Abstract
The tumor microenvironment affects malignancy in hepatocellular carcinoma (HCC) cells, and cancer‐associated fibroblasts (CAFs) play an important role in the microenvironment. As recent studies indicated a difference between CAFs isolated from chemoresistant and non‐resistant cancer tissues, therefore we investigated the intracellular mechanism in resistant HCC co‐cultured CAFs and interactions between these CAFs with cancer cells. We established a sorafenib‐resistant (SR) Huh7 (human HCC) cell line, and characterized it with cytokine assays, then developed CAFs by co‐culturing human hepatic stellate cells with resistant or parental Huh7 cells. The 2 types of CAFs were co‐cultured with parental Huh7 cells, thereafter the cell viability of these Huh7 cells was checked under sorafenib treatment. The SR Huh7 (Huh7SR) cells expressed increased B‐cell activating factor (BAFF), which promoted high expression of CAF‐specific markers in Huh7SR‐co‐cultured CAFs, showed activated BAFF, BAFF‐R, and downstream of the NFκB‐Nrf2 pathway, and aggravated invasion, migration, and drug resistance in co‐cultured Huh7 cells. When we knocked down BAFF expression in Huh7SR cells, the previously increased malignancy and BAFF/NFκB axis in Huh7SR‐co‐cultured CAFs reversed, and enhanced chemoresistance in co‐cultured Huh7 cells returned as well. In conclusion, the BAFF/NFκB pathway was activated in CAFs co‐cultured with cell‐culture medium from resistant Huh7, which promoted chemoresistance, and increased the malignancy in co‐cultured non‐resistant Huh7 cells. This suggests that the BAFF/NFκB axis in CAFs might be a potential therapeutic target in chemoresistance of HCC.
Keywords: B‐cell activating factor, cancer‐associated fibroblasts, drug resistance, hepatocellular carcinoma, sorafenib
In this study, we established a sorafenib‐resistant (SR) Huh7 (human hepatocellular carcinoma [HCC]) cell line, and developed cancer‐associated fibroblasts (CAFs) induced from resistant or parental Huh7 cells. We found that the B‐cell activating factor (BAFF)‐NFκB pathway was activated in sorafenib‐resistant Huh7‐co‐cultured CAFs, which promoted chemoresistance and increased malignancy in co‐cultured non‐resistant Huh7 cells. This suggests that the BAFF‐NFκB axis plays a crucial role between the interaction of SR HCC and CAFs, and it might be a potential therapeutic target in CAFs that caused chemoresistance of HCC.

1. INTRODUCTION
Activated cancer‐associated fibroblasts (CAFs) are critical to tumor development.1 CAFs may also affect cancer chemoresistance and cancer stem cells.2 When isolated fibroblasts from chemoresistant breast cancer tissues are co‐cultured with breast cancer cells, cancer cells reportedly sustain stemness and promote chemoresistance compared with cancer cells co‐cultured with CAFs isolated from chemosensitive (CS) cells or non‐co‐cultured controls.3 However, how the tumor microenvironment affects chemoresistance is still unclear.
B‐cell activating factor (BAFF), which belongs to the tumor necrosis factor family, is well studied in B‐cell biology and hematological diseases.4 In hepatocytes, activated BAFF and its receptor (BAFF‐R) decrease the expression of steatogenesis genes and increase steatosis.5 The BAFF–BAFF‐R axis has also been associated with cancer progression, apoptosis, and inflammation.6 Reducing BAFF activity by antibodies that selectively bind to BAFF‐R increases drug sensitivity in cancer cells.7 The NFκB signaling pathway is downstream of BAFF.8 Increasing negative regulator Kelch‐like ECH‐associated protein 1 (KEAP1) of Nuclear factor E2‐related factor 2 (Nrf2), the NFκB pathway activates the Nrf2/NADPH quinone oxidoreductase 1(NQO1) axis, which could also support drug resistance.9 Moreover, a permanently activated NFκB pathway and persistent nuclear expression of its downstream factor, p65, in CAFs are important in maintaining the secretion of IL6 and IL8, and the survival of pancreatic cancer stem cells.3 Other studies have found the balance of location (nuclear or cytoplasmic) of p65 is controlled by post‐translational modifications, and eventually affects MMP9, FGF4, and IL8 expression.6, 10 Hepatocellular carcinoma (HCC) cells with enhanced p16/IL6 pathways have low sensitivity to sorafenib; blocking IL6 in chemoresistant HCC cells reportedly upregulates sorafenib cytotoxicity.4
Here, we established a sorafenib‐resistant (SR) HCC cell line (Huh7SR), and found that CAFs stimulated by Huh7SR cells promoted invasion, migration, and chemoresistance in co‐cultured CS HCC cells, by increased BAFF secretion. We then investigated the effect of HCCSR cells on CAF malignant potential, including SR development in CS HCC cells, by the BAFF–BAFF‐R pathway.
2. MATERIALS AND METHODS
2.1. Cell culture
We purchased Huh‐7 (human HCC) cells from the Riken Cell Bank. The Huh7 cells were cultured in DMEM (Life Technologies Japan Ltd.) containing 10% FBS (Life Technologies Japan Ltd.). We developed the SR Huh7 (Huh7SR) cell line by culturing the cells in DMEM with 8.0 μM sorafenib (sc‐220125A; Santa Cruz Biotechnology), which was first dissolved in DMSO. The last concentration in DMSO was <0.1%. Lx2 human hepatic stellate cells were purchased from Merck Millipore (Tokyo, Japan). Huh7 and Huh7SR were cultured in DMEM for 48 h, then the cell‐culture medium was collected and centrifuged at 50g. Lx2 cells were co‐cultured with this conditioned medium or DMEM at a 1:1 ratio for at least 48 h (Figure 2A). Activation of CAFs was confirmed using real‐time‐PCR (RT‐PCR) of α‐smooth muscle actin (α‐SMA) and fibroblast activation protein (FAP).11
FIGURE 2.

