Abstract
Increased expression of developmentally silenced fetal globin (HBG) reduces the clinical severity of β-hemoglobinopathies. Benserazide has a relatively benign safety profile having been approved for 50 years in Europe and Canada for Parkinson’s disease treatment. Benserazide was shown to activate HBG gene transcription, in a high throughput screen and subsequent studies confirmed fetal hemoglobin (HbF) induction in erythroid progenitors from hemoglobinopathy patients, transgenic mice containing the entire human β-globin gene (β-YAC) and anemic baboons. The goal of this study is to evaluate efficacies and plasma exposure profiles of benserazide racemate and its enantiomers to select the chemical form for clinical development. Intermittent treatment with all forms of benserazide in β-YAC mice significantly increased proportions of red blood cells expressing HbF and HbF protein per cell with similar pharmacokinetic profiles and with no cytopenia. These data contribute to the regulatory justification for development of the benserazide racemate. Additionally, dose ranges and frequencies required for HbF induction using racemic benserazide were explored. Orally administered escalating doses of benserazide in an anemic baboon induced γ-globin mRNA up to 13-fold and establish an intermittent dose regimen for clinical studies as a therapeutic candidate for potential treatment of β-hemoglobinopathies.
Keywords: Beta hemoglobinopathies, benserazide, beta YAC transgenic mouse, fetal hemoglobin, anemic baboon
INTRODUCTION
Increased expression of endogenous silenced fetal globin genes (γ-globin, HBG) has been recognized to reduce anemia and clinical severity of the β-hemoglobinopathies, sickle cell disease (SCD) and β-thalassemia in a large body of clinical, biochemical, epidemiologic, genetic, and molecular studies for over > 6 decades, and therapies to increase HbF remain of interest [1–6]. Benserazide in combination with Levodopa (L-DOPA) has been used for treatment of Parkinson’s disease for over 50 years. For this indication, the drug inhibits DOPA decarboxylase and increases plasma levels of L-DOPA, which promotes its transport into the central nervous system. Benserazide is used in combination formulations to decrease adverse events associated with L-DOPA administration [7]. Recently, benserazide emerged as a lead compound in a high throughput screening campaign of approved drugs to identify HbF inducers to repurpose for the treatment of β-hemoglobinopathies. Screening was conducted in a cell line stably expressing a γ-globin promoter-driven enhanced green fluorescent protein reporter [8]. Benserazide produced 4-fold induction without cellular toxicity. Due to its benign safety profile, including lack of cytopenias with long-term administration in humans, it was considered for further preclinical development as a potential treatment candidate in the β-hemoglobinopathies [7]. Further studies demonstrated the ability of benserazide to increase fetal γ-globin gene transcription, assessed as 3.5-fold increase in HbF protein and proportions of cells expressing HbF (F-reticulocytes and F-cells) in erythroid progenitors from hemoglobinopathy patients [9, 10]. Benserazide was also evaluated for activity in the classic anemic baboon model (Papio anubis) and demonstrated increases from baseline in F-reticulocytes and in fetal globin mRNA with intermittent 2-week treatment courses. Similar effects were also observed in β-YAC transgenic mice (with increased F-cells and HbF protein levels of approximately twice that of hydroxyurea (HU) [8]. Molecular mechanisms of benserazide-mediated γ-globin activation in erythroid progenitors was shown to involve displacement and/or suppression of several known repressors from the γ globin gene promoter including BCL11A, LSD-1, KLF-1, and HDAC3 [3, 5, 9–24].
Benserazide in its racemic form was originally approved in combination with L-DOPA for use in Europe and Canada in the 1970s. Since then, a new guidance, Development of New Stereoisomeric Drugs, was issued in 1992 by the United States Food and Drug Administration (US FDA) [25]. The guidance outlines criteria including having different pharmacologic effects that could result in characterizing either isomer an active or inactive impurity. In any such event, the recommendation would be to develop the single, active on target isomer for human clinical trials. The existing commercial availability of clinical grade, Good Manufacturing Practice (GMP) active pharmaceutical ingredient (API) of racemic benserazide presented the opportunity to accelerate development of the single formulated drug, meeting the recommended criteria outlined by the US FDA guidance on the development of stereoisomeric drugs.
The purpose of this study was to provide a regulatory justification for the proposed clinical development of the racemate, by evaluating and comparing the efficacies of (R,S)-benserazide and its individual enantiomers, (R)- and (S)-benserazide as HbF inducers and to estimate doses suitable for initial clinical trials for HbF induction in β-hemoglobinopathy patients. Evaluation of the racemate (R,S)-benserazide, compared to its individual enantiomers was undertaken in a detailed efficacy study utilizing a single dose of each form of benserazide in the β-YAC mouse model. In vivo drug exposures in β-YAC mice for each form of the drug were also determined in plasma samples for drug concentration measurements at the end of efficacy studies. Evaluations of (R,S)-benserazide as an HbF inducer initially in anemic baboon and subsequently followed by a comparative analysis in large numbers of β-YAC mice was performed to identify an active dose and frequency of administration for this indication, distinct from the four daily doses used in Parkinson’s disease.
MATERIALS AND METHODS
Anemic Baboon Studies
Under a University of Oklahoma Institutional Animal Care and Use Committee (IACUC)-approved protocol, in the classic model for globin expression studies, baboons are phlebotomized daily (~3.7 to 5 mL/kg/day), to achieve and maintain stable anemia with a total hemoglobin of 7.0 to 7.5 g/dL to simulate the accelerated erythropoiesis that occurs in SCD and β-thalassemia, as previously described [8, 26]. After stabilization of hemoglobin, benserazide was administered intravenously (IV) through implanted indwelling vascular catheters or orally by orogastric administration under light sedation (~ 5mg/kg IV ketamine) with a wash-out period of 2–3 weeks between doses and treatments to allow γ globin mRNA to return to baseline. Daily oral doses (2 or 3 mg/kg/dose) of the racemic drug ((R,S)-benserazide) were administered by orogastric tube 2–3 times per week to phlebotomized anemic baboons. Blood samples were analyzed for γ-globin mRNA by quantitative RT-PCR (RT-qPCR). F-reticulocytes were also analyzed by flow cytometry as previously described [8].
Drug Treatment in β-YAC transgenic mice
The β-YAC transgenic mouse model was established with a 248 kb yeast artificial chromosome carrying a single copy of the entire human 81 kb β-globin locus bred on a C57BL genetic background from a single founder [27]. Under an Augusta University IACUC-approved protocol, 13–18 months old mice (n=4) in an initial study to confirm earlier findings [8] with the racemate and explore treatment with the enantiomers were treated with the racemic form, (R,S)-benserazide (EEY) or enantiomers EF2 (R)-benserazide), or EF3 (S)-benserazide) at a dose of 20 mg/kg by intraperitoneal (IP) injections, 3 times per week (MWF) for 4 weeks. Two control groups included water (VEH, a negative control) and hydroxyurea (HU; 100 mg/kg/dose, an established positive control), 5 days a week (M-F) for 4 weeks. A second powered study involving of each of the four treatment groups consisted of four β-YAC mice (6–8 weeks old) for three independent experimental replicates (n=12). Mice were weighed weekly and blood collected by tail vein into EDTA tubes at week 0, 2 and 4 for complete blood count (CBC) with differential, and flow cytometry for the proportions of red blood cells expressing HbF protein (F-cells) and mean fluorescent intensity (MFI) to quantify HbF protein per cell as previously described [8]; F-reticulocytes were assayed by flow cytometry using thiazole orange staining by published methods [28]. CBC were performed on the ABX Micro S60 machine (HORIBA Medical, Irvine, CA) as previously published [29]. In a second set of treatments (n=12), the dose response of (R,S)-benserazide (EEY) was also evaluated in young β-YAC mice (6–10 weeks old) treated with escalating doses of EEY of 5, 20, or 30 mg/kg/dose (EEY5, EEY20 and EEY30 respectively), 3 days per week (MWF) for 4 weeks. Studies involving benserazide and its enantiomers were conducted in a blinded fashion.
