Abstract
In Japan, the age‐adjusted incidence of cervical cancer has been increasing constantly and rapidly among younger women. We set out to accurately confirm the effectiveness of the HPV vaccine in Japan. Data were collected for women born in the fiscal year (FY) 1990 to 1997, who became eligible for their 20‐y‐old cervical cancer screening between the FY 2010 to 2017. The adjusted incidence of cervical intraepithelial neoplasia (CIN)1+ in women born in FY 1990 to 1993, that is those who reached the national vaccination target age prior to the introduction of publicly subsidized HPV vaccinations, referred here after as “the pre‐introduction generation”, was 1.42% (242/17 040). The incidence in the “vaccination generation” (women born in FY 1994 to 1997, that is those who were heavily vaccinated as a group when they were of the nationally targeted age of 13‐16) was 1.66% (135/8020). There was no significant difference between these incidence rates. However, our FY birth year‐by‐year analysis revealed that the incidence of CIN1+ was obviously lower than that predicted based on just the trend for CIN1+ seen in the pre‐introduction generation. Our analysis revealed that the incidence of CIN3+ was obviously lower in the vaccination generation than in the pre‐introduction generation (P = .0008). The incidence of CIN was already tending to increase in both the pre‐introduction and vaccination generations. The changes in CIN incidence by individual birth FY must be examined to accurately determine the actual effects of the HPV vaccine for reducing mild cervical lesions.
Keywords: birth year, cancer screening, cervical cancer, CIN, HPV vaccine
This is the first large‐scale study in Japan to show a significant preventive effect of HPV vaccine against CIN3+. The incidence of CIN1+ was the same in both the vaccination and the pre‐introduction generation. Because the incidence of CIN 1+ was tending to increase in both generations, the changes in CIN 1+ incidence by individual birth FY must be examined to accurately determine the actual effects of HPV vaccine for reducing mild cervical lesions.

Abbreviations
- FY
fiscal year
- HPV
human papillomavirus
1. INTRODUCTION
The number of cervical cancer cases has been declining in most parts of the world, but for the last 2 decades in Japan, since 1998, the age‐adjusted incidence of cervical cancers has been rapidly increasing, particularly among those aged 39 y or younger.1 This is in part because Japan has one of the lowest rates of cervical cancer screenings among all developed countries.2
This was not always the case. In FY 2010, public subsidies provided for HPV vaccination increased the vaccination rate of Japanese girls to c. 70% of the targeted age group (13‐16). However, in FY 2013, diverse symptoms alleged to be associated with recent HPV vaccination were reported. Japan's Health, Labour, and Welfare Ministry (MHLW) responded by announcing a suspension of its previous position of having a strong recommendation for HPV vaccination, leading to a sudden and dramatic decrease in Japan's HPV vaccination rate.3, 4, 5 As of April 2021 that recommendation suspension remains in effect. This has resulted in girls born in FY 2000‐2004 uniformly failing to participate in Japan's subsidized HPV vaccination program. It has been estimated that an additional 5500 of the vaccine‐suspension generation will die of cervical cancer, and that all of this loss of life could have been prevented by appropriate HPV vaccination.6
The life‐saving effect of the HPV vaccine, by preventing invasive cervical cancer, has been shown globally.7, 8 Preventing HPV infections decreases the numbers of HPV‐caused abnormal cytology. In Japan, a relative decrease in cervical intraepithelial neoplasia (CIN) findings has been reported for HPV vaccine recipients vs non‐recipients.9, 10, 11 In studies by Ozawa and by Konno et al, the vaccine recipients tended to be younger than the non‐recipients.9, 10 In comparison, over time in each birth FY, we have observed the vaccination rate and the incidence of abnormal cytology at age 20 (or at 21, in those who did not undergo screening at age 20). By only studying the results of screening at the age of 20, this enabled us to eliminate the bias of age distribution, HPV vaccination rates, and the higher rate of abnormal cytology. Among the women in their 20s, those in their early 20s who originally had a lower rate of abnormal cytology than those in their late 20s had the chance to receive HPV vaccine.
