Abstract
The use of a photon counting detector in CT (PCD CT) is currently the subject of intense investigation and development. In this review article, we will describe potential clinical applications of this technology with a particular focus on the experience of our own institution with a prototype PCD CT scanner. PCDs have three primary advantages over conventional, energy integrating detectors (EIDs): they provide spectral information without need for a dedicated dual energy protocol; they are immune to electronic noise; and they can be made very high resolution without significant compromises to quantum efficiency. These advantages translate into several clinical applications. Metal artifacts, beam hardening artifacts, and noise streaks from photon starvation can be better mitigated using PCD CT. Certain incidental findings can be better characterized using the spectral information from PCD CT. High-contrast, high-resolution structures such as the temporal bone can be better visualized using PCD CT and at greatly reduced dose. We also discuss new possibilities on the horizon, including new contrast agents, and how anticipated improvements in PCD CT will translate to performance in these applications.
Keywords: photon counting X-ray detectors, clinical applications, spectral CT
I. Introduction
SINGLE photon counting is an emerging capability in CT detectors. While energy-sensitive detection of individual photons has long been accomplished in other modalities such as PET, similar detectors in CT have been very challenging to create due to the demanding flux requirements in diagnostic CT, which can exceed a billion counts per second per mm2 for unattenuated beam. Existing detectors for diagnostic CT have accordingly been energy-integrating. Recent advances in semiconductor design, driven largely by Moore’s Law, have made it feasible to now resolve individual photons arriving on the detector in CT applications.
Research is being reported in this Special Issue and elsewhere on the introduction of photon counting detectors (PCDs) for CT. At the time of this writing, two whole body experimental PCD CT scanners (Siemens CounT) have now been placed in North America, one at the Mayo Clinic and one at NIH [7, 8], in addition to a system in Germany [10]. These two systems are built on a dual source CT gantry, with one of the EID subsystems replaced with a PCD subsystem so that PCD data can be acquired and compared to EID data. In Europe, a PCD CT scanner designed by another vendor (Philips) has been positioned in the University of Lyon [11]. Outside of whole body imaging, the MARS scanner [12, 13] has been tested on human extremities and small animals and the silicon strip detector [14] has been tested on human heads.
As the technical capabilities of PCDs continue to improve, it is natural to ask how these new PCD CT systems might improve existing clinical applications, or how they might create entirely new applications. The purpose of this review article is to attempt to address this question. The ultimate success of PCD CT within the imaging community will depend on its perceived value for improved patient diagnosis and downstream clinical outcomes.
At the time of this writing, our clinic is one of only three institutions that has acquired a whole-body PCD CT prototype system cleared to scan human volunteers. Much of this review will describe our own, single-institution experience, simply because there is very limited human data available to support other clinical applications. While several other PCD CT scanners are in development and have been used in preclinical or phantom scenarios, they have not yet been cleared by regulatory agencies for whole body human imaging.
Our paper is organized as follows. In Section II, we will briefly review the operating principles of PCD CT. Sections III, IV, and V will describe the various clinical applications of PCD CT. Section III focuses on improvements in routine imaging. Section IV describes new capabilities that will become possible because of PCD CT. Section V describes upcoming capabilities that are not yet practical but may become possible with future research and development. In Section VI, we will look ahead and connect ongoing research in improving PCD CT technical characteristics with their anticipated impact on clinical applications.
II. Technical Review
The physics of photon counting CT has been described at length elsewhere. A review of the technical principles, presented for a clinical audience, has been written by Willemink et al in Radiology [15]. A separate tutorial of PCDs including a description of the operating modes in the Siemens CounT has been written by Leng et al in Radiographics [16]. Reviews of the ASIC circuitry and the imaging algorithms are reported in this Special Issue [17, 18]. A survey of PCD research and development was written in 2013 [19] and later updated [20]. Some of these references also describe clinical applications [15, 16]. We will provide only a brief introduction into the operating principles of PCDs and direct the interested reader to these other works for deeper understanding.
All x-ray detectors used in CT, whether EID or PCD, must convert high-energy x-ray photons into electrical signal. In most EIDs, there is an intermediate step of converting the x-ray photons into visible light. This is most commonly performed using a scintillating ceramic material such as gadolinium oxysulfide (Gd2O2S). Once the energy is in the form of visible light, it can be channeled using reflective septa towards a pixelated photodiode. These photodiodes operate in a similar fashion to the pixels in digital cameras and convert the light directly into electrical signal. The amount of electrical signal produced by each x-ray photon is in proportion to its energy. Therefore, the detector is “energy integrating” and the high energy photons present greater statistical weight in an EID.
PCDs differ from EIDs by directly converting x-ray photons into electrical signal. A different type of material, a direct conversion semiconductor, is used that directly converts x-rays into electrons and holes. The most common direct conversion semiconductor being studied today is cadmium telluride or cadmium zinc telluride (CdTe or CZT), although silicon is also being studied [21]. The response time of these materials is very rapid so the charges are generated nearly instantaneously. The semiconductor is also subject to a strong bias voltage so that any liberated electrons are rapidly pulled to one end to be read out as electrical signal.
The magnitude of the electrical signal produced by the PCD is too large to be digitized and stored directly. Instead, on-chip electronics must rapidly process the signal and reduce it into a smaller number of integers. Because there is one processing circuit per pixel and hundreds of thousands of pixels in a PCD CT detector, the processing circuit must be simple. The most common mechanism for analysis is to apply a bank of comparators to the signal. A comparator, as its name implies, compares the electrical signal to a reference voltage level. Whenever the electrical signal exceeds the reference, the comparator activates and increments a counter. The reference voltage can be interpreted as an energy level in units of keV, and the corresponding counter can be interpreted as the number of photons greater than the reference energy level during that time interval. Fig. 1 shows an example of this process. While the bank of comparators operating independently in each pixel is currently the most popular analysis mechanism, other solutions are also possible.
Fig. 1.
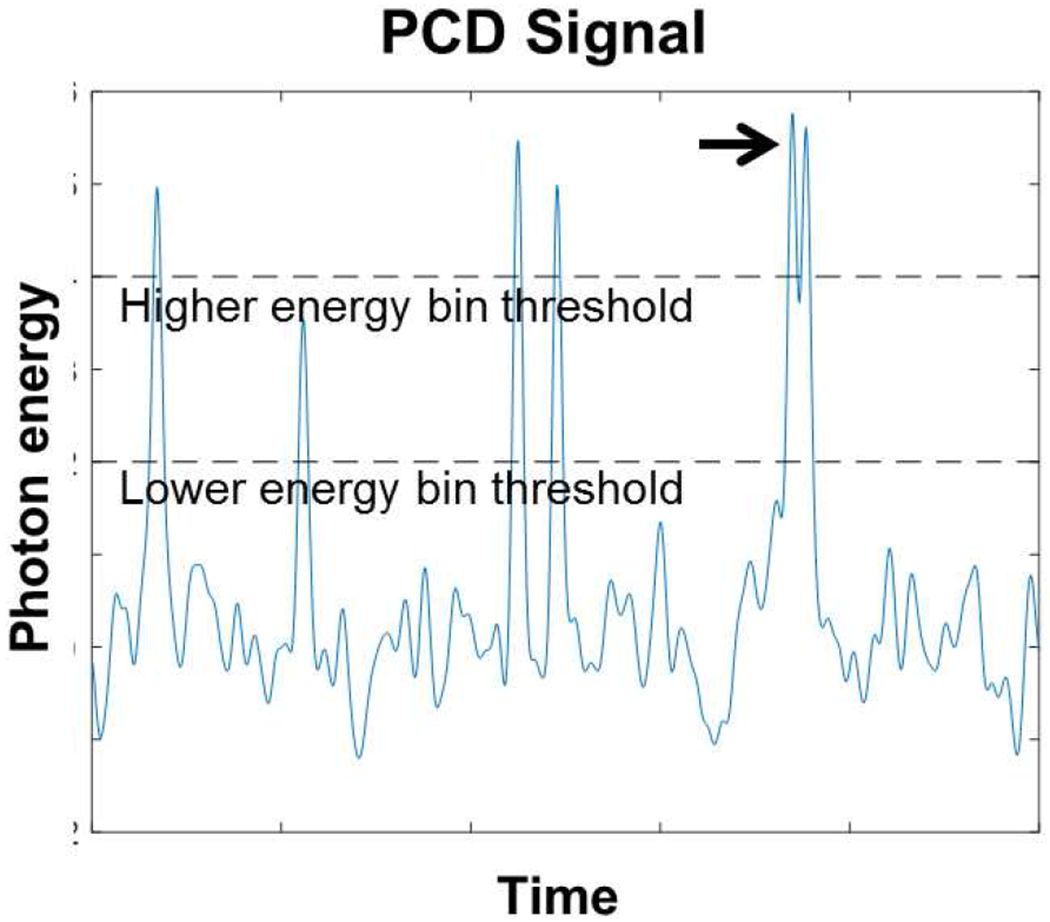
Simulated example of a signal detected by a PCD. Impinging photons deposit energy in the PCD, which creates a momentary signal with amplitude ideally proportional to the deposited energy. Six photons are present in this example, each one leading to a peak. If the amplitude exceeds preset comparator thresholds (dashed lines), the PCD increments the corresponding counter. The black arrow points to an example of pileup, wherein two photons arrive in close succession. In this case, the low energy bin is incremented only once even though two photons have arrived. Pileup and charge sharing (not shown here) are two major sources of error in PCDs. If the lower energy bin threshold is set sufficiently high, it will almost never be triggered from electronic noise (which appear as random fluctuations in this plot). Hence, the number of counts stemming from electronic noise can be close to zero in practice.