Huh7SR‐co‐cultured CAFs promoted invasion, migration, and drug resistance in HCC cells. A, CAFs were stimulated with cell‐culture medium (CM) of parental Huh7 or Huh7SR. B, C, PCR quantification of α‐SMA and FAP expressed in Lx2, Huh7‐co‐cultured CAFs and Huh7SR‐co‐cultured CAFs, 48 h cultured in DMEM (10% FBS) (n = 4). D, E, Invasion ability (D) was examined by transwell assay in Huh7, and Huh7 cells treated with the CM of Huh7‐co‐cultured CAFs or Huh7SR‐co‐cultured CAFs. Data analysis (E) was performed using GraphPad Prism 5 software (n = 4). F, G, Migration rate (F) of Huh7 treated with CM of Huh7, and Huh7 cells treated with the CM of Huh7‐co‐cultured CAFs or Huh7SR‐co‐cultured CAFs, performed by wound‐healing assay. ImageJ was used for quantification (G) of migration rate (n = 3). H, Colony‐forming assay was performed in Huh7, and Huh7 cells treated with the CM of Huh7‐co‐cultured CAFs or Huh7SR‐co‐cultured CAFs, incubated in DMEM (10% FBS, with 0, 3 or 5 μM sorafenib). (*, P < .05; **, P < .01; ***, P < .001.)
2.2. Establishment of SR cell line
Huh7SR cells were isolated from Huh7 cells according to a protocol of continuous exposure to increasing sorafenib concentrations and stepwise selection. Every 3‐4 d, cells were collected, passaged, and cultured in DMEM containing higher sorafenib concentrations. After development, cells could grow stably in the presence of sorafenib.
2.3. siRNA transfection
Huh7SR cells were transfected with BAFF siRNA (Life Technologies Japan Ltd.) or negative control RNA (Applied Biosystems, Select Negative Control #1 siRNA) with 0.01 µmol/L with Invitrogen Lipofectamine RNAiMax Transfection Reagent (Invitrogen, Thermo Fisher Scientific Inc) following the manufacturer's instructions.
2.4. Migration assay
We used transwell inserts (8‐µm‐pores, Corning) to evaluate cell migration. We seeded 0.5‐1 × 105 Huh7 cells in the upside chamber; they were attached after c. 12 h. The upper chambers were lightly washed in PBS twice, and liquid in the upper chambers was replaced with a conditioned medium of Lx2, CAFs co‐cultured with parental Huh7or CAFs co‐cultured with Huh7SR. The lower chambers were filled with the same conditioned medium (5% FBS). At 24 h after incubation, cells below the insert were fixed with 4% paraformaldehyde, stained with 0.2% crystal violet, and counted (stained cells in 5 random ×400 microscope fields).
2.5. Cell wound‐healing assay
Control and resistant Huh7 cells were plated in 6‐well plates. When the confluency of the cells reached c. 80%‐90%, a 10‐µl pipette was used to scratch a c. 0.5‐mm wound through the entire length of each well. We then changed the medium to DMEM, with or without sorafenib reagent, according to the test group. The wound was observed using a ×20 magnification microscope after 48 h.
2.6. Colony formation assay
The parental Huh7 cells were co‐cultured with supernatants from Lx2 cells, Huh7‐co‐cultured CAFs, or Huh7SR‐co‐cultured CAFs for 48 h at 37℃; and then seeded into 60‐mm cell‐culture dishes (Thermo Scientific) and cultured in DMEM with 0.0 µM (control), 3.0 µM, or 5.0 µM sorafenib for 3 wk. Cells were then fixed with paraformaldehyde (4%) and stained in 0.2% crystal violet. After washing, cell colony numbers were counted.
2.7. RNA isolation and RT‐PCR
We isolated total RNA with the RNeasy Mini Kit (Qiagen), and then synthesized complementary DNA with a reverse transcription kit (Applied Biosystems). RT‐PCR was performed in triplicate using the StepOnePlus Real‐Time PCR System (Applied Biosystems). Human GAPDH (4352339E, Applied Biosystems,) was used as an internal control.
2.8. Enzyme‐linked immunosorbent assay
BAFF secreted in conditioned medium was detected by the BAFF Quantikine ELISA kit (R&D Systems) following the manufacturer's instructions. Absorbance (450 nm, correction wavelength at 540 nm) was read by a plate reader.
2.9. Immunofluorescence
We first cultured the cells in chamber slides (Matsunami, Lot No. 191029) for 48 h. We then took off the fend on the chamber slides, washed the cells in the bottom of the slides with PBS (4℃), and fixed those slides with 4% paraformaldehyde. After washing in PBS, slides were treated with Triton X‐100 (Kanto Chemical Co., Inc Lot No. 308T1683), washed again, and incubated with anti‐p65 primary antibody (Ab16502, Abcam) overnight at 4℃. Next day, we washed and incubated the slides in fluorophore‐conjugated second antibody (Life Technologies Japan Ltd., Lot No. 1726587), and covered them in ProLong Gold antifade reagent with DAPI (Invitrogen). Finally, the slides were observed under a fluorescence microscope (Keyence Corporation). The integrated density of immunofluorescence was quantified using ImageJ software and analyzed with GraphPad Prism 5 software (GraphPad Software).
2.10. Immunohistochemical staining
The specimens were selected from patients who had undergone hepatectomy after sorafenib treatment in our institute, and all patients provided written informed consent. This study was approved by the ethics committee of our hospital, and the written informed consent for the use of their resected tissues was obtained from all patients (Approval no. 2900‐2). Sliced specimens were deparaffinized and rehydrated, then blocked with endogenous peroxidase. The samples were incubated with anti‐BAFF (Invitrogen, Thermo Fisher Scientific Inc), anti‐BAFF‐R (#s‐32774, Santa Cruz Biotechnology), and anti‐p65 (Ab16502, Abcam) primary antibodies for 1 h at room temperature. Then the slides were incubated with EnVision Dual Link System‐HRP secondary antibody (Dako), treated with diaminobenzidine (Wako), and Mayer's hematoxylin (Muto Pure Chemicals Co. Ltd.). Lastly the samples were observed under a fluorescence microscope (Keyence Corporation).