We have previously report that there were decreases in the incidence of atypical squamous cells of undetermined significance (ASC‐US)+ and low‐grade squamous intraepithelial lesions (LSIL)+ in women born between FY 1994 and FY 1995.12 We next reported that there was a decrease in the incidence of CIN3 in women born between FY 1994 and FY 1995.13 Our second report was the first to show the effect of HPV vaccination on preventing CIN3 in Japan, however our analysis was of only one municipality (with a population of approximately 510 000). Moreover, we could not confirm a decrease in the incidence of CIN1+. In our current study, we have analyzed data from 25 municipalities. We have also analyzed the data for girls born over 8 y between FY 1990 and FY 1997. We analyzed the effectiveness of HPV vaccination by accurately longitudinally observing and comparing the HPV vaccination rate and the incidence of abnormal cytology and CIN in each birth FY.
2. MATERIALS AND METHODS
We obtained data on the cumulative rate of vaccination of girls aged 16 or younger covered by public subsidies, the number of women receiving cervical cytology at age 20, and the results of cytology and biopsy for each birth FY woman's cervical cancer screening. These data were garnered from 25 different municipalities (Fukuoka, Hamamatsu, Iruma, Iwaki, Kawagoe, Kawasaki, Kishiwada, Kobe, Koka, Kumatori‐cho, Maebashi, Matsuyama, Mitake‐cho, Okayama, Ono, Osaka, Otsu, Oyama, Shikokuchuo, Suzuka, Takarazuka, Takatsuki, Toyonaka, Wako, Yao), with a total population of 12 950 000, corresponding to approximately one‐tenth of the total population of Japan. We focused on women born between FY 1990 and FY 1997 who had become age eligible for their publicly subsidized 20‐y‐old screening between FY 2010 and FY 2017. As described above, we eliminated biases based of age distribution, HPV vaccination rates, and abnormal cytology rates by analyzing only the results of screening at the age of 20 (although including those at age 21 if they had failed to undergo screening at age 20) so that a screening FY corresponded one‐to‐one with a single birth FY.
When the public subsidies for HPV vaccinations were introduced in FY 2010, the girls born between FY 1990 and FY 1993 were already aged 17 or older, so they could not receive their vaccination by public subsidies. Simply put, the vaccination rate was 0% for girls born between FY 1990 and FY 1993, and they are therefore here called the pre‐introduction generation. In contrast, because of the subsidized and officially recommended national vaccine program, the vaccination rate across the country varied from 55.5% to 78.8% in those girls born between FY 1994 and FY 1997, and were called the vaccination generation.14
First, we compared by Fisher exact test the rates of abnormal cervical cytology and abnormal histology between the pre‐introduction and the vaccination generations. Second, the change over time in the rate of cervical abnormalities in each group was examined by linear approximation using a simple linear regression analysis in MedCalc (MedCalc Software Ltd). An examination of the incidence of CIN showed that the rate of undergoing biopsy varied from 56.5% to 80.8% in accordance with the birth FY. The rate was adjusted by assuming that all girls with abnormal cytology underwent biopsy (Table 1). As we integrated the data provided by the municipalities, girls with inadequate data on the birth FY were excluded from the list. The level of statistical significance for our study was set at P = .05.
TABLE 1.
