and specificity of 0.974/0.818 (training/validation)[37].
Glypican 3 (GPC3), a type of heparan sulfate proteoglycan, is located on the cell surface. Previous studies have found that GPC3 is closely associated with postoperative metastasis and recurrence in patients with HCC[38]. Furthermore, GPC3 is considered a potential immunotherapeutic target for HCC therapy, especially in patients with unresectable HCC. Gu et al[39] constructed a radiomics signature from MRI and achieved good predictive efficacy, with an AUC of 0.879/0.871 (training/validation). Combining the radiomics signature with α-fetoprotein levels further improved the predictive performance, with an AUC of 0.926/0.914 (training/validation). Another molecular biomarker of HCC is the Ki-67 index. A high Ki-67 index is related to early recurrence and poor prognosis, thus making it a potential indicator of tumor aggressiveness. Recent studies have reported that the Ki-67 status can be predicted preoperatively using CT and MRI[40,41]. Prediction of molecular phenotypes via preoperative imaging can aid in the selection of individualized therapeutic strategies.
PREDICTION OF TREATMENT RESPONSE AND EARLY RECURRENCE AFTER CURATIVE TREATMENT
Individualized medical care depends on accurate risk stratification systems. These systems help select the proper treatment and evaluate treatment response. Pretreatment imaging acts as an important role in predicting the effects of treatment, helping clinicians choose the best individualized treatment strategy for patients.
Early recurrence, that is, recurrence within 1-2 years after resection or ablation, is a strong influencing factor of poor prognosis in patients with HCC. A radiomics nomogram derived from preoperative CT and MRI was established to predict early recurrence after surgery or curative ablation in HCC patients[42,43]. The radiomics nomogram comprising both the radiomics score and clinicoradiological risk factors demonstrated good performance, with an AUC of 0.785-0.844[44,45]. Prediction of early recurrence is critical for planning follow-up surveillance strategies and determining the necessity of further interventions after curative treatment. Wang et al[46] proposed a DL-based radiomics approach from multi-phase CT images to predict the early recurrence of HCC; it achieved an AUC of 0.825. Unfortunately, image-based radiomics models for early recurrent HCC have poor reproducibility among medical centers[47], limiting their application in clinical practice.
GUIDANCE OF TREATMENT SELECTION
Trans-arterial chemoembolization
Patients with unresectable HCC can undergo trans-arterial chemoembolization (TACE), local radiofrequency ablation, and systemic treatment with sorafenib. ML or DL models based on pretreatment CT or MRI have been recently considered as potential tools for predicting treatment response to TACE for HCC[48,49]. Morshid et al[50] used pretreatment quantitative CT image features and clinical factors to develop an ML model to predict treatment response to TACE; the model had an accuracy of 74.2%. Abajian et al[51] used MR imaging and clinical data to create an AI model for the prediction of TACE treatment response; the model had an overall accuracy of 78%. Similar results were obtained in other studies, confirming the usefulness of radiomics features extracted from pretreatment CT or MRI for the prediction of tumor response to TACE[52-54]. Peng et al[55] described a DL model of a residual CNN to predict the treatment response to TACE for HCC. The final model had a high accuracy in predicting four different therapy response types (complete response, partial response, stable disease, and progressive disease). Thus, it could help clinicians identify patients who will optimally benefit from TACE.
Immunotherapy
Immunotherapy has been shown to be a promising treatment for HCC; however, treatment response to immunotherapy remains low[56-58]. Therefore, it is necessary for clinicians to identify which patients will respond to immunotherapy. Treatment response to immunotherapy is highly dependent on the immune status of the tumor[59]. Tumor immunoprofiling is thus important in predicting its effect. A contrast-enhanced CT-based Rad score developed using the ML algorithm showed high predictive power for CD8+ T-cell infiltration, which is associated with the immunotherapy response[60]. Hectors et al[61] reported that MRI radiomics features were correlated with various immunohistochemical cell markers and the expression of certain immunotherapy targets. Chen et al[62] used clinical data and intratumoral and peritumoral radiomics features to build an ML model from Gd-EOB-DTPA-enhanced MRI. The model showed excellent performance in predicting the immunoscore, with an AUC of 0.926 (95%CI: 0.884-0.967). Collectively, these findings indicated that radiomics features extracted by ML methods might serve as noninvasive predictors of the immune characteristics of HCC and assist physicians in identifying patients who will benefit from immunotherapy.
