Abstract
There are no clinical reports of long‐term follow‐up after carbon‐ion radiotherapy (CIRT) using a dose of 51.6 Gy (relative biological effectiveness [RBE]) in 12 fractions for localized prostate cancer, or of a comparison of clinical outcomes between passive and scanning beam irradiation. A total of 256 patients with localized prostate cancer who received CIRT at a dose of 51.6 Gy (RBE) in 12 fractions using two different beam delivery techniques (passive [n = 45] and scanning [n = 211]), and who were followed for more than 1 year, were analyzed. The biochemical relapse‐free (bRF) rate was defined by the Phoenix definition, and the actuarial toxicity rates were evaluated using the Kaplan‐Meier method. Of the 256 patients, 41 (16.0%), 111 (43.4%), and 104 (40.6%) were classified as low, intermediate, and high risk, respectively, after a median follow‐up of 7.0 (range 1.1‐10.4) years. Androgen deprivation therapy was performed in 212 patients (82.8%). The 5‐year bRF rates of the low‐, intermediate‐, and high‐risk patients were 95.1%, 90.9%, and 91.1%, respectively. The 5‐year rates of grade 2 late gastrointestinal and genitourinary toxicities in all patients were 0.4% and 6.3%, respectively. No grade ≥3 toxicities were observed. There were no significant differences in the rates of bRF or grade 2 toxicities in patients who received passive irradiation versus scanning irradiation. Our long‐term follow‐up results showed that a CIRT regimen of 51.6 Gy (RBE) in 12 fractions for localized prostate cancer yielded a good therapeutic outcome and low toxicity rates irrespective of the beam delivery technique.
Keywords: biochemical relapse‐free rate, carbon‐ion radiotherapy, hypofractionation, prostate cancer, prostate‐specific antigen
A 51.6 Gy (relative biological effectiveness) carbon‐ion radiotherapy regimen in 12 fractions for localized prostate cancer with long‐term follow‐up yielded a good therapeutic outcome and low toxicity rates in both passive and scanning beam irradiation without significant difference.
![]()
1. INTRODUCTION
Radical treatments for patients with localized prostate cancer include radical prostatectomy or radiotherapy (RT).1, 2 In a recent RT series, a high dose of 70‐80 Gy (35‐40 fractions) administered over approximately 8 weeks is generally used for three‐dimensional conformal RT or intensity‐modulated RT (IMRT) for prostate cancer as conventional irradiation.1, 2 To shorten the long treatment duration schedule, hypofractionated RT has recently been used3 because hypofractionation is considered reasonable for prostate cancer in terms of improved efficacy due to tumor biological specificity, with a low α/β value based on the linear‐quadratic model.4 Several phase III trials involving X‐ray series showed that RT using moderate hypofractionation (20‐28 fractions, approximately 4 weeks) had equal efficacy and safety compared with conventional fractionation for localized prostate cancer.5, 6, 7, 8, 9, 10, 11
Carbon‐ion RT (CIRT) has physical and biological advantages compared with X‐ray RT.12 Several studies of CIRT using a moderate hypofractionation schedule (20 fractions, 5 weeks) for prostate cancer have been conducted since 1994 at QST Hospital (formerly the National Institute of Radiological Science Hospital).13 In addition, several attempts have been made to decrease the number of fractions while increasing the dose per fraction. After confirming the safety and efficacy of the 66 or 63 Gy (RBE) dose in 20 fractions,14, 15 a 16‐fraction schedule (57.6 Gy [RBE], 4 weeks) was implemented in 2005.16 Subsequently, a phase I/II clinical trial was performed to evaluate the feasibility of 51.6 Gy (RBE) in 12 fractions over 3 weeks in 46 patients who received CIRT from July 2010 to October 2011; the results confirmed the safety and efficacy of this regimen after a median follow‐up duration of 32.3 months.17 Consequently, 51.6 Gy (RBE) in 12 fractions was launched as the standard fractionation schedule for CIRT at our institute in 2013. On the other hand, at our institute in 2012, the beam delivery technique used for CIRT was switched from passive18 to scanning,19 therefore the patients in the above‐mentioned clinical trial17 received passive irradiation, whereas those who underwent CIRT after 2013 received scanning irradiation. As of 2020, a CIRT regimen of 51.6 Gy (RBE) in 12 fractions is performed as the standard therapy for localized prostate cancer in Japan.20, 21 However, there are no reports of the clinical outcomes of this regimen based on long‐term follow‐up data, or of a comparison of the clinical outcomes between the passive and scanning irradiation techniques.
The purposes of this study were to retrospectively review the clinical results of patients with localized prostate cancer treated with a CIRT regimen of 51.6 Gy (RBE) in 12 fractions based on more than 5 years of follow‐up data, and to compare the treatment outcomes between patients enrolled in the clinical protocol using passive irradiation (CPaI) and those enrolled in the standard therapy protocol using scanning irradiation (SScI) in our institution.