Changes over time (by birth FY) in the HPV vaccination rate and the incidence of abnormal cytology and CIN during cervical cancer screening at age 20
| Birth fiscal year | 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | |
| Screening fiscal year at age 20 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
| Number of municipalities | 14 | 17 | 20 | 21 | 17 | 16 | 17 | 11 | |
| Vaccination target | ー | ー | ー | ー | 16 357 | 26 536 | 28 306 | 14 433 | |
| Number of vaccinated girls | ー | ー | ー | ー | 11 049 | 18 480 | 21 023 | 11 024 | |
| Vaccination rate | 0.0% | 0.0% | 0.0% | 0.0% | 67.5% | 69.6% | 74.3% | 76.4% | |
| Number of screenings | 2676 | 3794 | 4913 | 5657 | 2482 | 2267 | 2186 | 1220 | |
| Number of abnormal cytology (ASC‐US+) | 41 | 97 | 201 | 225 | 73 | 72 | 77 | 46 | |
| Number of receiving biopsy | 24 | 59 | 113 | 149 | 59 | 59 | 52 | 26 | |
| Rate of receiving biopsy | 58.5% | 60.8% | 56.2% | 66.2% | 80.8% | 81.9% | 67.5% | 56.5% | |
| Number of CIN1 | 9 | 16 | 30 | 44 | 29 | 22 | 18 | 9 | |
| Number of CIN2 | 1 | 5 | 9 | 17 | 4 | 5 | 7 | 3 | |
| Number of CIN3 | 1 | 3 | 8 | 5 | 1 | 0 | 1 | 0 | |
| Rate of CIN1+ | 0.41% | 0.63% | 0.96% | 1.17% | 1.37% | 1.19% | 1.19% | 0.98% | |
| Rate of CIN2+ | 0.07% | 0.21% | 0.35% | 0.39% | 0.20% | 0.22% | 0.37% | 0.25% | |
| Rate of CIN3+ | 0.04% | 0.08% | 0.16% | 0.09% | 0.04% | 0.00% | 0.05% | 0.00% | |
| Adjusted number of CIN1+ | 19 | 39 | 84 | 100 | 42 | 33 | 39 | 21 | |
| Adjusted rate of CIN1+ (%) | 0.71% | 1.03% | 1.71% | 1.77% | 1.69% | 1.46% | 1.78% | 1.72% | |
| Adjusted rate of CIN1+ in each generation | 1.42% (242/17 040) | 1.66% (135/8020) | P = .15 | ||||||
| Adjusted number of CIN3+ | 2 | 5 | 14 | 8 | 1 | 0 | 1 | 0 | |
| Adjusted rate of CIN3+ (%) | 0.07% | 0.13% | 0.28% | 0.14% | 0.04% | 0.00% | 0.05% | 0.00% | |
| Adjusted rate of CIN3+ in each generation | 0.17% (29/17 040) | 0.02% (2/8155) | P = .0008 | ||||||
2.1. Informed consent and ethical approval
Informed consent was obtained by an opt‐out method on the website or the poster. This study was approved by the ethics committee of the Osaka University Hospital (#13261).
3. RESULTS
3.1. Effect on preventing abnormal cytology
The incidence of ASC‐US+ in the girls born in FYs 1990, 1991, 1992, and 1993 was 2.97% (120/4034), 3.08% (191/6211), 3.90% (223/5719), and 4.03% (297/7361), respectively. The decision coefficient (R²) was 0.89 and the regression coefficient was 0.40, which showed a strong correlation between the birth FY and the incidence of detecting ASC‐US+ and an increasing trend for ASC‐US+ (Figure 1). The vaccination rate for the girls born in FY 1994‐1997 was 65.4% (18 117/27 715), 71.4% (21 688/30 394), 76.2% (22 154/29 067), and 77.3% (13 059/16 899), respectively. The incidence of ASC‐US+ was 3.09% (126/4076), 3.05% (92/3021), 3.50% (119/3397), and 3.71% (87/2344), respectively. The R² was 0.86 and the regression coefficient was 0.23, which showed a strong correlation between the birth FY and the incidence of detecting ASC‐US+ and an increasing trend. The incidence of ASC‐US+ in the pre‐introduction generation was 3.56% (831/23 325), and in the vaccination generation was 3.30% (424/12 838), which showed no significant difference between the groups (P = .20). However, our FY birth year‐by‐year analysis revealed that the incidence of ASC‐US+ was obviously lower than that predicted based on just the trend for ASC‐US+ seen in the pre‐introduction generation.
FIGURE 1.
