Abstract
BACKGROUND
Gastric glomus tumor (GGT) is rare submucosal mesenchymal tumor that lacks specific clinical manifestations and is usually treated mainly by traditional surgical resection. This paper presents a case of a GGT, exhibited both intraluminally and extraluminally growth that was removed by laparoscopy-gastroscopy cooperative surgery.
CASE SUMMARY
A 52-year-old male presented with epigastric discomfort accompanied by a sense of fullness for 3 mo. Upper gastrointestinal endoscopy identified a submucosal lump located in the gastric antrum. Endoscopic ultrasonography identified a 2.4 cm × 1.8 cm lump located in the gastric antrum. It originated from the muscularis propria and exhibited both intraluminally and extraluminally growth, with hypoechoicity on the periphery, hyperechoicity in the middle, and unclear boundaries. Computed tomography showed nodular thickening of 3.0 cm × 2.2 cm in the gastric wall of the gastric antrum, and after enhancement, the lesion exhibited obvious enhancement We suspected that it was a gastrointestinal stromal tumor (glomus tumor and schwannoma were not excluded) and planned to perform laparoscopy-gastroscopy cooperative surgery. Immunohistochemical staining after the operation revealed that spinal muscular atrophy (+), h-caldesmon (+), cluster of differentiation 34 (CD34) (+), 2% Ki-67-positive rate, CD56, melanoma antigen, CD117, discovered on GIST-1, leukocyte common antigen, caudal type homeobox 2, cytokeratin, and S-100 were all negative. The tumor was finally diagnosed as a GGT.
CONCLUSION
GGTs are rare submucosal tumors of the stomach and should be considered in the differential diagnosis of gastric submucosal tumors. Laparoscopy-gastroscopy cooperative surgery is less invasive and more precise and could be an effective method for the treatment of GGTs.
Keywords: Gastric glomus tumor, Laparoscopy, Gastroscopy, Immunohistochemical staining, Operation method, Case report
Core Tip: Gastric glomus tumors (GGTs) lack specific clinical and endoscopic features. They are often mistaken as common gastrointestinal stromal tumors (GISTs), which means that they are difficult to diagnose preoperatively. Whereas laparoscopic resection can be performed in traditional surgery, it is difficult to accurately determine the location of the region growing into the cavity. Surgery is traumatic and invasive and can cause related complications. This paper presents a case of GGT that was removed by laparoscopy-gastroscopy cooperative surgery. We also reviewed the literature on computed tomography findings and traditional surgical methods of GISTs and GGTs.
INTRODUCTION
Glomus tumors are rare mesenchymal neoplasms arising from the neuromyoarterial canal or glomus body[1]. They can occur in different parts of the body, especially in the peripheral soft tissue, while other locations involve the sublingual area, nerves, nasal cavity, trachea, urogenital region, gastrointestinal (GI) tract, bile ducts and peritoneum; however, glomus tumors rarely occur in the GI tract[2]. Gastric glomus tumors (GGTs) account for approximately 1% of all GI soft tissue tumors, and the most common site of GI involvement is the stomach, especially the antrum[3,4]. GGTs are submucosal tumors that lack specific clinical and endoscopic features and are often mistaken for common GI stromal tumors (GISTs)[5].
This paper reports a case of GGTs that were resected by gastroscopy combined with laparoscopy.
CASE PRESENTATION
Chief complaints
A 52-year-old man experienced epigastric discomfort accompanied by a sense of fullness for 3 mo.
History of present illness
Three months prior, the patient developed epigastric discomfort, which was accompanied by a sense of fullness. He did not have fever, cough, chest tightness, or other discomfort.
History of past illness
He had never consumed alcohol or smoked cigarettes. He had no known food or drug allergies. He also had no history of blood transfusion or surgical trauma.
Personal and family history
The patient had no specific family history.
Physical examination
A body temperature of 36 °C, blood pressure of 120/94 mmHg, heart rate of 80 beats/min, and respiratory rate of 16 breaths/min were noted upon arrival. The abdomen was flat and soft, and the patient did not have abdominal tenderness or rebound pain.