PCDs, like all electronics, are still subject to electronic noise. However, if the reference voltage is placed high enough, then there will be nearly zero counts triggered due to electronic noise. This is in contrast to EIDs, where the electronic noise is always present. At high flux, Poisson noise dominates electronic noise, but at low flux (typically through thick regions of the patient or in low dose scans), electronic noise can exceed Poisson noise in EIDs. Unfortunately, the electronic noise in EIDs causes problems where the signal is already poorest, amplifying existing noise streaks. Electronic noise in PCDs does not amplify noise streaks in this fashion but instead increases the width of the photopeak in the spectral response.
Unfortunately, PCDs also have imperfections. When x-ray photons arrive near the boundary between two pixels, the cloud of electrons produced by the photon may be partially detected by both pixels. This phenomenon, known as charge sharing, can cause a single incident x-ray photon to be detected twice, both times at the wrong energy. Charge sharing can also occur from the emission of a characteristic photon. A separate problem is that two independent photons may arrive at the same pixel in very rapid succession. Their resulting signals will “pile up” and be interpreted by the processing electronics as a single photon. Mitigation for pileup and charge sharing is the subject of ongoing research. Some PCDs apply analysis mechanisms that can resist either pileup [22, 23] or charge sharing [24]. We will discuss the impact of these mitigation strategies on clinical applications in Section V.
In our brief overview of PCD operating principles, we have touched upon three advantages of PCDs, each of which translates into different clinical applications.
Decreased electronic noise.
Because PCDs have nearly zero electronic noise, they show reduced noise streaks. It is worth noting that some newer EIDs have been able to achieve lower electronic noise by redesign of their components, and this has been shown to reduce noise streaks [25, 26]. PCDs can be seen as a logical progression that can further suppress electronic noise.
Constant availability of spectral information.
PCDs always have the capability to sense the energy of each arriving photon. With the exception of the dual layer detector, existing dual energy solutions require protocol compromises in order to acquire dual energy data. With PCDs, dual energy information is always available. The dual layer detector is a related technology that always captures dual energy information for every protocol, but a weakness of the dual layer detector is that the thickness of each layer cannot be adjusted. This could lead to poor spectral noise characteristics for certain combinations of kVp and patient size. In PCDs, the energy bins can be tuned so that the spectral contrast is maximized for each kVp and patient size.
In some cases, the quality of the spectral information provided by PCDs is superior to conventional dual energy techniques. For simultaneous differentiation of k-edge and iodine contrast agents, the ability of PCDs to capture multi-energy information with three or more energy bins is essential. The ability to tune individual energy thresholds for the individual patient and task could further improve the spectral information provided by PCDs.
Increased resolution.
EIDs are limited by the reflective septa which must be placed around each pixel. Because the septa have a certain minimum size, decreasing pixel size leads to decreases in the fill factor of the EID and further resolution enhancements are not practical. PCDs can eliminate this tradeoff, enabling smaller pixels and thus higher resolution.
III. Improvements in Routine Imaging
A. Iodine CNR
The ability to sense the energy of individual photons can improve iodine CNR. Fig. 2 provides one example. An EID, by virtue of its energy integration, weights each photon by its incoming energy. High energy photons are therefore responsible for more signal than low energy photons, but for the task of iodine detection, low energy photons provide greater information. PCD CT can improve iodine CNR by providing greater weight to the lower energy bins.
Fig. 2.
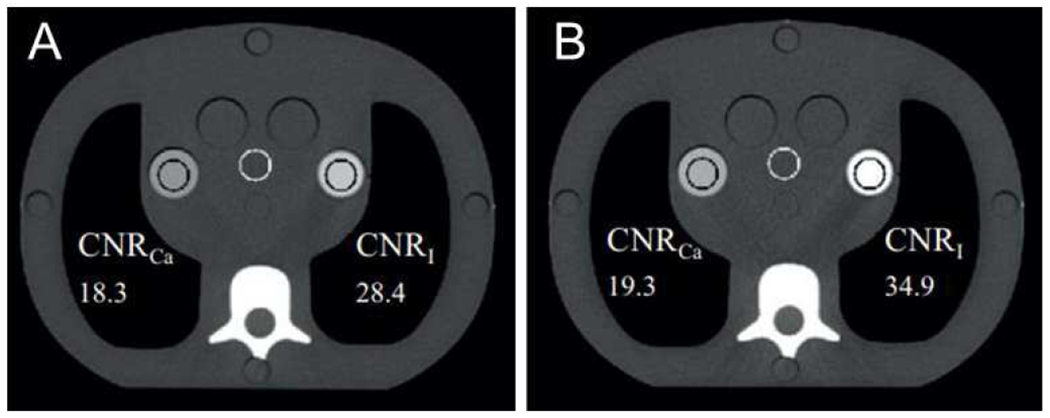
Improvement in CNR using a PCD, demonstrated in an anthropomorphic phantom. (Left) EID CT at 140 kVp. (Right) PCD CT with energy bin thresholds at 25 and 65 keV, scanned using the same protocol. The iodine contrast is improved. Adapted from Reference [4] with permission.
Improved iodine CNR can improve routine contrast enhanced exams. Because these exams are so common, this improvement would regularly improve patient care. For some patients, especially children, improved iodine CNR could be translated into reduced x-ray dose. For other patients, especially those with reduced kidney function, improved iodine CNR can translated into reduce contrast load and corresponding toxicity [27].
The improvement in iodine CNR is most significant at higher kVp. One study showed an 11% and 38% improvement in CNR at 80 and 140 kVp, respectively, compared to an EID system [7]. The exact improvement depends on the non-idealities of the PCD, especially the level of charge sharing present or background scatter [28].
B. Metal and beam hardening artifacts
Metal artifacts are common in CT and cause artifacts through several mechanisms, including beam hardening, scatter, photon starvation, and edge-gradient effects. The optimal correction strategy for metal artifacts depends partly on the metal responsible for the artifacts. Some metals, such as the mercury or gold used in dental fillings, absorb nearly all of the incident photons and therefore necessitate a software data replacement technique [29, 30]. However, data replacement can lead to other artifacts, including resolution loss. Other metals, such as titanium, generate artifacts primarily through beam hardening. In these cases the affected sinogram data can be corrected rather than replaced. In these cases, PCDs can provide a hardware solution.
When the cause of metal artifacts is due to photon starvation, PCD CT will provide no benefit because there is no useful data whether a PCD or EID is used. Lighter metals that do not cause photon starvation would instead benefit from PCD CT. While software-driven data replacement techniques could still be used for these lighter metals, they will have tradeoffs. In the long term, software algorithms that synergize with PCD CT may be able to able to nearly eliminate beam hardening artifacts caused from these lighter metals.
Whether from metal or from bone, beam hardening causes breakdown of the monoenergetic Beer-Lambert Law and leads to streaks. It preferentially affects low-energy photons. PCDs, which can discriminate between high energy and low energy photons, can simply discard all detected low energy photons and directly reduce beam hardening artifacts. Pre-patient filtration of the beam using a beam hardening filter can further reduce the effect of low-energy photons. Fig. 3 shows an example of metal artifact reduction using this strategy.
Fig. 3.

Anthropomorphic phantom showing improvement in metal artifacts from (left) EID CT to (right) high-threshold PCD CT with tin filtration. (WW, WL) = (400, 40) HU. Adapted from Reference [1] with permission.
Still better correction may be possible from basis material decomposition. Assuming the metal in question does not have a k-edge in the diagnostic energy range, the two-material basis decomposition applies. In theory, the measurements in the low and high energy bins can be perfectly inverted to recover equivalent basis material thicknesses, which can be recombined and reconstructed without artifact [31]. In practice, residual scatter or imperfect calibration could prevent perfect inversion.
PCDs can reduce other types of beam hardening artifacts in much the same fashion, either by filtering out low energy photons or by applying basis material decomposition techniques. These beam hardening artifacts are especially prevalent in head CT because of the subtle nature of the findings and the presence of dense bone in the skull.
C. Reduction of noise streaks
Electronic noise in EID CT adds noise to those parts of the sinogram that have poor Poisson statistics. Some newer detectors reduce this electronic noise through better design of the integrated circuit components [25, 26]. PCD CT can do still better in eliminating this electronic noise.
Fig. 4 shows a comparison between PCD CT and older EID CT scanners. Even with electronic noise eliminated, there are still noise streaks because of the limited counts present in the rays that must traverse the long axis of the patient. PCD CT can be used in conjunction with other strategies such as tube current modulation [32] or multi-dimensional filtering [33] which operate using different mechanisms to reduce these streaks.
Fig. 4.

Scan of an anthropomorphic phantom at 20 mAs with (left) EID and (right) PCD CT. The EID CT shows noise streaks and photon starvation near the center of the object. Adapted from Reference [2] with permission.
D. Brain imaging
As an example of the improvements possible in routine imaging, a prototype PCD CT scanner was compared against kV and mA matched EID CT for imaging human volunteers. Readers scored PCD CT better for gray-white matter differentiation and for image noise [34]. A related study compared contrast enhanced CT of the head and neck and showed that readers scored PCD CT better for image noise and artifacts [35].