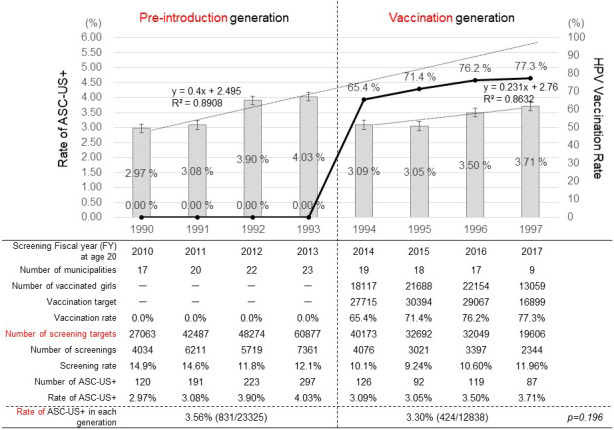
Changes over time (by birth FY) in the HPV vaccination rate and the incidence of ASC‐US+ cytology during cervical cancer screening at age 20. The line graph represents the cumulative rates of HPV vaccination in girls aged 16 y or younger, the bar graph represents the incidences of abnormal cytology (ASC‐US+). The regression lines and regression coefficients of changes in the incidence of abnormal cytology (ASC‐US+) in the pre‐introduction generation and the vaccination generation are graphically illustrated. For the pre‐introduction generation, x = 1 (FY 1990), x = 2 (FY1991), x = 3 (FY 1992), and x = 4 (FY1993). For the vaccination generation, x = 1 (FY 1994), x = 2 (FY1995), x = 3 (FY 1996), and x = 4 (FY1997)
The incidence of HSIL+ in the pre‐introduction girls, born in FYs 1990‐1993, was 0.20% (5/2531), 0.28% (13/4562), 0.29% (13/4468), and 0.38% (23/6114), respectively. The R² was 0.93 and the regression coefficient was 0.055, which showed a strong correlation between the birth FY and the incidence of detecting HSIL+ and a significantly increasing trend (Figure 2). The incidence of HSIL+ in women born in FY 1994‐1997 was 0.21% (6/2898), 0.11% (2/1893), 0.09% (2/2114), and 0.00% (0/966), respectively. The R² was .95 and the regression coefficient was −0.063, which showed a strong correlation between the birth FY and the incidence of detecting HSIL+ and had a significantly decreasing trend. The vaccination rate of the vaccination generation over those for the same 4 y was 61.5% (13 069/21 237), 69.6% (16 710/24 017), 75.5% (17 105/24 017), and 75.6% (7868/10 414), respectively.
FIGURE 2.
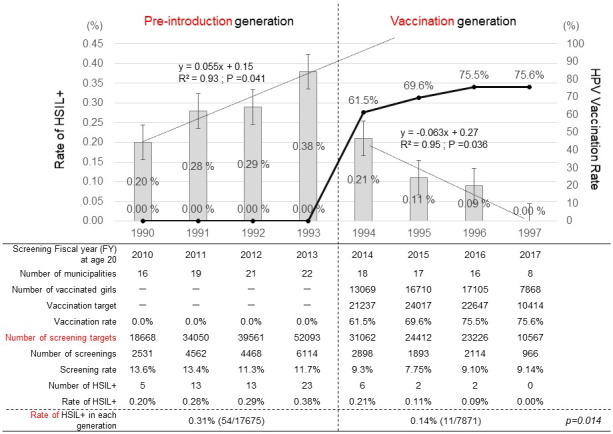
Changes over time (by birth FY) in the HPV vaccination rate and the incidence of HISL+ cytology during cervical cancer screening at age 20. The line graph represents the cumulative rates of HPV vaccination in girls aged 16 y or younger, and the bar graph represents the incidences of HSIL+. The regression lines and regression coefficients of changes in the incidence of HSIL+ in the pre‐introduction generation and the vaccination generation are graphically illustrated. For the pre‐introduction generation, x = 1 (FY 1990), x = 2 (FY1991), x = 3 (FY 1992), and x = 4 (FY1993). For the vaccination generation, x = 1 (FY 1994), x = 2 (FY1995), x = 3 (FY 1996), and x = 4 (FY1997)
The incidence of HISL+ in the overall combined pre‐introduction generation was 0.31% (54/17 675), and that in the overall combined vaccination generation was 0.14% (11/7871), which showed that the incidence of HSIL+ in the vaccination generation was significantly lower than that in the pre‐introduction generation (P = .014). By preventing HSIL+, HPV vaccination of the vaccination generation reversed the trend for increasing HISL+ that was seen in the pre‐introduction generation. The suppression of HISL+ was much stronger than the suppression seen for ASC‐US+.
