Abstract
CD38 expression on myeloma cells is a critical factor affecting the early response to the anti‐CD38 antibody daratumumab. However, factors affecting CD38 expression in untreated multiple myeloma are not fully elucidated. In this study, we found that CD38 expression was significantly lower in myeloma patients with the translocation t(11;14)‐associated immature plasma cell phenotype, and particularly in those expressing B–cell‐associated genes such as PAX5 and CD79A. CD138, a representative marker of plasmacytic differentiation, was also significantly lower in these patients, suggesting that CD38 expression may be associated with the differentiation and maturation stages of myeloma cells. Furthermore, the BCL2/BCL2L1 ratio, a response marker of the BCL2 inhibitor venetoclax, was significantly higher in patients with the immature phenotype expressing B–cell‐associated genes. The BCL2/BCL2L1 ratio and CD38 expression were significantly negatively correlated. We also confirmed that patients with translocation t(11;14) expressing B–cell‐associated genes were indeed less sensitive to daratumumab‐mediated direct cytotoxicity but highly sensitive to venetoclax treatment in ex vivo assays. Moreover, all‐trans‐retinoic acid, which enhances CD38 expression and induces cell differentiation in myeloma cells, reduced B‐cell marker expression and the BCL2/BCL2L1 ratio in myeloma cell lines, leading to reduced efficacy of venetoclax. Venetoclax specifically induces cell death in myeloma with t(11;14), although why patients with translocation t(11;14) show BCL2 dependence is unclear. These results suggest that BCL2 dependence, as well as CD38 expression, are deeply associated with the differentiation and maturation stages of myeloma cells. This study highlights the importance of examining t(11;14) and considering cell maturity in myeloma treatment strategies.
Keywords: BCL2, CD38, daratumumab, multiple myeloma, venetoclax
Here, we show that the expression of CD38 and BCL2 dependence are associated with the differentiation/maturation stage of myeloma cells. Indeed, myeloma patients with the t(11;14)‐associated immature phenotype, especially those expressing B–cell‐related genes, showed significantly lower CD38 levels and higher BCL2 dependence. Our data may reveal factors affecting CD38 expression in untreated myelomas, and explain why t(11;14) myelomas are BCL2 dependent and susceptible to venetoclax.
Abbreviations
- ADCC
antibody‐dependent cellular cytotoxicity
- ATRA
all‐trans‐retinoic acid
- BM‐MNCs
bone marrow mononuclear cells
- CDC
complement‐dependent cytotoxicity
- IMiDs
immunomodulatory drugs
- MFI
mean fluorescence intensities
- MM
multiple myeloma
- PIs
proteasome inhibitors
- Tregs
regulatory T‐cells
1. INTRODUCTION
Daratumumab, a fully human monoclonal antibody against CD38, has emerged as a promising agent for treating MM.1 It has shown substantial clinical activity in several clinical trials when used in combination with PIs or IMiDs.2, 3, 4, 5 Various mechanisms of action have been reported for daratumumab. Daratumumab has classic Fc‐dependent immune effector mechanisms, including CDC and ADCC.6 A previous study has demonstrated a significant positive association between CD38 expression levels in myeloma cells and the efficacy of daratumumab monotherapy.7 In addition, daratumumab targets CD38‐expressing immunosuppressive cells, including CD38‐positive (CD38+) regulatory T‐cells (Tregs), thereby inducing immunomodulatory effects. It has been shown previously that cytotoxic T‐cell number and clonality are increased after daratumumab treatment.8, 9
We recently showed that CD38 expression levels on myeloma cells and the frequency of circulating CD38+ Tregs are associated with the response to daratumumab.10 In the study, we demonstrated that the pretreatment levels of CD38 on myeloma cells may be a predictive marker for an early response to daratumumab treatment, reflecting the direct cytotoxicity of daratumumab. A previous study showed that IMiDs such as lenalidomide upregulate CD38 expression and prime myeloma cells for daratumumab‐mediated cytotoxicity by degrading the CD38 gene repressors, IKZF1 and IKZF3.11 Therefore, prior exposure to IMiDs may cause heterogenous CD38 expression in previously treated patients with MM. Daratumumab was recently approved for treating newly diagnosed patients with MM. however the factors affecting CD38 expression in untreated MM remain unclear. It is important to obtain a rapid response by daratumumab during initial treatment, as several reports have suggested that immunomodulatory effects are less likely to be induced if debulking of the tumor is not achieved.12, 13 Therefore, it may be clinically relevant to identify the determinants of CD38 expression. Therefore, we investigated the factors affecting CD38 expression in newly diagnosed patients with MM.
It has been shown that MM produces tumors at various stages of differentiation and maturation. Particularly, cases with translocation t(11;14) frequently show an immature phenotype, such as lymphoplasmacytic morphology,14, 15 B–cell‐associated gene expression,16, 17 low‐concentration of monoclonal proteins,18 and unmeasurable secretory status such as oligosecretory and nonsecretory types.19 In this study, we show that CD38 expression is significantly lower in patients with myeloma and the t(11;14)‐associated immature plasma cell phenotype. Moreover, we found that CD38 expression was negatively correlated with the BCL2/BCL2L1 ratio, and BCL2 dependence was significantly higher in patients with myeloma and the t(11;14)‐associated immature phenotype. These results suggest that BCL2 dependence, as well as CD38 expression, could be deeply associated with the differentiation and maturation stages of myeloma cells.