Drugs Administered
Syntheses of (R)- and (S)-benserazide HCl salts were performed at the National Center for Advancing Translational Sciences (NCATS) by published procedures with minor modifications (see Supplemental Materials and Methods for details of synthesis). For studies comparing (R)-, (S)- and (R,S)-benserazide HCl, the racemate was prepared by mixing equal amounts of (R)- and (S)-benserazide HCl salts in methanol and then recrystallizing from ethanol. R,S-benserazide HCl for other studies was procured from Sigma Aldrich Cat# B7183–5G, Lot# BCBL4153V). Hydroxyurea was purchased from Sigma (Cat#H8627–1G).
Reverse transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from blood using Trizol (Ambion, Carlsbad CA) and analyzed by RT-qPCR as previously published by our group [28]. Gene-specific primers were used to quantify mRNA levels for γ-globin, β-globin, and internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH); all mRNA levels were normalized to GAPDH before statistical analysis.
Flow Cytometry
Peripheral blood samples were washed in phosphate buffered saline and fixed in 4% paraformaldehyde for 40 min at 37°C. The cells were incubated for 1 min with ice-cold acetone/methanol and then incubated with FITC-conjugated anti-γ-globin antibody (Abcam, CAT#ab19365); isotype IgG was used as control. Stained and unstained cells were analyzed using a Becton Dickerson (BD) LSR-II flow cytometer (BD Bioscience) and the F-cell and MFI data analyzed with FACS Diva V6.1.3 and FlowJo v.X software as previously published [8].
Power and Statistical Analyses
Previously published data was used to determine the number of β-YAC mice necessary to appropriately power the experiments reported in the current work. See Supplemental Materials and Methods for analysis details which determined that ~5 animals per experiment was necessary to resolve HbF differences between HU or (R,S)-benserazide and VEH, and that n=10 animals per experiment was necessary to resolve HbF differences between (R,S)-benserazide and HU. These predicted animal numbers were used to inform the current experimental design of n=12 β-YAC mice per group.
For the statistical analyses, one-sample paired student’s t-tests were performed to determine differences in blood counts, reticulocytes, F-cells, and MFI at week 0 compared to week 2 and 4 within treatment groups (n=12). Baseline normalization was based on 100* [Activity (therapeutic) – mean activity (neg control)] / [Activity (pos control) – mean activity (neg control)]. To compare the effect across treatment groups, pairwise comparisons analysis of variance (ANOVA) was performed to identify the specific pair(s) with significant differences. The Tukey’s HSD (honest significant difference) test was performed, to adjust for the multiple pairwise comparisons. A level of p≤0.05 was utilized for statistical significance.
In Vivo Drug Exposure Measurements in β-YAC Transgenic Mice
To evaluate in vivo exposures in the β-YAC transgenic mice, drug concentrations were determined after the IP injection of EF3, EF2 and EEY at 20 mg/kg, at what would be the next scheduled dose administration, but following completion of the efficacy study in now 10–12 weeks old β-YAC mice. Following IP injections, blood samples were collected at 15, 30, 60, and 120 minutes after the last dose. Volumes of 0.5 – 0.6 mL blood was collected by cardiac puncture from three mice at each time point. Mice are euthanized before cardiac puncture by inhalation of CO2, followed by a secondary method (e.g., cervical dislocation) if required. Blood was collected in an EDTA tube containing 10–12 μL of 1.0 M ascorbic acid + 0.1% formic acid solution and immediately placed on ice. Blood samples were mixed well by inverting the tubes gently, placed on ice, until centrifugation. Samples were centrifuged at 4° C within 30 minutes of blood collection to harvest the plasma samples which were then stored at −80 °C until bioanalysis. Sample analysis was performed by a novel UPLC-MS/MS method.
Briefly, benserazide was extracted from plasma by protein precipitation using methanol/acetonitrile, with D3-Benserazide as the internal standard (IS). Analyses were carried out using a Waters Acquity I-Class UPLC interfaced with a Waters TQ-S mass spectrometer. The assay LLOQ was 2.50 ng/mL using a 30.0 μL aliquot of mouse plasma. Pharmacokinetic (PK) parameters for benserazide were calculated using the non-compartmental method (NCA; Model 200) of the pharmacokinetic software package Phoenix WinNonlin, version 7.1 (Certara, St. Louis, MO). PK parameters were calculated with the mean concentrations in three mice at each time point. The area under the plasma concentration versus time curve (AUC) was calculated using the linear trapezoidal method. Where warranted, the slope of the apparent terminal phase was estimated by log-linear regression using at least three data points and the terminal rate constant (λ) was derived from the slope. AUC0−∞ was estimated as the sum of the AUC0-t (where t is the time of the last measurable concentration) and Ct/λ. The apparent terminal half-life (t½) was calculated as 0.693/λ.
RESULTS
Benserazide induces γ-globin mRNA in a dose-dependent manner in an anemic baboon.
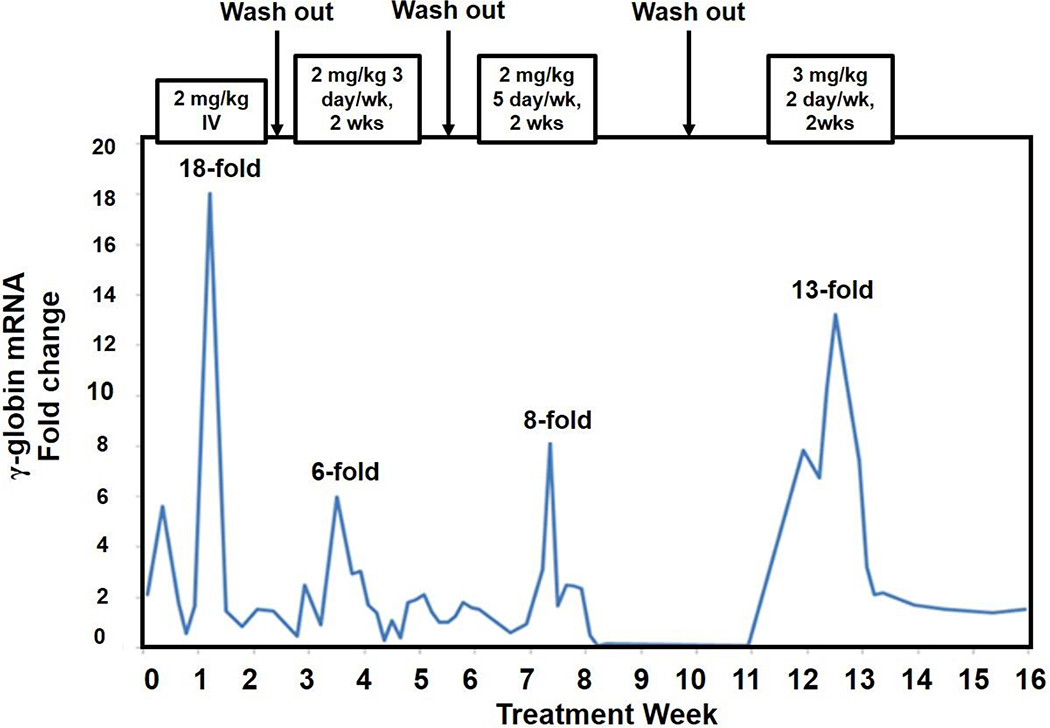
Escalating doses were evaluated to establish a basis for initial human equivalent doses for the new activity of inducing γ-globin expression. Shown in Figure 1 are representative results for RT-qPCR analysis of γ-globin mRNA with increasing doses of benserazide in an anemic baboon following intervals without drug to allow wash-out and a return to baseline. A single intravenous (IV) dose of the drug given at 2 mg/kg produced a peak 18-fold increase in γ-globin mRNA at 4–5 days after dosing, consistent with the time course of erythroid maturation to reticulocytes in the baboon. After wash-out periods between doses, following benserazide given orally at 2 mg/kg/dose twice per week, γ-globin mRNA increased by a peak of 6-fold over pre-treatment levels, and to 8-fold when administered 5 days/week, which lasted for approximately one week. Treatment with benserazide at 3 mg/kg/dose twice/week for 2 weeks induced γ-globin mRNA by a peak of 13-fold, with elevated levels observed for 2 weeks’ duration. These results demonstrated a dose-dependent increase in γ-globin mRNA levels with racemic benserazide administered intermittently over 14 weeks. F-reticulocytes also increased 2 to 3-fold with 2 mg/kg doses given 2 days/week from 15% to 30%, increasing further to 45% with treatment given 5 days/week, and to 55% following 3 mg/kg doses, 2 days/week, without returning to baseline levels following wash-out periods (data not shown). No adverse safety effects were observed on behavior, appetite, activity, chemistry panels, or hematologic counts; the only change was a rise in total hemoglobin of 1.2 grams/dl despite ongoing phlebotomy (data not shown). Based on metabolic differences between these juvenile non-human primates and humans, projections based on these brief courses for standard human dose equivalence are 1–1.5 mg/kg as starting doses for a dose-escalating clinical trial.
