Abstract
The purpose of this scoping review by the American Society for Parenteral and Enteral Nutrition (ASPEN) Coronavirus Disease 2019 (COVID-19) Nutrition Task Force was to examine nutrition research applicable to the COVID-19 pandemic. The rapid pace of emerging scientific information has prompted this activity to discover research/knowledge gaps. This methodology adhered with recommendations from the Joanna Briggs Institute. There were 2301 citations imported. Of these, there were 439 articles fully abstracted, with 23 main topic areas identified across 24 article types and sourced across 61 countries and 51 specialties in 8 settings and among 14 populations. Epidemiological/mechanistic relationships between nutrition and COVID-19 were reviewed and results mapped to the Population, Intervention, Comparator, Outcome, and Time (PICO-T) questions. The aggregated data were analyzed by clinical stage: pre–COVID-19, acute COVID-19, and chronic/post–COVID-19. Research gaps were discovered for all PICO-T questions. Nutrition topics meriting urgent research included food insecurity/societal infrastructure and transcultural factors (pre–COVID-19); cardiometabolic-based chronic disease, pediatrics, nutrition support, and hospital infrastructure (acute COVID-19); registered dietitian nutritionist counseling (chronic/post–COVID-19); and malnutrition and management (all stages). The paucity of randomized controlled trials (RCTs) was particularly glaring. Knowledge gaps were discovered for PICO-T questions on pediatrics, micronutrients, bariatric surgery, and transcultural factors (pre–COVID-19); enteral nutrition, protein-energy requirements, and glycemic control with nutrition (acute COVID-19); and home enteral and parenteral nutrition support (chronic/post–COVID-19). In conclusion, multiple critical areas for urgent nutrition research were identified, particularly using RCT design, to improve nutrition care for patients before, during, and after COVID-19.
Keywords: cardiometabolic risk, coronavirus disease 2019, diabetes, enteral nutrition, knowledge gap, knowledge translation, metabolic syndrome, nutrition, nutrition research, nutrition support therapy, obesity, parenteral nutrition, research gap, severe acute respiratory syndrome coronavirus-2, scoping review, social determinants of health
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and related coronavirus disease 2019 (COVID-19) reached pandemic dimensions on March 11, 2020.1 Based on the most recent epidemiological studies available, there are specific risk factors for severe COVID-19 (hospitalization, intensive care unit [ICU] admission, mechanical ventilation, or mortality) (Table 1).2–14 Each of these risk factors can be interpreted in terms of associational to causal relationships, depending on the nature of supporting scientific evidence (Table 2).15–19 By focusing on nutrition risk factors in COVID-19, specific clinically relevant Population, Intervention, Comparator, Outcome, and Time(PICO-T)–formatted questions can be formulated to identify gaps in the current literature. In this scoping review, 56 PICO-T questions were formulated as a group consensus (Table 3). Current evidence was mapped to these PICO-T questions to identify research and knowledge gaps (Table 4).
Table 1.
Risk Factors Associated with Severe COVID-19.
| Type | Risk factor | OR (reference) |
|---|---|---|
|
| ||
| Demographics | Increased age 41–60 years and mortality (Mexico) | 3.43 (CI 95% [2.69–4.37]) 6 |
| Increased age 61–80 years and mortality (Mexico) | 7.00 (CI 95% [5.49–8.93]) 6 | |
| Increased age > 80 years and mortality (Mexico) | 11.55 (CI 95% [8.95–14.91]) 6 | |
| Male and mortality (Mexico) | 1.43 (CI 95% [1.38–1.49]) 6 | |
| Social determinants | African American and hospitalization (USA) | 3.2 (CI 95% [1.80–5.80]) 7 |
| Asian and hospitalization (UK) | 2.1 (CI 95% [1.50–3.20]) 8 | |
| Low income and hospitalization (UK) | 2.00 (CI 95% [1.63–2.47]) 9 | |
| Disadvantaged education level and hospitalization (UK) | 2.05 (CI 95% [1.70–2.47]) 9 | |
| Lack of medical insurance and hospitalization (USA) | 2.8 (CI 95% [1.10–7.30]) 7 | |
| Cardiometabolic | ABCDa as obesity and hospitalization (USA) | 1.9 (CI 95% [1.10–3.30]) 7 |
| DBCDb as T2D and hospitalization (USA) | 3.1 (CI 95% [1.70–5.90]) 7 | |
| HTN and hospitalization (China) | 2.0 (CI 95% [1.30–3.20]) 10 | |
| Lower LDL with mortality (China) | 21.72 (CI 95% [1.40–337.54]) 11 | |
| Tobacco use and hospitalization (USA) | 2.3 (CI 95% [1.20–4.50]) 7 | |
| CMBCDc as CVD and mortality (China) | 12.83 (CI 95% [10.27–15.86]) 6 | |
| Immunity/chronic disease | Reduced CD4+ T cells with cancer and hospitalization (China) | 0·84 (CI 95% [0.71–0.98]) 12 |
| Reduced serum albumin level/globulin with cancer and hospitalization (China) | 0·12 (CI 95% [0·02–0·77]) 12 | |
| Cancer and hospitalization (China) | 3·61 (CI 95% [2·59–5·04]) 12 | |
| Underlying disease and mortality (Iran) | 1.53 (CI 95% [1.04–2.24]) 13 | |
| Lymphopenia and mortality (China) | 13.130 (CI 95% [1.63–105.66]) 14 | |
| Hypoalbuminemia and mortality (China) | 6.39 (CI 95% [1.32–31.09]) 14 | |
| Comorbidities and mortality (China) | 6.82 (CI 95% [1.36–34.13]) 14 | |
ABCD, adiposity-based chronic disease; CMBCD, cardiometabolic-based chronic disease; COVID-19, coronavirus disease 2019; CVD, cardiovascular disease; DBCD, dysglycemia-based chronic disease; HTN, hypertension; OR, odds ratio; T2D, type 2 diabetes.
Abnormal adiposity (in terms of amount [increased], distribution, and secretory function) 2.
Spectrum including insulin resistance, hyperglycemia from β-cell defect (prediabetes to diabetes), and cardiovascular complications 3.
Framework centering on abnormal adiposity, insulin resistance, and CVD4,5.
Table 2.
Proposed Mechanisms Linking Risk Factors With Severe COVID-19.
| Type | Risk factor | Proposed mechanism | References |
|---|---|---|---|
|
| |||
| Nonmodifiable | Increased age | Increased ACE2 expression, viral load, and proinflammatory immune responses | 15 |
| Male | Androgens modulate TMPRSS2 gene expression TMPRSS2 primes SARS-CoV-2 spike protein | 16 | |
| Modifiable | Social determinants of health | Disenfranchisement leads to decreased access to quality healthcare and increased exposure to unhealthy behaviors and environmental factors, leading to poorer health and increased risk factors for chronic disease and COVID-19 severity | 17 |
| Cardiometabolic | Based on the CMBCD model,1 there is increased ACE2 susceptibility and loss of cardioprotection, hypercytokinemia and insulin resistance, and SARS-CoV-2 and ACE2-mediated β-cell defect | 18 | |
| Immunity/chronic disease | Increased endothelial dysfunction, proinflammatory state, and abnormal innate immune response | 19 | |
ACE2, angiotensin-converting enzyme 2; CMBCD, cardiometabolic-based chronic disease; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane protease, serine 2.
Table 3.
Nutrition Issues Related to the COVID-19 Pandemic.a
| Stage 1: Pre–COVID-19 | Stage 2: Acute COVID-19 | Stage 3: Chronic/post–COVID-19 |
|---|---|---|
|
| ||
| Risk mitigation | Outpatient | Outpatient |
| Malnutrition | Nutrition risk assessment | Nutrition risk assessment |
| Cardiometabolicb | Healthy eating and lifestyle | Healthy eating and lifestyle |
| Immunity/chronic disease | Micronutrient nutriture and support | Standard nutrition and nutrition support |
| Social determinants of health | Inpatient: non-ICU or ICU | Micronutrient nutriture and support |
| Lifestyle | Nutrition risk assessment | Nutrition and physical therapy |
| Transcultural dietary factors | Standard nutrition | Complication-specific nutritiond |
| Nutrition support | Infrastructurec | |
| Nutrition, insulin, and glycemic control | Inpatient non-ICU or ICU | |
| Micronutrient nutriture and support | Nutrition risk assessment | |
| Infrastructurec | Chronic critical illness metabolic support | |
| Standard nutrition and nutrition support | ||
| Micronutrient nutriture and support | ||
| Nutrition and physical therapy | ||
| Complication-specific nutritiond | ||
| Infrastructurec | ||
COVID-19, coronavirus disease 2019; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
This 3-stage model is based on the presumed natural history of COVID-19 and applies to pediatric, adult, and geriatric populations, domestic (US) and global.
Cardiometabolic risk factors associated with increased severity of COVID-19 include: hypertension, obesity, diabetes, and cardiovascular disease.
Infrastructural changes needed to address shortages (eg, enteral pumps) and supply chain, redeployments, training, new programs, and adaptive protocols.
COVID-19 complications include encephalopathy, deconditioning, acute kidney injury and chronic kidney disease, hyperglycemia, hypercoagulable/prothrombotic state, cardiac injury, and pulmonary injury.