LIMITATIONS
The application of AI in different fields of medical research has demonstrated promising results; however, there are some limitations. First, nearly all the research was retrospective and included a relatively small sample size. Therefore, the performance of these predictive models should be validated in larger, multicenter, and prospective studies. Second, the predictive models had limited reproducibility for application in clinical practice, and image heterogeneity might be a significant influencing factor. Third, the AI calculation algorithm requires specialized software packages, leading to increased medical costs. The workflow includes imaging acquisition, segmentation, feature extraction, exploratory analysis, and modeling, making it complex and diverse, further limiting its clinical application.
While AI and radiomics have proven useful in various aspects of HCC, the underlying mechanisms have not been clearly stated, such as pathological correlation and relationship between radiomics and genomics. More research is needed to explore the relationships among imaging, pathophysiology, and prognosis.
CONCLUSION
AI has been applied in many studies on preoperative imaging of HCC. It can extract a large amount of quantitative information from images and reflect pathophysiological processes. Diagnostic and predictive models using AI algorithms have demonstrated promising results in the fields of segmentation, differential diagnosis, prediction of histology, and guidance for treatment selection. However, considering the limitations and complexity of AI, additional research is needed before it can be widely used in clinical practice. Some specific issues, such as reproducibility, heterogeneity of imaging acquisition, and lack of external multicenter validation, need to be considered. Further research will be crucial in improving the accuracy and reproducibility of diagnostic and predictive models, enabling their application for individualized treatment in patients with HCC.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: January 28, 2021
First decision: March 29, 2021
Article in press: July 27, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang SS S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Liu JH
Contributor Information
Bing Feng, Department of Diagnostic Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Xiao-Hong Ma, Department of Diagnostic Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China. maxiaohong@cicams.ac.cn.
Shuang Wang, Department of Diagnostic Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Wei Cai, Department of Diagnostic Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
Xia-Bi Liu, Beijing Laboratory of Intelligent Information Technology, School of Computer Science and Technology, Beijing Institute of Technology, Beijing 100081, China.
Xin-Ming Zhao, Department of Diagnostic Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China.
References
- 1.Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 2.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis S, Hectors S, Taouli B. Radiomics of hepatocellular carcinoma. Abdom Radiol (NY) 2021;46:111–123. doi: 10.1007/s00261-019-02378-5. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy J, Minsky ML, Rochester N, Shannon CE. A Proposal for the Dartmouth Summer Research Project on Artificial Intelligence. AI Mag. 1955;27:12–14. [Google Scholar]
