Abstract
Background
Corticosteroid‐induced osteoporosis is a cause of morbidity in patients with chronic obstructive lung disease, asthma, and rheumatologic disorders. Corticosteroid treatment causes bone loss by a variety of complex mechanisms. It has been shown that bone mineral loss at the hip averages 14% in the first year after starting corticosteroid therapy.
Objectives
To review the efficacy of calcitonin (subcutaneous or nasal) for the treatment and prevention of corticosteroid‐induced osteoporosis.
Search methods
We conducted a search of Medline, the Cochrane Controlled Trials Register and Embase using the Cochrane Musculoskeletal Group search strategy for randomized controlled trials (RCTs) up to May 1998. We also searched bibliographic references and consulted content experts.
Selection criteria
Two independent reviewers selected RCTs which met predetermined inclusion criteria.
Data collection and analysis
Two reviewers independently extracted data using predetermined forms and assessed methodological quality of randomization, blinding and dropouts. For dichotomous outcomes, relative risks (RR) were calculated. For continuous data, weighted mean differences (WMD) of the percent change from baseline were calculated. We decided a priori to use random effects models for all outcomes, because of uncertainty about whether a consistent true effect exists in such different populations.
Main results
Nine trials met the inclusion criteria, including 221 patients randomized to calcitonin and 220 to placebo. The median methodologic quality was two out of a maximum of five points. Calcitonin was more effective than placebo at preserving bone mass at the lumbar spine after six and 12 months of therapy with a WMD of 2.8% (95% CI: 1.4 to 4.3) and 3.2% (95% CI: 0.3 to 6.1). At 24 months, lumbar spine BMD was not statistically different between groups: WMD 4.5% (95% CI: ‐0.6 to 9.5)]. Bone density at the distal radius was also higher with calcitonin after six months of therapy, but bone density at the femoral neck was not different between placebo and calcitonin treated groups. The relative risk of fractures was not significantly different between calcitonin and placebo with a relative risk (RR) of 0.71 (95% CI: 0.26 to 1.89) for vertebral and 0.52 (95% CI: 0.14 to 1.96) for non vertebral fractures. The subgroup analyses of methodological quality and duration of corticosteroid therapy were confounded. Trials of patients who had been taking steroids for greater than three months (which were of low methodologic quality) demonstrated a larger effect of calcitonin on spine bone density (about 6%) than prevention trials (about 1%). There was no consistent effect of different dosages (50‐100 IU compared to 200‐400 IU). However, subcutaneous calcitonin showed substantially greater prevention of bone loss. Withdrawals due to side effects were higher in the calcitonin‐treated groups: RR 3.19 (95%CI: 0.66 to 15.47). Important side effects included nausea and facial flushing.
Authors' conclusions
Calcitonin appears to preserve bone mass in the first year of glucocorticoid therapy at the lumbar spine by about 3% compared to placebo, but not at the femoral neck. Our analysis suggests that the protective effect on bone mass may be greater for the treatment of patients who have been taking corticosteroids for more than three months. Efficacy of calcitonin for fracture prevention in steroid‐induced osteoporosis remains to be established.
Keywords: Humans, Calcitonin, Calcitonin/therapeutic use, Glucocorticoids, Glucocorticoids/adverse effects, Osteoporosis, Osteoporosis/chemically induced, Osteoporosis/drug therapy
Plain language summary
Calcitonin for preventing and treating corticosteroid‐induced osteoporosis
Long‐term corticosteroids are prescribed for a number of reasons, including inflammatory bowel disease, chronic obstructive lung disease and rheumatoid arthritis. Steroids cause bone loss by a variety of complex mechanisms. Calcitonin is an anti‐resorptive therapy that has been approved for the treatment of established osteoporosis. The purpose of this review was to evaluate calcitonin as a means of preventing bone loss with corticosteroid therapy. Nine randomized controlled trials were included in the review, with 221 patients randomized to calcitonin and 220 to placebo. The results showed that calcitonin prevents bone loss at the spine and forearm by about 3% after the first year of therapy. There was no effect on bone loss at the hip. Calcitonin was not statistically different from placebo at preventing fractures of the spine and long bones, such as hip fractures. Calcitonin was associated with four times the side effects of placebo, and these were mostly nausea and facial flushing.
Background
Osteoporosis and subsequent fracture are a major cause of morbidity and mortality. It is a disease state defined by low bone mass, and has many etiologies with different patterns of bone loss. Involutional or senile osteoporosis causes loss of both cortical and trabecular bone, whereas steroid‐induced osteoporosis is associated with rapid rates of spinal bone loss (Adachi 1999, Ruegsegger 1983). Because of this differential effect, vertebral collapse is the most common fracture in patients on steroids. Patients with inflammatory disorders such as rheumatoid arthritis, inflammatory bowel disease and sarcoidosis, are uniquely at risk for osteoporosis due to their underlying disease, as well as the frequent administration of corticosteroids.
A recent cohort study in the USA has identified that on average, 30‐40% of rheumatoid arthritis patients are prescribed prednisone (Ward 1998). They are usually maintained on steroid therapy for a mean of four years. As for polymyalgia rheumatica (PMR), steroids are the accepted method of treatment with greater than 95% of patients being treated in this manner. Therapy for this disorder is usually continued for one to three years. Additionally, steroids are prescribed for patients with chronic obstructive lung disease, asthma and inflammatory bowel disease.