Benchtop and phantom analyses has also been performed for dedicated stroke imaging. The lesions of interest in stroke imaging are small intracranial hemorrhage (millimeter sized) or occlusion of the major arteries. The smaller pixel size of PCD CT could improve detectability for these high frequency tasks. One detectability analysis suggested a 20% improvement for small intracranial hemorrhage [36]. A separate benchtop study showed advantages in CT angiography for detecting occlusion of mm-sized arteries in the brain [37].
IV. New Capabilities from PCD CT
A. Spectral characterization of incidental findings
With the exception of the dual layer detector, existing dual energy technologies require prospective selection of a dual-energy protocol. This is not a problem when the clinical indication for the CT scan is known a priori to require spectral information, such as classification of a suspected kidney stone. However, spectral information is sometimes desired for an incidental finding, and in these cases the ability of PCDs to provide retrospective spectral information is valuable.
One exemplar clinical application that we will discuss in detail is the incidental detection of a small renal mass. In contrast to previous eras where renal cancer was typically detected in an advanced stage from symptoms of hematuria, flank pain, and a palpable mass, today the majority of renal cancer is detected incidentally at an asymptomatic stage through cross-sectional imaging. When the proper protocol is applied, CT has the capacity to differentiate cancerous masses from benign cysts. An appropriately timed multiphase CT imaging is often sufficiently diagnostic to proceed directly to surgical extraction of the target mass without the need for a conformational biopsy [38]. On the other hand, a single-phase post-contrast CT scan may be insufficient to differentiate a cancerous mass from a harmless cyst. The telltale signature of a cancerous mass is that it enhances under iodine contrast because it retains a blood supply. The magnitude of this enhancement, however, may be only 10 HU.
Unfortunately, renal cysts are very common, with one study suggesting a prevalence of 27% [39]. In this context, when a possible renal mass is detected as an incidental finding, the radiologist must make a judgment. Ordering a follow-up scan would probably lead to anxiety and exposure of radiation but has the small probability of detecting a real cancer. PCD CT can eliminate the need for a follow-up scan by directly quantifying iodine content in each of these renal lesions to differentiate cancerous masses from benign cysts. Another application is in dedicated multi-phase renal scans. PCD CT could reduce pseudoenhancement, an effect where a cyst appears to undergo false enhancement because of beam hardening of the surrounding iodinated tissue [40].
PCDs can similarly characterize incidental adrenal, hepatic, and pancreatic lesions [41].
B. Improved resolution
PCD CT has the potential to double spatial resolution [42, 43]. The in-plane spatial resolution has been only minimally improved since 1990 [44]. PCDs offer the first significant increase of spatial resolution in several decades without substantially increasing the radiation dose, such as the combfilter based UHR approach used in clinical CT for imaging of temporal bone and extremities.
A common criticism is that smaller detector pixels will immediately lead to a large increase in noise as measured by standard deviation. However, this tradeoff is already present in EID CT and has been mitigated by use of a variety of kernels designed to reduce noise for specific tasks (e.g., body or soft kernels, compared to bone or sharp kernels). The same kernels could be extended to high resolution PCD CT. When soft or body kernels are used for the sake of low contrast lesions several mm in size, the spatial resolution is limited by the reconstruction kernel. Hence, the higher resolution of the PCD will not be easily visible in reconstructed images. However, when sharper kernels are used, the kernel can be designed in a way that reduces noise relative to standard resolution CT without changing the resolution characteristics (MTF) of the scanner. One simulation study suggested a 30% improvement in dose efficiency for imaging small objects via reduction of noise aliasing [45, 46]. Recently, investigations in phantoms and cadavers have shown that it is preferable to always acquire with smaller pixel size and reconstruct using standard kernels even when higher spatial resolution is not required. The reduction in radiation dose while maintaining the diagnostic image quality will benefit a wide range of patient sizes and exams [5]. PCD CT can be used to create “ultra-sharp” kernels that would fully exploit the smaller pixel size but which would indeed increase noise in the image. Current EID CT systems are capable of reaching ultra-sharp resolution using comb filters, which improve sampling characteristics by absorbing half or more of post-patient radiation [47].
The effect of PCD-CT imaging with smaller pixel sizes have been demonstrated by Klein et al. using a Siemens CounT in phantoms and cadavers [5]. An expected dose reduction of at least 10% in comparison to EID-CT has been reported. For applications requiring high-resolution capabilities such as temporal bone exams, da Silva et al. reported the characterization a prototype silicon-based PCD-CT capable of resolving up to 22 lp/cm in phantoms [48]. At our institution, exams of the temporal bone are performed using the comb filter. Phantom and cadaver studies showed that a 50% dose reduction could be achieved with PCD-CT due to the absence of comb filter [42]. Using a tin filter in addition to imaging with smaller pixel size, patient studies using PCD-CT have demonstrated substantial dose savings of about 80% for temporal bone exams (Fig. 5) [9].
Fig. 5.
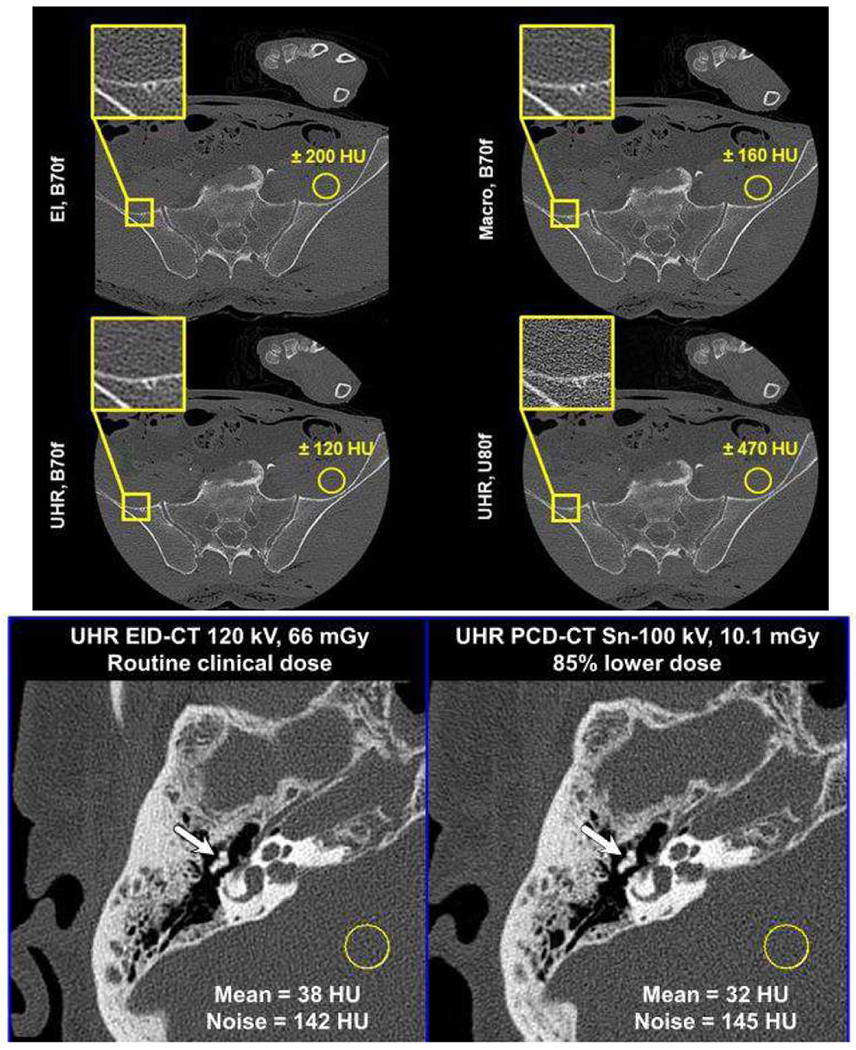
Comparison of dose efficiency for very high resolution tasks. (Top and center rows) Cadaver scan of a pelvis, comparing an energy-integrating detector (EI) with a PCD operating in either macro mode or UHR mode, with the UHR mode using smaller pixel size. All systems were scanned at the same dose. UHR mode shows improved noise using the B70f kernel. Adapted from [5] with permission. (Bottom row) The left and right columns shows EID and PCD CT, respectively. PCD CT reduces dose by (top row) 85% and (bottom row) 81% without reducing high-resolution detectability. The EID CT uses a comb filter, which reduces dose efficiency. (WW, WL) = (3200, 700) HU. Adapted from Reference [9] with permission.
Outside of the head, the higher resolution can also be useful in the evaluation of cardiac stents such as the detection of in-stent restenosis [47]. In the lung, the high resolution of PCD CT improves visualization of fine detail [49]. One example application is the evaluation of lung fissures completeness, which is necessary in making the decision to implant endobronchial valves for treatment of advanced emphysema [50]. Analysis of the margin of lung nodules might also aid in the differentiation of benign nodules and cancerous tumors, as spiculated nodules are known to be approximately 2.5 times more likely to be cancerous than those that are not [51].
C. High-resolution spectral imaging
Some spectral imaging tasks, like the detection of iodine in a large tumor, are low-resolution in nature and can be accomplished using a conventional dual energy CT system. Other spectral tasks are high-resolution in nature and also present very high contrast. For these tasks, the spectral imaging and high resolution capabilities of the PCD are synergistic.