2. MATERIAL AND METHODS
2.1. Patients
In total, 72 patients newly diagnosed with symptomatic MM at Kameda Medical Center between October 2017 and September 2020 were included. Of these, patients from whom sufficient RNA could be extracted were included in this study. All patients were diagnosed with symptomatic MM in accordance with International Myeloma Working Group criteria.20 Cytogenetic abnormalities including t(4;14), t(14;16), del(17p), t(11;14), del(13q), and 1q gain were examined in all patients using interphase fluorescence in situ hybridization, which was performed in accordance with the manufacturer's protocols at the Special Reference Laboratory (Hachiohji) using bone marrow plasma cells purified by CD138‐coated magnetic beads (Miltenyi Biotec). Written informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board of Kameda Medical Center (protocol number: 19‐014).
2.2. Flow cytometric analysis of bone marrow samples from patients
CD38 and CD138 expression levels were determined as MFI using flow cytometry. Flow cytometry was performed using the DURAClone RE PC antibody panel on a Navios cytometer, and the data were analyzed using Kaluza analysis software (all from Beckman Coulter). CD38 and CD138 MFI were accessed in the neoplastic plasma cell population (CD38+/CD138+/CD56+ or CD56−/CD19−) as previously described.10
2.3. Real‐time quantitative RT‐PCR analysis
Real‐time quantitative RT‐PCR analysis was performed using the TaqMan method (Applied Biosystems) on a Light Cycler Nano instrument (Roche). TaqMan probes for GAPDH (Hs02758991_g1), PAX5 (Hs01045955_m1), CD79A (Hs00998119_m1), BCL2 (Hs01048932_g1), BCL2L1 (Hs00236329_m1), and MCL1 (Hs01050896_m1) were purchased from Applied Biosystems. Quantitative analysis was performed by determining the threshold cycle (Ct) values during the exponential phase of amplification. The ΔC t value was calculated as the difference between the C t values for a specific mRNA and the C t value for GAPDH. Relative expression levels were presented as values. Total RNA was extracted using TRIzol reagent (Life Technologies) from bone marrow CD138‐purified plasma cells. Reverse transcription was performed using a Transcriptor First Strand cDNA Synthesis Kit (product no. 04379012001, Roche).
2.4. Myeloma cell lines and culture
MM cell lines (KMS12BM, NCU‐MM1, U266, MM.1S, H929, and RPMI8226) were cultured in RPMI 1640 medium (Invitrogen), supplemented with 10% fetal bovine serum (Sigma‐Aldrich).
2.5. Cell viability assay
Cell viability assays were carried out using an MTT‐based In Vitro Toxicology Assay Kit, in accordance with the manufacturer's protocol (Sigma‐Aldrich).
2.6. Flow cytometry‐based ex vivo CDC and ADCC assays
Among the 72 samples from patients with MM, bone marrow mononuclear cells (BM‐MNCs) derived from 48 patients with MM, containing 10‐67% CD138‐positive tumor cells but also autologous effector cells, were available for the ADCC and CDC assays. Lysis of MM cells by CDC and ADCC was measured using flow cytometry after measuring the percentage of propidium iodide‐positive cells, as previously reported.21 All cells were cultured at 37°C in a 5% CO2 in air atmosphere. MM cell lysis was determined after counting viable cells within the CD138‐positive cell population. For the CDC assay, BM‐MNCs were treated with daratumumab (10 µg/mL) and pooled human serum as source of complement for 1 h prior to flow cytometric analysis. For the ADCC assay, BM‐MNCs were treated with daratumumab (10 µg/mL) or control antibody (IgG1‐B12) for 48 h. The percentage of daratumumab‐mediated ADCC was then calculated using the following formula: % lysis = 1 − (absolute number of surviving CD138+ cells in the presence of daratumumab/absolute number of surviving CD138+ cells in the presence of control antibody) × 100%. Complement‐dependent lysis was calculated using the following formula: % lysis = 1 − (absolute number of surviving CD138+ cells in the presence of native human serum/absolute number of surviving CD138+ cells in the presence of heat‐inactivated serum) × 100%.
2.7. Reagents
Venetoclax was purchased from Selleck Chemicals. Daratumumab was obtained from Janssen Pharmaceuticals. ATRA was purchased from Sigma‐Aldrich.
2.8. Statistical analysis
Mann‐Whitney U tests, Kruskal‐Wallis test and Student t tests were used to examine significance. Correlations between variables were identified using the Spearman's rank correlation coefficient. P‐values below .05 were considered as significant. All statistical analysis was performed using GraphPad Prism 8 software (GraphPad, Inc).