Table 4.
A Priori Critical PICO-T Questions on COVID-19 and Nutrition and Mapping to Heat Map Nutrition Topics.a
| COVID-19 stage and issue | Critical PICO-T question | Heat map nutrition topic |
|---|---|---|
|
| ||
| Pre–COVID-19 | ||
| Risk mitigation Malnutrition | 1. In the adult and pediatric populations, are there any specific foods vs no specific foods that affect the risk of COVID-19 severity? | NONE |
| 2. In the adult population, what is the effect of micronutrient supplementation vs no supplementation on immune function–mediated risk for COVID-19 severity? | NONE | |
| 3. In adults with type 2 diabetes, does the use of nutraceuticals for those with poorly controlled blood glucose, compared with nonuse, confer any benefit on glycemic control during and after acute COVID-19? | MALNUTRITION | |
| 4. In patients aged >65 years with decreased activities of daily living (eg, isolation and decreased mobility), what is the effect of poor vs adequate nutrition intake and/or nutrition status on increased COVID-19 severity? | MALNUTRITION | |
| 5. What challenges do hospital organizations face regarding the safe handling and administration of expressed human milk, compared with use of standard commercial formulas, during the COVID-19 pandemic? | BREASTFEEDING CONSIDERATIONS | |
| 6. In neonates born to mothers with SARS-CoV-2 infection, does breastfeeding vs formula feeding increase neonatal risk for SARS-CoV-2 infection? | BREASTFEEDING CONSIDERATIONS | |
| 7. In infants born to mothers with SARS-CoV-2 infection, is donated human milk a safe source of nutrition, compared with mother’s breast milk, to mitigate risk for infection transmission and meet growth and development milestones? | BREASTFEEDING CONSIDERATIONS | |
| 8. In new breastfeeding mothers not infected with SARS-CoV-2, will infection mitigation procedures of mask wearing and hand and breast hygiene, compared with no mitigation procedures, offer sufficient protection from infection for newborns? | BREASTFEEDING CONSIDERATIONS | |
| Cardiometabolic | 9. In the adult population, does the presence vs absence or control vs noncontrol of any cardiometabolic risk factor (abnormal adiposity, dysglycemia, hypertension, cardiovascular disease, etc) alter the immune system, adversely affect nutrition status, and/or increase the risk of COVID-19 severity? | CARDIOMETABOLIC DISEASES |
| 10. In the adult population with obesity, does a change, vs no change, to a healthy diet reduce the risk of COVID-19 severity? | CARDIOMETABOLIC DISEASES | |
| 11. In the adult population with obesity, does having vs not having a history of bariatric procedure(s) increase the risk of COVID-19 severity? | NONE | |
| Social determinants of health Lifestyle | 12. In the adult population, do interventions that address food insecurity, vs no actions, affect the risk of increased COVID-19 severity? | FOOD INSECURITY/SOCIETAL INFRASTRUCTURE |
| 13. In school age children, does aggressive case finding for malnutrition and food insecurity in underserved populations, compared with not case finding, improve the clinical course of COVID-19? | FOOD INSECURITY/SOCIETAL INFRASTRUCTURE | |
| Transcultural | 14. In patients >65 years of age in the US, does being an ethnic minority vs Caucasian increase the negative effect of malnutrition on increased COVID-19 severity? | FOOD INSECURITY/SOCIETAL INFRASTRUCTURE |
| 15. Among high-risk ethnic minorities (eg, aged >60–65 years), do modifications in the nutrition assessment need to be performed, vs using standard protocols, to decrease the risk for COVID-19 severity? | MALNUTRITION | |
| 16. In the adult population, does accommodation of regional and cultural differences in food and lifestyle, compared with no accommodation, decrease the risk of COVID-19 severity? | NONE | |
| 17. In the adult population of uninfected new mothers in diverse geographic regions, does the presence vs absence of culturally sensitive recommendations for breastfeeding contribute to optimal neonatal outcomes and neonatal risk for COVID-19? | BREASTFEEDING CONSIDERATIONS | |
| Acute COVID-19 | ||
| Outpatient | 18. In patients with acute but nonsevere COVID-19 managed as an outpatient, does a formal nutrition assessment and counseling, with or without enteral supplements, vs no nutrition intervention, hasten recovery time and/or decrease the likelihood of severe COVID-19. | NONE |
| Inpatient: Non-ICU or ICU Nutrition assessment | 19. In patients hospitalized with COVID-19, does prehospitalization weight loss > 5% vs no weight loss modify the decision-making on timing, modality, and/or dosing of nutrition support, with respect to effects on clinical outcomes? | NUTRITION ASSESSMENT |
| Nutrition therapy | 20. In patients hospitalized with COVID-19 on high-flow nasal cannula and inadequate oral intake (<50% of estimated needs), does supplemental EN vs continued attempts with standard oral nutrition improve clinical outcomes (reduce infections, mortality, ICU days, ventilator days, and nosocomial infections)? | NUTRITION THERAPY |
| 21. In patients hospitalized with COVID-19 receiving EN, will increasing the head of bed to ≥10–25 degrees, compared with a supine position, be adequate to decrease aspirated gastric contents with intra-abdominal hypertension? | NUTRITION THERAPY | |
| 22. In patients hospitalized with COVID-19 and with obesity, does hypocaloric, high-protein nutrition, compared with standard care, improve clinical outcomes? | NUTRITION THERAPY | |
| 23. In patients hospitalized with COVID-19, does micronutrient supplementation (eg, vitamin A, B-complex, C, D, selenium, chromium, and/or zinc) vs no supplementation improve clinical outcomes (eg, reduce mortality, ICU days, and ventilator days)? | NUTRITION THERAPY | |
| 24. In patients hospitalized with COVID-19 and with severe hyperglycemia, does the infusion of IV chromium > 5 mcg/d, with or without PN, vs no or lower chromium (≤5 mcg/d) improve glycemic status? | NUTRITION THERAPY | |
| Infrastructure | 25. In patients hospitalized with COVID-19, does having an RDN and/or nutrition support team vs standard care on acute-care floors, or present in team rounds, increase adequacy of nutrition intake (standard/enteral/parenteral)? | HOSPITAL INFRASTRUCTURE |
| Inpatient: ICU only Nutrition assessment | 26. In patients with critical illness and receiving EN and/or PN, with vs without COVID-19, is there a higher risk for refeeding syndrome? | NUTRITION ASSESSMENT |
| 27. In patients with critical illness and COVID-19, is an energy deficit of <4000 kcal vs >4000 kcal by day 7 associated with improved clinical outcomes (eg, reduce infections, mortality, ICU days, ventilator days, and nosocomial infection)? | NUTRITION ASSESSMENT | |
| Nutrition support | 28. In patients with critical illness and COVID-19, does probiotic supplementation vs no supplementation improve clinical outcomes (eg, reduce infections, mortality, ICU days, and ventilator days)? | NUTRITION SUPPORT |
| 29. In patients on mechanical ventilation with COVID-19, does initiation of early EN (within 48 hours of ICU admission) compared with either not receiving EN, initiation of EN >48 hours, or early PN (within 48 hours of ICU admission) improve outcomes (eg, reduce infections, mortality, ICU days, ventilator days, and nosocomial infections)? | NUTRITION SUPPORT | |
| 30. In patients on mechanical ventilation with high nutrition risk (mNUTRIC ≥5 or NRS ≥3) and COVID-19, does full-dose early EN vs <70% prescribed EN improve clinical outcomes (eg, reduce infections, mortality, ICU days, and ventilator days)? | NUTRITION SUPPORT | |
| 31. In patients with critical illness and COVID-19, with or without prehospitalization sarcopenia, does higher (>1.2 g/kg/d ABW) vs lower (<1.2 g/kg/d ABW) protein delivery improve clinical outcomes (eg, reduce infections, mortality, ICU days, and ventilator days, and nosocomial infection)? | NUTRITION SUPPORT | |
| 32. In patients with critical illness and COVID-19 undergoing prone positioning with gastric delivery of EN, does monitoring vs not monitoring gastric residual volume increase enteral feeding tolerance and reduce adverse events, such as aspiration pneumonia? | NUTRITION SUPPORT | |
| 33. In patients with critical illness and COVID-19 undergoing prone positioning with gastric delivery of EN, does prokinetic use vs nonuse improve enteral feeding tolerance? | NUTRITION SUPPORT | |
| 34. In patients with critical illness and COVID-19 undergoing ECMO or VAD placement, is early EN vs no nutrition safe (eg, no nonocclusive bowel necrosis) and tolerated (eg, no vomiting, ileus, or diarrhea)? | NUTRITION SUPPORT | |
| 35. In patients with critical illness and COVID-19 receiving multiple vasopressor agents, does trophic or hypocaloric dosing of EN vs no EN increase the risk of nonocclusive bowel necrosis? | NUTRITION SUPPORT | |
| 36. In patients who are malnourished and critically ill with COVID-19, and also unable to tolerate early EN, does intervention with early PN (within 24–48 hours of demonstrated enteral intolerance), compared with either late or no PN, improve clinical outcomes (eg, reduce ICU mortality, ICU days, and ventilator days)? | NUTRITION SUPPORT | |
| 37. In patients with critical illness and COVID-19, do volume-based bolus feeds incidental to healthcare personnel at the patient’s bedside (due to enteral pump shortages) vs standard rate–based EN via enteral pump (when available) lead to more enteral feeding delivered and/or more feed intolerance (eg, diarrhea, vomiting, ileus) or complications (eg, pneumonia)? | NUTRITION SUPPORT | |