2.11. Statistical analysis
The research data were analyzed using GraphPad Prism 5 software (GraphPad Software) to conduct one‐way ANOVA and Mann‐Whitney U tests. All data are presented as the mean ± SD. A P‐value < .05 was considered significant.
3. RESULTS
3.1. BAFF secretion was enhanced in Huh7SR cultured medium
After development, the Huh7SR cells were cultured in the conditioned medium containing 8.0 µM sorafenib. To investigate differences between Huh7SR‐co‐cultured CAFs and CAFs co‐cultured with parental Huh7 cells, we performed cytokine assays in cell‐cultured medium from both Huh7 cell lines. The result suggested that, regarding the other cytokines, BAFF was much higher in the Huh7SR conditioned medium (Figure 1A). Then we confirmed that Huh7SR cells expressed greater BAFF mRNA and a much higher concentration of BAFF in conditioned medium than did parental Huh7 cells (Figure 1B,C). Expression of BAFF in the sorafenib‐sensitive and SR specimens from patients who underwent hepatectomy after sorafenib treatment was detected by immunohistochemistry stain. The results showed that BAFF was markedly stronger in SR cases (Figure 1D,E).
FIGURE 1.
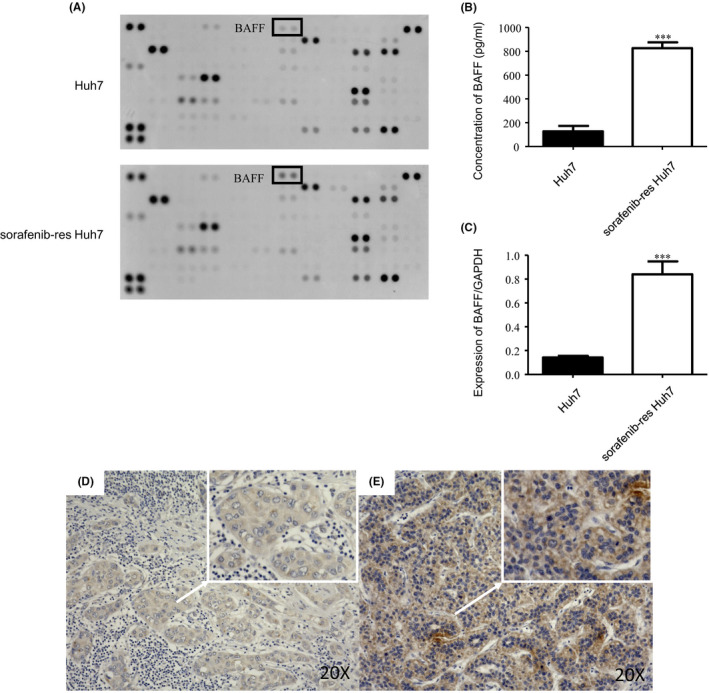
BAFF secretion is enhanced in the supernatant of sorafenib‐resistant Huh7. A, Cytokine assay of cell‐cultured medium in Huh7 and Huh7SR. B, ELISA test of BAFF secretion in cell‐cultured medium of Huh7 and Huh7SR (n = 3). C, PCR quantification of BAFF expressed in Huh7SR cells compared with Huh7 cells, expressed for 48 h cultured in DMEM (10% FBS) (n = 4). D, E, Immunohistochemical stain of BAFF in sorafenib‐sensitive and sorafenib‐resistant HCC cases. Low expression of BAFF in sensitive HCC samples (D). High expression of BAFF in resistant HCC samples (E). (***, P < .001.)
3.2. Huh7SR‐co‐cultured CAFs promoted invasion, migration, and drug resistance in CS Huh7 cells
As CAFs reportedly modulate drug sensitivity in the tumor microenvironment,2 we hypothesized that Huh7SR‐co‐cultured CAFs might affect chemosensitivity in HCC cells. In our study, Lx2 cells were cultured alone, or co‐cultured with parental Huh7 or Huh7SR cells to create 2 types of CAFs (Figure 2A). Then we checked CAF‐specific markers, such as α‐SMA and FAP in those groups. We found that α‐SMA and FAP expression levels were moderately higher in Huh7SR‐co‐cultured CAFs than in the CAFs co‐cultured with non‐resistant cells or Lx2 cells (Figure 2B,C). Next, we cultured parental Huh7 cells together with medium collected from Lx2 cells or the different types of CAFs, latterly performed transwell assay, wound‐healing assays and the colony‐forming assay (in medium with dose‐dependent sorafenib, at 0, 3 or 5 µM). In transwell assays, Huh7SR‐co‐cultured CAFs promoted invasiveness and migration capacity compared with CAFs treated with parental Huh7 culture medium (CM) (Figure 2D‐G), furthermore, Huh7SR‐co‐cultured CAFs enhanced drug resistance of HCCs in the colony‐forming assay (Figure 2H).
3.3. The BAFF‐R‐NFκB pathway was activated in Huh7SR‐co‐cultured CAFs
On the supposition of how BAFF expressed by Huh7SR cells influenced CAF‐driven malignancy, we checked a BAFF‐related pathway in CAFs. In Huh7SR‐co‐cultured CAFs, mRNA levels of BAFF and BAFF‐R were even higher than CAFs co‐cultured with the parental Huh7 subline or Lx2 cells (Figure 3A,B). One subset of CAFs reportedly expresses elevated levels of IL6 and IL8, and promotes chemoresistance and self‐renewal of cancer stem cells by those cytokines.3 As IL6 and IL8 are apparently involved in CAF‐driven chemoresistance,12, 13 we examined IL6 and IL8 mRNA expression in CAFs, and found that expression levels were higher in Huh7SR‐co‐cultured CAFs than in Huh7‐co‐cultured CAFs or Lx2 cells (Figure 3C,D). These results may be associated with the NFκB pathway involved in anti‐cancer drug resistance. Then we assessed the expression of nuclear NFκB p65, using immunofluorescence staining, and found that NFκB p65 nuclear expression in Huh7SR‐co‐cultured CAFs was notably stronger than in CAFs co‐cultured with the parental Huh7 cells (Figure 3E,F). Then we examined BAFF‐R and p65 expression in CAFs of SR and sorafenib‐sensitive HCC tissues, the results stated that, in SR samples, BAFF‐R was highly expressed on the cell membrane of CAFs in SR tissues, p65 level was also notably higher in the resistant cases (Figure 3G‐J). BAFF is reported to induce NFκB‐activated Nrf2 and its downstream molecule NQO1, which promotes survival and viability in chronic lymphocytic leukemia cells.9, 14 Therefore we checked gene expression of Nrf2 and downstream molecules, NQO1 and Heme oxygenase‐1 (HO‐1), in different types of CAFs, and found that Huh7SR‐co‐cultured CAFs expressed significantly higher levels of those genes (Figure 3L‐N), which suggested that BAFF/NFκB‐induced Nrf2 was activated in Huh7SR‐co‐cultured CAFs.