3.2. Vaccination effect on preventing CIN
The adjusted incidence of CIN1+ in the pre‐introduction girls born in FY 1990‐1993 was 0.71% (19/2676), 1.03% (39/3794), 1.71% (84/4913), and 1.77% (100/5657), respectively. The R² was 0.92 and the regression coefficient was 0.39, which showed a strong correlation between the birth FY and the incidence of detecting CIN1+, and a significantly increasing trend (Figure 3). The adjusted incidence of CIN1+ in the vaccination generation of girls, born in FY 1994‐1997, was 1.69% (42/2482), 1.46% (33/2267), 1.78% (39/2186), and 1.72% (21/1220), respectively. The R² was 0.14 and the regression coefficient was 0.041, which showed an increasing trend. The incidence of CIN1+ in the combined pre‐introduction generation was 1.42% (242/17 040), and that in the combined vaccination generation was 1.66% (135/8020), which showed no significant difference between the 2 groups (P = .15). However, our FY birth year‐by‐year analysis revealed that the incidence of CIN1+ was clearly lower than that predicted based on just the trend for A CIN1+ seen in the pre‐introduction generation.
FIGURE 3.
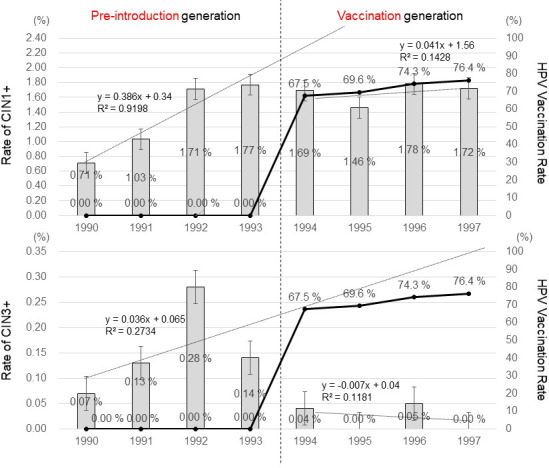
Changes over time (by birth FY) in the HPV vaccination rate and the incidence of CIN1+ and CIN3+ during cervical cancer screening at age 20. The line graph represents the cumulative rates of HPV vaccination in girls aged 16 y or younger, and the bar graph represents the incidences of CIN1+ and CIN3+. The regression lines and regression coefficients of changes in the incidence of CIN1+ and CIN3+ in the pre‐introduction generation and the vaccination generation are graphically illustrated. The rate of undergoing biopsy varied in accordance with the birth FY, for which the rate was adjusted by assuming that all girls with an abnormal cytology report subsequently underwent biopsy. For the pre‐introduction generation, x = 1 (FY 1990), x = 2 (FY1991), x = 3 (FY 1992), and x = 4 (FY1993). For the vaccination generation, x = 1 (FY 1994), x = 2 (FY1995), x = 3 (FY 1996), and x = 4 (FY1997)
The adjusted incidence of the more advanced lesions, CIN3+ in the pre‐introduction women, born in FY 1990‐1993, was 0.07% (2/2676), 0.13% (5/3794), 0.28% (14/4913), and 0.14% (8/5657), respectively. The R² was .27 and the regression coefficient was 0.036, which showed a weak correlation (Figure 3). The adjusted incidence of CIN3+ in the vaccination generation girls, born in FY 1994‐1997, was 0.04% (1/2482), 0.0% (0/2267), 0.05% (1/2186), and 0.00% (0/1220), respectively. The R² was 0.12 and the regression coefficient was −0.007, which showed no correlation. The incidence of CIN3+ in the entire pre‐introduction generation was 0.17% (29/17 040), and that in the entire vaccination generation was 0.02% (2/8155), which showed that the CIN3+ incidence in the vaccination generation was significantly lower than that in the pre‐introduction generation (P = .0008). HPV vaccination suppressed the incidence of CIN3+ much better than it prevented CIN1+.
4. DISCUSSION
We examined changes in the incidences of abnormal cervical cytology and biopsy resulting from the introduction of subsidized HPV vaccination in Japan. Previous studies have also examined the preventive effects of the HPV vaccine against cervical lesions only by comparing their incidence between the pre‐introduction generation and the vaccination generation.12, 13 In this study, we analyzed data on the vaccination generation in a new, more granular way, by looking at the results from individual birth FYs. The finer detail results revealed that the incidences of HSIL+ and CIN3+ were significantly lower in the vaccination generation than in the pre‐introduction generation, which corroborates the effectiveness of the HPV vaccine (P = .014, P = .0008) (Figure 2 and Table 1).