| 38. In patients with critical illness, COVID-19, and PN use, do fish oil–containing lipids, compared with pure soybean oil lipids or no lipids, improve viral clearance and/or clinical outcomes (eg, reduce infections, mortality, ICU days, and ventilator days)? | NUTRITION SUPPORT | |
| 39. In patients with critical illness, COVID-19, high nutrition risk (NUTRIC ≥5 or NRS ≥3), severe hyperglycemia (>300 mg/dl), and insulin resistance (>25 units/h), does permissive underfeeding with EN or PN to prioritize glycemic targets, vs continuing standard nutrition dosing, improve clinical outcomes (eg, reduce infections, mortality, ICU days, and ventilator days)? | NUTRITION SUPPORT | |
| 40. In patients with critical illness, COVID-19, and hyperglycemia (blood glucose > 180 mg/dL), does glycemic control to blood glucose 140–180 mg/dL, compared with other glycemic targets, improve clinical outcomes (eg, reduce infections, mortality, ICU days, and ventilator days)? | NUTRITION SUPPORT | |
| Infrastructure | 41. In patients on mechanical ventilation with COVID-19, does the presence of a feeding/nutrition protocol increase the number of patients receiving early EN? | HOSPITAL INFRASTRUCTURE |
| Chronic/Post–COVID-19 | ||
| Outpatient Nutrition assessment | 42. In patients who have had severe COVID-19, does the performance vs nonperformance of a nutrition risk assessment improve clinical outcomes? | DIET ADVICE |
| 43. In adults aged >65 years, with or without having had COVID-19, does poor mobility and isolation, when compared with socially active and mobile counterparts, result in poorer nutrition that leads to increased risk for COVID-19–related morbidity or mortality? | MALNUTRITION | |
| 44. In the general population during the COVID pandemic, what is the impact of stay-at-home quarantine schedules on weight and nutrition-related comorbidities? | QUARANTINE DEPRESSIONS/STRESS EATING, UNHEALTHY FOOD CHOICES, WEIGHT GAIN | |
| Nutrition therapy | 45. In patients with obesity who have had severe COVID-19, does weight loss vs weight maintenance or weight gain improve metrics of recovery? | DIET ADVICE |
| 46. In children recovering from COVID-19, does supplemental nutrition vs an ad lib diet, improve growth and weight parameters? | DIET ADVICE | |
| 47. In patients who have had severe COVID-19, does subsequent supplementation with micronutrients or other nutrition products vs no supplementation result in improved recovery? | DIET ADVICE | |
| 48. In patients who have had severe COVID-19, does nutrition management that achieves a priori protein-energy goals, vs standard care without goal-directed nutrition, decrease the risk of complications (eg, renal, pulmonary, cardiac, hematological, and neurological)? | DIET ADVICE AND MALNUTRITION | |
| 49. In patients who have had severe COVID-19, does nutrition supplementation (oral, EN, and/or PN) vs no supplementation improve achievement of rehabilitation goals? | DIET ADVICE AND MALNUTRITION | |
| Infrastructure | 50. In patients who have had severe COVID-19, does MNT provided by an RDN vs no MNT result in improved outcomes? | NUTRITION COUNSELING |
| 51. In patients who have had COVID-19, do outpatient nutrition follow-up protocols vs no protocols improve clinical outcomes? | NUTRITION COUNSELING | |
| 52. In patients receiving home PN and/or EN during the COVID-19 pandemic, does having access to care management by an RDN and/or nutrition support team impact nutrition adequacy and hospitalization due to COVID-19? | NUTRITION COUNSELING AND TELEHEALTH | |
| 53. In pediatric patients receiving home PN, do COVID-19–altered caregiver routines and schedules impact feeding and bodyweight? | DIET ADVICE AND NUTRITION COUNSELING | |
| 54. In patients who have had severe COVID-19, does subsequent food insecurity vs no food insecurity result in impaired recovery? | FOOD INSECURITY/SOCIETAL INFRASTRUCTURE | |
| Inpatient Nutrition support | 55. In patients who have had severe COVID-19 and remain in the ICU with a tracheostomy, do adjustments in nutrition care goals, vs continuing the same goals as with acute critical illness, improve clinical outcomes? | MALNUTRITION AND NUTRITION COUNSELING |
| 56. In patients who have had acute COVID-19 and discharged from the ICU, does meeting indirect calorimetry–determined nutrition goals vs standard care improve metrics of recovery and reduce rehospitalization due to COVID-19? | MALNUTRITION AND NUTRITION COUNSELING | |
ABW, adjusted body weight; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; EN, enteral nutrition; ICU, intensive care unit; MNT, medical nutrition therapy; mNUTRIC, modified nutrition risk in critically ill (score); NRS, nutrition risk screening; PICO-T, Population, Intervention, Comparator, Outcome, and Time; PN, parenteral nutrition; RDN, registered dietitian nutritionist; VAD, ventricular assist device.
PICO-T questions are based on nutrition issues with COVID-19 (Table 1) and the clinical experience of the expert writing group prior to analysis of the scoping results. PICO-T questions confer relevance to research and knowledge gaps and provide specific topics for systematic reviews to address practice gaps. Critical PICO-T questions are sorted by COVID-19 stage (pre-, acute, and post-/chronic) and deemed highly relevant to clinical nutrition practice and vetted by the primary authors and a member of the community. Each critical PICO-T question has a numerical designation to assist identification of the most relevant research and knowledge gaps discovered by this scoping review. Heat maps (Figure 3) are interpretable in terms of the PICO-T questions that map to the nutrition topics on the y-axes. PICO-T questions that do not map to nutrition topics (labeled as “NONE”) automatically qualify as research and knowledge gaps.
For this scoping review, the nutrition and COVID-19 space was considered as 3 stages: pre–, acute, and chronic/post–COVID-19. The pre–COVID-19 stage is the period prior to acute SARS-CoV-2 infection when both prevention strategies and considerations of COVID-19 risk factors are relevant. Key risk factors are directly nutrition-based20 or indirectly related to cardiometabolic drivers (eg, hypertension [HTN], type 2 diabetes [T2D], and obesity),21–23 sociodemographic factors (eg, minority communities—particularly counties in which the majority of residents are Black—with higher infection rates),24–26 or healthcare infrastructure (eg, food insecurity; stay-at-home unhealthy behaviors, particularly in children; global lockdowns; and interruption of supply chains).27–30 Hospitalization for COVID-19 was associated with adiposity-based (odds ratio [OR] 1.90; 95% CI, 1.10–3.30), dysglycemia-based (OR, 3.10; 95% CI, 1.70–5.90), and cardiometabolic-based (OR, 12.83; 95% CI, 10.27–15.86) chronic disease, as well as HTN (OR, 2.00; 95% CI, 1.30–3.20).31 The COVID-19 Impact Survey showed that about 34% of households (particularly in Black, Latino, and Native American households) in the US experience food insecurity, as compared with about 15% in 2018, and that, globally, about 265 million people could face a severe threat of food insecurity in 2020.30,31–33 The prevalence of malnutrition with COVID19 in Wuhan, China, was 52.7%,34 with detriment hypothesized to be due to nutrition deficiencies from abnormal eating patterns, leading to decreased immune function (innate and adaptive) and impaired resistance to infection.35–38 Further, obesity-associated insulin resistance interacts with aSARS-CoV-2–induced decrease in angiotensin-converting enzyme-2 and its resulting decrease in pancreatic β-cell reserve and counterregulatory renin-angiotensin system cardioprotective pathway, resulting in severe hyperglycemia, cardiovascular complications, and adverse outcomes.18,39
Acute COVID-19 can be nonsevere and initially managed at home in quarantine or severe (shortness of breath with hypoxemia) and initially managed in the hospital, with possible ICU admission for critical disease (respiratory failure, shock, or multiple organ dysfunction) and a higher likelihood of chronic/post–COVID-19 complications or mortality. Infrastructure is required for the effective care of patients with acute COVID-19 in the home setting or in the hospital and is challenged by social distancing, resource shortages, and the urgent need for adaptive protocols and policies. A formal nutrition assessment is recommended for all patients with COVID-19,40 with nutrition support needed in many cases. Older age, preexisting comorbidities, and adverse social/environmental factors increase the risk of malnutrition and refeeding syndrome.41 Among hospitalized patients with COVID-19, acquired malnutrition and sarcopenia can result from a protracted hospitalization, marred by immobility and prolonged mechanical ventilation.42 The role and dose of exogenous protein on muscle anabolism in critically ill patients with COVID-19 is unclear.43 Patients with COVID-19, pneumonia, and hypoxemic respiratory failure managed with awake prone positioning or extracorporeal membrane oxygenation are at increased risk for acquired malnutrition because these therapies can limit nutrient delivery. The value of early enteral nutrition (EN), ω−3 fatty acids, specialized proresolving mediators, and micronutrient supplementation on maintaining gut epithelial barrier and optimizing immune function has been demonstrated in non–COVID-19 patients but not clearly in COVID-19 patients with critical illness.44–51 Gastrointestinal symptoms related to COVID-19 and/or related therapies can impair nutrient delivery, digestion, and absorption, raising important questions about the specific use of parenteral nutrition (PN).