FIGURE 3.
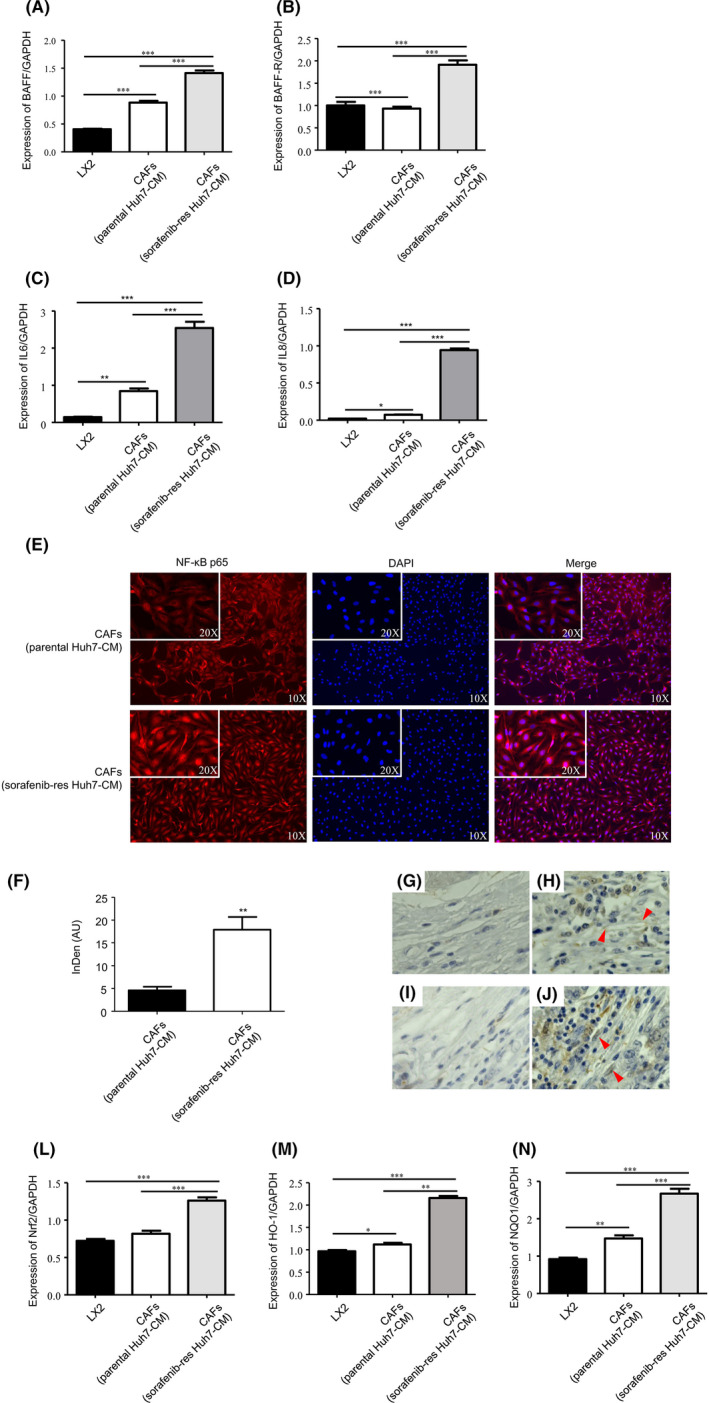
BAFF/NFκB was activated in Huh7SR‐co‐cultured CAFs. A‐D, PCR quantification of BAFF (A), BAFF‐R (B), IL6 (C) and IL8 (D) expressed in Lx2 cells, Huh7‐co‐cultured CAFs, or Huh7SR‐co‐cultured CAFs, expressed for 48 h cultured in DMEM (10% FBS) (n = 4). E, Immunofluorescence staining of NFκB p65 expression in Lx2 cells, Huh7‐co‐cultured CAFs, or Huh7SR‐co‐cultured CAFs. F, Integrated density of NFκB p65 in cell nucleus has been quantified. (AU, arbitrary units). G‐J, Immunohistochemical stain of BAFF‐R and p65 in HCC specimens. Low expression of BAFF‐R in sensitive HCC samples (G). High expression of BAFF‐R on cell membrane in resistant HCC samples (H, arrowheads: CAFs). Low expression of p65 in sensitive HCC samples (I). High expression of p65 in resistant HCC samples (J, arrowheads: CAFs). L‐N, PCR quantification of Nrf2 (L), HO‐1 (M) and NQO1 (N) expressed in Lx2 cells, Huh7‐co‐cultured CAFs or Huh7SR‐co‐cultured CAFs, expressed for 48 h cultured in DMEM (10% FBS). (n = 4) (*, P < .05; **, P < .01; ***, P < .001.)