Our examination of the incidence of abnormal cytology revealed that, while the incidence of ASC‐US+ in the pre‐introduction generation was 3.56%, that in the vaccination generation was 3.30%, which was not statistically different (P = .196) (Figure 1). In 1 previous study12 that analyzed data from only 7 municipalities, corresponding to less than one‐third of the number of the municipalities examined in this study, the incidences of ASC‐US+ in the pre‐introduction and vaccination generations was 3.96% vs 3.01%, which was a more significant decrease (P = .014) in ASC‐US+ than found by our group (P = .196) with a much larger population.
The previous study defined their “vaccination generation” as girls born in only 2 y, 1994 and 1995, and their pre‐introduction generation consisted of girls born during FY 1990‐1993). Using this more limited set of only 2 y instead of 4 y for the vaccination generation, a much more significant decrease in ASC‐US+ due to vaccination, from 3.56% to 3.07% (P = .047) was reported.
So, why did our simple expansion of the vaccination generation from FY 1994‐1995 to FY 1994‐1997 make the apparent decrease in the incidence of ASC‐US+ no longer statistically confirmable? Our larger study analyzed the data from 25 municipalities, and the much larger study population size enabled us to examine changes in the yearly incidence of ASC‐US+ using linear approximation. We envision that, as the incidence of ASC‐US+ in the vaccination generation tended to increase under all circumstances, no significant differences could be confirmed between the 2 groups, although the ASC‐US+ incidence in the vaccination generation was obviously lower than that predicted based on the increasing trend of ASC‐US+ in the pre‐introduction generation. It has been reported that increasingly more young females in Japan are now experiencing cervical cancer,1 and that the rate of the contribution of HPV types 16 and 18, which were targeted by the original vaccine, has been lower in Japan, at 58.5%, compared with the 70.7% rate shown in worldwide data.15, 16 AS the vast majority of cervical cancers are caused by at least 1 type of HPV 16/18, the remaining 31.5% of cervical cancers in women in Japan must be caused by different strains of HPV. The vaccination generation of girls received either a bivalent or a quadrivalent vaccine. However, this study observed that the incidence of ASC‐US+ tended to increase over the 4 y of the vaccination generation. We now theorize that this trending ASC‐US+ increase resulted from infections with HPV types other than 16/18 and that their vaccinations failed to prevent. It is clearly not appropriate to simply lump together the entire 4 y of the pre‐introduction generation and the entire 4 y of the vaccination generation. It is more accurate to compare the changes in incidence for each mild lesion in each specific birth FY.
The incidence of HSIL+ in the pre‐introduction generation was 0.31%, whereas in the vaccination generation it was 0.14%, which meant that vaccination caused a significant decrease (P = .014) (Figure 2). This suggests that HPV types 16 and 18 had a higher contribution to the more severe HISL+ lesions than for the lesser ASC‐US+ lesions17; therefore, a provable and adequate preventive effect was achieved for HISL+ by HPV vaccination.
While the adjusted incidence of CIN1+ in the pre‐introduction generation was 1.42%, that in the vaccination generation was 1.66%, and was not significantly different (P = .15) (Table 1). The previous study showed that the incidence in the pre‐introduction generation was 2.1%, and in their vaccination generation, defined as women born in FY 1994‐1996, was 1.4%, which was not a significant difference in incidence protection (P = .20).13 However, our FY birth year‐by‐year analysis revealed that the incidence of CIN1+ was clearly lower than that predicted based on just the trend for CIN1+ seen in the pre‐introduction generation.