Corticosteroid therapy is a contributor to the development of osteoporosis in these populations. A prospective cohort study has shown that most of the bone loss occurs in the first 5‐7 months of treatment (LoCasio 1984). Steroids cause bone loss by a variety of complex mechanisms (Manolagas 1999). They act to decrease absorption of calcium from the intestine, and increase urinary calcium loss. This leads to the development of secondary hyperparathyroidism, which results in bone resorption. Steroids may also directly inhibit osteoblasts from laying down new bone. Glucocorticoids suppress the hypthalmic‐pituitary‐gonadal axis which leads to functional hypogonadism (MacAdams 1998). Male rheumatoid arthritis patients on steroids have been shown to have lower testosterone levels, presumably on the basis of suppressed hypothalmic‐pituitary‐testicular axis (Adachi 1997R).
It has been shown in steroid treated sarcoid patients, that bone mineral loss at the hip averages 14% in the first year after starting corticosteroid therapy, and in steroid‐treated, early post‐menopausal women this figure increases to 22% (Montemurro 1990). One study has shown that even 5 mg of prednisone per day doubles the rate of bone loss in females and males over an eight year period (Saito 1995). It has been suggested that patients initiating steroids should receive therapy such as vitamin D, estrogens, calcitonin, fluoride or bisphosphonates (ACR 1996). Calcium should be considered as an adjunct to other agents (Adachi 1999). It is not yet common practice for patients to receive osteoporosis prophylaxis at the time they begin steroid therapy. In a recent study of 147 patients receiving long‐term oral corticosteroid therapy, 42% of postmenopausal women were receiving no preventive therapy (Buckley 1999).
Objectives
To determine the efficacy of calcitonin in the prevention and treatment of steroid induced osteoporosis.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) and controlled clinical trials (CCTs) meeting the inclusion criteria were included in this systematic review.
Types of participants
Men or women over the age of 18, with any underlying disease that requires therapy with systemic corticosteroids. Prevention was defined as initiating calcitonin therapy within three months of starting corticosteroid therapy. Treatment was defined by populations who were taking corticosteroids for at least three months prior to starting calcitonin therapy.
Types of interventions
Randomized controlled clinical trials that used calcitonin for the prevention or treatment of steroid induced osteoporosis. We accepted control groups that received a placebo or were untreated. Calcium and vitamin D were accepted in the control group if they were also given in equal doses to the treated group.
Types of outcome measures
The primary outcomes assessed were percent change in bone mineral density at six months, one and two years. We also looked at reduction in vertebral and non vertebral fracture incidence. Toxicity was assessed by tabulating side effects, withdrawals due to adverse events and withdrawals overall.
Search methods for identification of studies
MEDLINE, EMBASE and the Cochrane Controlled Trials Register (CCTR) were used to identify all clinical trials relating to calcitonin treatment of steroid‐induced osteoporosis. We used the MEDLINE search strategy adopted and modified for the Cochrane Musculoskeletal Group (Haynes 1994), and search the years 1966 ‐ August 1998. Keywords used for the search included: bone diseases, osteoporosis, anti‐inflammatory agents;steroidal, corticosteroid and calcitonin. The search strategy is provided below.
Database: Medline <1966 ‐ 1995>
Set Search Results ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp steroids/ 349986 2 steroid$.tw,rn. 73234 3 methylprednisone.tw,sh. 59 4 cortisol.tw,sh,tw. 19225 5 prednisone.tw,sh,rn. 21935 6 glucocorticoid.rn,tw,sh. 12798 7 exp adrenal cortex hormones/ 111945 8 exp cyclosporins/ 15453 9 cyclosporin$.tw,sh,rn. 18712 10 or/1‐9 421180 11 exp bone diseases, metabolic/ 18847 12 osteoporos#s.tw. 8369 13 bone density/ 5510 14 bone densit$.tw. 2662 15 bone mineral densit$.tw. 2234 16 exp "bone and bones"/ 215705 17 bone loss$.tw. 3564 18 osteomalacia.tw. 2107 19 osteodystrophy.tw. 1572 20 exp bone demineralization, pathologic/ 252 21 (bone adj demineralization).tw. 155 22 osteopenia.tw. 1367 23 bone mass.tw. 2622 24 exp densitometry/ 11119 25 densitometry.tw. 3064 26 dexa.tw. 299 27 exp fractures/ 60136 28 fracture$.tw. 55676 29 or/11‐28 288564 30 clinical trial.pt. 190918 31 randomized controlled trial.pt. 85487 32 tu.fs. 700826 33 dt.fs. 637888 34 random$.tw. 122317 35 (double adj blind$).tw. 39767 36 placebo$.tw. 43690 37 or/30‐36 993683 38 10 and 29 10827 39 calcitonin.sh,rn,tw. 13293 40 (calcitrin or thyrocalcitonin).tw. 705 41 miacalcin.tw. 1 42 or/39‐41 13337 43 38 and 42 579 44 37 and 43 260
Similar strategies were developed for searching EMBASE, although currently we were only able to search the years 1988 ‐ 1996.
All foreign language journals were included in the search.
An electronic search in Current Contents was done for the last six months up to Sept 1, 1999.
The lists of references were manually searched to add any citations missed by the electronic searches. Abstracts from the following scientific meetings were manually checked and included if the information was available in the body of the abstract: American Society for Bone and Mineral Research, American College of Rheumatology, Canadian Rheumatology Association.
Data collection and analysis
After fulfilling the initial criteria, the following criteria were also required:
Randomized allocation of patients into treatment groups. We looked for the words random and randomized in the methods of allocation of the trial and blinding of the study participants and investigators to the study group allocation.
We required an adequate description of the intervention medications in terms of dosage schedule and administration. We also required documentation of withdrawals and dropouts.
Methods used to collect data from included trials:
Data was extracted from the trials by two independent observers (AC,VW). Agreement between the two were assessed using the kappa statistic. In the case of disagreements, the two observers discussed the issue and attempted to reach a consensus. If necessary, a third observer was used as an adjudicator (BS).
Data was extracted for the following time points and outcomes: Time Points: Six months One year Two years
Outcomes: Efficacy Percent change in bone mineral density Incidence of vertebral fractures Incidence of non vertebral fractures Toxicity Number of patients with side‐effects Number of withdrawals due to side‐effects Withdrawals overall
Methods to synthesize data