3.4. Knocking down BAFF in Huh7SR cells reversed CAF stimulation
We wondered if BAFF in Huh7SR cells were responsible for the enhanced activity of Huh7SR‐co‐cultured CAFs, therefore siRNA was used to knock down BAFF expression in Huh7SR cells, which attenuated BAFF expression in these cells compared with control cells treated with nontargeting siRNA (Figure 4A). Then we co‐cultured Lx2 cells with conditioned medium from parental Huh7, Huh7SR, or BAFF‐knockdown Huh7SR cells, and compared them with non‐co‐cultured Lx2 cells. RT‐PCR showed that BAFF and BAFF‐R mRNA expression was decreased in CAFs from the BAFF‐knockdown cells (Figure 4B,C), and the formerly increased nuclear p65 expression was also reduced (Figure 4D,E). Nrf2, HO‐1, and NQO1 were also decreased in the BAFF‐knockdown‐Huh7‐co‐cultured CAFs (Figure 4F‐H). Upregulation of IL6 and IL8 expression was reversed as well (Figure 4I,J).
FIGURE 4.
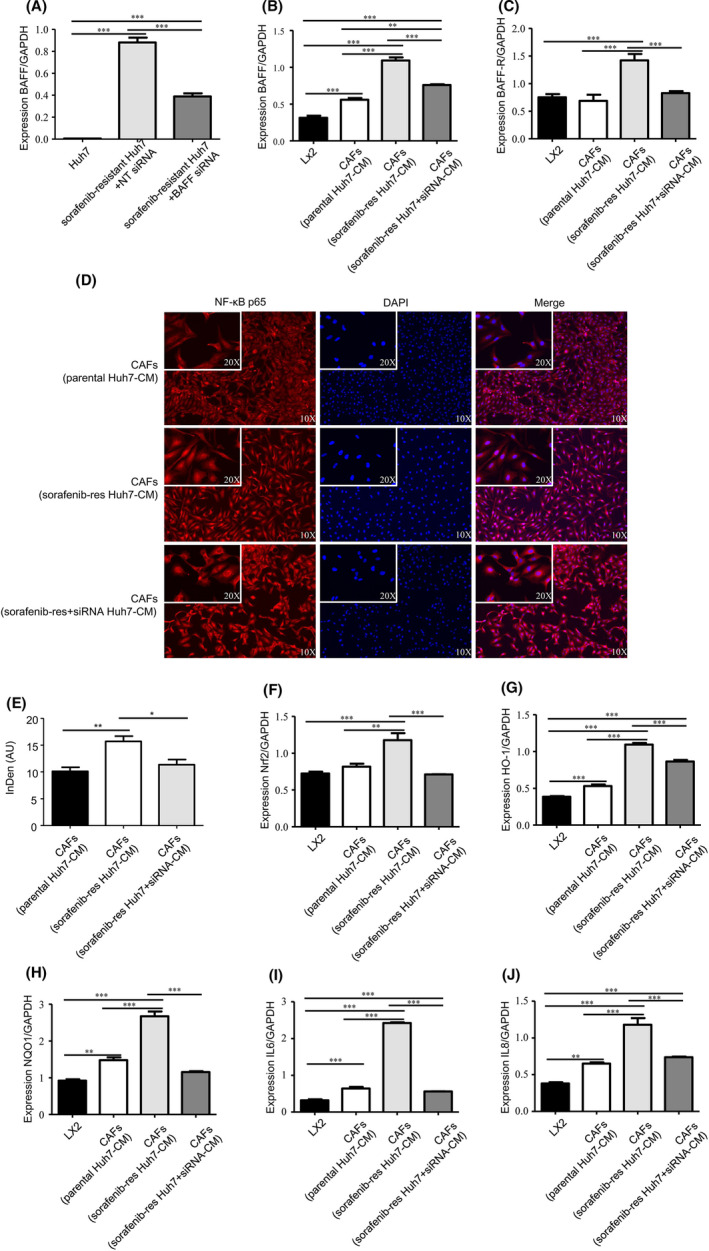
Knocking down BAFF expression in resistant Huh7 reversed the activated BAFF/NFκB axis in co‐cultured CAFs. A, PCR quantification of BAFF expression in Huh7, Huh7SR treated with nontargeting siRNA or BAFF siRNA, expressed for 48 h cultured in DMEM (10% FBS) (n = 4). B, C, PCR quantification of BAFF (B) and BAFF‐R (C) expressed in Lx2 cells, Huh7‐co‐cultured CAFs, Huh7SR‐co‐cultured CAFs, and BAFF siRNA‐treated Huh7SR‐co‐cultured CAFs, expressed for 48 h cultured in DMEM (10% FBS) (n = 4). D, Immunofluorescence staining of NFκB p65 expression in Lx2 cells, Huh7‐co‐cultured CAFs, Huh7SR‐co‐cultured CAFs, and BAFF siRNA‐treated Huh7SR co‐cultured CAFs, after co‐culturing for 48 h. E, Integrated density of NFκB p65 in cell nucleus quantified in each group. AU, arbitrary units. (*, P < .05; **, P < .01) F‐J, The mRNA expression of Nrf2 (F), HO‐1 (G), NQO1 (H), IL6 (I) and IL8 (J) were quantified by PCR in Lx2 cells, Huh7‐co‐cultured CAFs, Huh7SR‐co‐cultured CAFs, and BAFF siRNA‐treated Huh7SR‐co‐cultured CAFs, expressed for 48 h cultured in DMEM (10% FBS). (n = 4) (**, P < .01; ***, P < .001.)
Lastly, we co‐cultured Huh7 with different kinds of CAFs. The enhanced chemoresistance also partly returned in Huh7 which was co‐cultured with BAFF‐knockdown‐Huh7SR‐co‐cultured CAFs, compared with Huh7 co‐cultured with Huh7SR‐co‐cultured CAFs (Figure 5A). In transwell assays, the Huh7 cells co‐cultured with BAFF‐knockdown‐Huh7SR‐co‐cultured CAFs were less invasive than ones co‐cultured with Huh7SR‐co‐cultured CAFs (Figure 5B,C). In the wound‐healing assay, the increased migration ability in Huh7 cells induced by Huh7SR‐co‐cultured CAFs was reversed by knocking down BAFF expression in resistant cells (Figure 5D,E). These results suggested that blocking BAFF in Huh7SR cells reduced the migration and invasion promotion ability and the specific gene expression of co‐cultured CAFs.
FIGURE 5.