While the adjusted incidence of CIN3+ in the pre‐introduction generation was 0.17%, that in the vaccination generation was 0.02%, which showed a significant decrease in CIN3+ incidence due to vaccination (P = .0008) (Table 1). This more marked decrease in the incidence of CIN3+, compared with the vaccination effects on any of the lesser lesions, was attributable to a higher rate of CIN3+ lesions caused by the more oncogenic HPV types 16 and 18 in the young unvaccinated girls vs the fully protected vaccinated girls.18 In the J study, which was as nationwide as our study, the protective effect of the HPV vaccine against CIN3+ was found to be more marginal.19 In the study by Yagi et al,11 the effect of HPV vaccination for preventing CIN3+ was shown only for data from Matsuyama City. The study by Konno et al found similar results for protection from CIN3+ in Japan,.13 Their study showed that vaccine recipients and non‐recipients had greatly different age distributions, which strongly biased their results. Our present study analyzed only the results of cervical screenings conducted at age 20 y and we examined a much larger population than any previous study in Japan, which has enabled us to provide convincing evidence for a strong preventive effect of HPV vaccination against CIN3+, a significant precursor to malignant and sometimes deadly cervical cancer.
As the Japanese government's official recommendation for HPV vaccination is currently suspended (as it has been since 2013), girls born in 2000 or later are now defined as the “vaccine‐suspension generation”, and would have the same risk for cervical cancer as the pre‐introduction generation, except that the rise in earlier sexual relationships in teenagers has increased their exposure to HPV.6, 20 In Japan, the official recommendation for vaccination remains suspended, although the WHO and multiple academic and medical societies have released official encouragements to lift their suspension. The government is currently not taking any special measures to mitigate the heightened risk of cervical cancer in the vaccine‐suspension generation.
In addition, the 9‐valent HPV vaccine has been approved for use since FY 2020.21 It is still not too late to apply this vaccine, although it will be necessary to consider bold new strategies for effectively providing and encouraging vaccination opportunities for girls, and now women, who failed to receive an HPV vaccination due to the suspension of the governmental recommendation. We must also begin introducing HPV vaccines for young males, as most other modern countries have, and we must regain as early as possible the +70% level of vaccinations we once enjoyed. Our government and our medical, educational, and academic institutions must come together soon if we are ever to completely eliminate the scourge of cervical cancer.
This study had some limitations. First, unlike the J study, our study did not individually associate the woman's vaccination history with the results of abnormal cytology and biopsy. Second, the rate of undergoing biopsy may have varied in accordance with the birth FY. We have made an adjustment, however the adjusted rate may not reflect the actual measurement rate. Third, although the data were collected from 25 municipalities, the rates of abnormal cytology and CIN were relatively low. Moreover, the results of HPV tests were not included. Fourth, effectiveness of the HPV vaccine against invasive cervical cancer has not been shown in Japan. We have started a nationwide study, and these results will be described elsewhere in future.
We have found that previous simple comparisons between the pre‐introduction and the vaccination generations does not accurately reflect the preventive effects of the HPV vaccines in light of the increasing rates of cervical lesions and cervical cancers in Japanese patients. By collecting data from a larger population and observing the change over time in individual birth FYs, we provide convincing proof of the effectiveness of HPV vaccinations. Particularly, in Japan, this was the largest study to date to show a preventive effect of the HPV vaccine against CIN3+. Japan currently has no system of storing the results of cervical screening for local residents in a national database, so this study, conducted in cooperation with 25 different municipalities, is considered to be a landmark and highly valuable.
DISCLOSURE
This study was approved by the Institutional Review Board and Ethics Committee of the Osaka University Medical Hospital. The authors have no conflict of interest to declare. AY and TE have received a lecture fee from Merck Sharp & Dohme (MSD). YU and TK have received lecture fees and a research fund from MSD.
ACKNOWLEDGMENTS
We would like to thank Dr. GS Buzard for his constructive critique and editing of our manuscript. A grant (#17ck0106369s0101) from the Japanese Agency for Medical Research and Development and a Health and Labour Sciences Research Grant (grant number 20EA1025) from the MHLW supported this research.