- 5.Chlebus G, Schenk A, Moltz JH, van Ginneken B, Hahn HK, Meine H. Automatic liver tumor segmentation in CT with fully convolutional neural networks and object-based postprocessing. Sci Rep. 2018;8:15497. doi: 10.1038/s41598-018-33860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousabarah K, Letzen B, Tefera J, Savic L, Schobert I, Schlachter T, Staib LH, Kocher M, Chapiro J, Lin M. Automated detection and delineation of hepatocellular carcinoma on multiphasic contrast-enhanced MRI using deep learning. Abdom Radiol (NY) 2021;46:216–225. doi: 10.1007/s00261-020-02604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie P, Wang N, Pang J, Yang G, Duan S, Chen J, Xu W. CT-Based Radiomics Nomogram: A Potential Tool for Differentiating Hepatocellular Adenoma From Hepatocellular Carcinoma in the Noncirrhotic Liver. Acad Radiol. 2021;28:799–807. doi: 10.1016/j.acra.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Nie P, Yang G, Guo J, Chen J, Li X, Ji Q, Wu J, Cui J, Xu W. A CT-based radiomics nomogram for differentiation of focal nodular hyperplasia from hepatocellular carcinoma in the non-cirrhotic liver. Cancer Imaging. 2020;20:20. doi: 10.1186/s40644-020-00297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokrane FZ, Lu L, Vavasseur A, Otal P, Peron JM, Luk L, Yang H, Ammari S, Saenger Y, Rousseau H, Zhao B, Schwartz LH, Dercle L. Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur Radiol. 2020;30:558–570. doi: 10.1007/s00330-019-06347-w. [DOI] [PubMed] [Google Scholar]
- 10.Ponnoprat D, Inkeaw P, Chaijaruwanich J, Traisathit P, Sripan P, Inmutto N, Na Chiangmai W, Pongnikorn D, Chitapanarux I. Classification of hepatocellular carcinoma and intrahepatic cholangiocarcinoma based on multi-phase CT scans. Med Biol Eng Comput. 2020;58:2497–2515. doi: 10.1007/s11517-020-02229-2. [DOI] [PubMed] [Google Scholar]
- 11.Shi W, Kuang S, Cao S, Hu B, Xie S, Chen S, Chen Y, Gao D, Zhu Y, Zhang H, Liu H, Ye M, Sirlin CB, Wang J. Deep learning assisted differentiation of hepatocellular carcinoma from focal liver lesions: choice of four-phase and three-phase CT imaging protocol. Abdom Radiol (NY) 2020;45:2688–2697. doi: 10.1007/s00261-020-02485-8. [DOI] [PubMed] [Google Scholar]
- 12.Yasaka K, Akai H, Abe O, Kiryu S. Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-enhanced CT: A Preliminary Study. Radiology. 2018;286:887–896. doi: 10.1148/radiol.2017170706. [DOI] [PubMed] [Google Scholar]
- 13.Cao SE, Zhang LQ, Kuang SC, Shi WQ, Hu B, Xie SD, Chen YN, Liu H, Chen SM, Jiang T, Ye M, Zhang HX, Wang J. Multiphase convolutional dense network for the classification of focal liver lesions on dynamic contrast-enhanced computed tomography. World J Gastroenterol. 2020;26:3660–3672. doi: 10.3748/wjg.v26.i25.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Liu X, Chen J, Wei Y, Lee JM, Cao L, Wu Y, Duan T, Li X, Ma L, Song B. Man or machine? Cancer Imaging. 2019;19:84. doi: 10.1186/s40644-019-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhen SH, Cheng M, Tao YB, Wang YF, Juengpanich S, Jiang ZY, Jiang YK, Yan YY, Lu W, Lue JM, Qian JH, Wu ZY, Sun JH, Lin H, Cai XJ. Deep Learning for Accurate Diagnosis of Liver Tumor Based on Magnetic Resonance Imaging and Clinical Data. Front Oncol. 2020;10:680. doi: 10.3389/fonc.2020.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Khalvati F, Namdar K, Fischer S, Lewis S, Taouli B, Haider MA, Jhaveri KS. Can machine learning radiomics provide pre-operative differentiation of combined hepatocellular cholangiocarcinoma from hepatocellular carcinoma and cholangiocarcinoma to inform optimal treatment planning? Eur Radiol. 2021;31:244–255. doi: 10.1007/s00330-020-07119-7. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Long L, Wei J, Li Y, Xia Y, Zuo P, Chai X. Radiomics for diagnosis of dual-phenotype hepatocellular carcinoma using Gd-EOB-DTPA-enhanced MRI and patient prognosis. J Cancer Res Clin Oncol. 2019;145:2995–3003. doi: 10.1007/s00432-019-03062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jian W, Ju H, Cen X, Cui M, Zhang H, Zhang L, Wang G, Gu L, Zhou W. Improving the malignancy characterization of hepatocellular carcinoma using deeply supervised cross modal transfer learning for non-enhanced MR. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:853–856. doi: 10.1109/EMBC.2019.8857467. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Liu A, Cui J, Chen A, Song Q, Xie L. Radiomics-based classification of hepatocellular carcinoma and hepatic haemangioma on precontrast magnetic resonance images. BMC Med Imaging. 2019;19:23. doi: 10.1186/s12880-019-0321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao B, Zhang L, Ning P, Ding F, Wu F, Lu G, Geng Y, Ma J. Preoperative prediction for pathological grade of hepatocellular carcinoma via machine learning-based radiomics. Eur Radiol. 2020;30:6924–6932. doi: 10.1007/s00330-020-07056-5. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Tan H, Gao F, Hai J, Ning P, Chen J, Zhu S, Wang M, Dou S, Shi D. Predicting the grade of hepatocellular carcinoma based on non-contrast-enhanced MRI radiomics signature. Eur Radiol. 2019;29:2802–2811. doi: 10.1007/s00330-018-5787-2. [DOI] [PubMed] [Google Scholar]
- 22.Yang DW, Jia XB, Xiao YJ, Wang XP, Wang ZC, Yang ZH. Noninvasive Evaluation of the Pathologic Grade of Hepatocellular Carcinoma Using MCF-3DCNN: A Pilot Study. Biomed Res Int. 2019;2019:9783106. doi: 10.1155/2019/9783106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Z, Chen S, Xiao H, Wang Y, Li J, Mei J, Chen Z, Zhou Q, Feng S, Chen M, Qian G, Peng S, Kuang M. Microvascular Invasion as a Predictor of Response to Treatment with Sorafenib and Transarterial Chemoembolization for Recurrent Intermediate-Stage Hepatocellular Carcinoma. Radiology. 2019;292:237–247. doi: 10.1148/radiol.2019181818. [DOI] [PubMed] [Google Scholar]
- 24.Tang A. Using MRI to Assess Microvascular Invasion in Hepatocellular Carcinoma. Radiology. 2020;297:582–583. doi: 10.1148/radiol.2020203376. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Ruan S, Xiao W, Shao J, Tian W, Liu W, Zhang Z, Wan D, Huang J, Huang Q, Yang Y, Yang H, Ding Y, Liang W, Bai X, Liang T. Contrast-enhanced CT radiomics for preoperative evaluation of microvascular invasion in hepatocellular carcinoma: A two-center study. Clin Transl Med. 2020;10:e111. doi: 10.1002/ctm2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng J, Zhang J, Zhang Q, Xu Y, Zhou J, Liu L. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:121–127. doi: 10.5152/dir.2018.17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, Wang S, Zhao X, Tian J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595–3605. doi: 10.1007/s00330-018-5985-y. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Gu D, Wei J, Yang C, Rao S, Wang W, Chen C, Ding Y, Tian J, Zeng M. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer. 2019;8:373–386. doi: 10.1159/000494099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R, Xu L, Wen X, Zhang J, Yang P, Zhang L, Xue X, Wang X, Huang Q, Guo C, Shi Y, Niu T, Chen F. A nomogram based on bi-regional radiomics features from multimodal magnetic resonance imaging for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Quant Imaging Med Surg. 2019;9:1503–1515. doi: 10.21037/qims.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang YQ, Cao SE, Cao S, Chen JN, Wang GY, Shi WQ, Deng YN, Cheng N, Ma K, Zeng KN, Yan XJ, Yang HZ, Huan WJ, Tang WM, Zheng Y, Shao CK, Wang J, Yang Y, Chen GH. Preoperative identification of microvascular invasion in hepatocellular carcinoma by XGBoost and deep learning. J Cancer Res Clin Oncol. 2021;147:821–833. doi: 10.1007/s00432-020-03366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni M, Zhou X, Lv Q, Li Z, Gao Y, Tan Y, Liu J, Liu F, Yu H, Jiao L, Wang G. Radiomics models for diagnosing microvascular invasion in hepatocellular carcinoma: which model is the best model? Cancer Imaging. 2019;19:60. doi: 10.1186/s40644-019-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nebbia G, Zhang Q, Arefan D, Zhao X, Wu S. Pre-operative Microvascular Invasion Prediction Using Multi-parametric Liver MRI Radiomics. J Digit Imaging. 2020;33:1376–1386. doi: 10.1007/s10278-020-00353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol (NY) 2019;44:539–548. doi: 10.1007/s00261-018-1768-9. [DOI] [PubMed] [Google Scholar]