2. MATERIALS AND METHODS
2.1. Protocol and patients
All of the analyzed 260 patients received CIRT using a regimen of 51.6 Gy (RBE) in 12 fractions at our institution. Between July 2010 and October 2011, CPaI (protocol 1002) was performed as a phase I/II trial involving 46 patients, as described previously.17 SScI (protocol 9904 [4]) was performed for 214 patients from April 2013 to February 2014 using the same dose prescription as that in CPaI as advanced medical care. The eligibility and exclusion criteria applied in SScI were almost the same as those of previous reports.14, 15, 16 In brief, all eligible patients in both CPaI and SScI had biopsy‐proven adenocarcinoma and T1‐T3N0M0 disease, and the exclusion criteria were previous RT to the pelvis, a performance status of 3‐4, and the presence of active double cancers.
In both CPaI and SScI, the clinical stage was determined according to the TNM Classification of Malignant Tumors, 7th edition,22 using a digital rectal examination, ultrasonography, computed tomography (CT), magnetic resonance imaging, and isotope bone scanning. The Gleason score of each tumor was determined by the central pathologist before starting treatment. The risk categories of prostate cancer in this study were defined as follows: low‐risk group, initial prostate specific antigen (PSA) level <10 ng/mL, T1b‐T2bN0M0, and Gleason score ≤6; intermediate‐risk group, initial PSA level ≥10 and <20 ng/mL, and/or T2cN0M0, and/or Gleason score of 7; and high‐risk group, initial PSA level ≥20 ng/mL, or T3a‐T3bN0M0, or Gleason score ≥8. Patients with T4 disease or metastases in the lymph nodes or other organs were ineligible in both CPaI and SScI. Note that the patients with T1b‐T2bN0M0 disease and a Gleason score ≤6 but initial PSA level ≥10 and <20 ng/mL were categorized in the low‐risk group in SScI. The definitions of unfavorable intermediate risk and very high risk followed the NCCN guidelines, version 4.23 All patients satisfying the enrollment criteria signed an informed consent form before starting treatment, and the present study was approved by the National Institutes for Quantum and Radiological Science and Technology Certified Review Board (Study number: 20‐014, UMIN 000041880).
2.2. CIRT procedure
The treatment planning and setup methods in both protocols have been described previously.14, 15, 16, 17 The clinical target volume (CTV) was defined as the whole prostate and proximal one‐third of the seminal vesicles. In T3b cases, all seminal vesicles were included in the CTV. The planning target volume (PTV) 1 was defined as the CTV plus 5‐mm margins in the cranial, caudal, and posterior directions and 10‐mm margins in the right, left, and anterior directions. The PTV2 was created by adding 2‐3‐mm margins to the CTV in the dorsal direction but was identical to the CTV in the cranial and caudal directions and to the PTV1 in the right, left, and anterior directions; PTV2 was used for the last four times of the treatment course. The prophylactic area of the pelvic lymph nodes was not included in the PTV. Purgatives or enemas were used for rectal reproducibility in the CT simulation and as necessary during treatment. There was no use of metallic markers in the prostate to improve reproducibility or SpaceOAR to decrease the rectum dose in either CPaI or SScI. All patients were treated using resinous shells and an image‐guided irradiation system, and the images were compared with reference images and confirmed for bone matching with the digitally reconstructed radiographs, under shallow natural breathing. The treatment couch was moved to the matching position until the largest deviation of all measured points was less than 1 mm. The two‐fields technique (opposing lateral fields) was routinely used for CIRT planning in CPaI and SScI (Figure 1).
FIGURE 1.
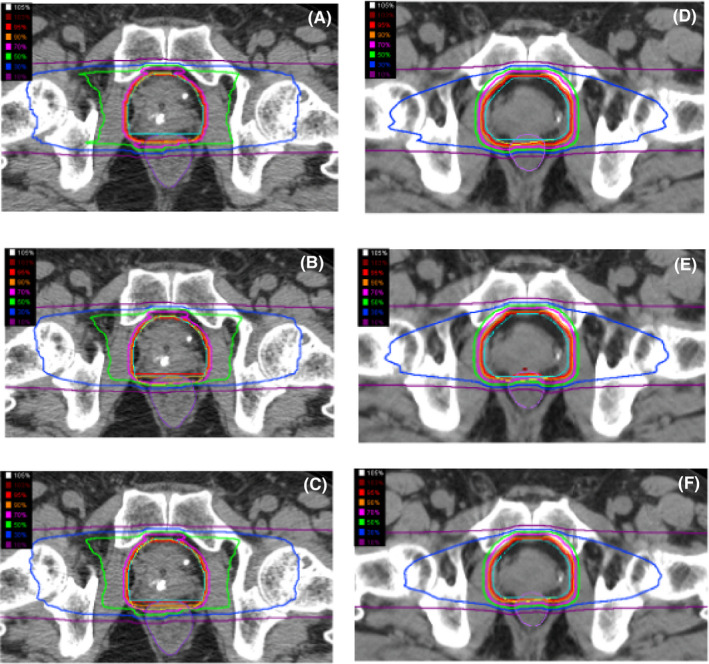
The dose distributions of the passive beam [(A) first 8 times to planning target volume (PTV) 1, (B) the remaining 4 times for PTV2, and (C) the fusion of total 12 times] and the scanning beam [(D) first 8 times to PTV 1, (E) the remaining 4 times for PTV2, and (F) the fusion of total 12 times] used with carbon‐ion radiotherapy (CIRT). The penumbra around the target volume with a high dose area (more than 70%) is slightly sharper for the passive beam than the scanning beam, however, the region of low to middle dose area (30% to 70%) of the passive beam is wider than that of the scanning beam.