All the trials were analyzed using Review Manager 4.0.3 and Metaview 3.1. For continuous variables such as bone density, we anticipated reports of mean differences. Thus, we did not calculate effect sizes. If absolute values were reported, we calculated mean differences. The mean difference for each intervention group was weighted by the sample size of the group.
Dichotomous results including fractures, dropouts and withdrawals were summarized as relative risks. The summary relative risk was obtained by weighting each individual relative risk by the inverse of the variance of the estimate for each trial.
The results for each trial were tested for heterogeneity using the chi square statistic.
We decided a priori to use random effects models throughout, due to the variety of patient populations and underlying diseases. We suspected that heterogeneity would be significant for most comparisons due to differences in the populations.
A sensitivity analysis was conducted to evaluate the robustness of the meta‐analysis results. This analysis examined the effects of methodological quality, duration of steroid therapy (prevention versus treatment) and potential differences in drug dosage and administration.
Results
Description of studies
The literature searches retrieved 303 articles. One abstract was located by searching abstract books (Emkey 1995). Of these articles, 12 were considered potential RCTs. Of these, nine randomized controlled trials were included (Adachi 1997, Bohning 1990, Emkey 1995, Healey 1996, Kotaniemi 1996, Luengo 1990, Luengo 1994, Ringe 1987, Sambrook 1993). Three trials were excluded due to lack of a placebo group (Garcia‐Delgado 1997), retrospective cohort design (Montemurro 1991), and one trial included a rheumatoid arthritis population which was not treated with corticosteroids (Dottori 1982).
The included trials involved 221 patients randomized to calcitonin and 220 to placebo. Of the included trials, four involved populations with polymyalgia rheumatica, temporal arteritis or rheumatoid arthritis (Adachi 1997, Healey 1996, Kotaniemi 1996, Sambrook 1993), two involved patients with chronic obstructive lung disease (COPD) (Bohning 1990, Ringe 1987) two involved asthmatic populations (Luengo 1994, Luengo 1990), and one did not specify the indication for steroids (Emkey 1995).
Subcutaneous calcitonin was given in three trials (Healey 1996, Ringe 1987, Luengo 1990).
Four trials were classified as prevention trials, defined by initiation of calcitonin therapy within three months of starting corticosteroid therapy (Adachi 1997, Healey 1996, Kotaniemi 1996, Sambrook 1993). The other trials were categorized as treatment trials because the patients had taken chronic steroids for greater than 3 months prior to initiating calcitonin therapy (Bohning 1990, Luengo 1990, Luengo 1994, Emkey 1995, Ringe 1987).
Two authors were contacted in July 1999 for further information (Emkey 1995, Kotaniemi 1996), but no replies have been received as yet.
Risk of bias in included studies
Quality was assessed using a validated, five point scale which included items relating to randomization (two points), blinding (two points), and description of withdrawals and dropouts (one point) (Jadad 1996). Two reviewers reached consensus on the scores (AC, VW).
The median quality score was two. One trial scored one point, four trials scored two points, two trials scored three points and one trial scored four points.
Effects of interventions
Bone density Heterogeneity was significant for most outcomes, confirming our a priori decision to use random effects models.
Bone density at the lumbar spine was increased relative to placebo at all time points from six to 24 months: six months WMD: 2.8% (95% CI: 1.4 to 4.3), 12 months WMD: 3.2% (95% CI: 0.3 to 6.1) and 24 months WMD: 4.5% (95% CI: ‐0.6 to 9.5). At the femoral neck, no difference was observed between treated and placebo groups at six, 12 or 24 months. At the distal radius, bone density was increased at six and 12 months with WMD of 2.9% (95%CI: 1.4 to 4.4) and 3.1% (95%CI: ‐0.6 to 6.7) respectively, but not at 24 months.
Fractures
Vertebral fractures were reported in five trials, which included 127 patients randomized to calcitonin and 129 to placebo (Healey 1996, Kotaniemi 1996, Luengo 1994, Ringe 1987, Sambrook 1993). The relative risk of vertebral fracture was reduced with calcitonin, but not significantly: RR 0.71 (95% CI: 0.26 to 1.89). Heterogeneity was not significant (chi‐square 2.61, df 4). An analysis of evaluable patients only (efficacy) found no difference in treatment effect.
The relative risk of non vertebral fractures was not statistically significant in the four trials with 102 patients randomized to calcitonin and 106 to placebo (RR 0.52, 95% CI: 0.14 to 1.96). Heterogeneity was not significant (chi square 2.23, df 3).
Toxicity
There was no difference in overall withdrawals, but withdrawals due to side effects was higher in the calcitonin group, though not statistically significant (RR 3.19, 95%CI: 0.66 to 15.47). Reported side effects that were increased in the calcitonin group relative to control were flushing or rashes (RR 5.0, 95% CI: 1.36 to 18.42) and nausea (RR 8.65, 95%CI: 1.61 to 46.54). Gastrointestinal symptoms, nasal symptoms and hypercalcemia were not different between calcitonin and placebo.
Sensitivity analysis
Methodological quality Low quality trials (less than three on Jadad's scale) showed a greater risk reduction for vertebral fractures (RR: 0.50, 95% CI: 0.12 to 2.06 compared to RR 0.91, 95% CI: 0.23 to 3.60), but the relative risk reduction was still not significant. Low quality trials also found a greater difference in lumbar bone density at 12 months (WMD 6.1, 95% CI: 4.5 to 7.8 compared to WMD 0.9, 95% CI: ‐1.9 to 3.7). However, this analysis was confounded since all of the low quality trials involved treatment populations who were already taking corticosteroids for greater than three months prior to starting calcitonin.
Dose of calcitonin No consistent differences in effect were observed for the different dosage categories used.
Route of administration of calcitonin (subcutaneous versus intranasal) Subcutaneous injection of calcitonin was used in one trial that reported bone density and it showed the greatest difference in lumbar bone density (WMD 6.5%, 95%CI: 4.6 to 8.4) (Luengo 1990). One trial with subcutaneous injection showed a greater vertebral fracture risk reduction (RR 0.14, 95% CI: 0.01 to 2.59) (Ringe 1987) than the four trials that used nasal calcitonin. However, this risk reduction was not significant.