Chronic/Post–COVID-19 refers to a disease trajectory lasting ≥3 months and may lead to an increase in chronic medical conditions (eg, depression, stroke, cardiac injury, chronicrenaldisease,andT2D),which could be exacerbated by unhealthy diets in vulnerable populations.52–54 This condition is now referred to as a myalgic encephalomyelitis–like illness55 and resembles chronic fatigue syndrome.56 Central sensitization occurs and is characterized by exaggerated hypersensitivity to stimuli, with variable gastrointestinal symptoms that can limit food intake and induce nutrition problems.57,58 This chronic inflammatory process can exacerbate catabolism and anorexia, aggravating malnutrition, impeding recovery, and leading to loss of independence, to disability, and to reduced quality of life.59 In an Italian series of 143 adults after acute COVID-19, the following conditions persisted, which have potential nutrition implications: HTN (35%), diabetes (7%), chronic heart disease (4.9%), and renal failure (2.1%).60 Children account for about 1%–5% of all diagnosed COVID-19 cases and often have mild or no symptoms but are at risk for chronic/post– COVID-19.61–63 One complication in those individuals aged <21 years is multisystem inflammatory syndrome in children, which occurs roughly 2–4 weeks after COVID-19 infection.61–63 Even though SARS-CoV-2 has not been isolated from human milk to date, and breastfeeding is encouraged for mothers with confirmed or suspected COVID-19, misinformation and disrupted health education may lead to discontinuation of breastfeeding and human milk usage.64 Infrastructure, screening protocols, and functional status tools for chronic care clinics and private healthcare settings will need to be modified to provide access to nutrition care as part of comprehensive healthcare services.65,66 Nutrition-related risk factors are aggravated by quarantine and lockdown (limiting access to nutritious, safe, and affordable foods), interruptions in nutrition support services, increased unemployment with limited financial resources, inaccessible school meals for vulnerable children, more sedentary lifestyles, and higher consumption of unhealthy foods.30,67 Patients with chronic/post–COVID-19 may require long-term EN or PN in skilled facilities or the home setting.68 Others are likely to require medical nutrition therapy to address underlying malnutrition and/or manage preexisting or newly developed comorbidities.36 Exercise programs and nutrition may increase the functional capacity of COVID-19 survivors and should start prior to discharge.69 Over the long term, weight gain during COVID-19–associated social distancing or quarantine can result from increased energy consumption and decreased physical activity.70
The purpose of this scoping review is to examine and analyze the terrain of scientific data across these COVID-19 stages and, further, to identify relevant and actionable research and knowledge gaps in clinical nutrition applicable to the COVID-19 pandemic.
Methods
General Comments
The American Society for Parenteral and Enteral Nutrition (ASPEN) President and Board of Directors mandated a scoping review on nutrition research applicable to the COVID-19 pandemic in Spring 2020. After appointing the Chair (J.M.), a diverse ASPEN COVID-19 Nutrition Task Force was assembled, including physician, nurse, dietitian, pharmacist, and scientific research experts in pediatric and adult clinical nutrition. A public health expert (T.E.P.) in food policy was also included to consult on issues of food infrastructure and patient advocacy, and a clinical nutrition epidemiologist (L.M.) was included as the methodologist. This task force was then divided into 3 teams, each corresponding to clinical stages: pre–, acute, and chronic/post–COVID-19. Weekly video conferencing was conducted for each team and the entire task force during the development of this document for training, troubleshooting, brainstorming, administrative logistics, and other discussions. Prior to submission to the Journal of Parenteral and Enteral Nutrition, the document was reviewed by additional ASPEN members and non-ASPEN experts and then approved by the ASPEN Board of Directors.
Search Strategy
The central scoping review question was “What is the current terrain of available knowledge to guide nutrition practice in patients with COVID-19?”PubMed was searched up to June 1, 2020, for Medical Subject Headings [of the US National Library of Medicine] and text terms relevant to nutrition in COVID-19. To be included, a citation had to contain ≥1 term from group 1 and be cross-referenced with ≥1 term from group 2 (Figure 1). Analogous search strategies were created for EMBASE, CINAHL, and Cochrane Central databases, as well as the following gray literature (identified through nonconventional/noncommercial publication channels) databases: Web of Science, Scopus, Opengrey, Wonder, and ClinicalTrials.gov.
Figure 1.
Final PubMED/MEDLINE search terms. To be included in the scoping review search, a citation must contain ≥1 term from group 1 and be cross-referenced with ≥1 term from group 2. COVID-19, coronavirus disease 2019; MeSH, Medical Subject Headings.
This scoping review methodology was performed in congruence with the recommendations from the Joanna BriggsInstitute.71 Specifically, the search strategy proceeded in 2 phases. Phase 1 included a preliminary citation search of PubMed and EMBASE. Phase 2 scanned the titles, abstracts, and keywords of articles culled from PubMed and EMBASE to identify those missing from the phase 1 search strategy. This was done to construct a more comprehensive search strategy that would span PubMed, EMBASE, CINAHL, Cochrane Central, and gray literature sources. Bias assessment is not standard practice in a scoping review72 and was not performed.
Article Inclusion and Exclusion
For an article to be included in the scoping review, 2 broad criteria needed to be met: (1) COVID-19 was directly discussed, and (2) there was some nutrition context in the discussion. This context may have involved nutrition assessment, nutrition requirements, forms of exogenous nutrition, nutrition practice recommendations, effects on nutrition physiology, and infrastructure directly related to nutrition. Articles were excluded if their discussion of COVID-19 was entirely inferential based on non–COVID-19 conditions. The nutrition and COVID-19 populations included in the search spanned pediatrics to adults to geriatrics and domestic (US) to global venues.
Screening Process
Two reviewers from the task force screened all citation titles and abstracts in duplicate based on inclusion criteria. Articles were downloaded if there was any indication that inclusion criteria may be met. Any article not meeting inclusion criteria was removed and the reason for removal recorded. The remaining (included) articles were then moved into the data extraction phase. A pilot screening process was conducted to ensure consensus understanding of the review criteria. Twenty-five random citations were given to the entire task force for screening. After screening, discrepancies were noted and discussed. If the agreement with inclusion criteria was <75%, then another 25 citations were given to the task force for repeat pilot screening, training, and discussion. Once the task force achieved 75% agreement, then full-citation screenings ensued.
Data Extraction
Data extraction was also performed in duplicate by 2 extractors and included the following attributes: author(s); year of publication; country of origin for the corresponding author or publication; specialty and subspecialty of the publication source; aims, purpose, or objective; population characteristics; sample size; methodology or methods; intervention type and description; comparator type and description; duration of intervention and/or study; outcomes and description of how they were measured; key findings that relate to the scoping review question; and COVID-19 stage.
Analysis
The analysis focused on comparing information from articles (frequencies stratified by topic, study design, population, setting, country/geography, and specialty) with PICO-T questions. PICO-T questions were formulated by task force members prior to article analysis to frame the most urgent issues related to safe and effective practice of clinical and community nutrition in patients with COVID-19. Hence, PICO-T questions reflected the experience of credentialed experts in the task force. Each PICO-T question in Table 4 was labeled with a numerical designation for mapping to nutrition topics.
The studies were separated into 2 groups: those that contained original data and those that did not. Studies that contained original data were deemed to fill a research gap for their respective topics. Studies that did not present original data were deemed to fill a knowledge gap; these studies made mention of both COVID-19 and some aspect of nutrition but exclusively drew their content from either clinical experience or a nonsystematic synthesis of the available literature. Nutrition topics in the COVID-19 literature were mapped to critical a priori PICO-T questions, and then research and knowledge gaps were identified in terms of critical PICO-T questions, nutrition topics, and other descriptors (ie, population and settings).
Results
General
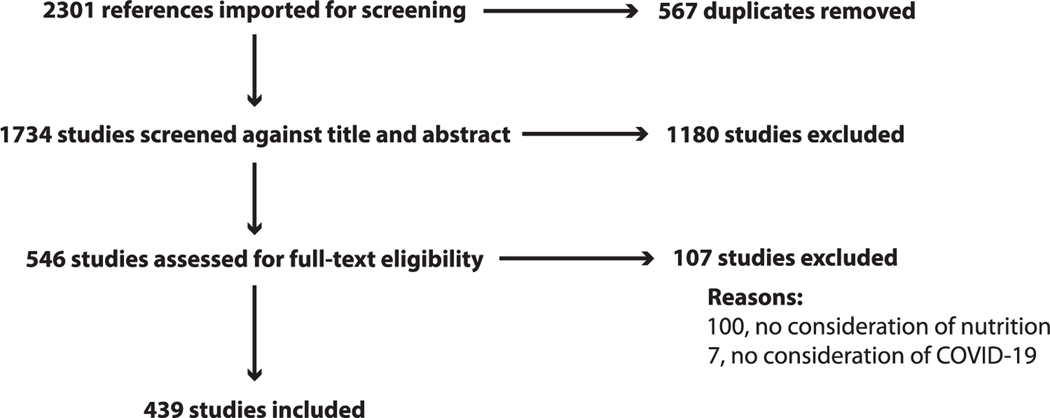
The combined search strategies yielded 2301 citations. After removal of duplicates, 1734 remained for title and abstract screening. Of these, 546 were moved forward for full-article screening. One-hundred seven were excluded for lacking information relevant either to nutrition (100 articles) or to COVID-19 (7 articles), leaving 439 articles for full-data abstraction (Figure 2).