In both CPaI and SScI, the dose prescription and dose constraints were the same. The irradiation dose is expressed as Gy (RBE; physical carbon ion dose [Gy] × RBE). The RBE value for CIRT was estimated to be 3.0 at the distal part of the spread‐out Bragg peak based on previous experience at our institution.22 CIRT was given once a day, 4 days a week (generally, Tuesday to Friday). The prescribed dose for all patients in this study was 51.6 Gy (RBE) administered in 12 fractions, and >95% of the dose was prescribed to the PTV2. The recommended dose constraints for the rectum are as follows: the rectal volume prescribed 53 Gy (RBE), 50 Gy (RBE), and 40 Gy (RBE) = 0%, ≤7%, and ≤16%, respectively. The dose constraints to other organs at risk were not defined.
The beam technique used for CIRT differed between CPaI and SScI. Compensators and multileaf collimators were used for each port individually in all patients enrolled in CPaI,18 whereas these devices were not needed in any of the patients in SScI.19
2.3. Androgen deprivation therapy
Androgen deprivation therapy consisted of medical (luteinizing hormone‐releasing hormone analogue) or surgical castration with or without anti‐androgen therapy. CPaI involved low‐risk patients who underwent ADT, and intermediate‐ or high‐risk patients who refused ADT or who underwent neoadjuvant ADT for more than 6 months.17 SScI involved low‐risk patients who received no ADT, and intermediate‐ and high‐risk patients who received neoadjuvant ADT for 2‐6 months. Adjuvant ADT was generally continued for a total duration of 6 and ≥24 months for intermediate‐risk and high‐risk patients, respectively. As described above, the patients with T1b‐T2bN0M0 disease, initial PSA level ≥10 and <20 ng/mL, and Gleason score ≤6, categorized as the low‐risk group in SScI, did not receive ADT, although these patients were categorized as intermediate risk in this study.
2.4. Follow‐up
Patients were generally followed‐up at 3‐ to 6‐month intervals during the first 5 years after CIRT and 6‐ to 12‐month intervals thereafter. Clinical records were collected in November 2020. Biochemical relapse was defined according to the Phoenix definition (PSA nadir plus 2.0 ng/mL).23 The biochemical relapse‐free (bRF) rate was measured from the start of CIRT to the date of progression, determined either clinically or by an increased PSA level, or to the last follow‐up. Death from other causes were not included. Toxicities were evaluated at ≥3 months after CIRT by patient interviews regarding their symptoms, urine analysis, stool analysis, cystoscopy, and colonoscopy and were graded according to the Common Terminology Criteria for Adverse Events, version 4.0.24 Late toxicities were counted as the highest grade of the adverse event at ≥3 month after CIRT.
2.5. Statistical analysis
Survival curves were generated by the Kaplan‐Meier method, and log‐rank tests were used to compare the bRF rates between CPaI and SScI according to risk group. Proportions were compared using the chi‐square test. Results were considered significant at P < .05. All statistical analyses were performed using SPSS software (version 20.0; IBM Japan, Ltd).
3. RESULTS
3.1. Patient characteristics
There were 46 and 214 patients enrolled in CPaI and SScI, respectively. Of the 260 total patients, four were excluded for the following reasons: one patient in CPaI experienced sudden death due to a ruptured aneurysm 5 months after CIRT,17 and three patients in SScI dropped out during the follow‐up period within 1 year after CIRT. The remaining 256, who were followed‐up for at least 12 months, were analyzed in this study. The characteristics of the analyzed patients are summarized in Table 1. The numbers of low‐, intermediate‐, and high‐risk patients were 41 (16.0%), 111 (43.4%), and 104 (40.6%), respectively. The number of patients categorized as unfavorable intermediate risk in the intermediate‐risk group were seven of 11 (63.6%) in CPaI and 74 of 100 (74%) in SScI (P = .927). The number of patients categorized as very high risk in the high‐risk group were five of 25 (20%) in CPaI and 25 of 79 (31.6%) in SScI, respectively (P = .263). The median (range) ADT durations in CPaI and SScI were 6 (5‐13) and 0 (0‐0) months in the low‐risk group, 9 (0‐28) and 6 (0‐8) months in the intermediate‐risk group, and 26 (15‐112) and 24 (10‐35) months in the high‐risk group, respectively.
TABLE 1.