Prevention versus Treatment As described above, this subgroup analysis was confounded since all of the prevention trials were of high quality (greater than or equal to three on Jadad's scale). However, the results suggest that there is a differential effect of calcitonin on prevention of bone loss in patients initiating steroid therapy (WMD spine: 0.9, 95%CI: ‐1.9 to 3.7) compared to those who have been treated for more than three months with steroids (WMD spine: 6.1, 95%CI: 4.5 to 7.8).
Discussion
Calcitonin has been approved by the FDA and the Health Protection Board (Canada) as an alternate therapy for the treatment of patients with established postmenopausal osteoporosis. Bisphosphonates and combinations of calcium and vitamin D have proven efficacy for the prevention of bone loss for patients with steroid‐induced osteoporosis (Homik 1999b, Amin 1999, Homik 1999a).
The recent PROOF trial demonstrated that calcitonin prevents vertebral fractures in women with post‐menopausal osteoporosis (Chesnut 1998). However, there was a large withdrawal rate from this trial (of approximately 30%), which raises concerns about generalizability.
The results of this meta‐analysis show that calcitonin prevents bone loss, as measured by bone density at the spine and distal radius, but not at the femoral neck. We found a non‐significant reduced relative risk for both vertebral and non vertebral fractures. The toxicity analysis demonstrated that calcitonin was associated with significantly more patient self‐reports of flushing and nausea.
All of these trials had small sample sizes (less than 40 per group). This sample size is insufficient to detect some evidence for fracture prevention, even with statistical pooling of several trials. The sample size required to detect a difference in vertebral fracture prevention is probably greater than 200, especially considering the mixed populations in these trials (Cranney 1999). The sample size required is lower in populations with prevalent vertebral fractures.
We used random effects models for all analyses because of concern about significant heterogeneity of results. The random effects model incorporates variation in treatment effect between trials, and results in wider, more conservative confidence intervals.
Low quality trials have been shown to overestimate the treatment effect for a variety of different interventions and populations (Moher 1998). In our analysis, low methodological quality trials showed a greater protective effect of calcitonin on bone density. However, these low quality trials all involved treatment populations (patients who were taking corticosteroids for greater than three months prior to starting calcitonin). Therefore, it is not possible to determine from this analysis whether the quality or the population characteristics are responsible for the observed difference in treatment effect. The treatment trials (which were all of low quality) found a 6% difference in spine bone density between calcitonin and control at 12 months. The prevention trials (which were all of high quality) found a nonsignificant difference of only 1% in 12 months spine bone density.
There was no dose‐response effect with calcitonin. However, the trial that used subcutaneous injections showed the greatest difference in bone density between treated and control groups. This may be due to greater bio‐availability of the drug when given by the subcutaneous route (Adachi 1997R).
In conclusion, calcitonin has not yet demonstrated effectiveness at preventing fractures associated with steroid‐induced osteoporosis. However, calcitonin appears to prevent bone loss at the lumbar spine by about 3% in the first year. The beneficial effect of calcitonin on bone density may be greater in patients who have been taking corticosteroids for more than three months. Longer term trials, with an adequate sample size, are necessary to confirm whether calcitonin continues to preserve bone mass beyond one year and whether calcition has a significant effect on fractures. The evidence from this meta‐analysis suggests that calcitonin can be considered as an alternative treatment for corticosteroid induced osteoporosis, particularly for patients who have been taking corticosteroids for greater than 3 months.
Authors' conclusions
Implications for practice.
Therapies with proven fracture efficacy, such as bisphosphonates, should remain the first line treatment for patients at risk of steroid‐induced osteoporosis, until further research becomes available.
Implications for research.
A large scale randomized controlled trial is necessary to determine whether calcitonin can prevent vertebral and non vertebral fractures in this population.
What's new
| Date | Event | Description |
|---|---|---|
| 28 May 2008 | Amended | Converted to new review format. CMSG ID C014‐R |
Acknowledgements
The authors would like to thank Jessie McGowan for help with the electronic search strategy and the Cochrane Musculoskeletal Review Group Editorial Team and the Osteoporosis Facilitators for their time and effort reviewing this document. A special thanks to Norma and Jim Davies and Jacqueline Tetroe for their comments and helpful suggestions.
Data and analyses
Comparison 1. Bone mineral density.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lumbar spine ‐ 6 months | 5 | 207 | Mean Difference (IV, Random, 95% CI) | 2.83 [1.37, 4.29] |
| 2 Lumbar spine ‐ 1 year | 6 | 256 | Mean Difference (IV, Random, 95% CI) | 3.17 [0.26, 6.07] |
| 3 Lumbar spine ‐ 2 year | 3 | 147 | Mean Difference (IV, Random, 95% CI) | 4.47 [‐0.56, 9.50] |
| 4 Femoral neck ‐ 6 months | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 1.16 [‐4.01, 6.33] |
| 5 Femoral neck ‐ 1 year | 3 | 136 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐2.22, 0.30] |
| 6 Femoral neck ‐ 2 year | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 1.93 [‐2.02, 5.88] |
| 7 Distal radius ‐ 6 months | 3 | 128 | Mean Difference (IV, Random, 95% CI) | 2.92 [1.41, 4.42] |
| 8 Distal radius ‐ 1 year | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 3.07 [‐0.60, 6.74] |
| 9 Distal radius ‐ 2 year | 1 | 63 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐9.38, 13.38] |
| 10 Lumbar spine ‐ 2 year ‐ without Sambrook | 2 | 84 | Mean Difference (IV, Random, 95% CI) | 5.04 [‐5.23, 15.32] |
1.1. Analysis.