Kidney stone classification is one such application. Kidney stones are a common source of pain and can arise from a wide variety of sources, from urinary tract infections to blood pH levels. The etiology and the treatment for kidney stones depends on the chemical composition of the stone. About 80% of kidney stones are calcium-based, but a minority of the stones are formed from uric acid. Pure stones which contain uric acid can be detected using dual energy techniques [52] and can be treated pharmacologically by alkanalization. For calcium-based stones, acute treatment options include shock wave lithotripsy or invasive endoscopic options that physically contact and destroy the stone. Existing dual energy techniques can do little to differentiate between subtypes of calcium stones, especially subtypes of calcium oxalate, which represent 75% of first time kidney stones [53].
Calcium oxalate stones can be divided into either monohydrate (COM) or dihydrate (COD) forms. Knowledge of stone composition could affect management because COM stones are known to be more difficult to fragment using shock wave lithotripsy. If it were known with high probability that a stone would be refractory to shock wave lithotripsy, the patient could be directed towards endoscopic intervention instead. High resolution micro-CT scans of ex vivo stones shows qualitative differences in stone morphology between COM and COD. While these differences can sometimes be inferred using the lower resolution of clinical CT, as Fig. 6 shows, the higher resolution of PCD CT could yield still better differentiation. Preliminary data suggests that PCD CT may be superior to EID CT for detecting and classifying small kidney stones [3].
Fig. 6.
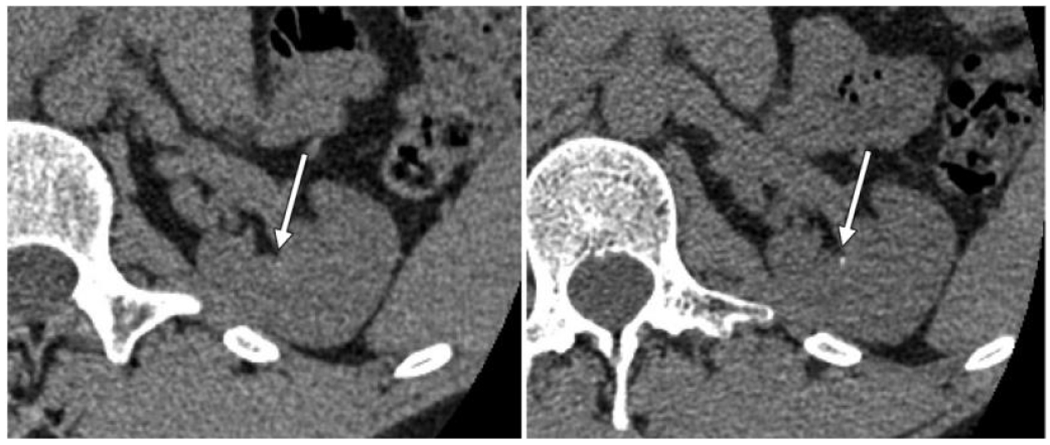
Detection of kidney stones with (left) EID and (right) PCD CT. The improved resolution of PCD enables better detectability for these small, high contrast objects. The enhanced spectral capabilities also enable better classification of the stone type. Adapted from Reference [3].
Another application area is atherosclerosis. Disease of the coronary arteries is a strong risk factor for myocardial infarction, and more precise quantification of this risk has been a longstanding goal of cardiology. Atherosclerotic plaques, once sufficiently advanced, develop calcium deposits that can be seen without the need for iodine contrast. The size and extent of these deposits can be converted to a standardized calcium score and used to estimate severity of atherosclerosis and the corresponding risk of myocardial infarct. The prognostic value of calcium scoring CT has been widely studied [54] but still remains controversial. There is an increase in risk between any visible calcium and zero calcium, with low amounts of coronary calcium (scores of 1-100 on the Agatston scale) typically showing four times the risk of those with zero calcium [54]. This is sometimes referred to as the “Power of Zero” and has also been said to confer a “warranty period” of up to 15 years [55]. The high resolution of PCD CT could enable further discrimination between patients with zero and nearly zero calcium, who might still harbor added risk.
Other areas that may benefit from high-resolution spectral imaging include musculoskeletal imaging. Dual energy may be particularly valuable in detecting edema, which is only apparent after subtracting out bone [56].
D. Stent imaging
Another specific area where the high resolution and spectral capabilities of PCD CT may be synergistic is in stent imaging. Visualization of cardiac stents is challenging because of the very high spatial and temporal resolution requirements. Under typical conditions and with current EID CT, the stent may appear as a bright cylinder within the vessel. PCD CT can enable the visualization of the individual struts of the stent. Fig. 7 compares the appearance of a stent using a prototype PCD CT scanner and a conventional EID CT scanner [6]. In vitro studies, with the stents placed in an iodinated vessel phantom, show that PCD CT is better able to visualize the lumen within the stent, including diameter or iodine concentration, than EID CT [57–59]. Similar results have also been reported with a recent high resolution EID CT scanner [47]. An added advantage of PCD CT is the ability to perform material decomposition to isolate the stent itself. One specific clinical application is the evaluation of in-stent restenosis. Even with drug eluting stents, in-stent restenosis can occur in 5% to 15% of patients [60]. This typically leads to further angina and the placement of a second stent to relieve symptoms, but some patients will present directly with acute myocardial infarction. The ability to evaluate in-stent restenosis is limited with current CT scanners because of the difficulty of visualizing subtle changes in lumen diameter in the presence of much stronger contrast of the stent itself and associated blooming artifacts. The high resolution of PCD CT could reduce blooming artifacts, and material decomposition could also be used to isolate the stent itself. Bratke et al. performed a phantom study showing that the lumen could be delineated in most cases with PCD CT but not with EID CT [61].
Fig. 7.

Comparison of a Promus Premier stent placed in a rabbit model, scanned using either a conventional EID CT scanner (Philips Brilliance 64) or with a prototype spectral photon counting CT (SPCCT) scanner, which is also capable of providing a platinum-only reconstruction. The details of the stent can be more cleanly visualized with SPCCT. Adapted from Reference [6] with permission.
E. Material decomposition
The multi-energy capabilities of energy-resolving PCDs have brought new attention to the material decomposition process. Possible techniques range from corrected linear estimators [62] to one-step inversion [63] to model-based material decomposition approach incorporating physical models (source, x-ray attenuation and detector) to account for system non-idealities. PCDs allow simultaneous acquisition of two or more energy bins, with user-defined energy thresholds. This facilitates customization of energy bins for the imaging task, such as setting the energy thresholds to capture the K-edge discontinuity of contrast materials such as gadolinium, bismuth, or gold nanoparticles. In addition, the ability to quantify the mass density of materials such as calcium, at high spatial resolution may improve calcium scoring. For instance, Juntunen et al. proposed a material decomposition framework (using regularized non-linear decomposition in projection space) to obtain quantitative calcium density score using PCD-CT as an alternative to the traditional Agatston scoring system widely employed in clinical coronary calcium scoring [64]. While the study was performed in static phantoms, the results demonstrate that a robust and improved calcium scoring method could be achieved using PCD-CT and material decomposition. Additionally, material decomposition is utilized to produce virtual non-contrast or virtual non-calcium images that are commonly used in clinical practice involving DECT. Improvements in the performance of basis decomposition techniques (quantification accuracy, noise properties, material separation) will likely translate to improved virtual image generation. Of note, the basis material decomposition techniques are inherently known to amplify image noise, and are prone to bias due to system non-idealities. Efforts to address these issues include using constrained regularization within the decomposition pipeline to suppress noise amplification [65] or using deep-learning framework to reduce bias in material decomposition [66]. Existing techniques are generally used for two- or three-material decomposition tasks. With the number of energy bins available on PCDs and its ability to simultaneously image multiple high-Z materials, active investigations are being pursued on material decomposition techniques for multicontrast imaging, as we will describe later in Section V. Existing literature and ongoing research directions are suggestive of the key role of material decomposition techniques in the application of PCD-CT for diagnostic imaging.
F. Universal protocol
Protocol optimization has been a goal of imaging physics since the earliest days of CT. Two of the largest advances in protocol optimization have been in the proper adaptation of adult CT protocols to the pediatric population through the Image Gently campaign [67] and the recognition that post-contrast scans may benefit from lower kVp [68]. While lower radiation dose is always desired, mistakes in protocol selection can lead to nondiagnostic images which do not benefit the patients being scanned. The advent of PCD CT would appear to further complicate this landscape by offering more parameters to tune in the form of bin thresholds.
Recent work by Zhou et al. [69] has suggested that protocol selection might counter-intuitively be simplified with PCD CT. Because PCD CT shows relatively improved performance at high kVp for iodine detection (due to its uniform photon weighting as explained in Section III), it may be possible to scan all patients at high kVp with fixed energy bin thresholds that do not need to be adapted to each patient. Because of the higher efficiency of PCD CT for detecting iodine at elevated kVp, this universal protocol would have only a modest dose penalty compared to a personalized protocol. This could improve and simplify the workflow for PCD CT.