Figure 2.
PRISMA flow chart for study selection. See http://www.prisma-statement.org/ (accessed on August 15, 2020) for the PRISMA. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
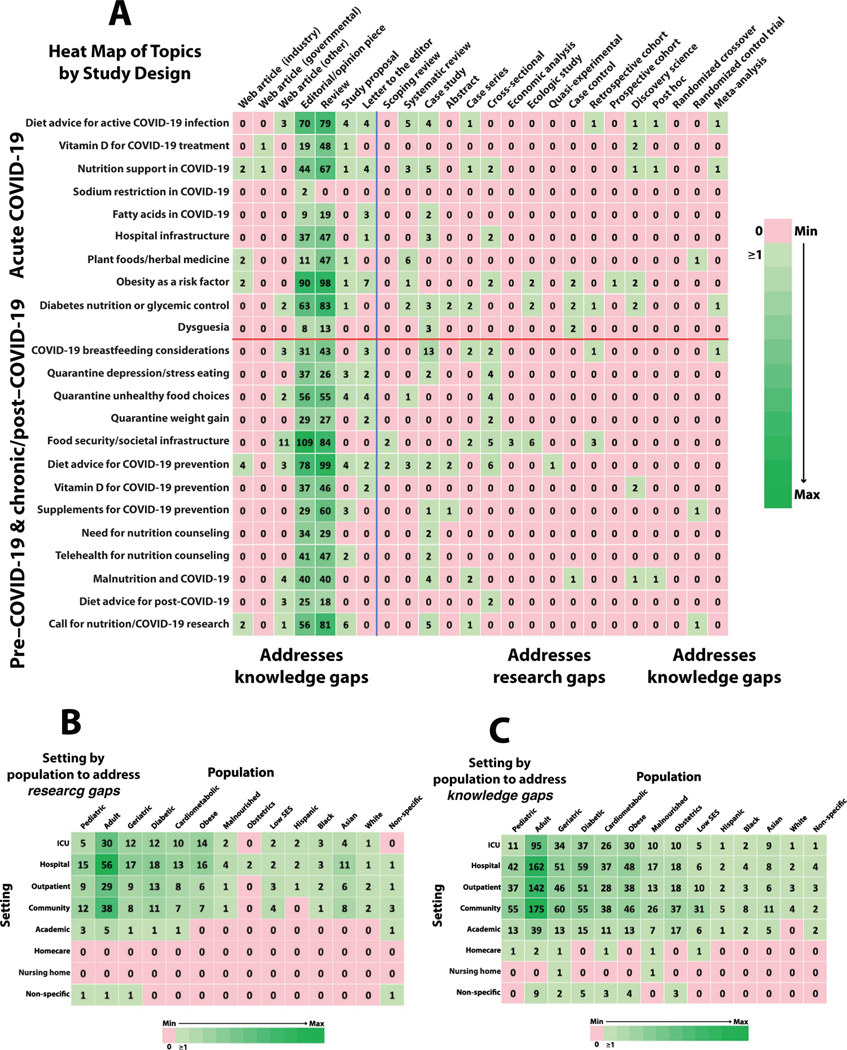
This review of the literature identified 23 main topic areas across 24 different article types, with editorial pieces and review papers accounting for the most mentions (Figure 3A). These articles were sourced across 61 countries and 51 specialties, with the US (88 [20.0%]), Italy (71 [16.2%]), and China (54 [12.3%]) and diabetes (56 [12.8%]), general medicine (56 [12.8%]), and nutrition (44 [10.1%]) accounting for the greatest representation, respectively. Eight different settings and 14 populations were identified and represented as heat maps for research and knowledge gaps (Figures 3B and 3C). Fifty-seven articles presented original data to satisfy a research gap. The remaining articles attempted to satisfy knowledge gaps by providing insights and advice based on clinical experience and/or prior literature. Relevance to clinical practice for research and knowledge gaps is conferred by the PICO-T questions that map to specific nutrition topics (y-axes of heat maps) and is presented in Table 4. PICO-T questions that do not map to nutrition topics found in the literature automatically correspond to research and knowledge gaps. A summary listing of research and knowledge gaps for aggregated and individual COVID-19 stages are given in Table 5. A detailed analysis by individual COVID-19 stage is provided below.
Figure 3.
Heat maps for clinical nutrition in patients during pre-, acute, and chronic/post- stages of COVID-19. (A) To the right of the vertical line are studies with original evidence (increasing strength left to right) exposing research gaps, and to the left are studies with nonoriginal evidence, referred to as mentions, exposing knowledge gaps; above the horizontal line are nutrition topics (that can be mapped to specific PICO-T questions for relevance) pertinent for the acute stage of COVID-19, and below are nutrition topics pertinent for the pre- and chronic/post-stages of COVID-19. (B) Setting by population to address research gaps. (C) Setting by population to address knowledge gaps. COVID-19, coronavirus disease 2019. ICU, intensive care unit. PICO-T, Population, Intervention, Comparator, Outcome, and Time.
Table 5.
Key Research and Knowledge Gaps for Clinical Nutrition in Patients During Pre-, Acute, and Chronic/Post- Stages of COVID-19.a
| COVID-19 stage | Research gaps | Knowledge gaps |
|---|---|---|
|
| ||
| Pre– and chronic/post–COVID-19 | PICO-T 1–11 (all) | PICO-T 1,2, 11, 16 |
| Pre–COVID-19 | Food insecurity/social infrastructure Malnutrition | None |
| Dietary management and cardiometabolic risk | ||
| Transcultural Factors | ||
| RCTs | ||
| Acute COVID-19 | PICO-T 19-41 (All) | PICO-T 20, 21, 24, 27, 31, 33, 37 |
| Obesity in ICU, community/outpatient/academic settings | None | |
| Pediatrics | ||
| Nutrition assessment (eg, prehospitalization weight loss) | ||
| Refeeding syndrome | ||
| Nutrition support, especially with glycemic control | ||
| Hospital infrastructure (eg, training, shortages, and protocols) | ||
| RCTs | ||
| Chronic/Post-COVID-19 | PICO-T 42–56 (All) | PICO-T 52, 53 |
| Nutrition risk assessment, outpatient | Home EN and PN support | |
| Weight management to improve recovery | Long-term nutrition effects | |
| Protein-energy targets to decrease complications | ||
| Impact of RDN counseling | ||
| RCTs | ||
| Aggregated COVID-19 stages | Homecare, nursing homes, and obstetrics (outpatient/ICU) | Homecare and nursing homes |
| Academic settings for obesity, malnutrition, obstetrics, low SES, and ethnic minorities | ||
COVID-19, coronavirus disease 2019; ICU, intensive care unit; PICO-T, Population, Intervention, Comparator, Outcome, and Time; RDN, registered dietitian nutritionist; RCT, randomized controlled trial; SES, socioeconomic status.
Key gaps are those interpreted by the task force members as critical for nutrition research and systematic reviews. Heat map of gaps in the nutrition topics vs study design in Figure 3A correspond to specific PICO-T questions listed in Table 4. Gaps discovered with more detailed analysis of data sets by the primary writer teams are presented in italics. Heat maps of gaps in settings vs populations in Figure 3B and 3C correspond to specific cross-tabulations, do not map to specific PICO-T questions, and are not specific to any given COVID-19 stage (presented as aggregated COVID-19 stages).
Stage 1: Pre–COVID-19
Research Gaps.
Eleven pre–COVID-19 nutritionally relevant topic areas were explored through original research. Food insecurity/societal infrastructure was explored in 21 articles; diet advice for COVID-19 prevention in 16 articles; diabetes nutrition/glycemic control in 17 articles; obesity as a risk factor in 10 articles; and malnutrition in 9 articles. It should be noted that the term “malnutrition,” here and in the rest of this document, conflates usage of the term as an official diagnosis with the looser definitions of malnutrition found in the gray literature. There were 21 studies in pediatrics, and 17 in geriatrics, on infection mitigation (mask wearing, safe hand hygiene, and breastfeeding practices). There were 14 studies, 3 studies, and 1 study addressing Asian, Black, and Latino/Hispanic populations, respectively, whereas 7 addressed socioeconomic status (SES), encompassing lifestyle constraints and access to food. Regardless of age, ethnicity, or SES, few original studies addressed identification and recognition of cultural practices, values, and belief systems that predispose underserved populations to noncommunicable conditions, increasing the risk and severity of COVID-19. Although there were some nutrition topics examined by observational studies, all a priori PICO-T questions were unanswered with original evidence.