Comparison 1 Bone mineral density, Outcome 1 Lumbar spine ‐ 6 months.
1.2. Analysis.

Comparison 1 Bone mineral density, Outcome 2 Lumbar spine ‐ 1 year.
1.3. Analysis.

Comparison 1 Bone mineral density, Outcome 3 Lumbar spine ‐ 2 year.
1.4. Analysis.

Comparison 1 Bone mineral density, Outcome 4 Femoral neck ‐ 6 months.
1.5. Analysis.

Comparison 1 Bone mineral density, Outcome 5 Femoral neck ‐ 1 year.
1.6. Analysis.
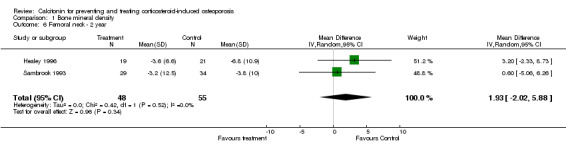
Comparison 1 Bone mineral density, Outcome 6 Femoral neck ‐ 2 year.
1.7. Analysis.

Comparison 1 Bone mineral density, Outcome 7 Distal radius ‐ 6 months.
1.8. Analysis.

Comparison 1 Bone mineral density, Outcome 8 Distal radius ‐ 1 year.
1.9. Analysis.

Comparison 1 Bone mineral density, Outcome 9 Distal radius ‐ 2 year.
1.10. Analysis.

Comparison 1 Bone mineral density, Outcome 10 Lumbar spine ‐ 2 year ‐ without Sambrook.
Comparison 2. Fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vertebral Fractures | 5 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.26, 1.89] |
| 2 Nonvertebral fractures | 4 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.14, 1.96] |
| 3 Vertebral fractures ‐ efficacy | 5 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.28, 1.98] |
2.1. Analysis.

Comparison 2 Fractures, Outcome 1 Vertebral Fractures.
2.2. Analysis.

Comparison 2 Fractures, Outcome 2 Nonvertebral fractures.
2.3. Analysis.

Comparison 2 Fractures, Outcome 3 Vertebral fractures ‐ efficacy.
Comparison 3. Toxicity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawals | 4 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.60, 2.33] |
| 2 Withdrawals due to side effects | 4 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 3.19 [0.66, 15.47] |
| 4 Flushing or rash | 5 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [1.36, 18.42] |
| 5 Nausea | 3 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 8.65 [1.61, 46.54] |
| 6 GI symptoms | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.16, 4.57] |
| 7 Nasal symptoms | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.54, 2.56] |
| 8 Hypercalcemia | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.50, 2.73] |
3.1. Analysis.

Comparison 3 Toxicity, Outcome 1 Withdrawals.
3.2. Analysis.

Comparison 3 Toxicity, Outcome 2 Withdrawals due to side effects.
3.4. Analysis.

Comparison 3 Toxicity, Outcome 4 Flushing or rash.
3.5. Analysis.

Comparison 3 Toxicity, Outcome 5 Nausea.
3.6. Analysis.

Comparison 3 Toxicity, Outcome 6 GI symptoms.
3.7. Analysis.

Comparison 3 Toxicity, Outcome 7 Nasal symptoms.
3.8. Analysis.

Comparison 3 Toxicity, Outcome 8 Hypercalcemia.
Comparison 5. Prevention vs Treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lumbar spine ‐ 1 year | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Prevention | 3 | 136 | Mean Difference (IV, Random, 95% CI) | 0.93 [‐1.87, 3.73] |
| 1.2 Treatment | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 6.14 [4.47, 7.80] |
| 2 Vertebral fractures | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Prevention | 3 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.22, 2.72] |
| 2.2 Treatment | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.08, 3.31] |
5.1. Analysis.

Comparison 5 Prevention vs Treatment, Outcome 1 Lumbar spine ‐ 1 year.
5.2. Analysis.

Comparison 5 Prevention vs Treatment, Outcome 2 Vertebral fractures.
Comparison 6. Low vs high quality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lumbar spine ‐ 1 year | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Quality < 3 | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 6.14 [4.47, 7.80] |
| 1.2 Quality => 3 | 3 | 136 | Mean Difference (IV, Random, 95% CI) | 0.93 [‐1.87, 3.73] |
| 2 Vertebral fractures | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Quality < 3 | 3 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.12, 2.06] |
| 2.2 Quality => 3 | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.23, 3.60] |
6.1. Analysis.

Comparison 6 Low vs high quality, Outcome 1 Lumbar spine ‐ 1 year.
6.2. Analysis.

Comparison 6 Low vs high quality, Outcome 2 Vertebral fractures.
Comparison 7. Subgroup analysis for dosage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lumbar spine | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcitonin 50 ‐ 100 IU | 3 | 126 | Mean Difference (IV, Random, 95% CI) | 3.48 [‐2.00, 8.97] |
| 1.2 Calcitonin 200‐400 IU | 3 | 130 | Mean Difference (IV, Random, 95% CI) | 2.44 [0.29, 4.59] |
| 2 Vertebral fractures | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Calcitonin 50 ‐ 100 IU | 4 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.19, 1.62] |
| 2.2 Calcitonin 200 ‐ 400 IU | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [0.22, 24.56] |
7.1. Analysis.