3. RESULTS
3.1. CD38 expression is significantly lower in t(11;14) myeloma, particularly in patients with the immature plasma cell phenotype and B‐cell‐associated gene expression
The clinical characteristics of the patients are shown in Table 1. First, we confirmed that CD38 MFI was heterogeneous in the newly diagnosed cases, as it is in relapsed/refractory cases (Figure 1A).7, 10 As previously reported, CD38 expression was not significantly associated with clinical parameters such as age, sex, lactate dehydrogenase levels, β2‐microglobulin levels, and the International Staging System stage (data not shown).7 In contrast, we found that CD38 MFI was significantly lower in cases with lymphoplasmacytoid morphology, which was identified by Hoyer et al14 (Figure 1B). In these patients, the plasma cells were small, with scant cytoplasm and without prominent nucleoli, suggesting a less differentiated plasma cell phenotype. This lymphoplasmacytoid morphology was reportedly associated with t(11;14).14, 15 Indeed, lymphoplasmacytoid morphology occurred only in patients with t(11;14) (in 50% of such cases), which was consistent with previous reports.14, 15 Furthermore, CD38 MFI was significantly lower in patients with t(11;14) compared with those without t(11;14) (Figure 1C). CD38 and CD138 expression levels increase during plasma cell development.22 Notably, CD138 MFI was also significantly lower in patients with lymphoplasmacytoid morphology and with t(11;14) (Figure 1D). Furthermore, CD38 and CD138 expression levels were significantly positively correlated (Figure 1E). However, not all patients with t(11;14) showed lower CD38 and CD138 MFI. Some patients with t(11;14) have been reported to express B–cell‐associated genes such as PAX5 and CD79A.16, 17 Silencing of PAX5 is essential for the terminal differentiation of B‐cells to plasma cells. However, in our study, PAX5 expression was detected more frequently in patients with t(11;14) (Figure 1F) and in those with lymphoplasmacytoid morphology (Figure 1G). Importantly, PAX5high cases showed significantly lower CD38 expression compared with PAX5low cases in t(11;14) patients (Figure 1G). Furthermore, CD38 MFI was significantly lower in patients with high CD79A expression (Figure 1G). PAX5high and CD79Ahigh mostly occurred together in patients, although some divergence was observed. PAX5 is a transcription factor of CD19, and therefore, we examined the surface expression of CD19. All our patient samples were negative for surface expression of CD19. We also examined the expression of CD19 mRNA. However, the expression of CD19 mRNA and CD38 MFI and PAX5 mRNA expression did not show a significant correlation. This may be because the expression of PAX5 in t(11;14) myeloma patients is relatively lower than that in normal B‐cells, and therefore it does not have sufficient ability to induce the expression of CD19. In the process of plasma cell development, the expression of CD38, CD138, IRF4, XBP1, and PRDM1 increases, whereas that of CD19, CD20, BCL6, PAX5, and CD79A decreases.23 Therefore, we also examined other differentiation markers such as CD20, BCL6, XBP1, IRF4, and PRDM1; however, none of these genes was significantly correlated with CD38 MFI. These results suggested that CD38 expression is affected by the t(11;14)‐associated immature plasma cell phenotype, particularly when B‐cell‐associated genes such as PAX5 and CD79A are also expressed.
TABLE 1.
Patient characteristics
| Parameter | Number of patients (N = 72) |
|---|---|
| Median age, years (range) | 76 (30‐97) |
| Male | 29 (52%) |
| Female | 43 (48%) |
| Paraprotein type | |
| IgG | 30 (42%) |
| IgA | 22 (31%) |
| IgD | 1 (1%) |
| LCD | 19 (26%) |
| International staging system | |
| I | 16 (22%) |
| II | 15 (21%) |
| III | 41 (57%) |
| Elevated LDH level (>ULN) | 26 (36%) |
| Elevated β2‐MG level (≥3.5 mg/L) | 47 (65%) |
| Cytogenetic abnormalities | |
| t(4;14) | 7 (10%) |
| del(17p) | 9 (13%) |
| t(14;16) | 2 (3%) |
| t(11;14) | 24 (32%) |
| del(13q) | 37 (51%) |
| 1q21 gains | 36 (50%) |
| Lymphoplasmacytoid morphology | 12 (16%) |
Abbreviations: LCD, light chain disease; LDH, lactate dehydrogenase; ULN, upper limit of normal; β2‐MG, β2‐microgulobulin.
FIGURE 1.