G. Extremity imaging
Dedicated extremity CT scanners have previously been studied for musculoskeletal applications [70]. Because of their high native resolution and spectral capabilities, PCDs could add value to extremity CT, particularly in the detection of gout. Current dual energy CT scanners have shown the ability to detect gout, but are only capable of detecting large, dense uric acid deposits and have poor sensitivity on the per-lesion level [71]. The greater spectral separation of PCD CT could enable more accurate classification of smaller uric acid deposits and also improve differentiation against pseudogout [72]. A prototype extremity PCD CT has been designed by MARS Bioimaging and tested on human volunteers. Fig. 8 shows a volumetric rendering with material classification [73]. Another application is the assessment of fracture risk by analyzing bone microstructure, which may provide clues beyond standard bone mineral density [74]. Another clinical application for this technology is imaging of cartilage in joints. Studies in excised human knees have shown quantitative capabilities to characterize the glycosaminoglycans (a marker of cartilage health) [75]. More recently, similar capabilities have been demonstrated in large-animal model of osteoarthritis using whole-body PCD-CT [76]. Additionally, monoenergetic images obtained using PCD-CT has been shown to facilitate detection of cartilage and meniscal defects at high spatial resolution [77]. Clinical value demonstrated in human subjects remains to be investigated.
Fig. 8.
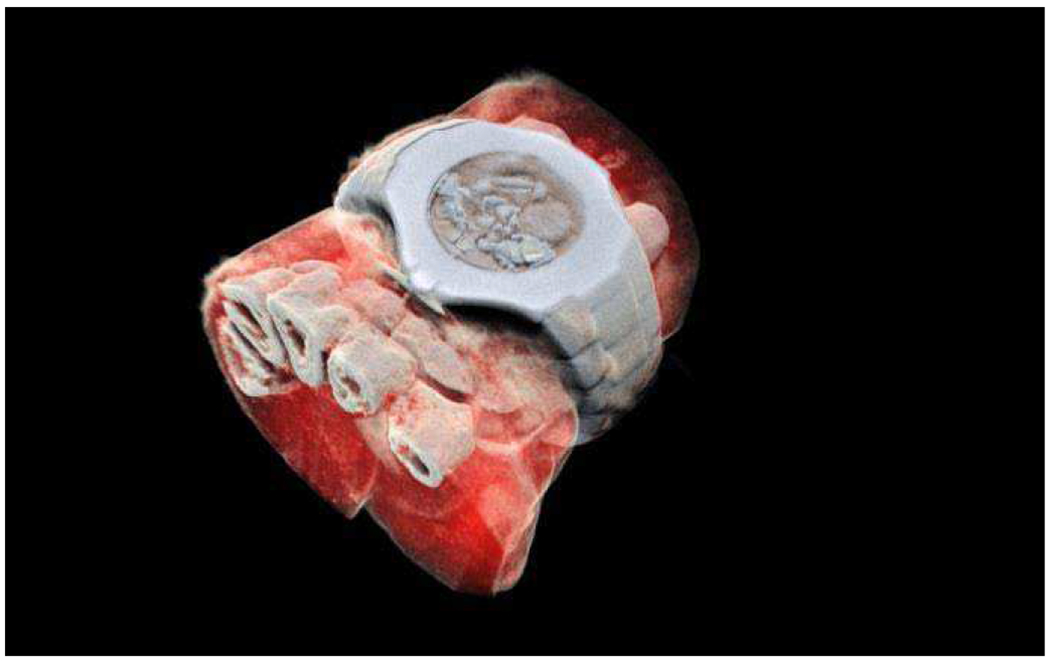
Volumetric rendering of an extremity PCD CT reconstruction of a human volunteer wrist including a wristwatch, scanned with the prototype MARS scanner. Image courtesy of MARS Bioimaging. Adapted from [RK Panta, IEEE 2018]. (©2018 IEEE).
V. Upcoming applications enabled by PCD CT
A. New contrast agents
PCD CT could provide new capabilities in contrast agents with molecular targeting. These contrast agents use k-edge metals such as gold nanoparticles that are embedded in a chemical structure that targets a specific biological process in the body. Material decomposition using PCD CT with three or more energy bins would allow isolation of the k-edge basis material. These molecular agents might identify preclinical atherosclerosis [78] or target cancer [79], including metastatic lesions that might otherwise be missed. Studies to date have been limited to preclinical models because of the substantial safety requirements needed for first in-human imaging.
A molecular contrast agent approved for human use in a common indication could energize the adoption of PCD CT into select patient populations. For example, if a targeted agent were developed that could highlight vulnerable plaque in atherosclerosis, PCD CT might become standard of care in determining optimal treatment for patients at high risk of myocardial ischemia. A targeted molecular agent for breast cancer could make PCD CT standard of care for breast cancer patients.
However, this vision is dependent on the development of such targeted agents. In some ways, PET is a more natural platform for molecular imaging. CT has limited sensitivity for contrast, with an estimated detection limit of 10−3 mol/l compared to 10−10 mol/l for PET [80]. Development of non-FDG contrast agents in PET has been slow, although very recently new agents have been approved for detection of metastatic prostate and neuroendocrine tumors [81]. While sensitivity is a challenge, the possible advantages of CT for molecular imaging are in higher resolution, faster scan times, and lower cost.
B. Multiple simultaneous contrast agents
Multi-phase imaging with a single scan is technically feasible with multi-bin PCD CT. In a conventional acquisition protocol, a non-contrast CT is first acquired prior to injection of iodine contrast, and then at different time points during perfusion, multiple post-contrast scans are acquired. In a multi-phase imaging protocol, different contrast agents are injected with different time delays and a single CT scan is performed. By calculating the amount of each contrast agent present in each voxel, multiple phases can be virtually synthesized. Multi-phase imaging is intuitively appealing but should be compared to virtual non-contrast (VNC) scanning with dual-energy CT which already provides two phases of imaging. VNC can theoretically simplify workflow but can also lead to image quality compromises. Basis material decomposition amplifies noise [82], and multi-phase CT will lead to even more noise amplification than dual phase CT. Advanced denoising using deep learning is one possibility [83] but this technique has not yet been fully validated. Multi-phase imaging also requires the development of new contrast agents besides iodine. Gadolinium is a natural candidate because of its use in MRI, but the doses required for adequate visualization in CT would be much higher than what is used in MRI.
Another promising direction is complementary modes of contrast. One example is in electronic cleansing for virtual colonoscopy. In patients unwilling to undergo laxative cleansing, a gadolinium-based solution could be swallowed and then differentiated from iodine contrast that is injected to highlight polyps [84]. Bismuth contrast could also be used and may be a safer alternative to gadolinium [85].
Another possible application is the quantification of iodine content in the microvessels surrounding the coronary arteries known as the vasa vasorum. Changes in these vessels can represent an early indication of atherosclerosis [86].
VI. Anticipated future developments
PCDs that are compatible with the high flux rates of CT have only recently appeared. While current prototype PCD CT scanners are capable of the flux rates required for human imaging, they have made several compromises in order to achieve this goal. As a result, multiple non-idealities are currently embedded in the current generation of PCDs. In the same way that the temporal resolution of CT rapidly improved after the first CT scanner was introduced by EMI in 1972, we might expect that the spectral resolution of PCD CT will rapidly improve in the years following the debut of clinical PCD CT. While the spectral response is partly limited by Compton scattering and k-escape, which are difficult to correct, substantial progress can be made by mitigation of charge sharing. We will briefly discuss current non-idealities and their mitigation strategies, and also look ahead to anticipate their impact on downstream clinical applications.
Charge sharing.
Modeling studies show that charge sharing causes degradations in spectral response [87]. One study estimated that these degradations in spectral response can translate to a dose efficiency penalty of approximately 50% [88]. Several schemes have been reported to reduce charge sharing, including charge summing [89] and digital schemes that operate after the comparator [90, 91]. Implementation of these corrections requires engineering effort but is scientifically feasible. Future generations of PCD CT may include these new technologies and benefit from reductions in charge sharing. Charge sharing harms conventional CT images because of the statistical effects of double counting photons, but it is especially harmful for material decomposition images. These corrections will especially benefit material decomposition applications such as imaging of k-edge agents.
Current studies [88] predict that conventional dual energy techniques, such as dual-source with added filtration on the high kV tube, can outperform PCD CT for spectral imaging tasks. For this reason, we have thus far characterized the main spectral advantage of PCD CT as providing retrospective availability, not higher image quality or spectral separation. When effective charge sharing compensation is introduced, PCD CT may outperform conventional dual energy CT in spectral imaging as well. An alternative path to improved spectral contrast is to dual source PCD CT with added filtration [92, 93]. While dual source PCD CT would be associated with higher infrastructure costs, it would also allow improved temporal resolution for cardiac scans.
Pileup.
Current prototype PCD CT systems have achieved low pileup at the expense of high charge sharing using small pixel sizes. The detrimental impact of pileup is not easily seen when imaging normal sized patients [94]. Improvements in counting speed could be used to increase the pixel size, which mediates the tradeoff between charge sharing and pileup [95]. This would allow the system to reduce both charge sharing and pileup simultaneously.
More bins.
The prototype PCD CT system present at our institution (Siemens CounT) allows only two energy bins per pixel. Four energy bins can be achieved by assigning different thresholds in each pixel in a chess pattern, but this is known to be dose inefficient. Many other PCDs in development include four or more energy bins per pixel. The introduction of more energy bins is a requirement before multiple contrast agents can be imaged simultaneously, and simulation studies show that it incrementally improves contrast-to-noise characteristics of spectral imaging more generally [88].