Laboratory examinations
No obvious abnormalities were found in routine blood tests, liver and kidney function tests, electrolytes or GI tumor indices.
Imaging examinations
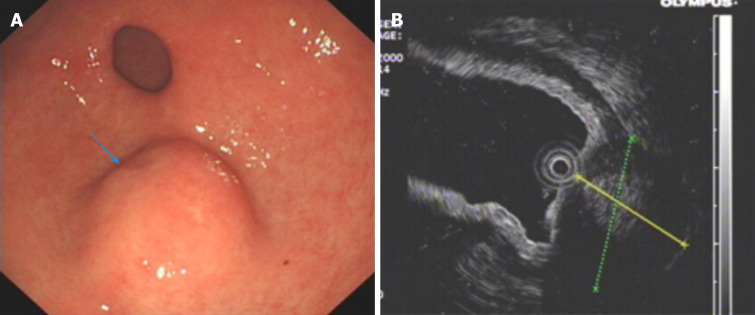
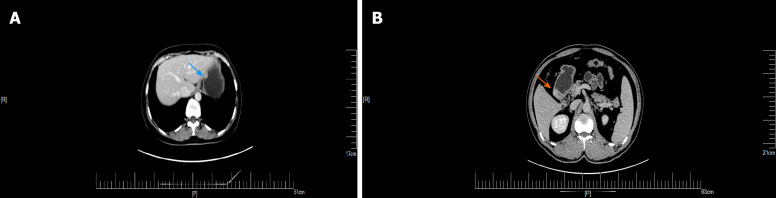
An upper GI endoscopy revealed a submucosal lump located in the gastric antrum (Figure 1A). Endoscopic ultrasonography identified a 2.4 cm × 1.8 cm lump located in the gastric antrum (Figure 1B). It originated from the muscularis propria and exhibited both intraluminally and extraluminally growth, with hypoechoicity on the periphery, hyperechoicity in the middle, and unclear boundaries. Computed tomography (CT) showed nodular thickening of 3.0 cm × 2.2 cm in the gastric wall of the gastric antrum, and after enhancement (Figure 2A), the lesion exhibited obvious enhancement (Figure 3A).
Figure 1.
Preoperative endoscopy and endoscopic ultrasonography. A: Endoscopy of the upper digestive tract revealed a submucosal tumor of great curvature in the gastric antrum; B: Endoscopic ultrasonography identified a 2.4 cm × 1.8 cm lump located in the gastric antrum originating from the muscularis propria with both intraluminal and extracavity growth.
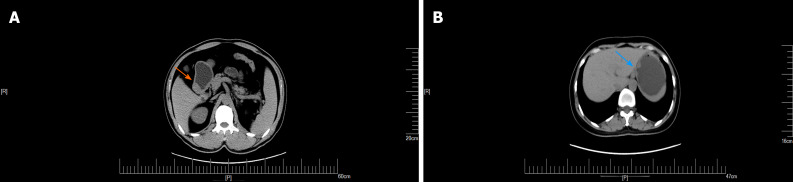
Figure 2.
Computed tomography plain scan of gastrointestinal stromal tumors and gastric glomus tumor. A: Gastric glomus tumor presented as submucosal masses with clear boundaries and uniform density (orange arrow); B: Plain computed tomography scan showed that the gastrointestinal stromal tumors were nodular (blue arrow).
Figure 3.
The arterial period of gastrointestinal stromal tumors and gastric glomus tumor. A: Enhanced computed tomography showed that the arterial phase of gastrointestinal stromal tumors presented mild heterogeneous enhancement (blue arrow); B: The arterial phase of gastric glomus tumor presented significant enhancement (orange arrow).
Preliminary diagnosis
We suspected that it was a GIST (neither glomus tumor nor schwannoma were excluded).