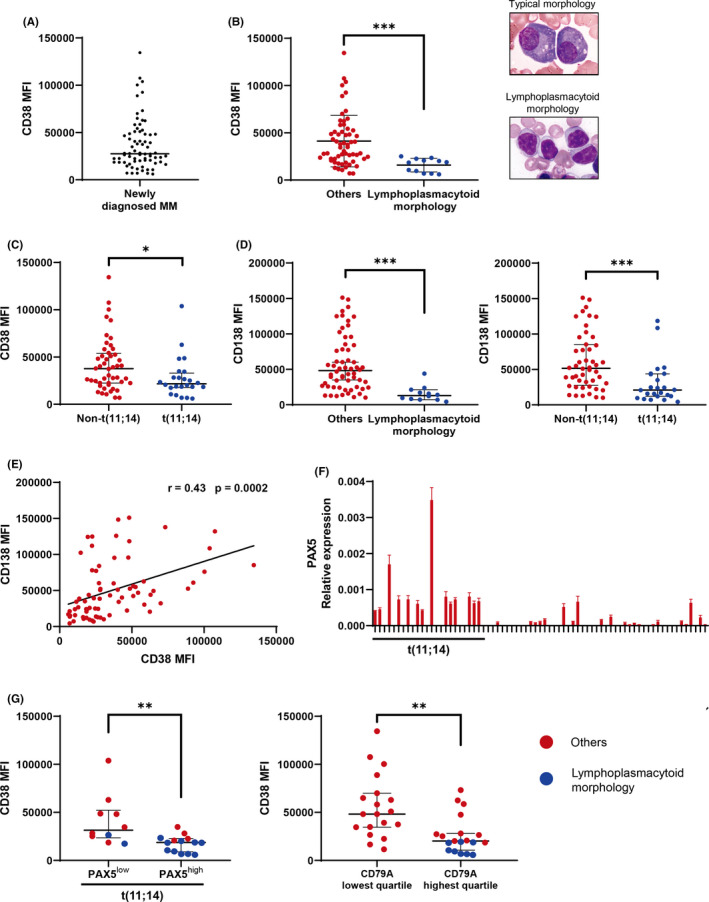
CD38 expression is significantly lower in t(11;14)‐associated immature plasma cell morphology, especially in cells expressing B–cell‐associated genes. Asterisks denote significant changes (* .01 ≤ P < .05, **.001 ≤ P < .01, and ***P < .001); ns, not significant. A, Flow cytometry analysis of CD38 MFI in newly diagnosed multiple myeloma patients. Bars indicate the median. B, Comparison of CD38 MFI in myeloma patients with or without lymphoplasmacytoid morphology. Representative typical plasma cell morphology and lymphoplasmacytoid morphology are shown. Lymphoplasmacytoid morphology type was defined as the cases with reduced cytoplasm (nuclear cytoplasmic [N/C] ratio exceeding 0.6) and >25% tumor cells showing lymphoplasmacytoid morphology. Bars indicate the median with interquartile range. Significance was assessed by Mann‐Whitney U test. C, CD38 MFI in patients with or without t(11;14). Bars indicate the median with interquartile range. Significance was assessed by Mann‐Whitney U test. D, CD138 MFI in myeloma patients with or without lymphoplasmacytoid morphology and t(11;14). Bars indicate the median with interquartile range. Significance was assessed by Mann‐Whitney U test. E, The correlation between CD38 and CD138 MFI is shown; r: correlation coefficient. Significance was assessed by Spearman test. F, Real‐time quantitative RT‐PCR (qRT‐PCR) analysis of PAX5 in newly diagnosed multiple myeloma patients. Expression levels were normalized to that of GAPDH. Specific mRNA relative expression levels are presented as . X‐axis: case numbers. Y‐axis: values for PAX5 mRNA expression. Results are representative of 3 different experiments. Error bars represent the mean ± standard error of 3 independent experiments. G, CD38 MFI in myeloma patients with PAX5high or PAX5low expression, as defined by the PAX5 median value of all patients. CD38 MFI by CD79A expression is also shown. Blue denotes the cases with lymphoplasmacytoid morphology. Significance was assessed by Mann‐Whitney U test
3.2. The BCL2/BCL2L1 ratio is inversely correlated with CD38 MFI and significantly higher in t(11;14) patients expressing B–cell‐associated genes
Venetoclax, a first‐in‐class BCL2 inhibitor, has shown clinical activity in MM, particularly in patients with t(11;14), who often show high BCL2 expression and low expression of MCL1 and BCL2L1 (encoding Bcl‐xL).24 However, not all t(11;14) patients showed high BCL2 expression, and it remains unclear why t(11;14) patients showed BCL2 dependence. Interestingly, BCL2 expression was significantly higher in PAX5high cases than in PAX5low cases among our t(11;14) patients (Figure 2A). In a previous study, it was shown that the expression of BCL2 family members in MM cells is heterogeneous, and upregulation of either MCL1 or BCL2L1 can confer resistance to venetoclax.25 Indeed, in a recent clinical study, high BCL2/BCL2L1 and/or BCL2/MCL1 mRNA ratios showed a good correlation with the response to venetoclax.24 Therefore, we analyzed these ratios in our patient samples. Importantly, the BCL2/BCL2L1 and BCL2/MCL1 ratios were also significantly higher in the PAX5high t(11;14) cases (Figure 2B). Furthermore, we confirmed that the BCL2/BCL2L1 ratio was significantly higher in the CD79Ahigh cases than in the CD79Alow cases (Figure 2C). Notably, the BCL2/BCL2L1 ratio, but not the BCL2/MCL1 ratio, showed a significant negative correlation with CD38 MFI (Figure 2D). These results suggested that BCL2 dependence, as well as CD38 expression, is deeply associated with the differentiation and maturation stages of myeloma cells.
FIGURE 2.