- 34.Mishra L, Banker T, Murray J, Byers S, Thenappan A, He AR, Shetty K, Johnson L, Reddy EP. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–329. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SH, Lee JS, Na GH, You YK, Kim DG. Immunohistochemical markers for hepatocellular carcinoma prognosis after liver resection and liver transplantation. Clin Transplant. 2017;31 doi: 10.1111/ctr.12852. [DOI] [PubMed] [Google Scholar]
- 36.Roncalli M, Park YN, Di Tommaso L. Histopathological classification of hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S228–S234. doi: 10.1016/S1590-8658(10)60510-5. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Gu D, Wei J, Ding Y, Yang L, Zhu K, Luo R, Rao SX, Tian J, Zeng M. A radiomics-based biomarker for cytokeratin 19 status of hepatocellular carcinoma with gadoxetic acid-enhanced MRI. Eur Radiol. 2020;30:3004–3014. doi: 10.1007/s00330-019-06585-y. [DOI] [PubMed] [Google Scholar]
- 38.Ning S, Bin C, Na H, Peng S, Yi D, Xiang-hua Y, Fang-yin Z, Da-yong Z, Rong-cheng L. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep. 2012;39:351–357. doi: 10.1007/s11033-011-0745-y. [DOI] [PubMed] [Google Scholar]
- 39.Gu D, Xie Y, Wei J, Li W, Ye Z, Zhu Z, Tian J, Li X. MRI-Based Radiomics Signature: A Potential Biomarker for Identifying Glypican 3-Positive Hepatocellular Carcinoma. J Magn Reson Imaging. 2020;52:1679–1687. doi: 10.1002/jmri.27199. [DOI] [PubMed] [Google Scholar]
- 40.Ye Z, Jiang H, Chen J, Liu X, Wei Y, Xia C, Duan T, Cao L, Zhang Z, Song B. Texture analysis on gadoxetic acid enhanced-MRI for predicting Ki-67 status in hepatocellular carcinoma: A prospective study. Chin J Cancer Res. 2019;31:806–817. doi: 10.21147/j.issn.1000-9604.2019.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H, Han X, Wang Z, Mo L, Liu W, Guo Y, Wei X, Jiang X. Prediction of the Ki-67 marker index in hepatocellular carcinoma based on CT radiomics features. Phys Med Biol. 2020;65:235048. doi: 10.1088/1361-6560/abac9c. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, He L, Huang Y, Chen S, Wu P, Ye W, Liu Z, Liang C. CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol (NY) 2017;42:1695–1704. doi: 10.1007/s00261-017-1072-0. [DOI] [PubMed] [Google Scholar]
- 43.Yuan C, Wang Z, Gu D, Tian J, Zhao P, Wei J, Yang X, Hao X, Dong D, He N, Sun Y, Gao W, Feng J. Prediction early recurrence of hepatocellular carcinoma eligible for curative ablation using a Radiomics nomogram. Cancer Imaging. 2019;19:21. doi: 10.1186/s40644-019-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu HB, Zheng ZY, Zhao H, Zhang J, Zhu H, Li YH, Dong ZY, Xiao LS, Kuang JJ, Zhang XL, Liu L. Radiomics-based nomogram using CT imaging for noninvasive preoperative prediction of early recurrence in patients with hepatocellular carcinoma. Diagn Interv Radiol. 2020;26:411–419. doi: 10.5152/dir.2020.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Jiang H, Chen J, Wei Y, Cao L, Ye Z, Li X, Ma L, Song B. Hepatocellular carcinoma: radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging. 2019;19:22. doi: 10.1186/s40644-019-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Chen Q, Iwamoto Y, Han X, Zhang Q, Hu H, Lin L, Chen YW. Deep Learning-Based Radiomics Models for Early Recurrence Prediction of Hepatocellular Carcinoma with Multi-phase CT Images and Clinical Data. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:4881–4884. doi: 10.1109/EMBC.2019.8856356. [DOI] [PubMed] [Google Scholar]
- 47.Hu HT, Shan QY, Chen SL, Li B, Feng ST, Xu EJ, Li X, Long JY, Xie XY, Lu MD, Kuang M, Shen JX, Wang W. CT-based radiomics for preoperative prediction of early recurrent hepatocellular carcinoma: technical reproducibility of acquisition and scanners. Radiol Med. 2020;125:697–705. doi: 10.1007/s11547-020-01174-2. [DOI] [PubMed] [Google Scholar]