FINAL DIAGNOSIS
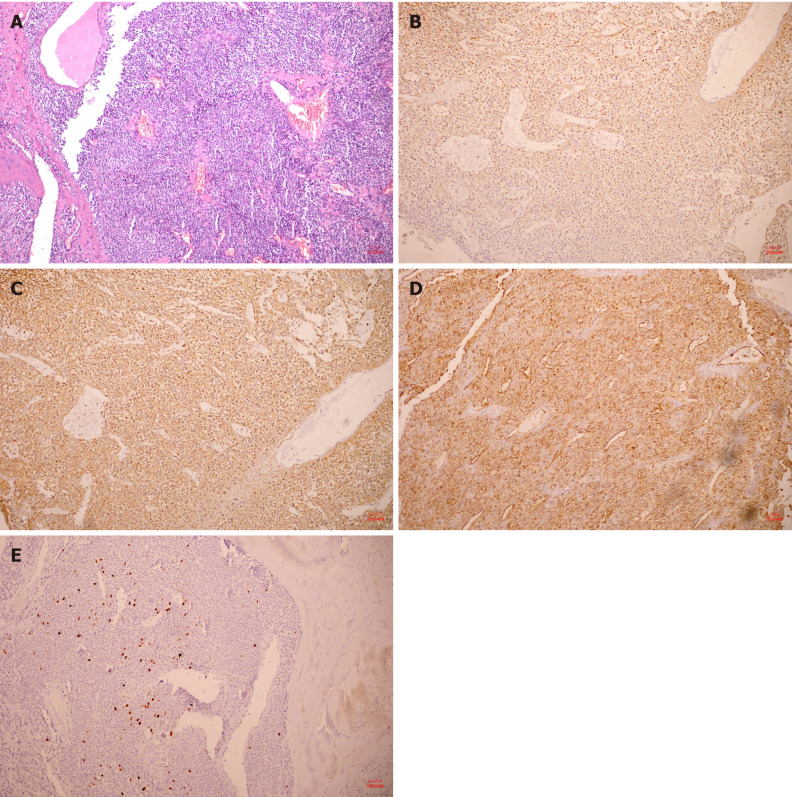
Histopathology confirmed that the tumor located in the submucosa had a clear boundary and was composed of aggregated round and fusiform cells surrounded by capillaries (Figure 4A). Immunohistochemistry showed that spinal muscular atrophy (SMA) (+; Figure 4B), h-caldesmon (+, Figure 4C), cluster of differentiation 34 (CD34) (+, Figure 4D), Ki-67-positive rate of 2% (Figure 4E), CD56, melanoma antigen, CD117, discovered on GIST-1, leukocyte common antigen, caudal type homeobox 2, cytokeratin, cytokeratin, and S-100 were all (-). The tumor was finally diagnosed as a GGT.
Figure 4.
Hematoxylin and eosin pathological sections and immunohistochemistry after operation. A: The tumor was composed of aggregated round and fusiform cells surrounded by capillaries (10 ×); B: Immunohistochemistry showed spinal muscular atrophy (+, 10 ×); C: H-caldesmon (+, 10 ×); D: cluster of differentiation 34 (+, 10 ×); E: A Ki-67 positive rate of 2% (10 ×).
TREATMENT
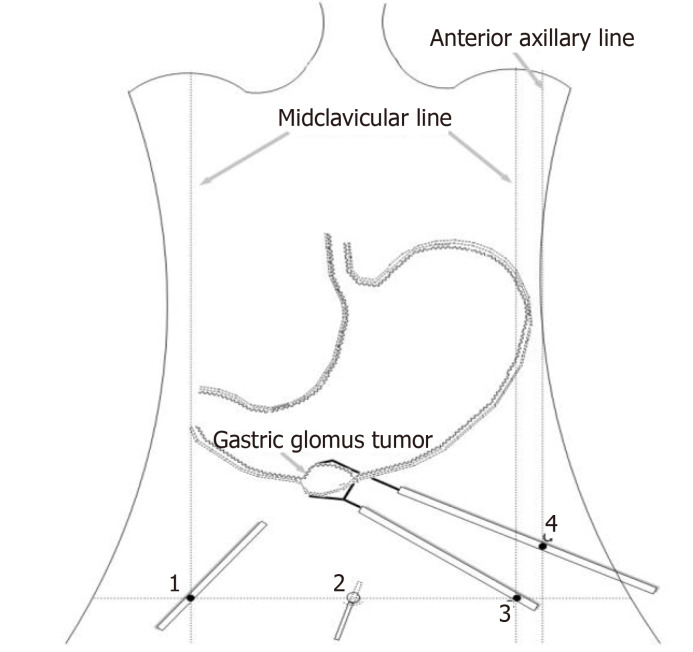
We planned to perform laparoscopy-gastroscopy cooperative surgery. First, endoscopic gastric mass extraction was performed under general anesthesia by endotracheal intubation; the great curvature of the gastric antrum exhibited a submucosal eminence approximately 3 cm in size, and the surface was smooth. We marked the edge of the lesion and injected methylene blue adrenal saline under the mucosa and then cut the mucosa to the lamina propria using a dual knife (Figure 5). The tumor was exposed, and then gradually peeled off to show most of it. A small amount of bleeding occurred during the operation, and electric hemostatic forceps were used to stop the bleeding. Since it is difficult to peel off the extraluminal part of the tumor using a dual knife, laparoscopic-assisted resection was performed to support the cut (Figure 6). The patient was in the supine position, and an arc-shaped small incision was made under his belly button (Figure 6B). We punctured the abdominal cavity using a pneumoperitoneum needle, and filled it with carbon dioxide gas to form a 12 mmHg pneumoperitoneum. Then the pneumoperitoneum needle was pulled out, the abdominal cavity was punctured using a 10 mm cannula needle, the inner core was pulled out, and the needle was inserted into a