Knowledge Gaps.
Among mentions without original evidence, 84 addressed malnutrition and 66 addressed cardiometabolic conditions in community/outpatient settings. Among just the reviews and editorials, 80 mentioned malnutrition, 188 mentioned obesity, 76 mentioned cardiometabolic issues, and 236 mentioned diabetes, many with discussions of potential mechanisms that may increase the risk of severe COVID-19. Breastfeeding, formula feeding, safe handling and administration of expressed human milk, and risk mitigation for infection transmission during breastfeeding accounted for 80 of the 103 obstetrics mentions. Obesity mentions were prominent, with 84 papers describing relevance to inflammation, food insecurity, and impact on healthcare (eg, postponed bariatric surgeries, poor glycemic control, quarantine-related unhealthy food choices, and mental health). Glycemic control mentions in the context of nutrition were also prominent, with 106 papers describing T2D in community/outpatient settings. Among papers addressing the impact of lower SES (a proxy for the effects of lifestyle and race/ethnicity on the disproportionate rate of severe COVID-19), 41 were in community/outpatient settings, with 7 in Latino/Hispanic, 11 in Black, and 17 in Asian populations. Mentions were distributed across the lifespan, with 92 for pediatrics, 317 for adults, and 106 for geriatrics in the community/outpatient setting. Mentions in editorials and reviews covered all the nutrition topics in the pre–COVID-19 stage, but this still left PICO-T questions 1, 2, 11, and 16 not mapping to nutrition topics and, therefore, representing knowledge gaps.
Stage 2: Acute COVID-19
Research Gaps.
Nineteen studies addressed breastfeeding, including 13 case studies, 2 case series, 2 cross-sectional studies, 1 retrospective cohort study, and 1 meta-analysis. All were limited to the assessment of vertical transmission of COVID-19 from mother to child. There were 14 studies that addressed dietary advice, including 5 systematic reviews, 4 case studies, 1 case series, 1 retrospective cohort study, 1 discovery science study, and 1 meta-analysis. Original research reports were primarily on adults, of whom 30 were critically ill, 56 were inpatient, 29 were outpatient, 38 were in the community, and 5 were in academic settings. There were 2 papers with original evidence on patients with obesity and acute COVID-19 in a hospital setting but none specifically in the ICU, outpatient, community, or academic settings. The most common study designs were case studies, with 33 mentions, 17 systematic reviews, and 8 discovery science studies, with only 1 randomized controlled trial (RCT). There were 14 original research studies specifically on nutrition support: 5 case studies, 3 systematic reviews, 2 cross-sectional studies, 1 case series, 1 discovery science study, 1 post hoc analysis or observational data, and 1 meta-analysis. Systematic reviews recommended nutrition support for those hospitalized, critically ill, and undergoing prone positioning. Healthcare infrastructure was a component of 2 of the 23 PICO-T questions dedicated to acute COVID-19. Five original data papers were on hospital infrastructure, of which 3 were case studies and 2 were cross-sectional studies. There was no original research on nutrition assessment, effects of prehospitalization weight loss, or the risk for refeeding syndrome for patients with acute COVID-19. Various case studies and series reported on gastrostomy feeding; the use of glutathione, α lipoic acid, zinc, and vitamin C; the timing of PN with respect to cholestasis prevention; and utilizing the geriatric nutrition risk index. A discovery science study recommended vitamin D supplementation because of an association of mortality with severe vitamin D deficiency. The need for adequate healthcare infrastructure was supported by 1 case study and 1 cross-sectional study. There was no evidence pertaining to the 18 nutrition support PICO-T questions. Overall, the 23 PICO-T questions for acute COVID-19 were unanswered with original evidence.
Knowledge Gaps.
There were 895 mentions without original evidence but related to nutrition and acute COVID-19: 501 were in review articles and 353 were in editorial/opinion pieces. There were 90 editorials and 98 review articles that addressed obesity as a risk factor for acute COVID-19, and 70 editorials, 79 review articles, 4 study proposals, 4 letters to the editor, and 3 web articles that addressed dietary advice. For adult vs pediatric patients, 95 vs 11 mentions were about the ICU and 162 vs 53 were about general hospital settings, respectively. There were 119 mentions related to nutrition support, of which 67 were in reviews and 44 in editorials. Other reviews recommended nutrition assessment and nutrition support in patients who were hospitalized. Another review discussed the role of diet on gut microbiota. Various reviews and editorials recommended a protocol to standardize nutrition approaches in patients in the ICU, and another recommended that all hospitalized patients receive nutrition support as early as possible, including the use of vitamin D, fish oil, whey proteins, and/or immunonutrients. Another review recommended early EN but not PN. For patients with diabetes, various editorials and reviews discussed glycemic control and worsening insulin resistance and then recommended fiber-rich, carbohydrate-based foods with a low glycemic index. Among the 85 mentions related to hospital infrastructure, 47 reviews, 37 editorial/opinion pieces, and 1 letter to the editor discussed self-care for members of the healthcare team. Some review papers discussed telehealth implementation to optimize nutrition status. The following PICO-T questions had no or just trivial mentions in the literature and, therefore, represented knowledge gaps: 20, 21, 24, 27, 31, 33, and 37.
Stage 3: Chronic/Post–COVID-19
Research Gaps.
No original research was found for nutrition risk assessment during outpatient follow-up; weight management (loss, maintenance, or gain) to improve recovery; the effect of targeted protein-energy goals on reducing risk of post–COVID-19 complications; the impact of registered dietitian nutritionist (RDN) counseling; energy expenditure and goal-directed nutrition therapy with prolonged critical illness and post-ICU care; access to and management of home EN and PN; caregiver routines and schedules for children on home nutrition support; and home care and nursing home settings. There was 1 report of a clinical trial underway in Greece to evaluate the impact of telehealth and telerehabilitation on the physical and psychological status of 19 patients following hospital discharge for COVID-19 and on how to cope with fatigue, muscle weakness, and eating difficulties. There were also significant gaps in higher-quality nutrition research, such as retrospective cohort studies, prospective cohort studies, RCTs, or meta-analyses, particularly on dietary advice, nutrition supplements, nutrition support, phytomedicine, food insecurity, quarantine-related behavior, and dietary and weight changes in children and adults with or without diabetes. There were 2 cross-sectional studies on quarantine weight gain, 4 on unhealthy diets, and 4 on eating patterns, with data predominantly based on self-reported survey responses. Five studies addressed food security and societal infrastructure. Among non-ICU populations, there were 15 studies in hospitalized patients, 12 in diabetic patients, 4 in cardiometabolic patients, and 5 in obese patients. The Asian population was the most studied, with 9 articles. This was followed by White, Black, and Latino/Hispanic populations with 1, 2, and 0 articles, respectively. Overall, there was inadequate original evidence for each of PICO-T questions for the chronic/post–COVID-19 stage.
Knowledge Gaps.
Although several studies mentioned food security, societal infrastructure, and nutrition concerns associated with quarantine (including depression, stress eating, unhealthy choices, and weight gain), no original evidence was presented on these topics. The quarantine topics included 7 study proposals that are currently being conducted or considered for future research. Of the articles that did not present original evidence, the topic of food security and societal infrastructure was the most prolific, with 204 mentions. Most of these were reviews or editorial reviews or editorials. The long-term nutrition effects of COVID-19 are generally unknown. The impact of COVID-19 on home EN and PN patients (PICO-T questions 52 and 53) represented a critical knowledge gap in the literature.
Discussion
This ASPEN scoping review has discovered gaping research and knowledge gaps in the clinical nutrition research literature applicable to COVID-19. The research gaps corresponded to all the a priori PICO-T questions. Some of the nutrition topics were of particular interest: food insecurity/societal infrastructure and transcultural factors (pre–COVID-19); obesity and cardiometabolic-based chronic disease, pediatrics, nutrition support, and hospital infrastructure (acute COVID-19); RDN counseling (chronic/post–COVID-19); and malnutrition and management (all stages). The knowledge gaps corresponded to a specific subset of PICO-T questions on pediatrics, micronutrients, bariatric surgery, and transcultural factors (pre–COVID-19); EN, protein-energy requirements, and glycemic control with nutrition (acute COVID-19); and home EN and PN support (chronic/post–COVID-19).
The PICO-T questions assembled for this review lie at the heart of an imperative to conduct high-quality and meaningful research. These a priori questions represent what a panel of experts on the COVID-19 front lines deemed to be critical to the safe and effective administration of nutrition in this population. These unanswered PICO-T questions must urgently guide updated recommendations for future nutrition and COVID-19–related research, especially strong study designs, such as large epidemiological studies, prospective cohort studies, clinical trials, and systematic reviews/meta-analyses. These recommendations are best considered according to COVID-19 stage.