Fig. 1. Increasing doses of racemic benserazide administered to an anemic baboon.
Fetal (γ)-globin mRNA levels relative to baseline in a baboon treated sequentially with racemic (R,S)-benserazide administered at an IV dose and at oral doses and regimens as shown above each peak. Baseline was assessed immediately before each dose regimen, following wash-out periods from the prior regimen. The increases in γ-globin mRNA compared to baseline were observed beginning at 3–4 days after oral dosing began; the maximal increase is noted above each peak. The duration of response is shown in width of each response. The highest peak followed the regimen of 3 mg/kg/dose orally and persisted for 2 weeks.
Benserazide and enantiomers activate HbF in β-YAC transgenic mice.
To achieve our study goal, age and sex matched controls were used for each treatment group to address the question about efficacy of benserazide enantiomers and to conduct pharmacokinetic studies, which required large numbers of transgenic mice. Therefore, we chose β-YAC mice as a preclinical model established by our group where we observed HbF induction by 5-azacytidine and a butyrate analog, which correlated with subsequent responses in sickle cell patients. Other single agents, including scriptaid [30] and tranylcypromine, [20] were reported to induce HbF in β-YAC. More recently, we reported HbF induction by a conjugate molecule AN-233 comprised of 5-aminolevulinic acid and butyrate, using this model [28] Collectively, these data demonstrate the β-YAC transgenic mouse containing the entire human HBB locus are a proven preclinical model to identify potential therapeutic agents for clinical trials.
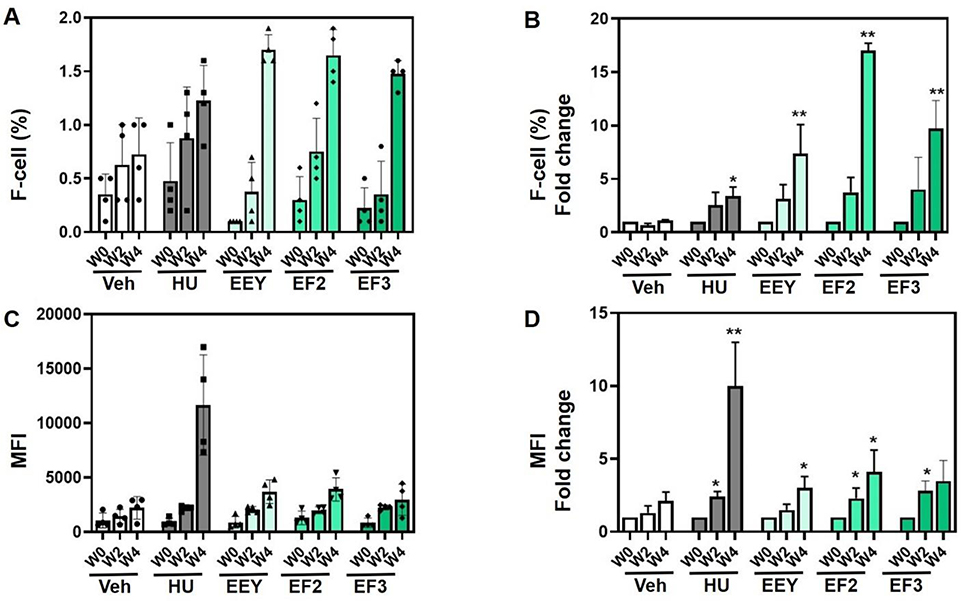
To compare the efficacy of (R,S)-benserazide (EEY) and its enantiomers, (R)-benserazide (EF3) and (S)-benserazide (EF2) in vivo, β-YAC transgenic mice ranging from 13–18 months old were initially treated at a dose of 20 mg/kg, 3 days per week (MWF), using 4 mice per treatment group. The positive and negative controls used in the study were HU (100 mg/kg) and the corresponding volume of water vehicle (VEH) respectively, administered daily, 5 days per week (M-F). Shown in Fig. 2A and 2B is the flow cytometry data generated for percent of F-cells with fold change after 2 and 4 weeks of treatment compared to baseline (week 0). In these mice, we observed a time-dependent increase in F-cells with a maximal 17-fold (p<0.001) increase by EEY (R,S)-benserazide) and 4-fold (p<0.01) and 7-fold (p<0.001) increase respectively for EF2 (R)-benserazide) and EF3 (S)-benserazide), after 4 weeks of dosing. At the 2-week time point, HbF levels were not significantly increased for any of the drugs tested. Of note, the positive control HU treatment at the dose and frequency administered increased F-cells 4-fold (p<0.05) at week 4. HbF protein level per cell displayed as mean fluorescence intensity (MFI) (Fig. 2C and 2D), demonstrated a 3- to 4-fold increase for all benserazide formulations, with EEY (p<0.05) and EF2 (p<0.05) maximal at 4 weeks; the positive control, HU produced a 10-fold (p<0.05) increase in HbF protein at 4 weeks. Notably, at two weeks, a modest but significant increase was also observed for the two enantiomers of benserazide and positive control, HU. Taken together, these data confirm previously published [8] efficacious induction of HbF protein by racemic benserazide and now, in this study, its individual enantiomers in vivo in 16 to 18-month old (adult stage) β-YAC mice.
Fig. 2. Benserazide increases HbF in older adult β-YAC mice.
β-YAC mice (13–18 months old) were treated with water (Veh) and hydroxyurea (HU) controls and (R,S)-benserazide HCL (EEY), (R)-benserazide HCL (EF2) or (S)-benserazide HCL (EF3) test formulations (n=4). Blood was collected for flow cytometry analysis (See Methods). A) Shown are raw data for F-cells percentage (%) at week 0 (W0), week 2 (W2) and week 4 (W4). B) Shown are the fold changes in F-cells over time quantified by flow cytometry. C) To quantify fetal hemoglobin level per cell, the mean fluorescence intensity (MFI) was collected by flow cytometry. Shown are the MFI means over time. D) Shown is the fold change in MFI over time. For all data analysis significance was set at p<0.01 (*) or p<0.001 (**)
A subsequent study was conducted in younger β-YAC mice 6–8 weeks old, an age range when fetal to beta globin switching is in progress and human fetal globin is being repressed. In this larger sample size (n=12) with equal sex distribution per treatment group, EEY, EF2, and EF3 given at 20 mg/kg, MWF for 4 weeks, weight gain remained normal and no apparent toxicity was observed (data not shown). Analysis of the complete blood counts demonstrated that all three drug forms resulted in a significantly increased MCV and/or hematocrit and Hb levels (Supplemental Materials Figure S1).