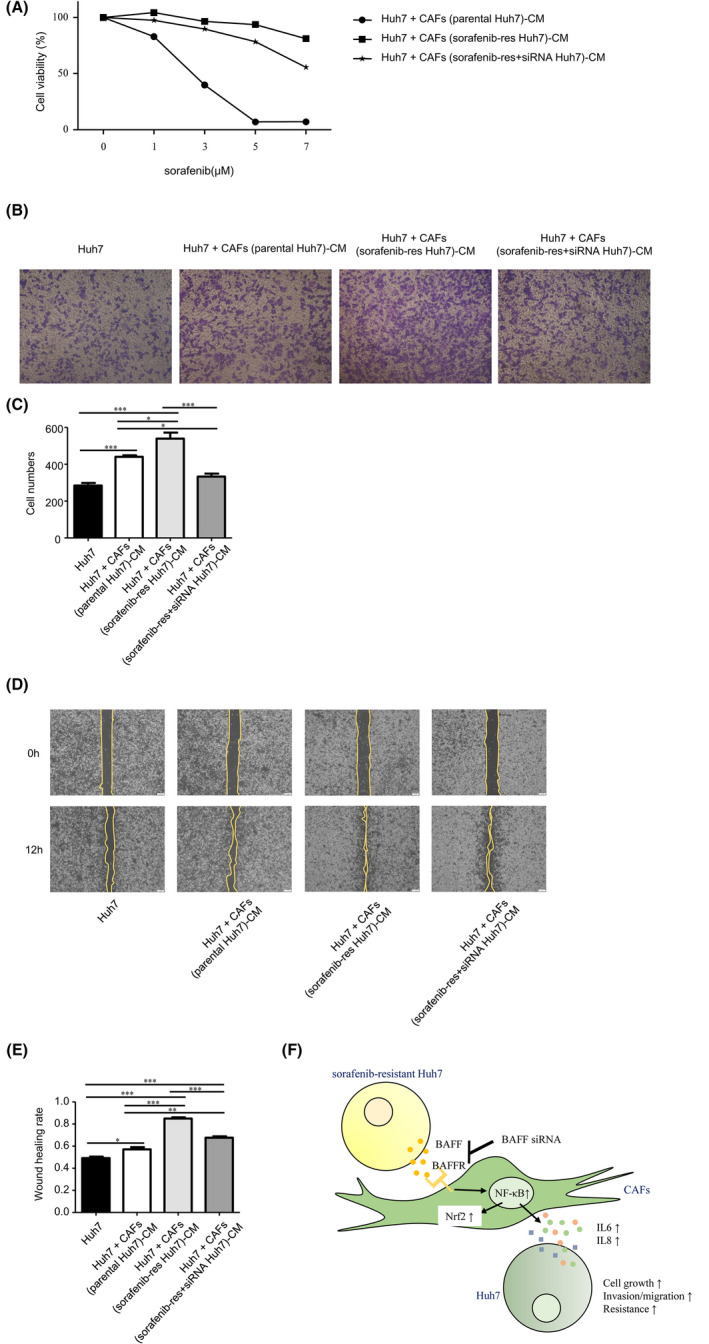
BAFF knocked down Huh7SR‐co‐cultured CAFs caused less malignancy and chemoresistance in Huh7. A, Cell viabilities of Huh7 co‐cultured with cell‐cultured medium of Huh7‐co‐cultured CAFs, Huh7SR‐co‐cultured CAFs, and BAFF siRNA‐treated resistant Huh7‐co‐cultured CAFs were checked by CCK8, incubated in DMEM (10% FBS) with increasing dose of sorafenib. Data analysis was performed using GraphPad Prism 5 software. B, C, Invasion ability (B) of Huh7, and Huh7 treated with the CM of Huh7‐co‐cultured CAFs, Huh7SR‐co‐cultured CAFs, and BAFF siRNA‐treated Huh7SR‐co‐cultured CAFs was detected by transwell assay. Data analysis (C) was performed using GraphPad Prism 5 software (n = 4). D, E, Migration rate (D) of above co‐cultured Huh7 and Huh7 only was detected by wound‐healing assay. ImageJ quantification (E) of migration rate (n = 3). F, Summary of BAFF/NFκB axis in the interaction between HCC cells with CAFs. (*, P < .05; **, P < .01; ***, P < .001.)
4. DISCUSSION
Sorafenib is an oral multi‐kinase inhibitor and is a common systemic therapy for HCC. It decreases tumor initiation and progression by controlling cell proliferation and angiogenesis.15, 16 Unfortunately, its use is limited by a low sensitivity rate. In our study, we developed an SR HCC cell line, Huh7SR, and found that Huh7SR cells not only gained greater invasiveness and cell growth ability, but also expressed more BAFF than Huh7 cells. Furthermore, we found that CAFs co‐cultured with resistant cells obtained stronger promotion ability with regards to invasiveness, migration, and chemoresistance in HCC cells compared with CAFs co‐cultured with parental cells, by the BAFF and its receptor BAFF‐R axes.
As a family member of the tumor necrosis cytokines, BAFF binds to 3 well‐known receptors expressed on B cells: B‐cell maturation antigen (BCMA), transmembrane activator and cyclophilin ligand interactor (TACI), and BAFF‐R.17, 18 In engineered cells, BAFF binding to these receptors affects diverse signaling pathways, including NFκB nuclear translocation, p38 mitogen‐activated kinase activation, and JNK phosphorylation for receptors.19, 20 Although BAFF is commonly associated with malignant B lymphocyte proliferation and differentiation,21, 22, 23 it is also expressed in an array of normal and cancer cell lines and specimens.24, 25, 26, 27 In liver cancer, BAFF and its receptors BCMA and BAFF‐R are reported to be expressed by HCC cell lines Hep3B, HepG2, and HCC tissues.28 In our study, the protein and mRNA expression of BAFF in Huh7SR cells were further upregulated compared with non‐resistant cells. Therefore, we investigated whether the enhanced BAFF/BAFF‐R axis in Huh7SR cells affected CAF characteristics.