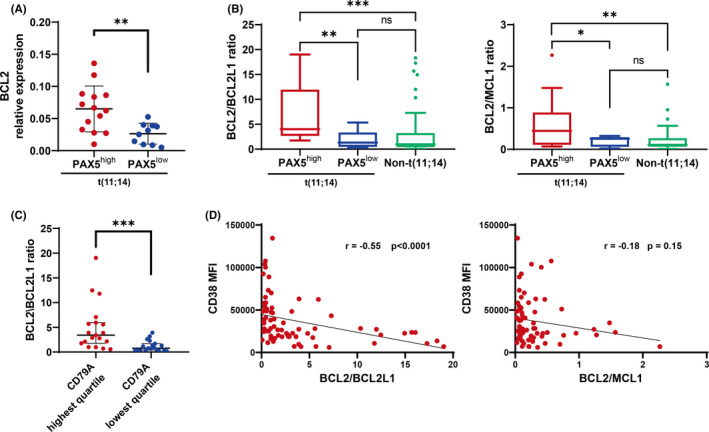
The BCL2/BCL2L1 ratio was inversely correlated with CD38 MFI, and was significantly higher in t(11;14) patients expressing B–cell‐associated genes. Asterisks denote significant changes (*.01 ≤ P < .05, **.001 ≤ P < .01, and ***P < .001); ns, not significant. A, BCL2 relative expression in myeloma patients with PAX5high or PAX5low expression. Bars indicate the median with interquartile range. Significance was assessed by Mann‐Whitney U test. Expression levels were normalized to that of GAPDH. Specific mRNA relative expression levels are presented as . B, The BCL2/BCL2L1 ratio in patients with t(11;14) plus PAX5high or PAX5low , or without t(11;14). The BCL2/MCL1 ratio is also shown. Bars indicate the median with interquartile range. Significance was assessed by Kruskal‐Wallis test. C, The BCL2/BCL2L1 ratio in patients by CD79A expression. Bars indicate the median with interquartile range. Significance was assessed by Mann‐Whitney U test. D, Correlations between CD38 MFI and the BCL2/BCL2L1 ratio, and CD38 MFI and the BCL2/MCL1 ratio; r, correlation coefficient. Significance was assessed by Spearman test
3.3. Patients with t(11;14) expressing B–cell‐associated genes are less sensitive to daratumumab‐induced direct cytotoxicity but highly sensitive to venetoclax
In Figures 1 and 2, we showed that PAX5high t(11;14) cases displayed lower expression of CD38 and a higher BCL2/BCL2L1 ratio. These results suggested that PAX5high t(11;14) cases may show different sensitivities to daratumumab and venetoclax. To determine whether patients with the t(11;14)‐associated immature plasma cell phenotype were indeed less sensitive to daratumumab‐mediated direct cytotoxicity, we conducted ex vivo CDC and ADCC assays using primary MM samples. Notably, PAX5high t(11;14) cases showed decreased sensitivity to daratumumab‐mediated ADCC and CDC compared with other cases (Figure 3A). In contrast, the PAX5high t(11;14) patients were highly sensitive to venetoclax and more so than PAX5low t(11;14) and other patients (Figure 3B). A similar trend was obtained when t(11;14)‐positive cases were divided by cell morphology, because almost all cases with PAX5 expression showed a lymphoplasmacytoid morphology (Figure S1). These results suggested that the t(11;14)‐associated immature phenotype can affect sensitivity to daratumumab and venetoclax.
FIGURE 3.
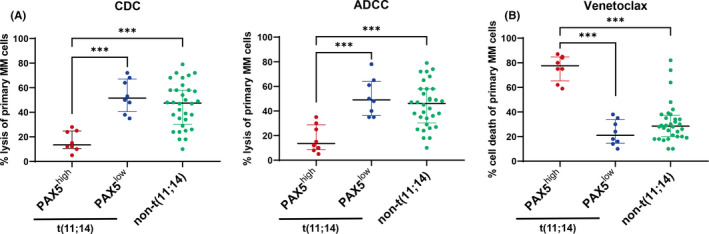
Patients with t(11;14) expressing B–cell‐associated genes are less sensitive to daratumumab‐induced direct cytotoxicity but highly sensitive to venetoclax. Asterisks denote significant changes (*.01 ≤ P < .05, **.001 ≤ P < .01, and ***P < .001); ns, not significant. ADCC, antibody‐dependent cellular cytotoxicity; BM‐MNC, bone marrow mononuclear cells; CDC, complement‐dependent cytotoxicity. A, Ex vivo CDC and ADCC assays using primary myeloma samples. BM‐MNCs from 48 patients with newly diagnosed MM were used in ADCC and CDC assays with 10 μg/ml daratumumab. ADCC and CDC assays were performed as described in Materials and Methods. Bars indicate the median with interquartile range. Significance was assessed by Kruskal‐Wallis test. B, Flow cytometry‐based ex vivo cell death assay in primary myeloma cells treated with venetoclax. BM‐MNCs from 48 patients with newly diagnosed MM were treated with 300 nM venetoclax or vehicle control (DMSO) for 24 h. Myeloma cells were identified by CD138 staining. Cell death was measured as the loss of CD138 staining. The percentage of venetoclax‐mediated cell death was calculated using the following formula: % cell death = 1 − (absolute number of surviving CD138+ cells in the presence of venetoclax/absolute number of surviving CD138+ cells in the presence of DMSO control) × 100%
3.4. ATRA induces CD38 expression and cell differentiation in MM cell lines but decreases the BCL2/BCL2L1 ratio and venetoclax efficacy
ATRA is known to enhance CD38 expression and induce cell differentiation in MM cells.26, 27 To clarify the relationship between the expression of CD38 and the B‐cell gene expression and myeloma cell differentiation, we conducted an experiment using ATRA. The expression of PAX5 and CD79A was confirmed only in the KMS12BM and NCU‐MM1 cell lines that harbored the t(11;14) translocation (Figure 4A). Interestingly, these cell lines showed an immature morphology; however, ATRA‐treated KMS12BM and NCU‐MM1 cells displayed the morphological characteristics of plasma cell differentiation, namely eccentric nuclei and a distinct clear perinuclear region of the cytoplasm (Figure 4B). In contrast, MM.1S, with neither t(11;14) nor B‐cell marker expression, exhibited mature plasma cell morphology, and showed no morphological changes following ATRA treatment. Furthermore, the fold increases in CD38 MFI and CD138 MFI were higher in KMS12BM and MCU‐MM1 than in other cell lines, as these cell lines showed lower baseline CD38 and CD138 expression levels (Figure 4C). We confirmed that the sensitivity to daratumumab was increased by ATRA in KMS12BM and NCU‐MM1 cells (Figure S2). In these cell lines, ATRA treatment downregulated PAX5 and CD79A expression (Figure 4D). Moreover, these cell lines showed a high baseline BCL2/BCL2L1 ratio, whereas ATRA exposure resulted in a lower ratio (Figure 4E). Consistent with these results, pretreatment with ATRA significantly reduced the efficacy of venetoclax in the KMS12BM and NCU‐MM1 cell lines (Figure 4F). These results indicated that the differentiation and maturation stages of myeloma cells, CD38 expression, and BCL2 dependence are closely related.