Patient characteristics according to the treatment protocol
| Total | CPaI | SScI | P value | |
|---|---|---|---|---|
| Number of patients | 256 | 45 | 211 | |
| Age, y | ||||
| Median (range) | 66 (50‐87) | 66 (54‐83) | 66 (50‐87) | .635 |
| T stage | ||||
| T1b | 0 | 0 | 0 | .097 |
| T1c | 89 (35%) | 17 (38%) | 73 (35%) | |
| T2a | 66 (26%) | 5 (11%) | 58 (27%) | |
| T2b | 13 (5%) | 3 (7%) | 7 (3%) | |
| T2c | 34 (13%) | 6 (13%) | 34 (16%) | |
| T3a | 44 (17%) | 13 (29%) | 31 (15%) | |
| T3b | 10 (4%) | 2 (4%) | 8 (4%) | |
| Gleason score | ||||
| 6 | 66 (26%) | 11 (24%) | 55 (26%) | .150 |
| 7 | 115 (45%) | 15 (33%) | 100 (47%) | |
| 8 | 27 (11%) | 8 (17%) | 19 (9%) | |
| 9 | 48 (19%) | 11 (24%) | 17 (8%) | |
| 10 | 0 | 0 | 0 | |
| Pretreatment PSA level, ng/mL | ||||
| <10 | 157 (61%) | 21 (47%) | 136 (64%) | .013 |
| 10‐20 | 64 (25%) | 12 (27%) | 52 (25%) | |
| >20 | 35 (14%) | 12 (27%) | 23 (11%) | |
| Risk group | ||||
| Low | 41 (16%) | 9 (20%) | 32 (15%) | .018 |
| Intermediate | 111 (43%) | 11 (24%) | 100 (47%) | |
| High | 104 (41%) | 25 (56%) | 79 (37%) | |
Abbreviations: ADT, androgen deprivation therapy; CPaI, clinical protocol using passive irradiation; SScI, standard therapy protocol using scanning irradiation.
3.2. Biochemical relapse‐free rates
The median follow‐up period was 7.0 (range 1.1‐10.4) years. The overall 5‐ and 7‐year bRF rates were 95.1% (95% confidence interval [CI] 81.9‐98.8) and 92.5% (95% CI 78.5‐97.5) in the low‐risk group, 90.9% (95% CI 83.7‐95.0) and 82.9% (95% CI 70.3‐90.5) in the intermediate‐risk group, and 91.1% (95% CI 83.6‐95.3) and 82.5% (95% CI 72.9‐89.0) in the high‐risk group, respectively. In CPaI, in which the median follow‐up was 9.3 (range 1.4‐10.4) years, the 5‐ and 7‐year bRF rates were 88.9% (95% CI 43.3‐98.4) and 88.9% (95% CI 43.3‐98.4) in the low‐risk group, 90.0% (95% CI 47.3‐98.5) and 80.0% (95% CI 40.9‐94.6) in the intermediate‐risk group, and 88.0% (95% CI 67.3‐96.0) and 79.2% (95% CI 56.9‐90.8) in the high‐risk group, respectively. In SScI, in which the median follow‐up was 6.9 years (range 1.1‐7.7 years), the 5‐ and 7‐year bRF rates were 96.9% (95% CI 79.8‐99.6) and 93.5% (95% CI 76.6‐98.3) in the low‐risk group, 91.0% (95% CI 83.4‐95.2) and 86.1% (95% CI 76.2‐92.1) in the intermediate‐risk group, and 90.9% (95% CI 83.3‐95.2) and 86.1% (95% CI 76.2‐92.1) in the high‐risk group, respectively. Figure 2 shows a comparison of the bRF rate curves between the CPaI and SScI patients according to risk group; no significant differences were observed. There were no prostate‐cancer specific deaths, but 13 patients (5.1%) died from other causes (bacterial pneumonia [n = 3], interstitial pneumonia [n = 1], chronic obstructive pulmonary disease [n = 1], dilated cardiomyopathy [n = 1], sepsis [n = 1], gastrointestinal bleeding [n = 1], pancreatic cancer [n = 1], gastric cancer [n = 1], parotid cancer [n = 1], suicide [n = 1], and accidental death [n = 1]). Concerning the case of death from gastrointestinal bleeding, the patient was followed for 5 years after treatment, during which there were no obvious complications, including rectal bleeding. Although details could not be obtained from the relevant hospital, irradiation of the gastrointestinal tract other than the ventral side of the rectum was not performed. Therefore, we considered that the gastrointestinal bleeding was not related to CIRT.
FIGURE 2.