Noise reduction.
The emergence of AI or deep learning reconstruction [96] may enable more realistic noise reduction [83]. High-resolution images may require advanced denoising because of the high levels of noise that would result from conventional reconstruction techniques. AI noise reduction might also enable concepts such as multi-phase scanning with reduced contrast load using a single CT scan. Compared to AI reconstruction for EID CT, one advantage in PCD CT will be the ability to capitalize on the high resolution capabilities of PCD CT without suffering from the high noise that is typically associated with very sharp kernels.
A wide variety of approaches are currently available for advanced reconstruction. Optimization-based approaches have been available for the past decade [97] and, while sometimes computationally intensive, can still be applied to PCD CT. Specific variations of this optimization can be posed with the particular goal of improving spectral contrast or reducing noise in spectral images ()[65, 98]. The correlations that result from basis material decomposition can be exploited [99]. Nonlocal means has been used for CT noise reduction and may be especially useful in spectral CT [100]. Increasingly, deep learning reconstruction is being used [101]. While deep learning can be applied to either EID or PCD CT, it may be especially necessary to control the high noise that would otherwise result from high resolution and sharp kernels.
Resolution.
With PCD CT, the resolution limit of a CT scanner will no longer lie on the detector but will shift to the x-ray source. The native resolution of PCD CT could be easily made to be four times better than current EID CT, but a similar reduction in tube spot size (a 16-fold reduction in focal spot area) would be very difficult because the concentration of power on such a small area would melt the anode. In the long term, more research is needed to develop high-brilliance x-ray sources with smaller focal spots. While PCD CT represents an enormous improvement over EID CT for in-plane resolution, still higher resolution could be clinically useful in the evaluation of cardiac stents.
Outlook.
Taken together, we expect substantial further improvement in the spectral capabilities of PCD CT. This would lead to still better iodine imaging so that contrast-enhanced exams could be performed at either lower radiation dose or lower iodine contrast dose. The improvements in spectral imaging will also improve the detectability of new contrast agents, particularly k-edge contrast agents. However, the chief challenge for the adoption of these new contrast agents remains the toxicity of these new agents at doses that are sufficient to accomplish their diagnostic task.
VII. Conclusions
PCDs present a variety of advantages over conventional EIDs. In this review, we have highlighted the primary benefits of this technology: higher spatial resolution, continuous retrospective availability of spectral information, and absence of electronic noise. Each of these three individual advantages has already been captured using appropriately configured energy-integrating systems: high-resolution EIDs have recently appeared in a clinical system [47], retrospective availability of spectral information is available with dual-layer detectors [102], and reduction in electronic noise has occurred with certain newer detectors, although it has not been fully eliminated as it could be with PCD CT [26]. It should not be surprising that most of the clinical applications of PCD CT can already be performed with certain EID CT systems. The value of PCD CT is that it incorporates all of these elements into a single acquisition platform.
For many routine applications, PCD CT may provide a modest improvement. Scans with metal hardware will become incrementally easier to read because of better artifact suppression. Scans with iodine contrast could show better detectability, leading to lower imaging dose or contrast dose, especially at high kVp.
New capabilities of PCD CT include simplification and consolidation of a standardized universal imaging protocol and the ability to classify small high-contrast lesions such as lung tumors and kidney stones. For examinations of high resolution structures such as the temporal bone or sinus, radiation dose could be reduced. Truly transformative capabilities may occur if targeted molecular contrast agents become clinically available.
PCD CT is only in its infancy, with the first human scanners only recently appearing. The next several years are sure to reveal a wealth of information on the potential applications of PCD CT.
Acknowledgments
Research reported in this work was supported by the National Institutes of Health under award number R01 EB016966 and C06 RR018898. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. We also acknowledge support from Siemens AG.
References
- [1].Zhou W et al. , “Reduction of metal artifacts and improvement in dose efficiency using photon-counting detector computed tomography and tin filtration,” Investigative radiology, vol. 54, no. 4, pp. 204–211, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yu Z et al. , “Noise performance of low-dose CT: comparison between an energy integrating detector and a photon counting detector using a whole-body research photon counting CT scanner,” Journal of Medical Imaging, vol. 3, no. 4, p. 043503, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marcus RP et al. , “Detection and Characterization of Renal Stones by Using Photon-Counting–based CT,” Radiology, vol. 289, no. 2, pp. 436–442, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu Z et al. , “Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array,” Physics in Medicine & Biology, vol. 61, no. 4, p. 1572, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Klein L et al. , “Effects of Detector Sampling on Noise Reduction in Clinical Photon-Counting Whole-Body Computed Tomography,” Investigative radiology, vol. 55, no. 2, pp. 111–119, 2020. [DOI] [PubMed] [Google Scholar]
- [6].Sigovan M et al. , “Feasibility of improving vascular imaging in the presence of metallic stents using spectral photon counting CT and K-edge imaging,” Scientific Reports, vol. 9, no. 1, pp. 1–9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gutjahr R et al. , “Human imaging with photon-counting-based CT at clinical dose levels: Contrast-to-noise ratio and cadaver studies,” Investigative radiology, vol. 51, no. 7, p. 421, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pourmorteza A et al. , “Abdominal imaging with contrast-enhanced photon-counting CT: first human experience,” Radiology, vol. 279, no. 1, pp. 239–245, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rajendran K et al. , “Dose Reduction for Sinus and Temporal Bone Imaging Using Photon-Counting Detector CT With an Additional Tin Filter,” Investigative radiology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Klein L et al. , “Effects of Detector Sampling on Noise Reduction in Clinical Photon-Counting Whole-Body Computed Tomography,” Investigative radiology, 2019. [DOI] [PubMed] [Google Scholar]
- [11].Si-Mohamed S et al. , “Review of an initial experience with an experimental spectral photon-counting computed tomography system,” Nuclear Instruments and Methods in Physics Research Section A : Accelerators, Spectrometers, Detectors and Associated Equipment, vol. 873, pp. 27–35, 2017. [Google Scholar]
- [12].Walsh M et al. , “First CT using Medipix3 and the MARS-CT-3 spectral scanner,” Journal of Instrumentation, vol. 6, no. 01, p. C01095, 2011. [Google Scholar]
- [13].Marfo E et al. , “Assessment of material identification errors, image quality and radiation doses using small animal spectral photon-counting CT,” IEEE Transactions on Radiation and Plasma Medical Sciences, 2020. [Google Scholar]
- [14].Persson M et al. , “Energy-resolved CT imaging with a photon-counting silicon-strip detector,” Physics in Medicine & Biology, vol. 59, no. 22, p. 6709, 2014. [DOI] [PubMed] [Google Scholar]
- [15].Willemink MJ, Persson M, Pourmorteza A, Pelc NJ, and Fleischmann D, “Photon-counting CT: technical principles and clinical prospects,” Radiology, vol. 289, no. 2, pp. 293–312, 2018. [DOI] [PubMed] [Google Scholar]
- [16].Leng S et al. , “Photon-counting Detector CT: System Design and Clinical Applications of an Emerging Technology,” RadioGraphics, vol. 39, no. 3, pp. 729–743, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang AS and Pelc NJ, “Spectral Photon Counting CT: Imaging Algorithms and Performance Assessment,” IEEE Transactions on Radiation and Plasma Medical Sciences, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ballabriga R et al. , “Photon Counting Detectors for X-ray Imaging with Emphasis on CT,” IEEE Transactions on Radiation and Plasma Medical Sciences, 2020. [Google Scholar]
- [19].Taguchi K and Iwanczyk JS, “Vision 20/20: Single photon counting x-ray detectors in medical imaging,” Medical physics, vol. 40, no. 10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Taguchi K, “Energy-sensitive photon counting detector-based X-ray computed tomography,” Radiological physics and technology, vol. 10, no. 1, pp. 8–22, 2017. [DOI] [PubMed] [Google Scholar]