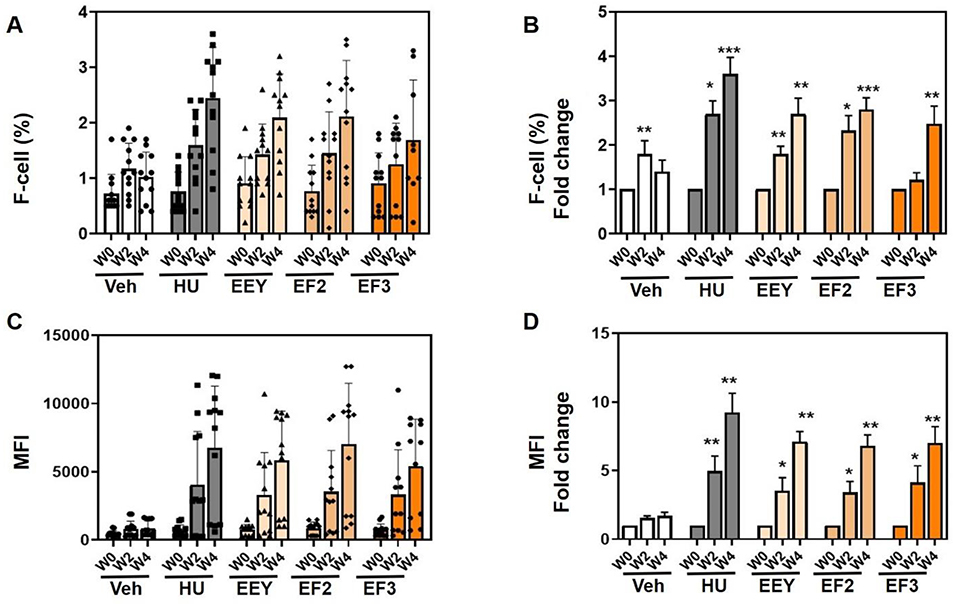
We examined the ability of benserazide to induce HbF in young β-YAC mice, which have higher baseline γ-globin gene expression than older mice and are in the process of switching-off human γ-globin gene expression. We first performed RT-qPCR and observed increases in γ-globin mRNA by week 4 for the racemate EEY, which was significant and for the EF2 derivative (Supplemental Materials Figure S2). These data confirm the ability of racemic benserazide to activate γ-globin transcription in young β-YAC mice. Flow cytometry analysis was also used to quantify the percentage of F-cells and HbF protein per cell (See Materials and Methods). A significant 2 to 2.5-fold increase in F-cells was observed for all benserazide derivatives at 4 weeks of treatment (Fig. 3A and 3B). Similarly, a significant, up to 4-fold increase in HbF protein (by MFI) was produced by EEY, EF2 and EF3 after 2-weeks of intermittent dosing and a further 7.5-fold induction after 4 weeks of treatment was observed for all forms of benserazide (Fig. 3C and 3D). These results support the ability of benserazide enantiomers (EF2 and EF3) to induce HbF levels comparable to the racemate (EEY) in β-YAC mice of different age groups.
Fig. 3. Benserazide increases HbF in young β-YAC mice.
β-YAC mice (6–8 weeks old) were treated with water (Veh) and hydroxyurea (HU) controls and the racemic (R,S)-benserazide HCL (EEY), (R)-benserazide HCL (EF2) and (S)-benserazide HCL (EF3) drug formulations. Three treatment replicates (n=12) were combined and analyzed as described and blood collected for flow cytometry analysis (See Methods). A) Shown are raw data for the F-cells percent (%) at week 0 (W0), week 2 (W2) and week 4 (W4). B) Shown is the fold change in F-cells over same time-period. C) To quantify mean fluorescence intensity (MFI) was quantified. Shown are the MFI mean over same time-period. D) Shown are fold changes in MFI. Statistical analysis as described in Fig. 1.
To address the recommendations of the FDA regulatory guidance on the development of stereoisomeric drugs [25] and provide a justification for continued development of commercially available GMP product of (R,S)-benserazide, further analysis of variance (ANOVA) was conducted on the parameters of the efficacy data. The objective was to determine whether any differences in the efficacy response for HbF induction between the benserazide racemate and its enantiomers occur that could cause regulatory consequences. The results obtained showed that in the younger mice, while the multiple comparison analyses yielded individual adjusted p-values of p<0.05 for each form of the drug compared to the vehicle control at week 4 for the MFI parameter, the individual comparisons between all forms yielded individual adjusted p-values >0.05. This confirmed that while the magnitude of the efficacy readout was significantly different from vehicle for each form of the drug, the magnitude of efficacy was not significantly different between groups of β-YAC mice treated with the benserazide racemate or its enantiomers. This data indicates similar efficacious responses, and that neither enantiomer could be considered an impurity, eliminating any requirement for developing a new synthetic scheme to manufacture a single enantiomeric API. Of note, in the older mice, the results by ANOVA were similar for the F-cell parameter. Therefore, these results provided sufficient justification for continued development of racemic benserazide to support clinical evaluation.
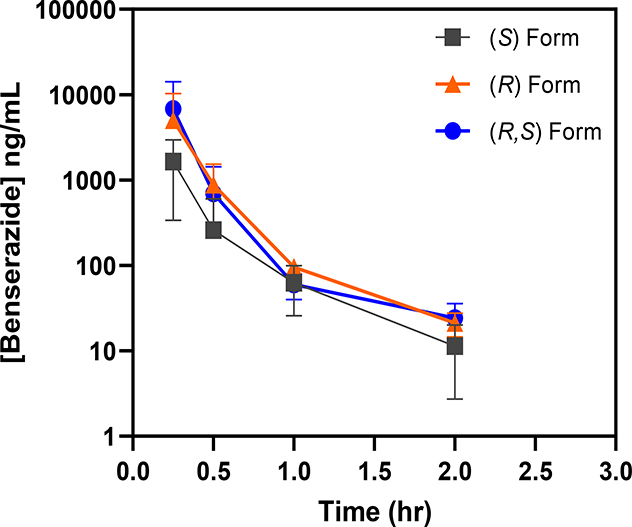
To evaluate in vivo exposures of benserazide and its enantiomers, the same β-YAC transgenic mice from the efficacy study were dosed at 20 mg/kg following the established dosing schedule. The average plasma concentrations of benserazide following the IP doses and related PK parameters are presented in Supplemental Materials Tables S1 and Table S2, respectively. It is noticed that there were large variations in plasma drug concentrations, especially for early time points, as indicated by relative high SD values (Table S1). The mean Cmax values were 6810, 1640 and 5030 ng/mL for EEY, EF2 and EF3, respectively, at the first sample collection point of 0.25 h. The corresponding mean AUC0−∞ values were 2030, 566 and 1680 ng∙hr/mL for EEY, EF2 and EF3, respectively. The terminal plasma t½ was 0.3 hrs for all forms of benserazide in this study. Plasma concentration versus time curves is shown in Figure 4.
Figure 4. Similar plasma exposure profile exhibited by racemic benserazide and its enantiomers.
After completion of the efficacy portion of the study, the assigned β-YAC mice now 10–12 weeks old were treated 2 days later with a single 20 mg/kg dose of the racemic (R,S)-benserazide HCL (EEY), (R)-benserazide HCL (EF2) and (S)-benserazide HCL (EF3) drug formulations. Blood samples were collected at designated timepoints and drug concentrations were analyzed as described (See Methods). Shown are the drug concentration-time curves for animals administered the (R)-, (S)- and (R,S)-benserazide.