laparoscope. In the visible range, a 12 mm cannula needle puncture was made on the left anterior axillary line belly button at 10 cm (Figure 6D). Then 5 mm and 5 mm cannula needle punctures were made at the left (Figure 6C) and right (Figure 6A) umbilical clavicle midlines. A 0.5 cm rupture was found in the anterior wall of the gastric antrum approximately 3 cm from the pylorus, with a small amount of bloody fluid around it. The gastric tumor was located on the side of the anterior wall of the gastric antrum and was approximately 3 cm × 3 cm in size. It was tough and exhibited intraluminal and extracavity growth the gastric cavity. The ultrasonic knife was used to carefully dissociate along the edge of the tumor and the broken mouth, and more attention was paid to protecting the gastric wall. After dissociation, the surgeon lifted the tumor with dissecting forceps in his left hand and removed the tumor along its periphery using a general endoscopic linear cutting stapler with his right hand. The gastric tumor was placed into the extraction bag and removed through the main surgical hole. There was a small amount of blood oozing from the anastomosis, and the wound was sutured continuously with 3-0 slippery thread to stop bleeding. Finally, the surgeons ensured that there was no bleeding in the abdominal cavity, removed the laparoscope, counted the instruments and gauze correctly, and sutured the skin and each puncture hole with silk thread. At the end of the operation, the size of the tumor was approximately 3 cm × 3 cm, and the capsule was intact.
Figure 5.
Cutting the mucosa to the lamina propria using a dual knife.
Figure 6.
Laparoscopic-assisted resection of the tumor.
OUTCOME AND FOLLOW-UP
The patient was able to get out of bed at 1 d after surgery, and he recovered and was discharged from the hospital at 1 wk after surgery.
DISCUSSION
Glomus tumors of the GI tract are much rarer, and the most common site of GI involvement is the stomach, especially the antrum[5,6]. GGT is usually a benign tumor and rarely undergoes malignant transformation. Epigastric discomfort is the most common symptom. However, it can also lead to bleeding that can be acute, even life-threatening, or anemia caused by chronic bleeding. It is estimated that the incidence of tumor bleeding is approximately 3%[7,8].