FIGURE 4.
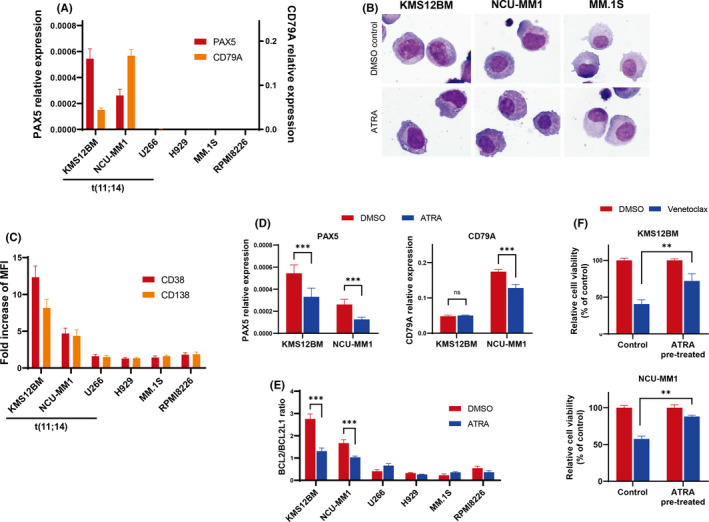
ATRA induces CD38 expression and cell differentiation in multiple myeloma cell lines, but decreases the BCL2/BCL2L1 ratio and venetoclax efficacy. Asterisks denote significant changes (*.01 ≤ P < .05, **.001 ≤ P < .01, and ***P < .001). ns; not significant. A, qRT‐PCR analysis of PAX5 and CD79A mRNA in multiple myeloma cell lines. Bars indicate the mean ± standard error of 3 independent experiments. B, Morphological changes in myeloma cell lines following exposure to ATRA. Cells were treated with ATRA (1 µM) or vehicle control (DMSO) for 72 h, at which time May‐Giemsa staining was performed. Photographs are representative of 3 independent experiments. C, Fold increases in CD38 and CD138 MFI in ATRA‐treated multiple myeloma cell lines. Cells were treated with ATRA (1 µM) or vehicle control (DMSO) for 72 h at which time flow cytometric analysis was performed. D, The qRT‐PCR analysis of PAX5 and CD79A in ATRA‐treated KMS12BM and NCU‐MM1 cell lines (1 μM for 72 h). Bars indicate the mean ± standard error of 3 independent experiments. The significance of differences between the indicated groups was assessed by Student t tests. E, qRT‐PCR analysis of BCL2/BCL2L1 ratio in ATRA‐treated myeloma cell lines (1 μM for 72 h). Bars indicate the mean ± standard error of 3 independent experiments. The significance of differences between the indicated groups was assessed by Student t tests. F, Cell viability assay of KMS12BM and NCU‐MM1 cell lines treated with ATRA and venetoclax. Cells were treated with 1 µM ATRA, or the DMSO control, for 72 h before venetoclax treatment. ATRA‐pretreated KMS12BM and NCU‐MM1 cells were treated with venetoclax (250 nM for KMS12BM and 500 nM for NCU‐MM1) or the DMSO control for 48 h, and cell viability was measured. Bars indicate the mean ± standard error of 3 independent experiments. Significance of differences between the indicated groups was assessed by Student t tests
4. DISCUSSION
Our findings suggested that it may be difficult to obtain rapid direct effects using daratumumab in patients with the t(11;14)‐associated immature phenotype, because of their decreased CD38 expression. Indeed, in the ALCYONE study, a randomized phase 3 trial of bortezomib, melphalan, and prednisone, with or without daratumumab, in patients with newly diagnosed MM, addition of daratumumab failed to improve survival in the non‐IgG type.4 It has been reported that the frequency of IgG type is relatively lower, whereas that of the non‐IgG type is relatively higher in t(11;14) cases.18, 28 Therefore, the t(11;14)‐associated immature phenotype may be more abundant in the non‐IgG type in the ALCYONE study, which may lead to a poor early response to daratumumab. However, the long‐term follow‐up data in the ALCYONE study showed improved efficacy for the non‐IgG type, suggesting that the durable response, reflecting the immunomodulatory effects, may be induced for cases with t(11;14)‐associated immature phenotype, as well. Therefore, we recommend the combination of daratumumab and IMiDs, rather than daratumumab, and PIs, for patients with the immature phenotype. This is because PIs are less effective and IMiDs are more effective in immature cases,29 and IMiDs enhance CD38 expression on myeloma cells and the immunomodulatory effect of daratumumab.8, 9, 11 Indeed, in the MAIA study, a randomized phase 3 trial of lenalidomide and dexamethasone, with or without daratumumab, patients with newly diagnosed MM demonstrated survival benefits in the non‐IgG type rather than in the IgG type.5