(R,S)-Benserazide produces a dose-dependent increase in HbF expression.
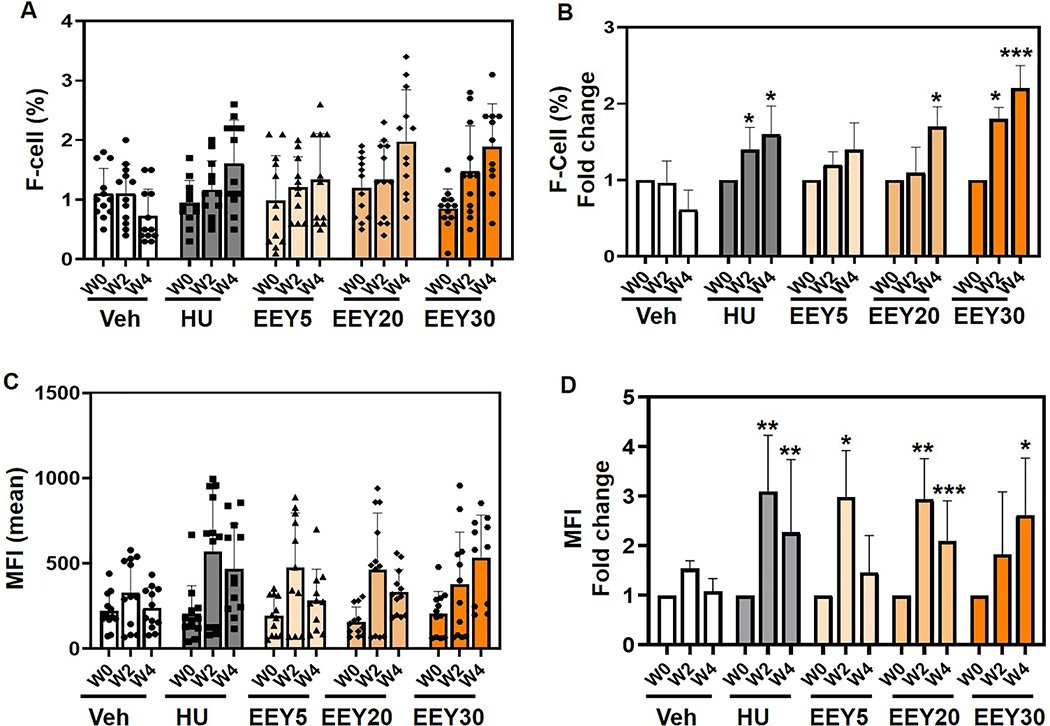
To further evaluate (R,S)-benserazide for clinical development, identification of active doses for HbF induction without bone marrow toxicity was needed, preferably by a single daily dose. A dose-response study was conducted, using doses projected from anemic baboon study treatments, which induced HbF without significant bone marrow toxicity, tachyphylaxis, cytopenias, or any other adverse side effects. Using the same experimental design, young β-YAC mice (6–10 weeks old; undergoing γ-globin gene silencing) of equal sex distribution were treated (n=12) with increasing concentrations of EEY at 5, 20 and 30 mg/kg/dose (EEY5, EEY20 and EEY30 respectively), 3 days per week, MWF.
In general, EEY did not produce significant changes in complete blood counts with differential although mild increase in MCV (EEY20), hematocrit (EEY30) and hemoglobin (EEEY30) were observed as shown in Supplemental Materials Figure S3. A detailed analysis of reticulocyte counts showed a slight but significant decrease for the EEY at 30 mg/kg dose. (Supplemental Materials Figure S4).
Finally, flow cytometry analysis demonstrated the proportions of F-cells increased in a dose-dependent manner by 1.4-fold, 1.7-fold, and 2.2-fold over baselines with three times weekly treatments for EEY5, EEY20, and EEY30 respectively (Fig. 5A and 5B). Although MFI levels were initially variable in dose response at the 2-week time point, by week 4 of treatment, the dose response was evident and significant 1.5-fold, 2.1-fold and 2.6-fold increases were observed with EEY5, EE20 and EE30 respectively (Fig. 5C and 5D).
Fig. 5. Racemic benserazide increases F-cells in a dose-dependent manner.
β-YAC mice (6–8 weeks old) were treated with water (Veh) and hydroxyurea (HU) controls and racemic (R,S)-benserazide HCL at 5, 20 and 30 mg/kg/dose (EEY5, EEY20, EEY30 respectively). Three treatment replicates (n=12) were combined and analyzed as described and blood collected for flow cytometry analysis (See Methods). Blood was collected for flow cytometry analysis. A) Shown are the raw data for the F-cells percent (%) at week 0 (W0), week 2 (W2) and week 4 (W4). B) Shown are fold changes in F-cells over same time-period. C) To quantify fetal hemoglobin level MFI was performed. Shown are MFI means over same time-period. D) Shown are fold changes in MFI over same time-period. Statistical analysis as described in Fig. 1; p<0.001 (***).
DISCUSSION
The β-globin disorders are an important global health burden, and cause multi-system disease only after the fetal globin genes are developmentally repressed [1, 31]. Fetal hemoglobin is expressed during development in utero, and the γ-globin genes are largely silenced by 12 months of age in children with normal hemoglobin production. However, in the β-hemoglobinopathies, hemoglobin switching occurs later with average 4–5% HbF levels persisting in adults with SCD, partly due to selective survival of red blood cells expressing HbF [32]. In children with SCD, as the level of hemoglobin S (HbS) rises, red cells with predominantly HbS and low HbF levels undergo hemoglobin polymerization with repeated cycles of deoxygenation and oxidant stress, developing abnormalities in nearly every structure and function of the red cells [33]. This results in hemolytic anemia and markedly reduced red cell lifespan, adhesion to endothelium, vaso-occlusive events, and other widespread clinical complications with consequent long-term morbidity and early mortality [34].
Many decades of biochemical, epidemiologic, clinical, genetic, and in vitro research have demonstrated the benefit of any incremental increase of HbF expression in patients with SCD. In phenotypes of β-thalassemia syndromes, small changes associated with minor genetic polymorphisms which reduce deficiency of non-alpha globin chains and globin chain imbalance can increase total Hb by 1 gram [11, 14, 35]. Survival in SCD is normalized at HbF levels ≥8.6% [13, 34, 36]. Higher proportions of both F-cells and HbF levels are important for ameliorating these anemias and reducing clinical complications of both conditions, and intracellular levels of HbF required for complete inhibition of HbS polymerization have been projected [1, 32, 37–42]. Augmentation of HbF has been shown to be particularly beneficial when HU treatment begins early, when baseline HbF levels are high [43–45]. Lower responses in adults are considered related to intolerance for optimal doses, hypothesized to be due to less active erythroid kinetics and lower marrow reserve related to prior infarcts [26, 46, 47]. Long-term follow-up strongly suggests that organ damage is reduced when HU therapy is begun in early childhood. Combination therapies with agents that act through different complimentary mechanisms are desirable for optimal HbF inducing treatment of these diseases [48].
The goal of this study was to evaluate the efficacy of (R,S)-benserazide compared to its enantiomers to identify an optimal form for eventual clinical development for the β-hemoglobinopathies including β-thalassemia and SCD. Doses of benserazide that induce HbF without toxicity in vivo were identified, and there were no consequential differences in the efficacious response for HbF induction between the benserazide racemate and its individual enantiomers. Therefore, the data indicate it was unlikely that a potential regulatory concern would arise for either enantiomer being classified as inactive, and therefore an impurity, requiring its exclusion from the drug product. Similarly, the plasma elimination half-lives of all three forms of the drug were determined to be similar. Therefore, the results provide support for future clinical studies leveraging a commercially available GMP API of the racemate for evaluation for HbF induction. This affords a significant time and cost savings for therapeutic development efforts compared to the daunting prospect of developing a new synthetic process for scale-up manufacturing of a single enantiomeric API.