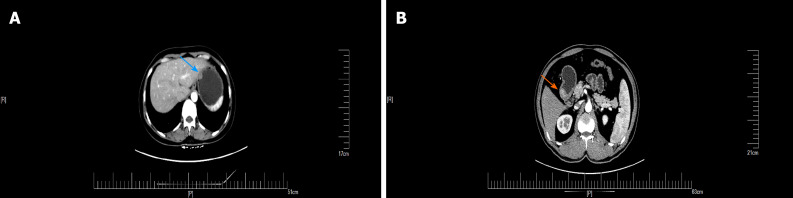
GGTs are rare submucosal mesenchymal tumors that lack specific clinical and endoscopic features and are often mistaken for common GISTs[9]. It is often difficult to diagnose GGT by routine imaging examinations such as CT, ultrasound, and magnetic resonance imaging before surgery. Endoscopic ultrasonography revealed a hypoechoic mass in the third or fourth layer of the submucosa. Its boundary was clear, the internal echo was not uniform, and a dot-like strong echo was found[10,11]. Electron microscopy and immunohistochemical observation showed characteristics of smooth muscle differentiation. Under an electron microscope, dense sarcolemmal bodies were seen in the cytoplasm, and there was a connecting structure between adjacent cells and the basement membrane around the cells. Immunohistochemical staining showed that SMA, vimentin, H-caldesmon, and calponin were positive in tumor cells, while synuclein and CD34 might be positive[12]. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is an effective method to obtain pathological specimens of gastric submucosal tumors. Miettinen et al[7] and Aoba et al[13] reported that the correct rate diagnosis was 95.6%[7,13]. Considering the possibility of benign tumors, it was not necessary to arrange EUS-FNA for this patient. He chose surgical resection directly. Finally, after the operation, pathology confirmed a diagnosis of GGT. Through analysis of the CT images of GGT and gastric stromal tumor, CT plain scan of GGT showed a well-defined and homogeneous submucosal tumor (Figure 2A). Enhanced CT revealed enhancement in the arterial phase (Figure 3B) and continuous enhancement in the delayed phase (Figure 4B). Gastric stromal tumors were nodular or lobulated (Figure 2B). Blood flow in the tumor was not abundant. The arterial phase exhibited heterogeneous mild enhancement, and the degree of enhancement was significantly lower than the GGT (Figures 3 and 7). These findings provide some guidance for the imaging diagnosis of GGT.
Figure 7.
The delay period of gastrointestinal stromal tumors and gastric glomus tumor. Gastric glomus tumor (orange arrow) was more obvious than gastrointestinal stromal tumors (blue arrow) during the delay period. A: Gastrointestinal stromal tumors; B: Gastric glomus tumor.
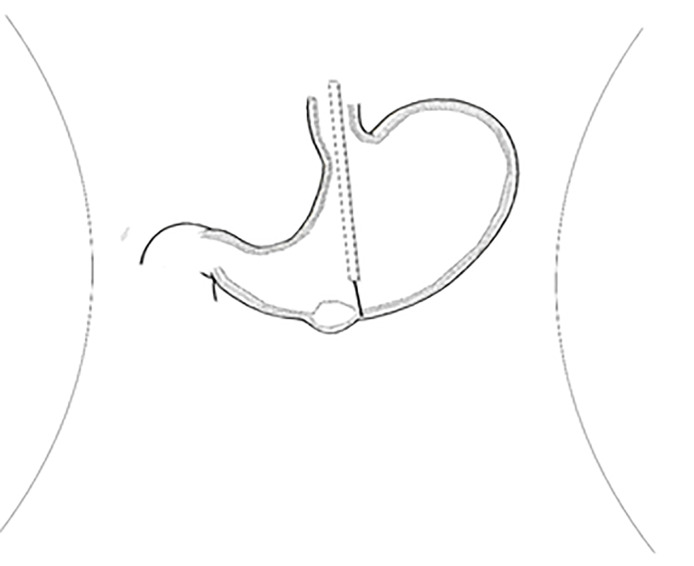
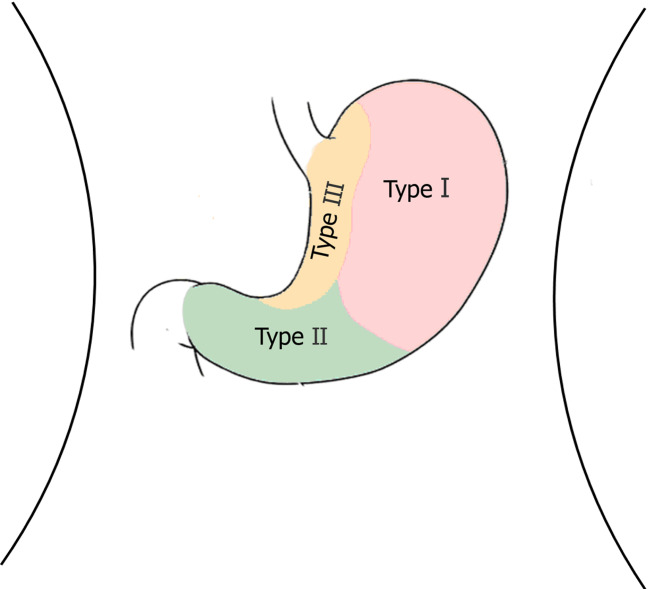
Laparoscopic resection can be used in the treatment of gastric hemangioma (Figure 8), but it is difficult to accurately determine the location of the region growing into the cavity. Since surgery is traumatic and invasive for treatment and may cause some corresponding complications and is usually used for the treatment of malignant GGT[13,14], endoscopic tumor exhumation can be used in some patients with small tumors. However, bleeding and perforation are the most common complications[15,16]. The nature of the antral tumor in this patient was not clear before the operation. Considering the possibility of stromal tumors, the volume of the tumor was relatively large, with intraluminal and extracavity growth, combined with enhanced CT. It can be found that the blood