Furthermore, the BCL2/BCL2L1 ratio was negatively correlated with CD38 expression. Considering that plasma cells are BCL2L1 dependent, and that post‐germinal center B‐cells are BCL2 dependent during B‐cell differentiation,30 these findings appear to be compatible. That is, less differentiated plasma cells may show lower BCL2L1 expression and higher BCL2 expression, thereby exhibiting BCL2 dependence and high sensitivity to venetoclax. Consistent with this, Cleynen et al reported that BCL2/BCL2L1, but not BCL2/MCL1, separated t(11;14) patients into 2 groups.31 These findings are also consistent with a report that the BCL2/BCL2L1 ratio is a better marker for venetoclax sensitivity than the BCL2/MCL1 ratio.32 Patients with t(11;14) showed BCL2 dependence, possibly because the frequency of the immature phenotype in t(11;14) is relatively high. Recently, Gupta et al also reported that B‐cell gene expression is associated with the response to venetoclax.33 Our results confirmed their findings; in addition to the presence of t(11;14) and the expression of B–cell associated genes, the immature morphology and lower CD38 expression may be useful for predicting the response to venetoclax.
CD20 is a representative B‐cell marker, however, in this study, there was no significant difference in CD38 MFI with or without CD20 expression. In fact, Grigoriadis et al34 showed that CD20‐positive MM exhibits heterogenous clinical features, morphology, immunophenotype, and cytogenetics. Furthermore, it has been reported that the expression levels of CD20 and PAX5 are not concordant.35 Therefore, CD20 may be expressed independently of the differentiation and maturation stages in MM. We also found no association between CD38 MFI and other differentiation markers such as BCL6, XBP1, and PRDM1. In this study, we identified a population with particularly low CD38 expression. High CD38 expression may be associated with other factors; this requires further investigation.
In conclusion, we found that CD38 expression was significantly lower in patients with MM with the t(11;14)‐associated immature plasma cell phenotype, particularly those expressing B–cell‐associated genes such as PAX5 and CD79A. Furthermore, the BCL2/BCL2L1 ratio was negatively correlated with CD38 expression and higher in patients with MM with the immature phenotype accompanied by B–cell‐associated genes. Our findings may be useful for determining the optimal combination in daratumumab‐based treatment, as well as for predicting the treatment response to venetoclax. Despite being the most frequent chromosomal abnormality in MM, t(11;14) has not been widely examined. Our findings highlight the importance of evaluating t(11;14) and considering the MM differentiation and maturation stages in treatment strategies.
DISCLOSURE
AK: Honoraria, Janssen. NT: Honoraria, Pfizer, Otsuka, and Novartis.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the technicians of the Clinical Laboratory Department, Kameda Medical Center; Ms Hiromi Kataho, Yuko Chiba, and Yukiko Abe (Department of Hematology, Nephrology, and Rheumatology, Akita University) for their outstanding technical assistance; Editage (www.editage.jp), for English language editing; and Kensuke Kojima (Department of Hematology, Kochi University) for his helpful advice. This work was supported by the Japan Society for the Promotion of Science KAKENHI (Grant‐in‐Aid for Scientific Research) (AK).