In the pre–COVID-19 stage, critical, unanswered PICO-T questions 1–17 covered biological and social determinants of health that impact the risk for severe COVID-19 and, therefore, require scientific evidence on the following:
Specific foods, macronutrients, and micronutrients to avoid or incorporate to reduce overall risk for severe COVID-19;
The use of dietary supplements and nutraceuticals in patients with diabetes to prevent severe hyperglycemia with acute COVID-19;
Nutrition interventions in patients aged >65 years, or with frailty, to prevent severe COVID-19;
All aspects of breastfeeding during the COVID-19 pandemic to prevent SARS-CoV-2 transmission and infection;
The interaction of nutrition and cardiometabolic risk on the severity and outcomes of COVID-19;
The effect of food insecurity on COVID-19 severity in adults and children; and
The effect of malnutrition and role of nutrition assessment on COVID-19 in different ethnic minorities.
In the acute COVID-19 stage, critical, unanswered PICO-T questions 18–41 covered nutrition management issues, primarily in the hospital, that impact clinical outcomes for severe COVID-19 and, therefore, require scientific evidence on the following:
The impact of routine nutrition assessment, counseling, intervention, and team approaches (eg, MDRDN-RN-PharmD) on the clinical course of nonsevere COVID-19;
The impact of prehospitalization weight loss on decision-making for nutrition support;
The impact of high-flow nasal cannula, nonrebreather mask, continuous/bilevel positive airway pressure, proning, vasopressor, and mechanical circulatory support/extracorporeal membrane oxygenation on decision-making for EN and/or PN support;
The optimization of EN/PN support timing and delivery, protocols, and management of complications;
Nutrition support for patients with obesity and severe COVID-19;
Specific uses of micronutrient supplements, probiotics, and other nutrition pharmacological agents as part of standard nutrition or nutrition support, specifically intravenous chromium for very severe insulin resistance and hyperglycemia, fish oils, and antioxidants in the ICU, to improve clinical outcomes;
Optimal protein-energy doses in nutrition support to avoid refeeding syndrome, overfeeding, or prolonged underfeeding;
Optimal nutrition in patients with very severe hyperglycemia, especially judicious, hypocaloric (especially low-carbohydrate), high-protein feeding; and
The impact of infrastructural changes in hospital nutrition policies and protocols on logistical ease, patient and personnel safety, economics, and clinical outcomes.
In the chronic/post–COVID-19 stage, critical, unanswered PICO-T questions 42–56 covered nutrition management issues in the outpatient and inpatient setting and, therefore, require scientific evidence on the following:
The role of nutrition risk assessment, medical nutrition therapy (under RDN supervision), and/or nutrition delivery protocols after acute COVID-19 on clinical course;
The mechanisms behind and interactions of frailty, physical activity and/or rehabilitation, and nutrition (eg, standard, supplemental, and/or enteral) on recovery and functional outcomes after COVID-19;
The effect of quarantine models and routines on body weight and composition, and nutrition-related comorbidities;
The effects of weight change (increase, none, decrease) in patients with obesity on recovery after COVID-19;
The effects of nutrition interventions beyond an ad lib dietary pattern on growth (height and weight) in children after COVID-19;
The effects of nutrition interventions, including micronutrients and other supplements, on the clinical course of COVID-19–related myalgic encephalomyelitis–like illness;
Protein-energy prescriptions to optimize clinical outcomes and minimize complications after COVID-19;
The impact of care management teams, RDN involvement, and caregiver routines on clinical course afterCOVID-19inpediatricandadultpatientsreceiving home EN and/or PN support therapy; and
Specialized nutrition care for patients with chronic critical illness (tracheostomy and mechanical ventilation) on clinical course after acute COVID-19.
Several other scoping reviews have been published on nutrition and COVID-19. Infusino et al73 surveyed PubMed for articles on dietary supplements, probiotics, and nutraceuticals but were unable to find any specific data, especially clinical trials. Rozga et al74 performed a scoping review through a literature search of the MEDLINE database and hand search of the Cochrane Database of systematic reviews and were unable to find any direct evidence supporting a role for specific micronutrients or conditional amino acids in patients with COVID-19. With the paucity of evidence on nutrition and COVID-19 that can guide clinical practice,41 representing distinct and pervasive research gaps, the role for education and dissemination of extant information becomes paramount, especially during a global crisis. Thus, knowledge gaps are addressed with articles based on theory, experience, prior evidence for extrapolation, and judgment, especially white papers and those in the gray literature. ASPEN20,75 and the European Society for Clinical Nutrition and Metabolism76 have already published reports on nutrition management with COVID-19, with a follow-up review by Minnelli et al,77 but it is the strategic planning of nutrition studies to generate original, high-quality data to close research gaps, which can efficiently advance knowledge and promote optimal, evidence-based, comprehensive care.
The strengths of this scoping review are strict adherence with an accepted and rigorous methodology (under the leadership of a dedicated methodologist [L.M.]), interpretation of identified articles by experts in clinical nutrition from high-volume centers caring for patients with COVID-19, successfully meeting an ambitious expedited timeline (3 months), and the inclusion of both Chinese and Italian translators. Limitations include the exclusion of certain databases and gray literature due to foreign language restrictions and the continued explosion of literature, which made true currency of the literature search an impossibility.
In conclusion, research gaps exposed by this scoping review need to inform revision of relevant PICO-T questions for a subsequent systematic review, as well as exploration by academic and clinical scientists in the clinical nutrition space for the urgent design and execution of clinical investigations. The knowledge gaps must be closed with effective educational programs in medical institutions, professional medical societies, and other settings to achieve prompt and uniform distribution of critical information for all stakeholders caring for patients before, during, and after COVID-19. These mandates for nutrition researchers and educators must be facilitated and implemented to keep pace with the rapid, and at times unpredictable, tempo and trajectory of the ongoing COVID-19 pandemic and future viral pandemics.
Acknowledgements
We acknowledge and greatly appreciate the expert work of Sara Fleming, Wanda Johnson, CMP, CAE, FACEHP, and the ASPEN staff in the organization and logistical support of this ASPEN document.
Footnotes
Supplementary Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Financial disclosure: Salvatore Carbone was supported by a Career Development Award (19CDA34660318) from the American Heart Association and by the Clinical and Translational Science Awards Program (UL1TR002649) from the National Institutes of Health to Virginia Commonwealth University. Any recommendation in this paper does not constitute medical or other professional advice and should not be taken as such. To the extent that the information published herein may be used to assist in the care of patients, this is the result of the sole professional judgment of the attending healthcare professional whose judgment is the primary component of quality medical care. The information presented here is not a substitute for the exercise of such judgment by the healthcare professional. Circumstances in clinical settings and patient indications may require actions different from those recommended in this document, and in those cases, the judgment of the treating professional should prevail. This paper was approved by the ASPEN Board of Directors.
Conflicts of interest: Jeffrey I. Mechanick received honoraria from Abbott Nutrition for lectures and program development and is on the advisory board of GoodSugar+. Ryan T. Hurt is a consultant for the Nestlé Nutrition Institute Clinical Fellowship Program. Kris M. Mogensen received honoraria from Baxter for lectures at the Baxter iCAN conference and is on the advisory board of Baxter Indirect Calorimetry. Juan B. Ochoa Gautier received honoraria from Baxter, Fresenius Kabi, and Nestlé Health Science for lectures and program development. Jayshil Patel is a consultant for Nestlé Health Science and on the advisory board of Baxter. Martin Rosenthal received honoraria from Fresenius Kabi and Nestlé Health Science for lectures. All other authors declare they have no conflicts of interest to disclose.