In the HCC microenvironment, hepatic stellate cells are regarded as the majority; they are activated by tumor cells to become CAFs, and express various cytokines to tumor progression and invasiveness in HCC.29, 30, 31, 32 CAFs also create the extracellular matrix structure, mediate tumor microenvironment metabolism, and contribute to chemotherapy resistance.33, 34 High IL6 expression in the tumor microenvironment can also promote cancer progression and chemoresistance,35 but chemoresistance derived from CAFs has rarely been studied. In this context, we co‐cultured the Huh7SR cells and parental Huh7 cells with Lx2 cells to develop 2 types of CAFs. These Huh7SR‐co‐cultured CAFs expressed high levels of CAF‐specific markers. Furthermore, Huh7SR cells could induce CAFs highly tumor‐promoting properties through the BAFF/BAFF‐R axis.
The BAFF/BAFF‐R axis has been observed to modestly activate canonical NFκB signaling, leading to rapid phosphorylation and nuclear translocation of NFκB p65,36, 37 and also enhanced expression of Nrf2 and its related genes, such as NQO1.8 We noticed that in Huh7SR‐co‐cultured CAFs, more BAFF‐R was activated than usual, leading to upregulated the nuclear location of NFκB p65 in Huh7SR‐co‐cultured CAFs. The activated NFκB promoted IL6 and IL8 expression in Huh7SR‐co‐cultured CAFs and led to elevated tumor malignancy promotion ability of CAFs; Nrf2 and its downstream molecules, NQO1 and HO‐1, were similarly increased. High IL6 and IL8 expression are reportedly related to chemoresistance in cancers.5, 10, 12 When we treated Huh7 cells with CM from Huh7SR‐co‐cultured CAFs, the Huh7 cells developed greater invasiveness, and sorafenib resistance compared with Huh7 treated with the CM of Huh7‐co‐cultured CAFs or the Lx2 cells. However, when we knocked down BAFF in sorafenib‐resistant cells, the previous effects in Huh7SR‐co‐cultured CAFs, the increased BAFF/NFκB activation and the upregulation of Nrf2, HO‐1, IL6, and IL8, were all reversed. The enhanced chemoresistance in Huh7 cells co‐cultured with Huh7SR‐co‐cultured CAFs also partly attenuated.
BAFF inhibitors are now widely used in clinical treatment. Belimumab, a kind of BAFF inhibitor approved by the FDA, is mainly used in rheumatologic disease, and some anti‐BAFF antibodies as well as anti‐BAFF‐R antibodies are used for leukemia.38, 39, 40 These inhibitors are potential agents against chemoresistance in nonhematologic solid tumors, such as HCC. Although this study only used in vitro experiments and tested resistant HCC cells, in the future we will develop animal models for drug‐resistant HCC to investigate the role of BAFF in the tumor microenvironment in vivo.
Together, the current and previous findings support our hypothesis (Figure 5F). In the Huh7 cells, when resistance to sorafenib was strengthened, BAFF was also elevated, stimulating the CAFs to overexpress a BAFF receptor, BAFF‐R, and to activate the NFκB pathway including more p65 located in the nucleus. This resulted in the upregulation of Nrf2 and its related genes, and promoted IL6 and IL8 expression by CAFs. This cascade could induce drug resistance in non‐resistant HCC cells when treated by CM from HCCSR‐co‐cultured CAFs.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
PATIENT CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTERESTS
We state the potential conflicts of interest with regard to our study as follows: Mitsuo Shimada received grant support from Bayer Yakuhin, Co. Ltd., Japan. Other authors have no conflict of interest.
ACKNOWLEDGMENTS
This study was partly supported by Research Program on Hepatitis from Japanese Foundation for Multidisciplinary Treatment of Cancer, the Japan Agency for Medical Research and Development (AMED) Grant Numbers JP19fk0210048 and JP20fk0210048, and Grant‐in‐Aid for Scientific Research (Grant no. 20K08957 to Yuji Morine).
Gao L, Morine Y, Yamada S, et al. The BAFF/NFκB axis is crucial to interactions between sorafenib‐resistant HCC cells and cancer‐associated fibroblasts. Cancer Sci. 2021;112:3545–3554. 10.1111/cas.15041
REFERENCES
- 1.Öhlund D, Handly‐Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeulen L, Felipe De Sousa EM, Van Der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12(5):468‐476. [DOI] [PubMed] [Google Scholar]
- 3.Su S, Chen J, Yao H, et al. CD10+ GPR77+ cancer‐associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172(4):841‐856. [DOI] [PubMed] [Google Scholar]
- 4.Hengeveld PJ, Kersten MJ. B‐cell activating factor in the pathophysiology of multiple myeloma: a target for therapy? Blood Cancer J. 2015;5:e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawasaki K, Abe M, Tada F, et al. Blockade of B‐cell‐activating factor signaling enhances hepatic steatosis induced by a high‐fat diet and improves insulin sensitivity. Lab Invest. 2013;93(3):311‐321. [DOI] [PubMed] [Google Scholar]
- 6.Chou CH, Ho CM, Lai SL, et al. B‐cell activating factor enhances hepatocyte‐driven angiogenesis via B‐cell CLL/lymphoma 10/nuclear factor‐kappaB signaling during liver regeneration. Int J Mol Sci. 2019;20(20):5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McWilliams EM, Lucas CR, Chen T, et al. Anti–BAFF‐R antibody VAY‐736 demonstrates promising preclinical activity in CLL and enhances effectiveness of ibrutinib. Blood Adv. 2019;3(3):447‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo T, Nishio M, Enzler T, et al. BAFF and APRIL support chronic lymphocytic leukemia B‐cell survival through activation of the canonical NF‐κB pathway. Blood. 2007;109(2):703‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez‐Lopez E, Ghia EM, Antonucci L, et al. NF‐κB‐p62‐NRF2 survival signaling is associated with high ROR1 expression in chronic lymphocytic leukemia. Cell Death Differ. 2020;27(7):2206‐2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo S‐H, Yeh P‐Y, Chen L‐T, et al. Overexpression of B cell–activating factor of TNF family (BAFF) is associated with Helicobacter pylori–independent growth of gastric diffuse large B‐cell lymphoma with histologic evidence of MALT lymphoma. Blood. 2008;112(7):2927‐2934. [DOI] [PubMed] [Google Scholar]