supply of the tumor is abundant; thus, simple endoscopic treatment has three difficult problems. First, there would be more bleeding during the operation. Second, if part of it grows in the extracavity, it will inevitably perforate and will be difficult to close under endoscopy due to the large perforation area. Third, if the cutting edge of the tumor is positive, additional surgery will be required. Traditional surgery is relatively risky and traumatic. After consultation with surgery and pathology departments, gastroscopy combined with laparoscopy was used to remove the tumors in this case. The intraluminal tumor was removed under gastroscopy, and then the extracavity growth was resected and sutured with the assistance of laparoscopy. This combination achieved a seamless connection, which fully exposed the intraluminal and extracavity tumor, reducing accidental injury and redundant resection, and fully increasing the resection rate. The final pathology was as follows: (gastric) glomus tumor is a benign tumor with a negative incisal margin. After the surgery, the patient was treated with acid inhibition, fluid replacement, diet restriction and nutritional support. The patient was able to get out of bed 1 d after the surgery, and he recovered and was discharged from the hospital 1 wk after the surgery. One month after the operation, the patient did not feel any uncomfortable, and after 2 years of follow-up, the patient was no any recurrence or discomfort.
Figure 8.
Tumor location and corresponding traditional surgical technique. Type I: Laparoscopic partial gastrectomy; Type II: Laparoscopic distal gastrectomy; Type III: Laparoscopic transgastric resection.
CONCLUSION
This case describes the detailed process of using gastroscopy combined with laparoscopy to remove GGTs. GGTs are a rare type of gastric submucosal tumor that should be considered in the differential diagnosis of gastric submucosal tumors. There is a slight difference in the imaging diagnosis of this kind of tumor; therefore, immunohistochemical analysis is necessary for the diagnosis. Therefore, gastroscopy combined with laparoscopy is an effective choice for tumor resection.
Footnotes
Informed consent statement: The patient's informed consent has been obtained in this case.
Conflict-of-interest statement: The authors have no financial disclosures or conflicts of interest to declare.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: January 20, 2021
First decision: May 13, 2021
Article in press: July 6, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chetty R, Gallo G, Pinheiro RN S-Editor: Fan JR L-Editor: Filipodia P-Editor: Xing YX
Contributor Information
Wen-Hao Wang, Clinical Medical College, Weifang Medical University, Weifang 261042, Shandong Province, China.
Ting-Ting Shen, Clinical Medical College, Weifang Medical University, Weifang 261042, Shandong Province, China.
Zhi-Xing Gao, Department of Gastroenterology, Affiliated Hospital of Weifang Medical University, Weifang 261042, Shandong Province, China.
Xin Zhang, The Plastic Surgery Hospital of Weifang University, Weifang Medical University, Weifang 261042, Shandong Province, China.