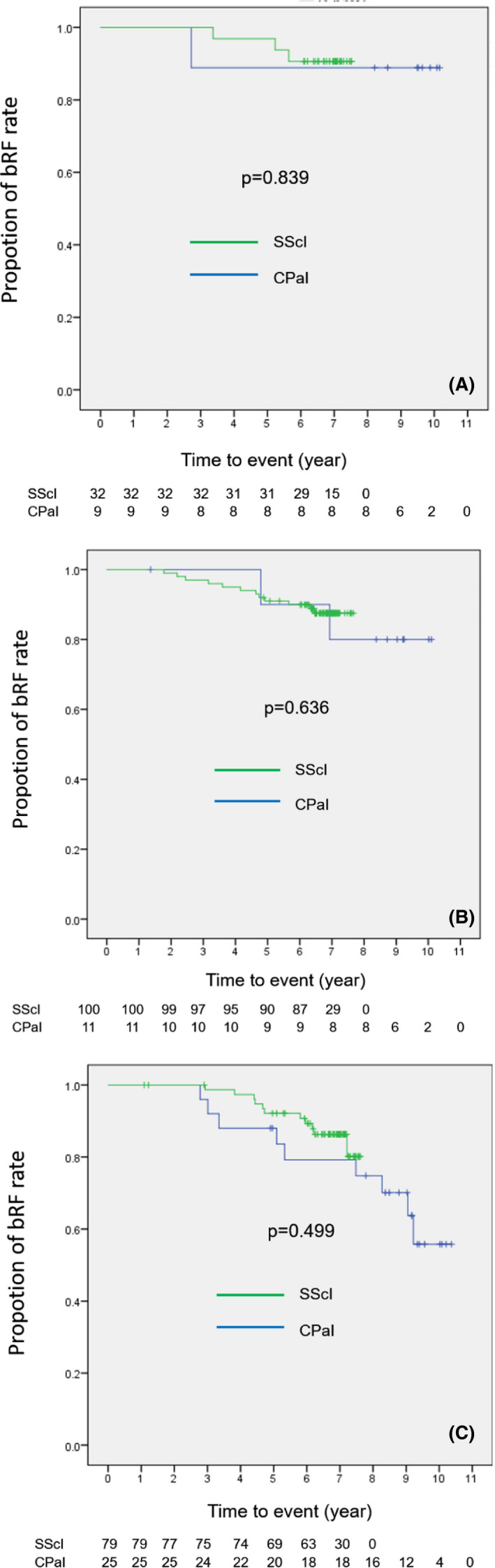
Kaplan‐Meier curves for biochemical relapse free (bRF) rates between clinical trial by use of passive irradiation (CPaI) and standard therapy by use of scanning irradiation (SScI) of (A) low‐, (B) intermediate‐, and (C) high‐risk group after carbon‐ion radiotherapy. There were no significant differences between the two protocols in each risk group.
3.3. Toxicities
No grade ≥3 gastrointestinal (GI) or genitourinary (GU) toxicities were observed. A late grade 2 GI toxicity was observed in one patient (0.4%), who was diagnosed with rectal bleeding 3.6 years after CIRT, which improved after 4 months by conservative medical management. Late grade 2 GU toxicities were observed in 18 patients (7.0%), of whom 8 had symptoms of dysuria, urinary frequency, or pain urinating during the treatment period and required continuous drug treatment. Of the remaining 10 patients who suffered late grade 2 GU, drugs were newly required for dysuria in three, for urinary frequency in three, and for bleeding in one, and urinary pads were required for incontinence in the remaining three patients. The patient with grade 2 hematuria had a prior history of urolithiasis and hematuria before CIRT. Thereafter, hematuria was observed intermittently, but it resolved spontaneously or by temporary use of hemostatic agents. The 5‐year actuarial rates of late grade 2 GI and GU toxicities were 0.4% and 6.3%, respectively. There was no significant difference in the cumulative rate of late grade 2 GI or GU toxicities between CPaI and SScI (Table 2).
TABLE 2.
Late grade 2 adverse events of the patients in CPaI and SScI
| Symptom | Treatment | CPaI (n = 45) | SScI (n = 211) | P value | |
|---|---|---|---|---|---|
| Late grade 2 GI toxicities | Rectal bleeding | Oral drug | 0 | 1 | .646 |
| Total | 0 (0%) | 1 (0.5%) | |||
| Late grade 2 GU toxicities | Hematuria | Oral drug | 1 | 0 | .780 |
| Frequent urination | 1 | 4 | |||
| Dysuria | 2 | 7 | |||
| Incontinence | Urinary pads | 0 | 3 | ||
| Total | 4 (8.9%) | 14 (6.6%) | |||
Abbreviations: CPaI, clinical protocol using passive irradiation; GI, gastrointestinal; GU, genitourinary; SScI, standard therapy protocol using scanning irradiation.
4. DISCUSSION
The outcomes of the CIRT regimen of 51.6 Gy (RBE) in 12 fractions for 3 weeks in patients with localized prostate cancer enrolled in CPaI and SScI were analyzed. CPaI was performed as a phase I/II study of CIRT using passive beam delivery with a median follow‐up duration of 9.3 years. SScI was conducted as clinical practice using scanning CIRT with a median follow‐up duration of 6.9 years. Although some of the methods differed between CPaI and SScI, including the ADT duration, this is the first study to analyze the clinical outcomes of CIRT at 51.6 Gy (RBE) in 12 fractions for prostate cancer, with more than 5 years of follow‐up data, and to compare the outcomes of carbon‐ion beam passive and scanning irradiation.