Preclinical studies in β-YAC mice demonstrated tolerability and safety profiles for racemic (R,S)-benserazide, the form in long-term clinical use for Parkinson’s disease, as well as its enantiomers (R)-, and (S)-benserazide. We observed similar HbF induction by all forms that were well-tolerated by β-YAC mice, from 6–8 weeks of age to older animals at 13–18 months of age, long after hemoglobin switching is complete. Subsequent dose range studies of racemic benserazide, demonstrated increasing HbF induction from 5 to 30 mg/kg treatments, without an upper dose limit found for toxicity. In vivo exposure showed a similar plasma half-life across the three drug forms. Target doses estimated for producing in vivo activity in patients are projected to be 1.0 to 4.3 mg/kg from calculations of human equivalent doses from these and prior studies in transgenic mice and juvenile baboons.
Hydroxyurea, the first disease-modifying therapeutic, was clearly proven to reduce the frequency of painful episodes and acute chest syndrome in adult patients with sickle cell anemia in the Multicenter Study of Hydroxyurea [49], which justified US FDA approval in 1998. When taken daily at the recommended dose, primary effects of HU are increases in HbF, by a mean of approximately 3% above baseline in adult subjects, total hemoglobin, and in MCV, with dilution of HbS and consequent decreases in vascular adhesion [37]. Subsequent clinical study of HU showed safety and efficacy in children with a decrease in vaso-occlusive events and was subsequently FDA-approved for children ≥2 years old in 2017 [43, 44]. Long-term follow up of adults treated with HU documented the prevention of acute vaso-occlusive events, and improved survival in those patients in whom HbF is increased, although whether HU prevents chronic organ damage at doses which are suboptimal when adjusted for cytopenias is unknown [13]. It has been demonstrated that children are more responsive to HU therapy, perhaps related to increased marrow cellularity and proliferation kinetics, and as the γ-globin gene are not as suppressed compared to adults [42, 44, 46]. Hydroxyurea produced higher HbF protein per cell by MFI, in β-YAC mice. Both effects are beneficial in β-hemoglobinopathies. [32, 37–39] [10]
It is important to note that active erythroid proliferation, in contrast to low erythropoietic proliferation rates in non-anemic subjects, and select timing of exposure to erythroid progenitors, or intermittent exposure, is required for activity of many HbF inducers, [46, 50–53] and other regimens than 3 times weekly dosing may require exploration. Intermittent exposure or exposure at specific times has been shown to be required for HbF induction by other candidates in vitro [52] and pulsed dosing of other candidates has been shown to induce higher HbF and total Hb in β-thalassemia and sickle cell patients than has daily dosing with the same agent [51–53]
Previous studies have identified mechanisms by which benserazide induces HbF expression. Studies by Perrine and colleagues demonstrated loss of four important repressor proteins [10, 11, 14–20] binding to the γ-globin gene promoter and new histone acetylation and methylation patterns consistent with gene activation in erythroid progenitors treated with benserazide [9]. Benserazide has not demonstrated any hematologic toxicity in Parkinson’s disease patients treated for many years or in animal toxicology studies administering very high doses [7]. As added F-cell effects have been observed in progenitors of SCD patients treated with HU compared to progenitors of untreated SCD subjects [10], benserazide may well have additive activity with HU and with recently approved therapeutics which act through different mechanisms to improve red cell health.
Recently, after more than 20 years without a second drug approval, three therapeutics, Endari™ (L-glutamine), Adakveo® (crizanlizumab), and Oxbryta® (voxelotor) were US FDA-approved for the treatment of SCD in 2017 and 2019, respectively. Endari™ improves clinical symptoms of SCD by reducing oxidative stress in red blood cells with resulting decreased adhesion of sickle red cells to endothelial cells [54, 55]. Oxbryta® inhibits hemoglobin S polymerization through increased oxygen affinity and increases total hemoglobin levels [56]. Adakveo® (crizanlizumab), a monoclonal antibody, binds to P-selectin on the surface of activated endothelial cells and platelets, [57] reduces interactions among endothelial cells and blood components. No single agent eliminates all clinical events and hematologic abnormalities. A HbF inducing agent without overlapping toxicities could be combined with any or all of these agents for greater efficacy in reducing progressive organ damage [47, 48].
Benserazide is attracting attention in repurposing work due to its considered benign safety, negative mutagenicity profiles, and as it is reported to be well-tolerated at doses as high as 700–1300 mg/day in Parkinson’s disease patients [7, 58]. Based on these findings, extended toxicology studies, and with almost 50 years of patient use in the EU, Canada, and other countries, a clinical supply was acquired and manufactured as a single formulation without L-DOPA. A Phase 1b clinical trial (NCT04432623) has been initiated to identify doses that safely increase F-cells and HbF/cell initially in patients with β-thalassemia intermedia, with expansion to patients with SCD if indicated.
CONCLUSION
Studies described herein confirm that racemic benserazide modulates in vivo HbF expression and merits clinical evaluation in patients with β-hemoglobinopathies. The studies determined that neither enantiomer, (R)- or (S)-benserazide, can be characterized as an impurity, providing justification for further clinical development of (R,S)-benserazide. This therapeutic offers the potential to be developed as a repurposed non-chemotherapy agent and/or combined with HU or decitabine to increase the magnitude of HbF and proportions of F-cells through complimentary mechanisms. Furthermore, benserazide should produce additive effects without myelosuppression, and could be combined with other therapies which act on red cell oxidant stress, oxygenation, energy levels, or inhibition of vascular adhesion [47, 48, 54–58].
Supplementary Material
Highlights.
Fetal hemoglobin induction via pharmacologic approaches remains the most feasible treatment for most individuals living with sickle cell disease.
Racemate (R,S)-Benserazide and its enantiomers induce fetal hemoglobin expression in vivo in β-YAC transgenic mice dosed three times weekly without myelosuppression.
A clinical trial to determine the safety and efficacy of Benserazide is in progress in patients with thalassemia and sickle cell disease.
ACKNOWLEDGEMENTS
We would like to thank Mrs. Natasha Alford for arranging conference calls to review the data and completing minutes of meetings for the mouse studies. We thank David W. Carey and D. Nicole Reuter for their dedication and expertise with baboon care and handling.
FUNDING
This β-YAC mouse study was supported by the Intramural Research Program of the National Center for Advancing Translational Sciences, NIH under Contract No. HHSN261200800001E from the National Cancer Institute, NIH to BSP (Augusta University). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The work related to the baboon study was supported by R01 DK-52962 and R42 HL-110727 (SPP and RFW).
Footnotes
DECLARATION OF COMPETING INTEREST
BSP, BL, LM, HX, MT, SJH, XX, AW, JH, AA, WZ, GT, LT, JS, AF and RFW have no competing interest to declare. SP is an inventor on patents related to this work.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Weatherall DJ, The inherited diseases of hemoglobin are an emerging global health burden, Blood, 115 (2010) 4331–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steinberg MH, Rodgers GP, Pharmacologic modulation of fetal hemoglobin, Medicine (Baltimore), 80 (2001) 328–344. [DOI] [PubMed] [Google Scholar]
- [3].Wilber A, Nienhuis AW, Persons DA, Transcriptional regulation of fetal to adult hemoglobin switching: new therapeutic opportunities, Blood, 117 (2011) 3945–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bauer DE, Kamran SC, Orkin SH, Reawakening fetal hemoglobin: prospects for new therapies for the β-globin disorders, Blood, 120 (2012) 2945–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bank A, Regulation of human fetal hemoglobin: new players, new complexities, Blood, 107 (2006) 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Perrine SP, Pace BS, Faller DV, Targeted fetal hemoglobin induction for treatment of beta hemoglobinopathies, Hematol Oncol Clin North Am, 28 (2014) 233–248. [DOI] [PubMed] [Google Scholar]
- [7].Prolopa Product Monograph, (2019). https://www.rochecanada.com/content/dam/rochexx/roche-ca/products/ConsumerInformation/MonographsandPublicAdvisories/Prolopa/Prolopa_PM_E.pdf. pp. 1–30.