- [21].Bornefalk H and Danielsson M, “Photon-counting spectral computed tomography using silicon strip detectors: a feasibility study,” Physics in Medicine & Biology, vol. 55, no. 7, p. 1999, 2010. [DOI] [PubMed] [Google Scholar]
- [22].Loeliger T, Brönnimann C, Donath T, Schneebeli M, Schnyder R, and Trüb P, “The new PILATUS3 ASIC with instant retrigger capability,” in 2012 IEEE Nuclear Science Symposium and Medical Imaging Conference Record (NSS/MIC), 2012, pp. 610–615: IEEE. [Google Scholar]
- [23].Hsieh SS and Pelc NJ, “Improving pulse detection in multibin photon-counting detectors,” Journal of Medical Imaging, vol. 3, no. 2, p. 023505, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ballabriga R, Campbell M, Heijne E, Llopart X, Tlustos L, and Wong W, “Medipix3: A 64 k pixel detector readout chip working in single photon counting mode with improved spectrometric performance,” Nuclear Instruments and Methods in Physics Research Section A : Accelerators, Spectrometers, Detectors and Associated Equipment, vol. 633, pp. S15–S18, 2011. [Google Scholar]
- [25].Browne JE, Bruesewitz MR, Vrieze TJ, McCollough CH, and Yu L, “Increased Photon Starvation Artifacts at Low Helical Pitch in Ultra-Low-Dose CT,” Medical Physics, 2019. [DOI] [PubMed] [Google Scholar]
- [26].Duan X et al. , “Electronic noise in CT detectors: impact on image noise and artifacts,” American Journal of Roentgenology, vol. 201, no. 4, pp. W626–W632, 2013. [DOI] [PubMed] [Google Scholar]
- [27].Mehran R, Dangas GD, and Weisbord SD, “Contrast-associated acute kidney injury,” New England Journal of Medicine, vol. 380, no. 22, pp. 2146–2155, 2019. [DOI] [PubMed] [Google Scholar]
- [28].Schmidt TG, “CT energy weighting in the presence of scatter and limited energy resolution,” Medical physics, vol. 37, no. 3, pp. 1056–1067, 2010. [DOI] [PubMed] [Google Scholar]
- [29].Boas FE and Fleischmann D, “Evaluation of two iterative techniques for reducing metal artifacts in computed tomography,” Radiology, vol. 259, no. 3, pp. 894–902, 2011. [DOI] [PubMed] [Google Scholar]
- [30].Meyer E, Raupach R, Lell M, Schmidt B, and Kachelrieß M, “Normalized metal artifact reduction (NMAR) in computed tomography,” Medical physics, vol. 37, no. 10, pp. 5482–5493, 2010. [DOI] [PubMed] [Google Scholar]
- [31].Lehmann L et al. , “Generalized image combinations in dual KVP digital radiography,” Medical physics, vol. 8, no. 5, pp. 659–667, 1981. [DOI] [PubMed] [Google Scholar]
- [32].Gies M, Kalender WA, Wolf H, Suess C, and Madsen MT, “Dose reduction in CT by anatomically adapted tube current modulation. I. Simulation studies,” Medical physics, vol. 26, no. 11, pp. 2235–2247, 1999. [DOI] [PubMed] [Google Scholar]
- [33].Kachelriess M, Watzke O, and Kalender WA, “Generalized multi-dimensional adaptive filtering for conventional and spiral single-slice, multi-slice, and cone-beam CT,” Medical physics, vol. 28, no. 4, pp. 475–490, 2001. [DOI] [PubMed] [Google Scholar]
- [34].Pourmorteza A et al. , “Photon-counting CT of the brain: in vivo human results and image-quality assessment,” American Journal of Neuroradiology, vol. 38, no. 12, pp. 2257–2263, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Symons R et al. , “Photon-counting CT for vascular imaging of the head and neck: first in vivo human results,” Investigative radiology, vol. 53, no. 3, p. 135, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ji X, Zhang R, Chen G-H, and Li K, “Task-driven optimization of the non-spectral mode of photon counting CT for intracranial hemorrhage assessment,” Physics in Medicine & Biology, vol. 64, no. 21, p. 215014, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Harvey EC et al. , “Impacts of photon counting CT to maximum intensity projection (MIP) images of cerebral CT angiography: theoretical and experimental studies,” Physics in Medicine & Biology, vol. 64, no. 18, p. 185015, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Campbell S et al. , “Renal mass and localized renal cancer: AUA guideline,” The Journal of urology, vol. 198, no. 3, pp. 520–529, 2017. [DOI] [PubMed] [Google Scholar]
- [39].Tada S, Yamagishi J, Kobayashi H, Hata Y, and Kobari T, “The incidence of simple renal cyst by computed tomography,” Clinical radiology, vol. 34, no. 4, pp. 437–439, 1983. [DOI] [PubMed] [Google Scholar]
- [40].Maki DD, Birnbaum BA, Chakraborty DP, Jacobs JE, Carvalho BM, and Herman GT, “Renal cyst pseudoenhancement: beam-hardening effects on CT numbers,” Radiology, vol. 213, no. 2, pp. 468–472, 1999. [DOI] [PubMed] [Google Scholar]
- [41].Wortman JR, Bunch PM, Fulwadhva UP, Bonci GA, and Sodickson AD, “Dual-energy CT of incidental findings in the abdomen: can we reduce the need for follow-up imaging?,” American Journal of Roentgenology, vol. 207, no. 4, pp. W58–W68, 2016. [DOI] [PubMed] [Google Scholar]
- [42].Leng S et al. , “Dose-efficient ultrahigh-resolution scan mode using a photon counting detector computed tomography system,” Journal of Medical Imaging, vol. 3, no. 4, p. 043504, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Leng S et al. , “150-μm spatial resolution using photon-counting detector computed tomography technology: technical performance and first patient images,” Investigative radiology, vol. 53, no. 11, pp. 655–662, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kalender WA, “X-ray computed tomography,” Physics in Medicine & Biology, vol. 51, no. 13, p. R29, 2006. [DOI] [PubMed] [Google Scholar]
- [45].Baek J, Pineda AR, and Pelc NJ, “To bin or not to bin? The effect of CT system limiting resolution on noise and detectability,” Physics in Medicine & Biology, vol. 58, no. 5, p. 1433, 2013. [DOI] [PubMed] [Google Scholar]
- [46].Kachelrieß M and Kalender WA, “Presampling, algorithm factors, and noise: Considerations for CT in particular and for medical imaging in general,” Medical physics, vol. 32, no. 5, pp. 1321–1334, 2005. [DOI] [PubMed] [Google Scholar]
- [47].Onishi H et al. , “Phantom study of in-stent restenosis at high-spatial-resolution CT,” Radiology, vol. 289, no. 1, pp. 255–260, 2018. [DOI] [PubMed] [Google Scholar]
- [48].da Silva J et al. , “Resolution characterization of a silicon-based, photon-counting computed tomography prototype capable of patient scanning,” Journal of Medical Imaging, vol. 6, no. 4, p. 043502, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bartlett DJ et al. , “High-Resolution Chest Computed Tomography Imaging of the Lungs: Impact of 1024 Matrix Reconstruction and Photon-Counting Detector Computed Tomography,” Investigative radiology, vol. 54, no. 3, pp. 129–137, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sciurba FC et al. , “A randomized study of endobronchial valves for advanced emphysema,” New England Journal of Medicine, vol. 363, no. 13, pp. 1233–1244, 2010. [DOI] [PubMed] [Google Scholar]
- [51].McWilliams A et al. , “Probability of cancer in pulmonary nodules detected on first screening CT,” New England Journal of Medicine, vol. 369, no. 10, pp. 910–919, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Primak AN et al. , “Noninvasive differentiation of uric acid versus non–uric acid kidney stones using dual-energy CT,” Academic radiology, vol. 14, no. 12, pp. 1441–1447, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Singh P et al. , “Stone composition among first-time symptomatic kidney stone formers in the community,” in Mayo Clinic Proceedings, 2015, vol. 90, no. 10, pp. 1356–1365: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Detrano R et al. , “Coronary calcium as a predictor of coronary events in four racial or ethnic groups,” New England Journal of Medicine, vol. 358, no. 13, pp. 1336–1345, 2008. [DOI] [PubMed] [Google Scholar]
- [55].Valenti V et al. , “A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals,” JACC: Cardiovascular Imaging, vol. 8, no. 8, pp. 900–909, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Baffour FI et al. , “Clinical utility of virtual noncalcium dual-energy CT in imaging of the pelvis and hip,” Skeletal radiology, pp. 1–10, 2019. [DOI] [PubMed] [Google Scholar]
- [57].von Spiczak J et al. , “Photon counting computed tomography with dedicated sharp convolution kernels: tapping the potential of a new technology for stent imaging,” Investigative radiology, vol. 53, no. 8, pp. 486–494, 2018. [DOI] [PubMed] [Google Scholar]
- [58].Mannil M et al. , “Photon-counting CT: high-resolution imaging of coronary stents,” Investigative radiology, vol. 53, no. 3, pp. 143–149, 2018. [DOI] [PubMed] [Google Scholar]
- [59].Symons R et al. , “Quarter-millimeter spectral coronary stent imaging with photon-counting CT: initial experience,” Journal of cardiovascular computed tomography, vol. 12, no. 6, pp. 509–515, 2018. [DOI] [PubMed] [Google Scholar]
- [60].Dibra A et al. , “Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients,” New England Journal of Medicine, vol. 353, no. 7, pp. 663–670, 2005. [DOI] [PubMed] [Google Scholar]
- [61].Bratke G et al. , “Spectral Photon-Counting Computed Tomography for Coronary Stent Imaging: Evaluation of the Potential Clinical Impact for the Delineation of In-Stent Restenosis,” Investigative radiology, vol. 55, no. 2, pp. 61–67, 2020. [DOI] [PubMed] [Google Scholar]