Recent clinical outcomes of RT using moderate hypofractionation and conventional fractionation are shown in Table 3. The treatment efficacy of CIRT using 51.6 Gy (RBE) in 12 fractions after 5 years was equivalent to that of moderately hypofractionated/conventional X‐ray RT or that of CIRT using 16 or 20 fractions. These results might be because the BED of 51.6 Gy (RBE) in 12 fractions was calculated as the equivalent BED of the previous dose prescriptions at α/β = 1.5 (Table 3). Furthermore, the rates of grade ≥2 GI and GU toxicities in this study were equivalent to or less than those of the previous reports. Especially, grade 2 GI and GU bleeding was observed only in one patient each among all 256 patients, including the 27 (10.5%) who used anticoagulant drugs without SpaceOAR or metallic markers in the prostate, even though previous reports from our institution reported that the grade 2 adverse events after CIRT consisted mainly of bleeding.14, 15, 16, 25 The reason for the low rate of grade 2 bleeding in both CPaI and SScI might be attributed to not only the relatively low BED or sharp penumbra but also unknown factors associated with the dose prescription of 51.6 Gy(RBE) in 12 fractions. Further studies with longer follow‐up periods are needed to confirm the low rate of adverse events, especially bleeding, after CIRT using 51.6 Gy (RBE) in 12 fractions.
TABLE 3.
Comparison of the treatment outcomes after hypofractionated radiotherapy at approximately 5 years of follow‐up
| Type of radiation | Dose (Gy)/no. of fractions | BED | No. of pts | Median f/u (years) | 5‐year biochemical relapse‐free rate (%) | 5‐year toxicity rates (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α/β | Late GI | Late GU | ||||||||||
| 1.5 | 3.0 | Low | Int. | High | Grade 2 | Grade 3 | Grade 2 | Grade 3 | ||||
| X‐ray radiotherapy | ||||||||||||
| CHHiP5 | 74/37 | 172 | 123 | 1065 | 5.2 | 96.7 | 86.8 | 86.5 | 13.7a | 2 | 9.1a | 3 |
| 60/20 | 180 | 120 | 1074 | 96.6 | 90.2 | 84.2 | 11.9a | 3 | 11.7a | 6 | ||
| 57/19 | 171 | 114 | 1077 | 90.9 | 86 | 78.3 | 11.3a | 4 | 6.6a | 3 | ||
| Fox Chase6 | 78/36 | 182 | 130 | 152 | 5.7 | 79.6b | 20.5a | 2c | 13.4a | 3.3c | ||
| 70.2/26 | 196 | 133 | 151 | 76.7b | 16.1a | 2c | 21.5a | 4c | ||||
| RTOG 04157 | 73.8/41 | 162 | 118 | 543 | 5.8 | 91.9 | — | — | 11.4c | 2.6 c , d | 20.5c | 2.3 c , d |
| 70/28 | 187 | 128 | 550 | 94.3 | — | — | 18.3c | 4.1c | 26.2c | 3.5c | ||
| HYPRO8, 9 | 78/39 | 182 | 130 | 410 | 5.0 | 77.1 b , g | 17a (3y) | 2.6e (3y) | 39a (3y) | 19e (3y) | ||
| 64.6/19 | 211 | 138 | 410 | 80.5 b , g | 21.9a (3y) | 3.3e (3y) | 41.3a (3y) | 12.9e (3y) | ||||
| PROFIT10 | 78/39 | 182 | 130 | 608 | 6.0 | 85% b , f , g | — | 11c | 2.9 c , d , e | 19 | 3.0 c , d , e | |
| 60/20 | 180 | 120 | 598 | 85% b , f , g | — | 7.4c | 1.5c | 20 | 2.2 c , d , e | |||
| MD Anderson11 | 75.6/42 | 166 | 121 | 102 | 8.5 | 91.7f | — | 4c (8y) | 1c (8y) | 15c (8y) | 1c (8y) | |
| 72/30 | 187 | 130 | 104 | 91.8f | — | 10c (8y) | 2c (8y) | 15c (8y) | 0 | |||
| Carbon‐ion radiotherapy | ||||||||||||
| Okada (passive)16 | 66 (RBE)/20 | 211 | 138 | 250 | 4.9 | 90 | 97 | 88 | 3.2c | 0 | 15.2 | 0 |
| 63 (RBE)/20 | 193 | 128 | 216 | 2.3c | 0 | 8.2 | 0 | |||||
| 57.6 (RBE)/16 | 195 | 127 | 274 | 1.5c | 0 | 5.0 | 0 | |||||
| Kawamura (passive)29 | 57.6 (RBE)/16 | 195 | 127 | 304 | 5.0 | 91.7 | 93.4 | 92 | 0.3c | 0 | 9c | 0.3c |
| Present study | 51.6 (RBE)/12 | 200 | 126 | 256 | 7.0 | 95.1 | 90.9 | 91.1 | 0.4 | 0 | 6.3 | 0 |
Abbreviations: BED, biologically effective dose; fr, fraction; GI, gastrointestinal, GU, genitourinary; Gy, gray; int., intermediate; Pt, patient.
Rate of ≥grade 2 toxicities.
Not available in each risk group.
Crude rate.
Including one case of grade 4 toxicity.
Rate of ≥grade 3 toxicities.