Kitadate A, Terao T, Narita K, et al. Multiple myeloma with t(11;14)‐associated immature phenotype has lower CD38 expression and higher BCL2 dependence. Cancer Sci. 2021;112:3645–3654. 10.1111/cas.15073
REFERENCES
- 1.van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131:13‐29. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319‐1331. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Chanan‐Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754‐766. [DOI] [PubMed] [Google Scholar]
- 4.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378:518‐528. [DOI] [PubMed] [Google Scholar]
- 5.Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380:2104‐2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nijhof IS, Groen RW, Noort WA, et al. Preclinical evidence for the therapeutic potential of CD38‐targeted immuno‐chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin Cancer Res. 2015;21:2802‐2810. [DOI] [PubMed] [Google Scholar]
- 7.Nijhof IS, Casneuf T, van Velzen J, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128:959‐970. [DOI] [PubMed] [Google Scholar]
- 8.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T‐cell expansion, and skews T‐cell repertoire in multiple myeloma. Blood. 2016;128:384‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casneuf T, Adams HC 3rd, van de Donk NWCJ, et al. Deep immune profiling of patients treated with lenalidomide and dexamethasone with or without daratumumab. Leukemia. 2021;35:573‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitadate A, Kobayashi H, Abe Y, et al. Pre‐treatment CD38‐positive regulatory T cells affect the durable response to daratumumab in relapsed/refractory multiple myeloma patients. Haematologica. 2020;105:e37‐e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedele PL, Willis SN, Liao Y, et al. IMiDs prime myeloma cells for daratumumab‐mediated cytotoxicity through loss of Ikaros and Aiolos. Blood. 2018;132:2166‐2178. [DOI] [PubMed] [Google Scholar]
- 12.Guisier F, Cousse S, Jeanvoine M, Thiberville L, Salaun M. A rationale for surgical debulking to improve anti‐PD1 therapy outcome in non small cell lung cancer. Sci Rep. 2019;9:16902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oppel F, Görner M, Sudhoff H. The potential of tumor debulking to support molecular targeted therapies. Front Oncol. 2020;10:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyer JD, Hanson CA, Fonseca R, Greipp PR, Dewald GW, Kurtin PJ. The (11;14)(q13;q32) translocation in multiple myeloma. A morphologic and immunohistochemical study. Am J Clin Pathol. 2000;113:831‐837. [DOI] [PubMed] [Google Scholar]
- 15.Garand R, Avet‐Loiseau H, Accard F, Moreau P, Harousseau JL, Bataille R. t(11;14) and t(4;14) translocations correlated with mature lymphoplasmacytoid and immature morphology, respectively, in multiple myeloma. Leukemia. 2003;17:2032‐2035. [DOI] [PubMed] [Google Scholar]
- 16.Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An G, Xu Y, Shi L, et al. t(11;14) multiple myeloma: a subtype associated with distinct immunological features, immunophenotypic characteristics but divergent outcome. Leuk Res. 2013;37:1251‐1257. [DOI] [PubMed] [Google Scholar]
- 18.Fonseca R, Blood EA, Oken MM, et al. Myeloma and the t(11;14)(q13;q32); evidence for a biologically defined unique subset of patients. Blood. 2002;99:3735‐3741. [DOI] [PubMed] [Google Scholar]
- 19.Avet‐Loiseau H, Garand R, Lodé L, et al. Translocation t(11;14)(q13;q32) is the hallmark of IgM, IgE, and nonsecretory multiple myeloma variants. Blood. 2003;101:1570‐1571. [DOI] [PubMed] [Google Scholar]
- 20.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538‐e548. [DOI] [PubMed] [Google Scholar]
- 21.de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840‐1848. [DOI] [PubMed] [Google Scholar]
- 22.Jourdan M, Caraux A, Caron G, et al. Characterization of a transitional preplasmablast population in the process of human B cell to plasma cell differentiation. J Immunol. 2011;187:3931‐3941. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro‐Shelef M, Calame K. Regulation of plasma‐cell development. Nat Rev Immunol. 2005;5:230‐242. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130:2401‐2409. [DOI] [PubMed] [Google Scholar]
- 25.Punnoose EA, Leverson JD, Peale F, et al. Expression profile of BCL‐2, BCL‐XL, and MCL‐1 predicts pharmacological response to the BCL‐2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther. 2016;15:1132‐1144. [DOI] [PubMed] [Google Scholar]
- 26.Nijhof IS, Groen RW, Lokhorst HM, et al. Upregulation of CD38 expression on multiple myeloma cells by all‐trans retinoic acid improves the efficacy of daratumumab. Leukemia. 2015;29:2039‐2049. [DOI] [PubMed] [Google Scholar]
- 27.Kawano Y, Kikukawa Y, Fujiwara S, et al. Hypoxia reduces CD138 expression and induces an immature and stem cell‐like transcriptional program in myeloma cells. Int J Oncol. 2013;43:1809‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdallah N, Rajkumar SV, Greipp P, et al. Cytogenetic abnormalities in multiple myeloma: association with disease characteristics and treatment response. Blood Cancer J. 2020;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furukawa Y, Kikuchi J. Molecular basis of clonal evolution in multiple myeloma. Int J Hematol. 2020;111:496‐511. [DOI] [PubMed] [Google Scholar]
- 30.Dai J, Luftig MA. Intracellular BH3 profiling reveals shifts in antiapoptotic dependency in human B cell maturation and mitogen‐stimulated proliferation. J Immunol. 2018;200:1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleynen A, Samur M, Perrot A, et al. Variable BCL2/BCL2L1 ratio in multiple myeloma with t(11;14). Blood. 2018;132:2778‐2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez‐Bougie P, Maiga S, Tessoulin B, et al. BH3‐mimetic toolkit guides the respective use of BCL2 and MCL1 BH3‐mimetics in myeloma treatment. Blood. 2018;132:2656‐2669. [DOI] [PubMed] [Google Scholar]
- 33.Gupta VA, Barwick BG, Matulis SM, et al. Venetoclax sensitivity in multiple myeloma is associated with B‐cell gene expression. Blood. 2021;137(26):3604‐3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grigoriadis G, Gilbertson M, Came N, et al. Is CD20 positive plasma cell myeloma a unique clinicopathological entity? A study of 40 cases and review of the literature. Pathology. 2012;44:552‐556. [DOI] [PubMed] [Google Scholar]
- 35.Lin P, Mahdavy M, Zhan F, Zhang HZ, Katz RL, Shaughnessy JD. Expression of PAX5 in CD20‐positive multiple myeloma assessed by immunohistochemistry and oligonucleotide microarray. Mod Pathol. 2004;17:1217‐1222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