Comparison 7 Subgroup analysis for dosage, Outcome 1 Lumbar spine.
7.2. Analysis.

Comparison 7 Subgroup analysis for dosage, Outcome 2 Vertebral fractures.
Comparison 8. Route of administration: intranasal vs subcutaneous.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lumbar spine ‐ 1 year | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Intranasal | 5 | 216 | Mean Difference (IV, Random, 95% CI) | 2.13 [‐0.38, 4.63] |
| 1.2 Subcutaneous injection | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 6.5 [4.61, 8.39] |
| 2 Vertebral fractures | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Intranasal | 4 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.31, 2.47] |
| 2.2 Subcutaneous injection | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.59] |
8.1. Analysis.

Comparison 8 Route of administration: intranasal vs subcutaneous, Outcome 1 Lumbar spine ‐ 1 year.
8.2. Analysis.

Comparison 8 Route of administration: intranasal vs subcutaneous, Outcome 2 Vertebral fractures.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adachi 1997.
| Methods | Double blind, minimized, placebo controlled trial; Prevention Sample size at entry: SCT 16; placebo 15 Withdrawals: SCT 4; Placebo 2 Trial duration: 12 months | |
| Participants | Polymyalgia rheumatica with or without temporal arteritis, over 50 years of age, started prednisone within 1 month at does > 10 mg/d Mean age: 71 years Women/Men: SCT 9/7; Placebo 9/6 Prednisone dose: 17.2 mg/d Lumbar spine (mg/cm2): SCT 1.06; Placebo: 1.11 | |
| Interventions | Intranasal salmon calcitonin, 200 IU daily Calcium supplemented if baseline calcium intake < 800 mg/d |
|
| Outcomes | DXA lumbar spine, femoral neck, trochanter, Total body, withdrawals | |
| Notes | Quality score = 4 (R 1, B 2, W 1) | |
Bohning 1990.
| Methods | Randomized, double‐blind trial Treatment Trial duration: 12 months Sample size at entry: 36 Withdrawals: not reported | |
| Participants | Chronic obstructive lung disease with permanent steroid intake of at least 2 years previously with a mean dose of 10 mg/d prednisone Age: 56 (44‐67) Men/Women: 26/10 Mean prednisone dose: 16 mg (12.5 to 25) Mean prednisone duration: 6 years (2‐12) Lumbar bone mineral content (g/cm): SCT 3.44; Placebo: 3.61 | |
| Interventions | Intranasal Salmon Calcitonin 200 IU/day vs Placebo nasal spray | |
| Outcomes | Bone mineral content at spine and forearm (g/cm), spinal deformity index, biochemical markers | |
| Notes | Quality = 2 (R 1, B 1, W 0) | |
Emkey 1995.
| Methods | Randomized, double blind, controlled trial Treatment Sample size at entry: 75 Withdrawals: 19 Trial duration: 6 months | |
| Participants | Patients taking average daily dose of at least 10 mg prednisone for 3 months prior to study entry Age range: 24‐70 years | |
| Interventions | Calcitonin 50 IU by subcutaneous injection versus placebo Both groups received calcium to bring total intake to 1500 mg/d and vitamin D 400 IU/d |
|
| Outcomes | DEXA lumbar spine, SPA forearm BMC | |
| Notes | Quality = not applicable‐ abstract | |
Healey 1996.
| Methods | Randomized, controlled, double blind trial Prevention Sample size at entry: SCT 25; Pl 23 Withdrawals: SCT 6; Pl 2 Trial duration: 24 months | |
| Participants | 48 patients with newly diagnosed temporal arteritis, PMR Mean age: SCT 71.6; Pl 71.7 years Women/Men: SCT 16/9; Pl 20/3 Cumulative dose steroid: SCT 5371 mg; Pl 4680 mg Lumbar BMD(g/cm2): SCT 1.008 (0.212); Pl 0.987 (0.227) | |
| Interventions | Subcutaneous calcitonin 100 IU every other day versus
Placebo injections Both groups received calcium 1500 mg/d + Vitamin D3 400 IU |
|
| Outcomes | DXA Lumbar spine, Femoral neck BMD, vertebral fractures | |
| Notes | Quality score = 3 (R 1, B 1, W 1) | |
Kotaniemi 1996.
| Methods | Randomized, open parallel Prevention Sample size at entry: SCT 32; Placebo 31 Withdrawals: SCT 6; Placebo 8 Trial duration: 12 months | |
| Participants | Participants with RA (ACR criteria), using glucocorticoids > 7.5 mg/d prednisolone ordered for > 3 months Mean age: 49.2 years Number postmenopausal: SCT 15; Placebo 19 Lumbar BMD (g/cm2): SCT 1.036; Placebo 1.023 | |
| Interventions | Intranasal calcitonin 100 IU/day (Miacalcin) versus untreated 500 mg Calcium in both groups |
|
| Outcomes | BMD spine, femoral neck, trochanter, vertebral fractures, nonvertebral fractures, side effects | |
| Notes | Quality score =2 (R 1, B 0, W 1) | |
Luengo 1990.
| Methods | Randomized, open, controlled trial Treatment Sample size at entry: SCT 31; Ca 31 Withdrawals: SCT 11; Ca none, but only 20 used in analysis Trial duration: 12 months | |
| Participants | 62 steroid dependent asthmatics who had received steroids for at least 1 year Mean age: SCT 58.9; Pl 60.1 years Women/Men: SCT 8/12; Pl 8/12 Prednisone dose: SCT 10.5; Pl 10.9 mg/day Lumbar spine BMD, g/cm2: SCT 0.89 ; Pl 1.03 | |