Almost all patients were categorized as low or intermediate risk.
Biochemical relapse‐free survival rate.
CPaI and SScI were performed using different CIRT beam‐delivery techniques, and the CIRT beam technique was changed from passive beam to scanning beam between the completion of CPaI to the start of SScI at our institution. Thus, we were able to compare the two techniques by comparing the outcomes of CPaI and SScI. An advantage of the scanning over passive beam technique is no requirement for expensive equipment such as a compensating bolus or patient collimator, which produces secondary neutrons.26 Moreover, the scanning beam technique is associated with high radiation accuracy and flexible treatment planning as well as a lower dose to organs at risk, compared with the passive beam technique.26 Therefore, the transition to the scanning beam technique is becoming more widespread with the evolution of CIRT. However, Fossati et al reported that the scanning beam is less robust in terms of set‐up error and organ motion compared with the passive beam.27 In addition, the penumbra in the high‐dose area (≥70% dose) around the target volume appeared slightly sharper for the passive beam than the scanning beam (Figure 1). Therefore, clinical studies with long term follow‐up are needed to confirm how beam conversion has affected the clinical outcomes. In this analysis, there were no significant differences in the bRF rate between CPaI and SScI in any prostate cancer risk group. Moreover, over the relatively long‐term follow‐up period, there were no severe adverse events and low rates of grade 2 adverse events in both trials, as well as no significant differences in the rates of late grade 2 adverse events between the two patient populations. Thus, this study suggested that conversion to the scanning beam has resulted in similar clinical outcomes compared with the passive beam, which might be attributed to the similar dose distributions in the prostate and surrounding organs between the two irradiation techniques due to a simple method of irradiation from two opposite directions. On the other hand, for more complicated irradiation techniques involving multiple directions, scanning CIRT can reduce the high‐dose area in the proximal side, which may contribute to flexible planning and improved clinical results.28
In Japan, because CIRT for localized prostate cancer is covered by the Japanese national health insurance system as of April 2018, patients will not incur expensive treatment fees for CIRT treatment, similar to other RT methods such as IMRT and brachytherapy. Currently, there are seven institutions in Japan that provide CIRT treatment,29 including a new (opened in 2021) CIRT facility at Yamagata University that uses the scanning beam. Therefore, the number of prostate cancer patients treated with CIRT in Japan is expected to increase. We consider that challenging to ultrahypofraction as well as X‐ray series is an important strategy for CIRT in the future, not only to improve clinical results but to decrease the patients’ burden and improve throughput. Prospective clinical trials in our institution are being conducted for prostate cancer patients categorized as low/intermediate risk (UMIN000032340) and high risk (JRCTs032190180).
There are some limitations to this study. First, this study was a retrospective analysis conducted at a single institution. To investigate the efficacy and safety of CIRT in 12 fractions with more robust evidence, prospective multi‐institutional clinical trials of CIRT in Japanese patients with high‐risk prostate cancer treated with 51.6 Gy (RBE) in 12 fractions were conducted in April 2017 (UMIN000025921). Second, the patients were treated with ADT for various durations, and the patient number, especially in CPaI, was insufficient. Concerning the number of patients, only 46 prostate cancer patients were treated with passive beam CIRT of 51.6 Gy (RBE) in 12 fractions at QST Hospital, all of whom were included in this study. Since passive CIRT using the same dose prescription is performed in other hospitals in Japan, such as Gunma University30 and SAGA HIMAT, the future clinical outcomes of patients in these hospitals might support those of this study. Third, although the scanning beam technique used is a hybrid scanning technique,31 energy scanning, which is a more sophisticated beam technique without a range shifter, has been used for all CIRT treatments for prostate cancer since 2015 at QST Hospital. The outcomes of this study were therefore evaluated carefully in terms of the differences from the current treatment method. Fourth, a longer median follow‐up duration (10 years or more) is required for more accurate evaluation of patients with prostate cancer.
In conclusion, the CIRT regimen of 51.6 Gy (RBE) in 12 fractions for localized prostate cancer had a relatively good therapeutic effect and low rate of toxicities after more than 5 years of follow‐up, regardless of the beam delivery method. Moreover, no significant differences in the rates of bRF or grade 2 toxicities were observed between the two CIRT beam‐delivery techniques, which suggested that the conversion from passive to scanning irradiation in our institution was successful, based on the clinical results.
Sato H, Kasuya G, Ishikawa H, et al; the Working Group for Genitourinary Tumors . Long‐term clinical outcomes after 12‐fractionated carbon‐ion radiotherapy for localized prostate cancer. Cancer Sci. 2021;112:3598–3606. 10.1111/cas.15019
Funding information
This work was supported by the Research Project with Heavy Ions at the National Institute of Radiological Sciences, Japan.