Zhao-Hui Zhai, Plastic Surgery Institute of Weifang Medical University, Yuhe Campus of Weifang Medical University, Weifang 261042, Shandong Province, China.
Yu-Li Li, Plastic Surgery Institute of Weifang Medical University, Yuhe Campus of Weifang Medical University, Weifang 261042, Shandong Province, China. lily19791002@126.com.
References
- 1.McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397–1400. doi: 10.1016/j.jhsa.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Liu C, Ao W, An Y, Zhang W, Niu Z, Jia Y. Differentiation of gastric glomus tumor from small gastric stromal tumor by computed tomography. J Int Med Res. 2020;48:300060520936194. doi: 10.1177/0300060520936194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma YH, Li P, Jiang GZ, Jin RJ, Li WC. [Gastrointestinal glomus tumors: a clinicopathological analysis of fifteen cases] Zhonghua Bing Li Xue Za Zhi. 2020;49:22–27. doi: 10.3760/cma.j.issn.0529-5807.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Vassiliou I, Tympa A, Theodosopoulos T, Dafnios N, Fragulidis G, Koureas A, Kairi E. Gastric glomus tumor: a case report. World J Surg Oncol. 2010;8:19. doi: 10.1186/1477-7819-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabowski M, Paszkowski A, Skotarczak J, Dorobisz T, Leśniak M, Janczak D. Glomus Tumor of the Stomach - A Case Report and A Literature Review. Pol Przegl Chir. 2016;88:356–358. doi: 10.1515/pjs-2016-0076. [DOI] [PubMed] [Google Scholar]
- 6.Fang HQ, Yang J, Zhang FF, Cui Y, Han AJ. Clinicopathological features of gastric glomus tumor. World J Gastroenterol. 2010;16:4616–4620. doi: 10.3748/wjg.v16.i36.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301–311. doi: 10.1097/00000478-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Sethu C, Sethu AU. Glomus tumour. Ann R Coll Surg Engl. 2016;98:e1–e2. doi: 10.1308/rcsann.2016.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Kumar A, Singh V. Gastric Glomus Tumor. Niger J Surg. 2020;26:162–165. doi: 10.4103/njs.NJS_8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu XD, Lu XH, Ye GX, Hu XR. Immunohistochemical analysis and biological behaviour of gastric glomus tumours: a case report and review of the literature. J Int Med Res. 2010;38:1539–1546. doi: 10.1177/147323001003800438. [DOI] [PubMed] [Google Scholar]
- 11.Mago S, Pasumarthi A, Miller DR, Saade R, Tadros M. The Two Challenges in Management of Gastric Glomus Tumors. Cureus. 2020;12:e9251. doi: 10.7759/cureus.9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Shen J, Yue H, Li Q, Cheng Y, Zhou M. Gastric Glomus Tumor: A Clinicopathologic and Immunohistochemical Study of 21 Cases. Biomed Res Int. 2020;2020:5637893. doi: 10.1155/2020/5637893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoba T, Kato T, Hiramatsu K, Shibata Y, Yoshihara M, Yamaguchi N, Kamiya T. A case of gastric glomus tumor resection using laparoscopy endoscopy cooperative surgery (LECS) Int J Surg Case Rep. 2018;42:204–207. doi: 10.1016/j.ijscr.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsahwan AG, Alfaraj ZM, AlSafwani J, Bunaiyan AH, AlKhalifah RH, Al-Saba'a SA, Al-Momen SA, Aldolah Q. Rare gastric neoplasm: Malignant glomus tumor of the stomach. A case report. Int J Surg Case Rep. 2021;81:105802. doi: 10.1016/j.ijscr.2021.105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato S, Kikuchi K, Chinen K, Murakami T, Kunishima F. Diagnostic utility of endoscopic ultrasound-guided fine-needle aspiration biopsy for glomus tumor of the stomach. World J Gastroenterol. 2015;21:7052–7058. doi: 10.3748/wjg.v21.i22.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Privette A, McCahill L, Borrazzo E, Single RM, Zubarik R. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc. 2008;22:487–494. doi: 10.1007/s00464-007-9493-4. [DOI] [PubMed] [Google Scholar]