- 11.Zou B, Liu X, Gong Y, et al. A novel 12‐marker panel of cancer‐associated fibroblasts involved in progression of hepatocellular carcinoma. Cancer Manag Res. 2018;10:5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niu L‐L, Cheng C‐L, Li M‐Y, et al. ID1‐induced p16/IL6 axis activation contributes to the resistant of hepatocellular carcinoma cells to sorafenib. Cell Death Dis. 2018;9(9):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zender L, Rudolph KL. Keeping your senescent cells under control. Aging. 2009;1(5):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo SH, Tsai HJ, Lin CW, et al. The B‐cell‐activating factor signalling pathway is associated with Helicobacter pylori independence in gastric mucosa‐associated lymphoid tissue lymphoma without t (11; 18)(q21; q21). J Pathol. 2017;241(3):420‐433. [DOI] [PubMed] [Google Scholar]
- 15.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48(6):2047‐2063. [DOI] [PubMed] [Google Scholar]
- 16.Niu L, Liu L, Yang S, Ren J, Lai PB, Chen GG. New insights into sorafenib resistance in hepatocellular carcinoma: responsible mechanisms and promising strategies. Biochim Biophys Acta Rev Cancer. 2017;1868(2):564‐570. [DOI] [PubMed] [Google Scholar]
- 17.Lied GA, Berstad A. Functional and clinical aspects of the B‐cell‐activating factor (BAFF): a narrative review. Scand J Immunol. 2011;73(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 18.Khlaiphuengsin A, Chuaypen N, Sodsai P, et al. Decreased of BAFF‐R expression and B cells maturation in patients with hepatitis B virus‐related hepatocellular carcinoma. World J Gastroenterol. 2020;26(20):2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G, Boone T, Delaney J, et al. APRIL and TALL‐1 and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1(3):252‐256. [DOI] [PubMed] [Google Scholar]
- 20.Hatzoglou A, Roussel J, Bourgeade M‐F, et al. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor‐associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF‐κB, elk‐1, c‐Jun N‐terminal kinase, and p38 mitogen‐activated protein kinase. J Immunol. 2000;165(3):1322‐1330. [DOI] [PubMed] [Google Scholar]
- 21.Benson MJ, Elgueta R, Noelle RJ. B cell survival: an unexpected mechanism of lymphocyte vitality. Immunol Cell Biol. 2008;86(6):485‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B‐lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100(8):2973‐2979. [DOI] [PubMed] [Google Scholar]
- 23.Novak AJ, Darce JR, Arendt BK, et al. Expression of BCMA, TACI, and BAFF‐R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103(2):689‐694. [DOI] [PubMed] [Google Scholar]
- 24.Sun B, Wang H, Wang X, et al. A proliferation‐inducing ligand: a new biomarker for non‐small cell lung cancer. Exp Lung Res. 2009;35(6):486‐500. [DOI] [PubMed] [Google Scholar]
- 25.Pelekanou V, Notas G, Theodoropoulou K, et al. Detection of the TNFSF members BAFF, APRIL, TWEAK and their receptors in normal kidney and renal cell carcinomas. Anal Cell Pathol. 2011;34(1, 2):49‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langat DL, Wheaton DA, Platt JS, Sifers T, Hunt JS. Signaling pathways for B cell‐activating factor (BAFF) and a proliferation‐inducing ligand (APRIL) in human placenta. Am J Pathol. 2008;172(5):1303‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelekanou V, Kampa M, Kafousi M, et al. Expression of TNF‐superfamily members BAFF and APRIL in breast cancer: immunohistochemical study in 52 invasive ductal breast carcinomas. BMC Cancer. 2008;8(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Notas G, Alexaki VI, Kampa M, et al. APRIL binding to BCMA activates a JNK2–FOXO3–GADD45 pathway and induces a G2/M cell growth arrest in liver cells. J Immunol. 2012;189(10):4748‐4758. [DOI] [PubMed] [Google Scholar]
- 29.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Zijl F, Mair M, Csiszar A, et al. Hepatic tumor–stroma crosstalk guides epithelial to mesenchymal transition at the tumor edge. Oncogene. 2009;28(45):4022‐4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang N, Gores GJ, Shah VH. Hepatic stellate cells: partners in crime for liver metastases? Hepatology. 2011;54(2):707‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R, Gao X, Zuo J, et al. STMN1 upregulation mediates hepatocellular carcinoma and hepatic stellate cell crosstalk to aggravate cancer by triggering the MET pathway. Cancer Sci. 2020;111(2):406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner EF. Fibroblasts for all seasons. Nature. 2016;530(7588):42‐43. [DOI] [PubMed] [Google Scholar]
- 34.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Shen J, Lu K. IL‐6 and PD‐L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun. 2017;486(2):239‐244. [DOI] [PubMed] [Google Scholar]
- 36.Zheng N, Wang D, Ming H, Zhang H, Yu X. BAFF promotes proliferation of human mesangial cells through interaction with BAFF‐R. BMC Nephrol. 2015;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19(3–4):263‐276. [DOI] [PubMed] [Google Scholar]
- 38.Wu W, Li S, Zhang W, Sun J, Ren G, Dong Q. A novel VHH antibody targeting the B cell‐activating factor for B‐cell lymphoma. Int J Mol Sci. 2014;15(6):9481‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rihacek M, Bienertova‐Vasku J, Valik D, Sterba J, Pilatova K, Zdrazilova‐Dubska L. B‐cell activating factor as a cancer biomarker and its implications in cancer‐related cachexia. Biomed Res Int. 2015;2015:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parameswaran R, Lim M, Fei F, et al. Effector‐mediated eradication of precursor B acute lymphoblastic leukemia with a novel Fc‐engineered monoclonal antibody targeting the BAFF‐R. Mol Cancer Ther. 2014;13(6):1567‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]