REFERENCES
- 1.Kuban DA, Tucker SL, Dong L, et al. Long‐term results of the M. D. Anderson randomized dose‐escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67‐74. [DOI] [PubMed] [Google Scholar]
- 2.Swisher‐McClure S, Mitra N, Woo K, et al. Increasing use of dose‐escalated external beam radiation therapy for men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2014;89:103‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kupelian PA, Willoughby TR, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity‐modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland clinic experience. Int J Radiat Oncol Biol Phys. 2007;68:1424‐1430. [DOI] [PubMed] [Google Scholar]
- 4.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose‐fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9‐2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82:e17‐e24. [DOI] [PubMed] [Google Scholar]
- 5.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high‐dose intensity‐modulated radiotherapy for prostate cancer: 5‐year outcomes of the randomised, non‐inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external‐beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860‐3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low‐risk prostate cancer. J Clin Oncol. 2016;34:2325‐2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open‐label, phase 3 trial. Lancet Oncol. 2016;17:1061‐1069. [DOI] [PubMed] [Google Scholar]
- 9.Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non‐inferiority, phase 3 trial. Lancet Oncol. 2016;17:464‐474. [DOI] [PubMed] [Google Scholar]
- 10.Catton CN, Lukka H, Gu C‐S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884‐1890. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose‐escalated, intensity‐modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36:2943‐2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy‐ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201‐210. [DOI] [PubMed] [Google Scholar]
- 13.Kamada T, Tsujii H, Blakely EA, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16:e93‐e100. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji H, Yanagi T, Ishikawa H, et al. Hypofractionated radiotherapy with carbon ion beams for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1153‐1160. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa H, Tsuji H, Kamada T, et al. Carbon ion radiation therapy for prostate cancer: results of a prospective phase II study. Radiother Oncol. 2006;81:57‐64. [DOI] [PubMed] [Google Scholar]
- 16.Okada T, Tsuji H, Kamada T, et al. Carbon ion radiotherapy in advanced hypofractionated regimens for prostate cancer: from 20 to 16 fractions. Int J Radiat Oncol Biol Phys. 2012;84:968‐972. [DOI] [PubMed] [Google Scholar]
- 17.Nomiya T, Tsuji H, Maruyama K, et al. Phase I/II trial of definitive carbon ion radiotherapy for prostate cancer: evaluation of shortening of treatment period to 3 weeks. Br J Cancer. 2014;110:2389‐2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minohara S, Fukuda S, Kanematsu N, et al. Recent innovations in carbon‐ion radiotherapy. J Radiat Res. 2010;51:385‐392. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa T, Inaniwa T, Sato S, et al. Performance of the NIRS fast scanning system for heavy‐ion radiotherapy. Med Phys. 2010;37:5672‐5682. [DOI] [PubMed] [Google Scholar]
- 20.Takakusagi Y, Katoh H, Kano K, et al. Preliminary result of carbon‐ion radiotherapy using the spot scanning method for prostate cancer. Radiat Oncol. 2020;15:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori S, Takei Y, Shirai T, et al. Scanned carbon‐ion beam therapy throughput over the first 7 years at National Institute of Radiological Sciences. Phys Med. 2018;52:18‐26. [DOI] [PubMed] [Google Scholar]
- 22.International Union Against Cancer (UICC) . TNM Classification of Malignant Tumours, 7th edn. New York, NY: Wiley‐Blackwell; 2011. [Google Scholar]
- 23.National Comprehensive Cancer Network (NCCN) . Clinical Practice Guidelines in Oncology. Version 2. 2020 Prostate Cancer. https://www.nccn.org/professionals/physician_gls/pdf/prostate. pdf. Accessed June 25, 2021. [DOI] [PubMed]
- 24.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 2010. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010‐06‐14_QuickReference_5x7.pdf. Accessed June 25, 2021.
- 25.Kasuya G, Ishikawa H, Tsuji H, et al. Cancer‐specific mortality of high‐risk prostate cancer after carbon‐ion radiotherapy plus long‐term androgen deprivation therapy. Working Group for Genitourinary Tumors. Cancer Sci. 2017;108:2422‐2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yonai S, Matsufuji N, Akahane K. Monte Carlo study of out‐of‐field exposure in carbon‐ion radiotherapy with a passive beam: organ doses in prostate cancer treatment. Phys Med. 2018;51:48‐55. [DOI] [PubMed] [Google Scholar]
- 27.Fossati P, Matsufuji N, Kamada T, et al. Radiobiological issues in prospective carbon ion therapy trials. Med Phys. 2018;45:e1096‐e1110. [DOI] [PubMed] [Google Scholar]
- 28.Shiomi M, Mori S, Shinoto M, et al. Comparison of carbon‐ion passive and scanning irradiation for pancreatic cancer. Radiother Oncol. 2016;119:326‐330. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa H, Tsuji H, Murayama S, et al. Particle therapy for prostate cancer: the past, present and future. Int J Urol. 2019;26:971‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawamura H, Kubo N, Sato H, et al. Moderately hypofractionated carbon ion radiotherapy for prostate cancer; a prospective observational study “GUNMA0702”. BMC Cancer. 2020;20:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inaniwa T, Furukawa T, Kanematsu N, et al. Evaluation of hybrid depth scanning for carbon‐ion radiotherapy. Med Phys. 2012;39:2820‐2825. [DOI] [PubMed] [Google Scholar]