- [8].Boosalis MS, Sangerman JI, White GL, Wolf RF, Shen L, Dai Y, White E, Makala LH, Li B, Pace BS, Nouraie M, Faller DV, Perrine SP, Novel Inducers of Fetal Globin Identified through High Throughput Screening (HTS) Are Active In Vivo in Anemic Baboons and Transgenic Mice, PLoS One, 10 (2015) e0144660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dai Y, Sangerman J, Luo HY, Fucharoen S, Chui DH, Faller DV, Perrine SP, Therapeutic fetal-globin inducers reduce transcriptional repression in hemoglobinopathy erythroid progenitors through distinct mechanisms, Blood Cells Mol Dis, 56 (2016) 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dai Y, Sangerman J, Nouraie M, Faller AD, Oneal P, Rock A, Owoyemi O, Niu X, Nekhai S, Maharaj D, Cui S, Taylor R, Steinberg M, Perrine S, Effects of hydroxyurea on F-cells in sickle cell disease and potential impact of a second fetal globin inducer, Am J Hematol, 92 (2017) E10–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lettre G, Sankaran VG, Bezerra MA, Araújo AS, Uda M, Sanna S, Cao A, Schlessinger D, Costa FF, Hirschhorn JN, Orkin SH, DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease, Proc Natl Acad Sci U S A, 105 (2008) 11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mankidy R, Faller DV, Mabaera R, Lowrey CH, Boosalis MS, White GL, Castaneda SA, Perrine SP, Short-chain fatty acids induce gamma-globin gene expression by displacement of a HDAC3-NCoR repressor complex, Blood, 108 (2006) 3179–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Steinberg MH, McCarthy WF, Castro O, Ballas SK, Armstrong FD, Smith W, Ataga K, Swerdlow P, Kutlar A, DeCastro L, Waclawiw MA, The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up, Am J Hematol, 85 (2010) 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, Chen W, Usala G, Busonero F, Maschio A, Albai G, Piras MG, Sestu N, Lai S, Dei M, Mulas A, Crisponi L, Naitza S, Asunis I, Deiana M, Nagaraja R, Perseu L, Satta S, Cipollina MD, Sollaino C, Moi P, Hirschhorn JN, Orkin SH, Abecasis GR, Schlessinger D, Cao A, Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia, Proc Natl Acad Sci U S A, 105 (2008) 1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sedgewick AE, Timofeev N, Sebastiani P, So JCC, Ma ESK, Chan LC, Fucharoen G, Fucharoen S, Barbosa CG, Vardarajan BN, Farrer LA, Baldwin CT, Steinberg MH, Chui DHK, BCL11A is a major HbF quantitative trait locus in three different populations with beta-hemoglobinopathies, Blood Cells Mol Dis, 41 (2008) 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Borg J, Papadopoulos P, Georgitsi M, Gutiérrez L, Grech G, Fanis P, Phylactides M, Verkerk AJ, van der Spek PJ, Scerri CA, Cassar W, Galdies R, van Ijcken W, Ozgür Z, Gillemans N, Hou J, Bugeja M, Grosveld FG, von Lindern M, Felice AE, Patrinos GP, Philipsen S, Haploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobin, Nat Genet, 42 (2010) 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang X, Thein SL, Switching from fetal to adult hemoglobin, Nature Genetics, 50 (2018) 478–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nuinoon M, Makarasara W, Mushiroda T, Setianingsih I, Wahidiyat PA, Sripichai O, Kumasaka N, Takahashi A, Svasti S, Munkongdee T, Mahasirimongkol S, Peerapittayamongkol C, Viprakasit V, Kamatani N, Winichagoon P, Kubo M, Nakamura Y, Fucharoen S, A genome-wide association identified the common genetic variants influence disease severity in beta0-thalassemia/hemoglobin E, Hum Genet, 127 (2010) 303–314. [DOI] [PubMed] [Google Scholar]
- [19].Zhou D, Liu K, Sun CW, Pawlik KM, Townes TM, KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching, Nat Genet, 42 (2010) 742–744. [DOI] [PubMed] [Google Scholar]
- [20].Shi L, Cui S, Engel JD, Tanabe O, Lysine-specific demethylase 1 is a therapeutic target for fetal hemoglobin induction, Nat Med, 19 (2013) 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Manwani D, Bieker JJ, KLF1: when less is more, Blood, 124 (2014) 672–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen Z, Luo HY, Steinberg MH, Chui DH, BCL11A represses HBG transcription in K562 cells, Blood Cells Mol Dis, 42 (2009) 144–149. [DOI] [PubMed] [Google Scholar]
- [23].Li X, Hu X, Patel B, Zhou Z, Liang S, Ybarra R, Qiu Y, Felsenfeld G, Bungert J, Huang S, H4R3 methylation facilitates β-globin transcription by regulating histone acetyltransferase binding and H3 acetylation, Blood, 115 (2010) 2028–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Menzel S, Garner C, Gut I, Matsuda F, Yamaguchi M, Heath S, Foglio M, Zelenika D, Boland A, Rooks H, Best S, Spector TD, Farrall M, Lathrop M, Thein SL, A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15, Nat Genet, 39 (2007) 1197–1199. [DOI] [PubMed] [Google Scholar]
- [25].U.S. Food and Drug Administration. Center for Drug Evaluation and Research. (1992, May). Development of New Stereoisomeric Drugs. Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-new-stereoisomeric-drugs. pp. 1–8. [Google Scholar]
- [26].Centis F, Tabellini L, Lucarelli G, Buffi O, Tonucci P, Persini B, Annibali M, Emiliani R, Iliescu A, Rapa S, Rossi R, Ma L, Angelucci E, Schrier SL, The importance of erythroid expansion in determining the extent of apoptosis in erythroid precursors in patients with beta-thalassemia major, Blood, 96 (2000) 3624–3629. [PubMed] [Google Scholar]
- [27].Peterson KR, Clegg CH, Huxley C, Josephson BM, Haugen HS, Furukawa T, Stamatoyannopoulos G, Transgenic mice containing a 248-kb yeast artificial chromosome carrying the human beta-globin locus display proper developmental control of human globin genes, Proc Natl Acad Sci U S A, 90 (1993) 7593–7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Oseghale AR, Zhu X, Li B, Peterson KR, Nudelman A, Rephaeli A, Xu H, Pace BS, Conjugate prodrug AN-233 induces fetal hemoglobin expression in sickle erythroid progenitors and beta-YAC transgenic mice, Blood Cells Mol Dis, 79 (2019) 102345. [DOI] [PubMed] [Google Scholar]
- [29].Cai Y, Pi W, Sivaprakasam S, Zhu X, Zhang M, Chen J, Makala L, Lu C, Wu J, Teng Y, Pace B, Tuan D, Singh N, Li H, UFBP1, a Key Component of the Ufm1 Conjugation System, Is Essential for Ufmylation-Mediated Regulation of Erythroid Development, PLoS genetics, 11 (2015) e1005643–e1005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Johnson J, Hunter R, McElveen R, Qian XH, Baliga BS, Pace BS, Fetal hemoglobin induction by the histone deacetylase inhibitor, scriptaid, Cell Mol Biol (Noisy-le-grand), 51 (2005) 229–238. [PubMed] [Google Scholar]
- [31].Vichinsky EP, MacKlin EA, Waye JS, Lorey F, Olivieri NF, Changes in the Epidemiology of Thalassemia in North America: A New Minority Disease, Pediatrics, 116 (2005) e818. [DOI] [PubMed] [Google Scholar]