- [62].Alvarez RE, “Estimator for photon counting energy selective x-ray imaging with multibin pulse height analysis,” Medical physics, vol. 38, no. 5, pp. 2324–2334, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schmidt TG, Barber RF, and Sidky EY, “A spectral CT method to directly estimate basis material maps from experimental photon-counting data,” IEEE transactions on medical imaging, vol. 36, no. 9, pp. 1808–1819, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Juntunen MA et al. , “Framework for Photon Counting Quantitative Material Decomposition,” IEEE transactions on medical imaging, 2019. [DOI] [PubMed] [Google Scholar]
- [65].Tao S, Rajendran K, McCollough CH, and Leng S, “Material decomposition with prior knowledge aware iterative denoising (MD-PKAID),” Physics in Medicine & Biology, vol. 63, no. 19, p. 195003, 2018. [DOI] [PubMed] [Google Scholar]
- [66].Lu Y et al. , “A learning-based material decomposition pipeline for multi-energy x-ray imaging,” Medical physics, vol. 46, no. 2, pp. 689–703, 2019. [DOI] [PubMed] [Google Scholar]
- [67].Goske MJ et al. , “The Image Gently campaign: working together to change practice,” American Journal of Roentgenology, vol. 190, no. 2, pp. 273–274, 2008. [DOI] [PubMed] [Google Scholar]
- [68].Yu L, Li H, Fletcher JG, and McCollough CH, “Automatic selection of tube potential for radiation dose reduction in CT: a general strategy,” Medical physics, vol. 37, no. 1, pp. 234–243, 2010. [DOI] [PubMed] [Google Scholar]
- [69].Zhou W, Abdurakhimova D, Bruesewitz M, Halaweish A, McCollough CH, and Leng S, “Impact of photon counting detector technology on kV selection and diagnostic workflow in CT,” in Medical Imaging 2018: Physics of Medical Imaging, 2018, vol. 10573, p. 105731C: International Society for Optics and Photonics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Carrino JA et al. , “Dedicated cone-beam CT system for extremity imaging,” Radiology, vol. 270, no. 3, pp. 816–824, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Melzer R, Pauli C, Treumann T, and Krauss B, “Gout tophus detection—a comparison of dual-energy CT (DECT) and histology,” in Seminars in arthritis and rheumatism, 2014, vol. 43, no. 5, pp. 662–665: Elsevier. [DOI] [PubMed] [Google Scholar]
- [72].Stamp LK et al. , “Clinical utility of multi-energy spectral photon-counting computed tomography in crystal arthritis,” Arthritis & rheumatology, vol. 71, no. 7, pp. 1158–1162, 2019. [DOI] [PubMed] [Google Scholar]
- [73].Panta RK et al. , “First human imaging with MARS photon-counting CT,” in 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), 2018, pp. 1–7: IEEE. [Google Scholar]
- [74].Matanaghi A et al. , “Semi-automatic quantitative assessment of site-specific bone health using spectral photon counting CT,” Journal of Nuclear Medicine, vol. 60, no. supplement 1, pp. 1297–1297, 2019. [Google Scholar]
- [75].Rajendran K et al. , “Quantitative imaging of excised osteoarthritic cartilage using spectral CT,” European radiology, vol. 27, no. 1, pp. 384–392, 2017. [DOI] [PubMed] [Google Scholar]
- [76].Rajendran K et al. , “Quantitative Knee Arthrography in a Large Animal Model of Osteoarthritis Using Photon-Counting Detector CT,” Investigative Radiology, vol. 55, no. 6, pp. 349–356, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chappard C et al. , “Feasibility of spectral computed tomography to assess knee cartilage,” Osteoarthritis and Cartilage, vol. 28, pp. S277–S278, 2020. [Google Scholar]
- [78].Cormode DP et al. , “Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles,” Radiology, vol. 256, no. 3, pp. 774–782, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Reuveni T, Motiei M, Romman Z, Popovtzer A, and Popovtzer R, “Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study,” International journal of nanomedicine, vol. 6, p. 2859, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Roessl E, Brendel B, Engel K-J, Schlomka J-P, Thran A, and Proksa R, “Sensitivity of Photon-Counting Based ${\rm K} $-Edge Imaging in X-ray Computed Tomography,” IEEE transactions on medical imaging, vol. 30, no. 9, pp. 1678–1690, 2011. [DOI] [PubMed] [Google Scholar]
- [81].Clarke BN, “PET Radiopharmaceuticals: What’s new, what’s reimbursed, and what’s next?,” Journal of nuclear medicine technology, vol. 46, no. 1, pp. 12–16, 2018. [DOI] [PubMed] [Google Scholar]
- [82].Ren L, Rajendran K, McCollough CH, and Yu L, “Radiation dose efficiency of multi-energy photon-counting-detector CT for dual-contrast imaging,” Physics in Medicine & Biology, vol. 64, no. 24, p. 245003, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Poirot MG et al. , “physics-informed Deep Learning for Dual-energy computed tomography image processing,” Scientific reports, vol. 9, no. 1, pp. 1–9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Muenzel D et al. , “Spectral photon-counting CT: Initial experience with dual–contrast agent K-edge colonography,” Radiology, vol. 283, no. 3, pp. 723–728, 2016. [DOI] [PubMed] [Google Scholar]
- [85].Symons R et al. , “Photon-counting CT for simultaneous imaging of multiple contrast agents in the abdomen: an in vivo study,” Medical physics, vol. 44, no. 10, pp. 5120–5127, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sheikhzadeh M et al. , “Impact of Effective Detector Pixel and CT Voxel Size on Accurate Estimation of Blood Volume in Opacified Microvasculature,” Academic radiology, vol. 26, no. 10, pp. 1410–1416, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Taguchi K, Stierstorfer K, Polster C, Lee O, and Kappler S, “Spatio-energetic cross-talk in photon counting detectors: Numerical detector model (Pc TK) and workflow for CT image quality assessment,” Medical physics, vol. 45, no. 5, pp. 1985–1998, 2018. [DOI] [PubMed] [Google Scholar]
- [88].Faby S et al. , “Performance of today’s dual energy CT and future multi energy CT in virtual non-contrast imaging and in iodine quantification: a simulation study,” Medical physics, vol. 42, no. 7, pp. 4349–4366, 2015. [DOI] [PubMed] [Google Scholar]
- [89].Koenig T et al. , “How spectroscopic x-ray imaging benefits from interpixel communication,” Physics in Medicine & Biology, vol. 59, no. 20, p. 6195, 2014. [DOI] [PubMed] [Google Scholar]
- [90].Hsieh SS, “Coincidence counters for charge sharing compensation in spectroscopic photon counting detectors,” IEEE transactions on medical imaging, 2019. [DOI] [PubMed] [Google Scholar]
- [91].Hsieh SS and Sjolin M, “Digital count summing vs analog charge summing for photon counting detectors: A performance simulation study,” Medical physics, vol. 45, no. 9, pp. 4085–4093, 2018. [DOI] [PubMed] [Google Scholar]
- [92].Tao S, Rajendran K, McCollough CH, and Leng S, “Feasibility of Multi-Contrast Imaging on Dual-Source Photon Counting Detector (PCD) CT: An Initial Phantom Study,” Medical Physics, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tao A, Huang R, Tao S, Michalak GJ, McCollough CH, and Leng S, “Dual-source photon counting detector CT with a tin filter: a phantom study on iodine quantification performance,” Physics in Medicine & Biology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kappler S, Henning A, Kreisler B, Schoeck F, Stierstorfer K, and Flohr T, “Photon counting CT at elevated X-ray tube currents: contrast stability, image noise and multi-energy performance,” in Medical Imaging 2014: Physics of Medical Imaging, 2014, vol. 9033, p. 90331C: International Society for Optics and Photonics. [Google Scholar]
- [95].Hsieh SS, Rajbhandary PL, and Pelc NJ, “Spectral resolution and high-flux capability tradeoffs in CdTe detectors for clinical CT,” Medical physics, vol. 45, no. 4, pp. 1433–1443, 2018. [DOI] [PubMed] [Google Scholar]
- [96].Zhu B, Liu JZ, Cauley SF, Rosen BR, and Rosen MS, “Image reconstruction by domain-transform manifold learning,” Nature, vol. 555, no. 7697, p. 487, 2018. [DOI] [PubMed] [Google Scholar]
- [97].Thibault JB, Sauer KD, Bouman CA, and Hsieh J, “A three-dimensional statistical approach to improved image quality for multislice helical CT,” Medical physics, vol. 34, no. 11, pp. 4526–4544, 2007. [DOI] [PubMed] [Google Scholar]
- [98].Tao S, Rajendran K, Zhou W, Fletcher JG, McCollough CH, and Leng S, “Improving iodine contrast to noise ratio using virtual monoenergetic imaging and prior-knowledge-aware iterative denoising (mono-PKAID),” Physics in Medicine & Biology, vol. 64, no. 10, p. 105014, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Leng S, Yu L, Wang J, Fletcher JG, Mistretta CA, and McCollough CH, “Noise reduction in spectral CT: Reducing dose and breaking the trade-off between image noise and energy bin selection,” Medical physics, vol. 38, no. 9, pp. 4946–4957, 2011. [DOI] [PubMed] [Google Scholar]
- [100].Li Z, Leng S, Yu L, Manduca A, and McCollough CH, “An effective noise reduction method for multi-energy CT images that exploit spatio-spectral features,” Medical physics, vol. 44, no. 5, pp. 1610–1623, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Missert AD, Leng S, McCollough CH, Fletcher JG, and Yu L, “Simulation of CT images reconstructed with different kernels using a convolutional neural network and its implications for efficient CT workflow,” in Medical Imaging 2019: Physics of Medical Imaging, 2019, vol. 10948, p. 109482Y: International Society for Optics and Photonics. [Google Scholar]
- [102].Ozguner O, Dhanantwari A, Halliburton S, Wen G, Utrup S, and Jordan D, “Objective image characterization of a spectral CT scanner with dual-layer detector,” Physics in Medicine & Biology, vol. 63, no. 2, p. 025027, 2018. [DOI] [PubMed] [Google Scholar]