| Interventions | Subcutaneous salmon calcitonin 100 IU every other day versus untreated Both groups received Calcium 1 g/d |
|
| Outcomes | Lunar DPA Lumbar spine, side effects | |
| Notes | Quality score = 1 (R 0, B 0, W 1) | |
Luengo 1994.
| Methods | Randomized, open parallel trial Treatment Sample size at entry: SCT 22; Ca 22 Withdrawals: SCT 5; Ca 5 Trial duration: 24 months | |
| Participants | Asthma, steroid dependent patients, received steroids for at least 1 year Mean age: 58.9 years Dose prednisone: 10 mg/d Duration steroid treatment: 9.7 years Women/Men: SCT 19/3; Ca 19/3 Prevalent fractures: SCT 4/22; Ca 4/22 Lumbar BMD (% predicted): SCT 88; Ca 91 | |
| Interventions | Intranasal calcitonin 200 IU q 2 days versus untreated Both groups received Calcium 1 gm/d |
|
| Outcomes | Lunar DPA: lumbar spine, fractures side effects | |
| Notes | Quality score = 2 (R 1, B 0, W 1) | |
Ringe 1987.
| Methods | Randomized, open, controlled study Treatment Sample size at entry: SCT 19; C 19 Withdrawals: SCT 1; C 1 Trial duration: 6 months | |
| Participants | Thirty‐six patients with steroid‐dependent COPD Mean age: SCT 49; C 50 years Duration steroid use: SCT 67; C 75 months Women/Men: SCT 15/3; C 14/4 Baseline prednisone mg/d: SCT 22.6; C 17.2 Prednisone dose during study mg/d: SCT 16.2; C 16.8 Lumbar BMD: unavailable | |
| Interventions | Calcitonin 100 IU salmon calcitonin subcutaneously every other day versus control Control group received analgesics on demand and physiotherapy |
|
| Outcomes | Distal radius 1/3 and 1/10, nonvertebral and vertebral fractures, pain | |
| Notes | Quality score = 2 (R 1, B 0, W 1) | |
Sambrook 1993.
| Methods | Double‐blind randomized trial; Prevention Sample size at entry: SCT+D 29; D 34; Pl 29 Withdrawals: 11 Trial duration: 12 months | |
| Participants | 103 particpants newly started on steroids Mean age: 52 years Women/Men: SCT+D 23/6; D 27/7 Baseline calcium: SCT+D 810 mg/d; D 938 mg/d Mean prednisone mg/d: 25 mg Mean dose prednisone in first year: 13.5 mg/d Mean dose prednisone in second year: 7.5 mg/d Lumbar BMD: SCT+D 1.13; D 1.18 | |
| Interventions | 1. Intranasal salmon calcitonin 400 IU per day plus calcitriol 0.5‐1.0 ug/d
2. Calcitriol 0.5‐1.0 ug plus placebo spray
3. Double placebo (placebo calcitriol and nasal spray) All groups received calcium 1000 mg/d |
|
| Outcomes | Lunar DPA: spine, femoral neck, distal radius, vertebral fractures, nonvertebral fractures, side effects | |
| Notes | Quality score = 3 (R 1, B 2, W 0) | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dottori 1982 | Rheumatoid arthritis patients, not all taking corticosteroids |
| Garcia‐Delgado 1997 | No placebo. Randomized, comparative trial, Intranasal salmon calcitonin 100 IU/d versus cyclical etidronate vs calcifediol 32,000 IU/wk |
| Montemurro 1991 | not an RCT, retrospective cohort design |
Contributions of authors
AC, VW and JDA are responsible for the content, protocol and interpretation of results. GW assisted with the statistical analysis. BS, PT, JH, MES helped with the protocol development and commented on draft versions of the review. BS, PT and GW helped with the development of the extraction forms and quality assessment forms.
Sources of support
Internal sources
Loeb Research Institute, Clinical Epidemiology Unit, Canada.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Adachi 1997 {published data only}
- Adachi JD, Bensen WG, Bell MJ, et al. Salmon calcitonin nasal spray in the prevention of corticosteroid‐induced osteoporosis. Br J Rheumatol 1997;36:255‐59. [DOI] [PubMed] [Google Scholar]
Bohning 1990 {published data only}
- Bohning W, Ringe JD, Weizel D, Bode V. Intranasales lachscalcitonin zur prophylaxe des knochenmineralverlustes bei steroid‐bedurftigen, chronisch‐obstruktiven atemwegserkrankungen. Drug Res 1990;40:1000‐1003. [PubMed] [Google Scholar]
Emkey 1995 {published data only}
- Emkey R, Reading W, Procaccini R, Gaich G and Steroid Induced Osteoporosis Study Group. The Effect of calcitonin on bone mass in steroid‐induced osteoporosis. Arthr Rheum 1994;37(9):S183. [Google Scholar]
Healey 1996 {published data only}
- Healey JH, Paget SA, Williams‐Russo P, et al. a randomized controlled of salmon calcitonin to prevent bone loss in corticosteroid‐trated temporal arteritis and polymyalgia rheumatica. Calcif Tissue Int 1996;58:73‐80. [DOI] [PubMed] [Google Scholar]
Kotaniemi 1996 {published data only}
- Kotaniemi A, Piirainen H, Paimela L, et al. Is continuous intranasal salmon calcitonin effective in treating axial bone loss in pateints with active rheumatoid arthritis receiving low dose glucocorticoid therapy?. J Rheumatol 1996;23:1875‐9. [PubMed] [Google Scholar]
Luengo 1990 {published data only}
- Luengo M, Picado C, Rio L, Guanabens N, Montserrat JM, Setoain J. Treatment of steroid‐induced osteopenia with calcitonin in corticosteroid‐ dependent asthma. Am Rev Resp 1990';142:104‐7. [DOI] [PubMed] [Google Scholar]