- [32].Franco RS, Yasin Z, Palascak MB, Ciraolo P, Joiner CH, Rucknagel DL, The effect of fetal hemoglobin on the survival characteristics of sickle cells, Blood, 108 (2006) 1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bunn HF, Pathogenesis and Treatment of Sickle Cell Disease, New England Journal of Medicine, 337 (1997) 762–769. [DOI] [PubMed] [Google Scholar]
- [34].Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP, Mortality in sickle cell disease. Life expectancy and risk factors for early death, N Engl J Med, 330 (1994) 1639–1644. [DOI] [PubMed] [Google Scholar]
- [35].Gallo E, Massaro P, Miniero R, David D, Tarella C, The Importance of the Genetic Picture and Globin Synthesis in Determining the Clinical and Haematological Features of Thalassaemia Intermedia, British Journal of Haematology, 41 (1979) 211–221. [DOI] [PubMed] [Google Scholar]
- [36].Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M, Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment, JAMA, 289 (2003) 1645–1651. [DOI] [PubMed] [Google Scholar]
- [37].Steinberg MH, Chui DH, Dover GJ, Sebastiani P, Alsultan A, Fetal hemoglobin in sickle cell anemia: a glass half full?, Blood, 123 (2014) 481–485. [DOI] [PubMed] [Google Scholar]
- [38].Boyer SH, Dover GJ, Serjeant GR, Smith KD, Antonarakis SE, Embury SH, Margolet L, Noyes AN, Boyer ML, Bias WB, Production of F cells in sickle cell anemia: regulation by a genetic locus or loci separate from the beta-globin gene cluster, Blood, 64 (1984) 1053–1058. [PubMed] [Google Scholar]
- [39].Urio F, Lyimo M, Mtatiro SN, Cox SE, Mmbando BP, Makani J, High prevalence of individuals with low concentration of fetal hemoglobin in F-cells in sickle cell anemia in Tanzania, Am J Hematol, 91 (2016) E323–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fucharoen S, Siritanaratkul N, Winichagoon P, Chowthaworn J, Siriboon W, Muangsup W, Chaicharoen S, Poolsup N, Chindavijak B, Pootrakul P, Piankijagum A, Schechter AN, Rodgers GP, Hydroxyurea increases hemoglobin F levels and improves the effectiveness of erythropoiesis in beta-thalassemia/hemoglobin E disease, Blood, 87 (1996) 887–892. [PubMed] [Google Scholar]
- [41].Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ, Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial, Blood, 85 (1995) 43–49. [PubMed] [Google Scholar]
- [42].Singer ST, Kuypers FA, Olivieri NF, Weatherall DJ, Mignacca R, Coates TD, Davies S, Sweeters N, Vichinsky EP, Fetal haemoglobin augmentation in E/beta(0) thalassaemia: clinical and haematological outcome, Br J Haematol, 131 (2005) 378–388. [DOI] [PubMed] [Google Scholar]
- [43].Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, Rana S, Thornburg CD, Rogers ZR, Kalpatthi RV, Barredo JC, Brown RC, Sarnaik SA, Howard TH, Wynn LW, Kutlar A, Armstrong FD, Files BA, Goldsmith JC, Waclawiw MA, Huang X, Thompson BW, Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG), Lancet, 377 (2011) 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maier-Redelsperger M, Labie D, Elion J, Long-term hydroxyurea treatment in young sickle cell patients, Current Opinion in Hematology, 6 (1999). [DOI] [PubMed] [Google Scholar]
- [45].Noguchi CT, Rodgers GP, Serjeant G, Schechter AN, Levels of fetal hemoglobin necessary for treatment of sickle cell disease, N Engl J Med, 318 (1988) 96–99. [DOI] [PubMed] [Google Scholar]
- [46].Pootrakul P, Sirankapracha P, Hemsorach S, Moungsub W, Kumbunlue R, Piangitjagum A, Wasi P, Ma L, Schrier SL, A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in thai patients with thalassemia, Blood, 96 (2000) 2606–2612. [PubMed] [Google Scholar]
- [47].Steinberg MH, Clinical trials in sickle cell disease: adopting the combination chemotherapy paradigm, Am J Hematol, 83 (2008) 1–3. [DOI] [PubMed] [Google Scholar]
- [48].Minniti CP, L-Glutamine and the Dawn of Combination Therapy for Sickle Cell Disease, N Engl J Med, 379 (2018) 292–294. [DOI] [PubMed] [Google Scholar]
- [49].Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR, Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia, N Engl J Med, 332 (1995) 1317–1322. [DOI] [PubMed] [Google Scholar]
- [50].Santos MEHP, Olops L, Vendrame F, Tavares AHJ, Leonardo DP, de Azevedo PC, Piovesana LG, Costa FF, Fertrin KY, Benserazide as a potential novel fetal hemoglobin inducer: an observational study in non-carriers of hemoglobin disorders, Blood Cells, Molecules, and Diseases, 87 (2021) 102511. [DOI] [PubMed] [Google Scholar]
- [51].Atweh GF, Sutton M, Nassif I, Boosalis V, Dover GJ, Wallenstein S, Wright E, McMahon L, Stamatoyannopoulos G, Faller DV, Perrine SP, Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease, Blood, 93 (1999) 1790–1797. [PMC free article] [PubMed] [Google Scholar]
- [52].Krivega I, Byrnes C, de Vasconcellos JF, Lee YT, Kaushal M, Dean A, Miller JL, Inhibition of G9a methyltransferase stimulates fetal hemoglobin production by facilitating LCR/γ-globin looping, Blood, 126 (2015) 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ikuta T, Atweh G, Boosalis V, White GL, Da Fonseca S, Boosalis M, Faller DV, Perrine SP, Cellular and molecular effects of a pulse butyrate regimen and new inducers of globin gene expression and hematopoiesis, Ann N Y Acad Sci, 850 (1998) 87–99. [DOI] [PubMed] [Google Scholar]
- [54].Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, Gordeuk VR, Viswanathan K, Sarnaik S, Osunkwo I, Guillaume E, Sadanandan S, Sieger L, Lasky JL, Panosyan EH, Blake OA, New TN, Bellevue R, Tran LT, Razon RL, Stark CW, Neumayr LD, Vichinsky EP, A Phase 3 Trial of l-Glutamine in Sickle Cell Disease, N Engl J Med, 379 (2018) 226–235. [DOI] [PubMed] [Google Scholar]
- [55].Niihara Y, Matsui NM, Shen YM, Akiyama DA, Johnson CS, Sunga MA, Magpayo J, Embury SH, Kalra VK, Cho SH, Tanaka KR, L-glutamine therapy reduces endothelial adhesion of sickle red blood cells to human umbilical vein endothelial cells, BMC Blood Disord, 5 (2005) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vichinsky E, Hoppe CC, Ataga KI, Ware RE, Nduba V, El-Beshlawy A, Hassab H, Achebe MM, Alkindi S, Brown RC, Diuguid DL, Telfer P, Tsitsikas DA, Elghandour A, Gordeuk VR, Kanter J, Abboud MR, Lehrer-Graiwer J, Tonda M, Intondi A, Tong B, Howard J, A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease, N Engl J Med, 381 (2019) 509–519. [DOI] [PubMed] [Google Scholar]
- [57].Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, Gualandro S, Colella MP, Smith WR, Rollins SA, Stocker JW, Rother RP, Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease, N Engl J Med, 376 (2017) 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li W, Zheng M, Wu S, Gao S, Yang M, Li Z, Min Q, Sun W, Chen L, Xiang G, Li H, Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2, J Exp Clin Cancer Res, 36 (2017) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.