Yagi A, Ueda Y, Nakagawa S, et al. A nationwide birth year‐by‐year analysis of effectiveness of HPV vaccine in Japan. Cancer Sci. 2021;112:3691–3698. 10.1111/cas.15060
REFERENCES
- 1.Yagi A, Ueda Y, Kakuda M, et al. Epidemiologic and clinical analysis of cervical cancer using data from the population‐based osaka cancer registry. Cancer Res. 2019;79:1252‐1259. [DOI] [PubMed] [Google Scholar]
- 2.The Ministry of Health, Labour and Welfare . Comprehensive survey of living conditions 2016 https://www.mhlw.go.jp/toukei/saikin/hw/k‐tyosa/k‐tyosa16/index.html (accessed on May 1, 2020)
- 3.Ueda Y, Enomoto T, Sekine M, Egawa‐Takata T, Morimoto A, Kimura T. Japan's failure to vaccinate girls against human papillomavirus. Am J Obstet Gynecol. 2015;212:405‐406. [DOI] [PubMed] [Google Scholar]
- 4.Sekine M, Kudo R, Adachi S, et al. Japanese crisis of HPV vaccination. Int J Pathol Clin Res. 2016;2:39. [Google Scholar]
- 5.Hanley SJ, Yoshioka E, Ito Y, Kishi R. HPV vaccination crisis in Japan. Lancet. 2015;385:2571. [DOI] [PubMed] [Google Scholar]
- 6.Yagi A, Ueda Y, Nakagawa S, et al. Potential for cervical cancer incidence and death resulting from Japan's current policy of prolonged suspension of its governmental recommendation of the HPV vaccine. Sci Rep. 2020;10:15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV‐associated cancers. Int J Cancer. 2018;142:2186‐2187. [DOI] [PubMed] [Google Scholar]
- 8.Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340‐1348. [DOI] [PubMed] [Google Scholar]
- 9.Ozawa N, Ito K, Tase T, Metoki H, Yaegashi N. Beneficial effects of human papillomavirus vaccine for prevention of cervical abnormalities in miyagi. Japan. Tohoku J Exp Med. 2016;240:147‐151. [DOI] [PubMed] [Google Scholar]
- 10.Kudo R, Yamaguchi M, Sekine M, et al. Bivalent human papillomavirus vaccine effectiveness in a japanese population: high vaccine‐type‐specific effectiveness and evidence of cross‐protection. J Infect Dis. 2019;219:382‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konno R, Konishi H, Sauvaget C, Ohashi Y, Kakizoe T. Effectiveness of HPV vaccination against high grade cervical lesions in Japan. Vaccine. 2018;36:7913‐7915. [DOI] [PubMed] [Google Scholar]
- 12.Ueda Y, Yagi A, Nakayama T, et al. Dynamic changes in Japan's prevalence of abnormal findings in cervical cervical cytology depending on birth year. Sci Rep. 2018;8:5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yagi A, Ueda Y, Ikeda S, et al. Evaluation of future cervical cancer risk in Japan, based on birth year. Vaccine. 2019;37:2889‐2891. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa S, Ueda Y, Yagi A, Ikeda S, Hiramatsu K, Kimura T. Corrected human papillomavirus vaccination rates for each birth fiscal year in Japan. Cancer Sci. 2020;111:2156‐2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz N, Bosch FX, Castellsagué X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278‐285. [DOI] [PubMed] [Google Scholar]
- 16.Miura S, Matsumoto K, Oki A, et al. Do we need a different strategy for HPV screening and vaccination in East Asia? Int J Cancer. 2006;119:2713‐2715. [DOI] [PubMed] [Google Scholar]
- 17.Onuki M, Matsumoto K, Iwata T, et al. Human papillomavirus genotype contribution to cervical cancer and precancer: implications for screening and vaccination in Japan. Cancer Sci. 2020;111:2546‐2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto K, Yaegashi N, Iwata T, et al. MINT Study Group. Reduction in HPV16/18 prevalence among young women with high‐grade cervical lesions following the Japanese HPV vaccination program. Cancer Sci. 2019;110:3811‐3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda S, Ueda Y, Hara M, et al. Human papillomavirus vaccine to prevent cervical intraepithelial neoplasia in Japan: a nationwide case‐control study. Cancer Sci. 2021;112:839‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka Y, Ueda Y, Egawa‐Takata T, Yagi A, Yoshino K, Kimura T. Outcomes for girls without HPV vaccination in Japan. Lancet Oncol. 2016;17:868‐869. [DOI] [PubMed] [Google Scholar]
- 21.The Ministry of Health, Labour and Welfare . https://www.mhlw.go.jp/stf/shingi2/0000203023_00007.html (accessed on January 18, 2021)