Luengo 1994 {published data only}
- Luengo M, Pons F, Martinez de Osaba MJ, Picado C. Prevention of further bone mass loss by nasal calcitonin in patients on long term glucocorticoiod therapy for asthma: a two year follow up study. Thorax 1994;49(11):1099‐1102. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ringe 1987 {published data only}
- Ringe JD, Welzel D. Salmon calcitonin in the therapy of corticoid‐induced osteoporosis. Eur J Clin Pharmacol 1987;33:35‐9. [DOI] [PubMed] [Google Scholar]
Sambrook 1993 {published data only}
- Sambrook P, Birmingham J, Kelly P, et al. Prevention of corticosteroid‐induced osteoporosis. NEJM 1993;328(24):1747‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dottori 1982 {published data only}
- Dottori L, D'Ottavio D, Brundisini B. Calcifediol and calcitonin in the therapy of rheumatoid arthritis. A short‐term controlled study. Minerva Medica 1982;73(43):3033‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Garcia‐Delgado 1997 {published data only}
- Garcia‐Delgado I, Prieto S, Gil‐Fraguas L, Robles E, Rufolanchas JJ, Hawkins F. Calcitonin, etidronate, and calcidiol treatment in bone loss after cardiac transplantation. Calcif Tissue Int 1997;60:155‐159. [DOI] [PubMed] [Google Scholar]
Montemurro 1991 {published data only}
- Montemurro L, Schiraldi G, Fraioli P, Tosi G, Riboldi A, Rizzato G. Prevention of corticosteroid‐induced osteoporosis with salmon calcitonin in sarcoid patients. Calcif Tissue Int 1991;49:71‐6. [DOI] [PubMed] [Google Scholar]
Additional references
ACR 1996
- Anonymous. Recommendations for the prevention and treatment of glucocorticoid‐induced osteoporosis. American College of Rheumatology Task Force on Osteoporosis Guidelines. Arthritis & Rheum 1996;39(11):1791‐1801. [DOI] [PubMed] [Google Scholar]
Adachi 1997R
- Adachi JD. Corticosteroid‐induced osteoporosis. Am J Med Sci 1997;313(1):41‐49. [DOI] [PubMed] [Google Scholar]
Adachi 1999
- Adachi JD, Rostom A. Metabolic bone disease in adults with inflammatory bowel disease. Inflamm Bowel Dis 1999;5(3):200‐11. [DOI] [PubMed] [Google Scholar]
Adachi 1999b
- Adachi JD, Ioannidis G. Calcium and vitamin D therapy in corticosteroid‐induced bone loss: what is the evidence?. Calcif Tissue Int 1999;65(4):335‐36. [DOI] [PubMed] [Google Scholar]
Amin 1999
- Amin S, LaValley MP, Simms RW, Felson DT. The role of vitamin D in corticosteroid‐induced osteoporosis: a meta‐analytic approach. Arthritis Rheum 1999;42(8):1740‐51. [DOI] [PubMed] [Google Scholar]
Buckley 1999
- Buckley LM, Marquez M, Feezor R, Ruffin DM, Benson LL. Prevention of corticosteroid‐induced osteoporosis: results of a patient survey. Arthritis Rheum 1999;42(8):1736‐9. [DOI] [PubMed] [Google Scholar]
Chesnut 1998
- Chestnut C, Silverman SL, Andriano K, et al. Salmon calcitonin nasal spray reduces the rate of new vertebral fractures independently of known major pre‐treatment risk factors: accrued five year analysis of the PROOF study. J Bone Mineral Research 1998;8:S290. [Google Scholar]
Cranney 1999
- Cranney A, Welch V, Tugwell P, Wells G, Adachi JD, McGowan J, Shea B. Responsiveness of endpoints in osteoporosis clinical trials ‐ an update. J Rheumatol 1999;26(1):222‐8. [PubMed] [Google Scholar]
Haynes 1994
- Haynes RB, Wilczynski N, McKibbon KA, Walker CJ, Sinclair JC. Developing optimal search strategies for detecting clinically sound studies in MEDLINE. J Am Med Informatics Assoc 1994;1:447‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Homik 1999a
- Homik J, Suarez‐Almazor ME, Shea B, et al. Calcium and vitamin D for corticosteroid‐induced osteoporosis (Cochrane Review). IN The Cochrane Library, Issue 1, 1999. Update Software. [DOI] [PMC free article] [PubMed]
Homik 1999b
Jadad 1996
- Jadad A, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
LoCasio 1984
- LoCasio V, Bonucci E, Imbimbo B, et al. Bone loss after glucocorticoid therapy. Calcif Tissue Int 1984;36:435. [DOI] [PubMed] [Google Scholar]
MacAdams 1998
Manolagas 1999
- Manolagas SC, Weinstein RS. New developments in the pathogenesis and treatment of steroid‐induced osteoporosis. J Bone Miner Res 1999;14(7):1061‐6. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Montemurro 1990
- Montemurro L, Fraioli P, Riboldi A, Delpiano S, Zanni D, Rizzato G. Bone loss in prednisone treated sarcoidosis: a two year follow‐up. Annali Italiani di Medicina Interna 1990;5(3 Pt 1):164‐8. [PubMed] [Google Scholar]
Ruegsegger 1983
Saito 1995
- Saito JK, Davis JW, Wasnich RC, Ross PD. Users of low dose glucocorticoids have increased bone loss rates: a longitudinal study. Calcif Tissue Int 1995;57:115‐9. [DOI] [PubMed] [Google Scholar]
Ward 1998
- Ward MM, Fries JF. Trends in antirheumatic medication use among patients with rheumatoid arthritis, 1981‐1996. J Rheumatol 1998;25(3):408‐16. [PubMed] [Google Scholar]