References
- 1.Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Bio Med. 2020;91(1):157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mechanick JI, Hurley DL, Garvey WT. Adiposity-based chronic disease as a new diagnostic term: American Association of Clinical Endocrinologists and the American College of Endocrinology position statement. Endocr Pract. 2017;23(3):372–378 [DOI] [PubMed] [Google Scholar]
- 3.Mechanick JI, Garber AJ, Grunberger G, Handelsman Y, Garvey WT. Dysglycemia-based chronic disease: An American Association of Clinical Endocrinologists position statement. Endocr Pract. 2018;24(11):995–1011. [DOI] [PubMed] [Google Scholar]
- 4.Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic-based chronic disease – adiposity and dysglycemia drivers. J Am Coll Cardiol. 2020;75(5):525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic-based chronic disease – addressing knowledge and clinical practice gaps in the preventive care plan. J Am Coll Cardiol. 2020;75(5):539–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. Published online August 14, 2020. doi: 10.1016/j.annepidem.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Killerby ME, Link-Gelles R, Haight SC, et al. Characteristics associatedwithhospitalizationamongpatientswithCOVID-19–Metropolitan Atlanta, Georgia, March-April, 2020. MMWR. 2020;69(25):790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel AP, Paranjpe MD, Kathiresan NP, et al. Race, socioeconomic deprivation, and hospitalization for COVID-19 in English participants of a national biobank. Int J Equity Health. 2020;19(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batty GD, Deary IJ, Luciano M, et al. Psychosocial factors and hospitalisations for COVID-19: prostpective cohort study based on a community sample. Brain Behav Immun. 2020; 89:569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan J, Wang H, Ye G, et al. Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020; 107:154243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicenter, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikpouraghdam M, Jalali Farahani A, Alishiri G, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: a single center study. J Clin Virol. 2020;127:104378. 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Cheng A, Kumar R, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. Published May 14, 2020. 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wambier CG, Goren A, Vano-Galvan S, et al. Androgen sensitivitygatewaytoCOVID-19 disease severity. Drug Dev Res. Published online May 15, 2020. 10.1002/ddr.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollston R, Galea S. COVID-19 and the social determinants of health. Am J Health Promotion. 2020;34(6):687–689. [DOI] [PubMed] [Google Scholar]
- 18.Mechanick JI, Rosenson RS, Pinney SP, et al. Coronavirus and Cardiometabolic Syndrome. J Am Coll Cardiol.2020;76(17):2024–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Chen S, Liu M, et al. Comorbid chronic diseases are strongly correlated with disease severity among COVID-19 patients: a systematic review and meta-analysis. Aging Dis. 2020;11(3):668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells Mulherin D, Walker R, Holcombe B, et al. ASPEN report onnutritionsupportpracticeprocesseswithCOVID-19: the first response. Nutr Clin Pract. 2020;35(5):783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yehya A, Carbone S. Managing type 2 diabetes mellitus duringCOVID-19 pandemic: the bittersweet. Diabetes Metab Res Rev. Published online June 3, 2020. 10.1002/dmrr.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muscogiuri G, Pugliese G, Barrea L, et al. Commentary. Obesity: the “Achilles heel” for COVID-19? Metabolism. 2020;108:154251. 10.1016/j.metabol.2020.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Rio C, Malani P. Translating science on COVID-19 to improve clinical care and support the public health response. JAMA. 2020; 323(24):2464–2465. 10.1001/jama.2020.9252. [DOI] [PubMed] [Google Scholar]
- 25.Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and COVID-19 in patients presenting with in fluenzalike symptoms. Int Forum Allergy Rhinol. 2020;10(7):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price-Haywood EG, Burton J, Fort D, et al. Hospitalization and mortality among black patients and white patients with COVID-19. N Engl J Med. 2020;382(26):2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn CG, Kenney E, Fleischhacker SE, et al. Feeding low-income children during the COVID-19 pandemic. N Engl J Med. 2020;382(18):e40. 10.1056/NEJMp2005638. [DOI] [PubMed] [Google Scholar]
- 28.Wolfson JA, Leung CW. Food insecurity and COVID-19: disparities in early effects for US adults. Nutrients. 2020;12(6):1648. 10.3390/nu12061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huizar MI, Arena R, Laddu DR. The global food syndemic: the impact of food insecurity, malnutrition and obesity on the healthspan amid the COVID-19 pandemic. Prog Cardiovasc Dis. Published online July 10, 2020. 10.1016/j.pcad.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paslakis G, Dimitropoulos G, Katzman DK, et al. A call to action to address COVID-19-induced global food insecurity to prevent hunger, malnutrition, and eating pathology. Nutr Rev. Published online July 11, 2020. 10.1093/nutrit/nuaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belanger MJ, Hill MA, Angelidi AM, et al. COVID-19 and disparities in nutrition and obesity. N Engl J Med. 2020;383:11–e69. [DOI] [PubMed] [Google Scholar]
- 32.Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7(3):398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Food Security Information Network. Global report on food crises 2019. http://www.fao.org/emergencies/resources/documents/resources-detail/en/c/1187704/ (Accessed on August 24, 2020).
- 34.Li T, Zhang Y, Gong C, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74(6):871–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calder PC, Carr AC, Gombart AF, et al. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Handu D, Moloney L, Rozga M, et al. Malnutrition care during the COVID-19 pandemic: considerations for Registered Dietitian Nutritionists. J Acad Nutr Diet. Published online May 14, 2020. 10.1016/j.jand.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zabetakis I, Lordan R, Norton C, et al. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12(5):1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler MJ, Barrientos RM. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immunol. 2020;87:53–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rayman G, Lumb A, Kennon B, et al. Guidance on the management of diabetic ketoacidosis in the exceptional circumstances of the COVID-19 pandemic. Diab Med. 2020;37(7):1214–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang P, He Z, Yu G, et al. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin Nutr. Published on June 5, 2020; 10.1016/j.clnu.2020.05.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martindale R, Patel JJ, Taylor B, et al. Nutrition therapy in critically ill patients with coronavirus disease 2019. J Parenter Enteral Nutr. Published online May 27, 2020; 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369: m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenthal MD, Bala T, Wang Z, et al. Chronic critical illness patients fail to respond to current evidence-based intensive care nutrition secondarily to persistent inflammation, immunosuppression, and catabolic syndrome. J Parenter Enteral Nutr. Published online February 6, 2020; 10.1002/jpen.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schorghuber M, Fruhwald S. Effects of enteral nutrition on gastrointestinal function in patients who are critically ill. Lancet Gastroenterol Hepatol. 2018;3(4):281–287. [DOI] [PubMed] [Google Scholar]
- 45.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J Parenter Enteral Nutr. 2016;40(2):159–211. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. [DOI] [PubMed] [Google Scholar]
- 48.Putzu A, Belletti A, Cassina T, et al. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. J Crit Care. 2017;38:109–114. [DOI] [PubMed] [Google Scholar]
- 49.Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Critical Care Med. 2001;29(12):2264–2270. [DOI] [PubMed] [Google Scholar]
- 50.Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, vitamin c, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 2017;151(6):1229–1238. [DOI] [PubMed] [Google Scholar]
- 51.Marik PE. Vitamin C for the treatment of sepsis: the scientific rationale. Pharmacol Ther. 2018;189:63–70. [DOI] [PubMed] [Google Scholar]
- 52.Lyons D, Frampton M, Naqvi S, et al. Fallout from the Covid-19 pandemic – should we prepare for a tsunami of post viral depression? Irish J Psychologic Med. Published online May 15, 2020. 10.1017/ipm.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perrin R, Walther A, Mukherjee A, et al. Into the looking glass: post-viral syndrome post COVID-19. Med Hypothesis. 2020;144:110055. 10.1016/j.mehy.2020.110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butler MJ, Barrientos RM. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immun. 2020;87;53–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmer K. Could COVID-19 trigger chronic disease in some people? The Scientist. 2020. https://www.the-scientist.com/news-opinion/could-covid-19-trigger-chronic-disease-in-some-people-67749 (Accessed on August 8, 2020). [Google Scholar]
- 56.Stam HJ, Stucki G, Bickenbach J. COVID-19 and post intensive care syndrome: a call for action. J Rehabil Med 2020;52(4):jrm00044. [DOI] [PubMed] [Google Scholar]
- 57.Batheja S, Nields JA, Landa A, et al. Post-treatment lyme syndrome and central sensitization. J Neuropsychiatry Clin Neurosci. 2013;25(3):176–186. [DOI] [PubMed] [Google Scholar]
- 58.Mehr SE, Barbul A, Shibao CA. Gastrointestinal symptoms in postural tachycardia syndrome: a systematic review. Clin Auton Res. 2018;28(4):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gemelli Against COVID-19 Post-Acute Care Study Group. PstCOVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carfi A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020;324(6):603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levin M. Childhood multisystem inflammatory syndrome – a new challenge in the pandemic. N Engl J Med. 2020;383(4):393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giuliani C, Li Volsi P, Brun E, et al. Breastfeeding during the COVID-19 pandemic: suggestions on behalf of woman study group of AMD. Diabetes Res Clin Pract. 2020;165:108239. 10.1016/j.diabres.2020.108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klok FA, Boon GJAM, Barco S, et al. The post-COVID-19 functional status scale: a toll to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1):2001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krznaric Z, Bender DV, Laviano A, et al. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin Nutr. 2020;39(7):1983–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akseer N, Kandru G, Keats EC, et al. COVID-19 pandemic and mitigation strategies: implications for maternal and child health and nutrition. Am J Clin Nutr. 2020;112(2):251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matras P, Klek S, Folwarski M, et al. Home medical nutrition duringSARS-CoV-2 pandemic – a position paper. Clin Nutr. 2020;38:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaffri A, Jaffri UA. Post-intensive care syndrome and COVID-19: crisisafter a crisis? Heart & Lung. Published online June 18, 2020; 10.1016/j.hrtlng.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pearl RL. Weight stigma and the “Quarantine-15”. Obesity. 2020;28(7):1180–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aromataris E, Munn Z (Editors). Joanna Briggs Institute Reviewer’s Manual, JBI, 2017. [Google Scholar]
- 72.Munn Z, Peters MD, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Infusino F, Marazzato M, Mancone M, et al. Diet supplementation, probiotics, and nutraceutical in SARS-CoV-2 infection: a scoping review. Nutrients. 2020;12(6):1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rozga M, Cheng FW, Moloney L, et al. Effects of micronutrients orconditional amino acids on COVID-19-related outcomes: an evidence analysis center scoping review. J Acad Nutr Dietet. Published online May 20, 2020. 10.1016/j.jand.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.American Society for Parenteral and Enteral Nutrition. Resources for clinicians caring for patients with coronavirus. http://www.nutritioncare.org/COVID19Resources/ (Accessed on August 16,2020).
- 76.Barazzoni R, Bischoff SC, Breda J, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutrition. 2020;39(6):1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Minelli N, Gibbs L, Larrivee J, et al. Challenges of maintaining optimal nutritional status in COVID-19 patients in intensive care settings. J Parenter Enteral Nutr. Published online August 16, 2020. 10.1002/jpen.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]