- 48.Kim J, Choi SJ, Lee SH, Lee HY, Park H. Predicting Survival Using Pretreatment CT for Patients With Hepatocellular Carcinoma Treated With Transarterial Chemoembolization: Comparison of Models Using Radiomics. AJR Am J Roentgenol. 2018;211:1026–1034. doi: 10.2214/AJR.18.19507. [DOI] [PubMed] [Google Scholar]
- 49.Liu QP, Xu X, Zhu FP, Zhang YD, Liu XS. Prediction of prognostic risk factors in hepatocellular carcinoma with transarterial chemoembolization using multi-modal multi-task deep learning. EClinicalMedicine. 2020;23:100379. doi: 10.1016/j.eclinm.2020.100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morshid A, Elsayes KM, Khalaf AM, Elmohr MM, Yu J, Kaseb AO, Hassan M, Mahvash A, Wang Z, Hazle JD, Fuentes D. A machine learning model to predict hepatocellular carcinoma response to transcatheter arterial chemoembolization. Radiol Artif Intell. 2019;1 doi: 10.1148/ryai.2019180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abajian A, Murali N, Savic LJ, Laage-Gaupp FM, Nezami N, Duncan JS, Schlachter T, Lin M, Geschwind JF, Chapiro J. Predicting Treatment Response to Intra-arterial Therapies for Hepatocellular Carcinoma with the Use of Supervised Machine Learning-An Artificial Intelligence Concept. J Vasc Interv Radiol. 2018;29:850–857.e1. doi: 10.1016/j.jvir.2018.01.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song W, Yu X, Guo D, Liu H, Tang Z, Liu X, Zhou J, Zhang H, Liu Y. MRI-Based Radiomics: Associations With the Recurrence-Free Survival of Patients With Hepatocellular Carcinoma Treated With Conventional Transcatheter Arterial Chemoembolization. J Magn Reson Imaging. 2020;52:461–473. doi: 10.1002/jmri.26977. [DOI] [PubMed] [Google Scholar]
- 53.Abajian A, Murali N, Savic LJ, Laage-Gaupp FM, Nezami N, Duncan JS, Schlachter T, Lin M, Geschwind JF, Chapiro J. Predicting Treatment Response to Image-Guided Therapies Using Machine Learning: An Example for Trans-Arterial Treatment of Hepatocellular Carcinoma. J Vis Exp. 2018 doi: 10.3791/58382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng XP, Wang YC, Ju S, Lu CQ, Zhong BY, Ni CF, Zhang Q, Yu Q, Xu J, Ji J, Zhang XM, Tang TY, Yang G, Zhao Z. Radiomics Analysis on Multiphase Contrast-Enhanced CT: A Survival Prediction Tool in Patients With Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Front Oncol. 2020;10:1196. doi: 10.3389/fonc.2020.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng J, Kang S, Ning Z, Deng H, Shen J, Xu Y, Zhang J, Zhao W, Li X, Gong W, Huang J, Liu L. Residual convolutional neural network for predicting response of transarterial chemoembolization in hepatocellular carcinoma from CT imaging. Eur Radiol. 2020;30:413–424. doi: 10.1007/s00330-019-06318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, Hack SP, Spahn J, Liu B, Abdullah H, Wang Y, He AR, Lee KH GO30140 investigators. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 58.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 59.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 60.Liao H, Zhang Z, Chen J, Liao M, Xu L, Wu Z, Yuan K, Song B, Zeng Y. Preoperative Radiomic Approach to Evaluate Tumor-Infiltrating CD8+ T Cells in Hepatocellular Carcinoma Patients Using Contrast-Enhanced Computed Tomography. Ann Surg Oncol. 2019;26:4537–4547. doi: 10.1245/s10434-019-07815-9. [DOI] [PubMed] [Google Scholar]
- 61.Hectors SJ, Lewis S, Besa C, King MJ, Said D, Putra J, Ward S, Higashi T, Thung S, Yao S, Laface I, Schwartz M, Gnjatic S, Merad M, Hoshida Y, Taouli B. MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur Radiol. 2020;30:3759–3769. doi: 10.1007/s00330-020-06675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S, Feng S, Wei J, Liu F, Li B, Li X, Hou Y, Gu D, Tang M, Xiao H, Jia Y, Peng S, Tian J, Kuang M. Pretreatment prediction of immunoscore in hepatocellular cancer: a radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur Radiol. 2019;29:4177–4187. doi: 10.1007/s00330-018-5986-x. [DOI] [PubMed] [Google Scholar]