Abstract
Objectives:
To assess the prevalence, changes in and prognostic importance of B-lines, a pulmonary congestion measure, using a simplified lung ultrasound (LUS) method in acute heart failure (AHF).
Background:
Pulmonary congestion is an important finding in AHF, however, traditional methods for its detection are insensitive.
Methods:
In a two-site, prospective, observational study 4-zone LUS was performed early during hospitalization for AHF (LUS1) and at discharge (LUS2). B-lines were quantified offline, blinded to clinical findings and outcomes by a core laboratory.
Results:
Among 349 patients (median age 75, 59% men, mean EF 39%) the sum of B-lines in 4 zones ranged from 0 to 18 (LUS1). The risk of an adverse in-hospital event increased with rising B-line number on LUS1: odds ratio for each B-line tertile 1.82 (95% CI 1.14–2.88, P=0.011). B-line count decreased from a median of 6 (LUS1) to 4 (LUS2; P<0.001) over 6 days (median). In 132 patients with LUS2 images, the risk of HF hospitalization or all-cause death was greater in patients with a higher B-line number at discharge. This relationship was stronger closer to discharge: unadjusted HR 60 days: 3.30, 95% CI 1.52–7.17, P=0.002; 90 days: 2.94, 1.46–5.93, P=0.003; 180 days: 2.01, 1.11–3.64, P=0.021. The association between B-line number and short and long-term outcomes persisted after adjusting for important clinical variables, including NT-proBNP.
Conclusions:
Pulmonary congestion using a simplified 4-zone LUS method was common in AHF and improved with therapy. A higher B-line number at baseline and discharge identified patients at increased risk for adverse events.
Keywords: Acute heart failure, pulmonary congestion, lung ultrasound, prognosis
Introduction
Pulmonary congestion in acute heart failure (AHF) is both a common and important finding. However, current methods for its detection, such as auscultation and chest x-ray (CXR), are insensitive.1 Lung ultrasound (LUS) has been gaining increasing attention over the past decade as a non-invasive tool in the detection and quantification of pulmonary congestion in both ambulatory and hospitalized patients with heart failure (HF).2 LUS could potentially play an important role in the monitoring of pulmonary congestion during an AHF hospitalization and improve risk assessment. Specifically, LUS could aid clinicians in tailoring HF diuretic therapy during and following a hospitalization, and potentially improve discharge timing. Currently, adjustment of inpatient diuretic therapy and timing of discharge are based on symptom improvement, physical examination findings, urine output and weight loss. Detection of clinical signs of congestion is dependent on physician examination skills and highly variable, and urine volumes and weights may not be measured reliably. Consequently, patients can be under- or over-diuresed at the time of discharge, with potentially increased morbidity post-discharge.3–5 LUS might offer a more reliable method of assessing pulmonary congestion in patients with AHF. However, comprehensive data on the prevalence, dynamic changes and prognostic importance of pulmonary congestion on LUS in AHF are sparse. Prior studies have predominately used time-intensive 28-zone imaging protocols and lacked central, off-line ultrasound image analysis.2, 6–8
We therefore sought to assess the prevalence and prognostic importance of pulmonary congestion detected with a simplified 4-zone LUS method on both short- and long-term adverse events. A secondary aim was to examine the dynamic changes of B-lines with treatment for AHF.
Methods
Patient population
This was a prospective, pre-planned, two-center, observational study in adults hospitalized for AHF (HF signs and symptoms, and requiring intravenous diuretics), irrespective of left ventricular ejection fraction (EF). Main exclusion criteria were: Important lung disease potentially impacting LUS findings (e.g. pulmonary fibrosis, pneumonia), dialysis, isolated right HF, pregnancy, BNP<100 pg/ml in Glasgow, NT-proBNP <1,400 pg/ml in Boston if known or suspected HFpEF (see Supplements). Patients were recruited from inpatient units (both sites) and Emergency Department (ED) Observation Units (Boston) of two academic hospitals between January 2013 and November 2014 in Glasgow, and April 2015 and August 2017 in Boston. Details of the Glasgow study protocol have been previously published.9 After obtaining informed consent an investigator not involved in the patient’s clinical care performed the first LUS (LUS1) at the time of echocardiography early during the hospitalization (median number of days since admission: Glasgow 1 day [IQR 1, 2], Boston 1 day [IQR 1, 1]. A second LUS (LUS2) was performed prior to hospital discharge. In cases of unexpected delays in discharge, a 3rd LUS was performed (n=14), if patients agreed. The LUS closest to hospital discharge was used as LUS2 for the long-term outcome analysis (median number of days between pre-discharge LUS and hospital discharge: Glasgow 0 days [IQR 0, 1], Boston 0 days [IQR 0, 1]. Due to logistical reasons (investigator and ultrasound machine availability), the LUS2 was not feasible in Glasgow, and LUS2 data collection was stopped at this site. Clinical providers were blinded to LUS findings at both sites. This study complies with the Declaration of Helsinki and was approved by the local institutional review committees. Written informed consent was obtained from all participants.
Lung ultrasound protocol and image analysis
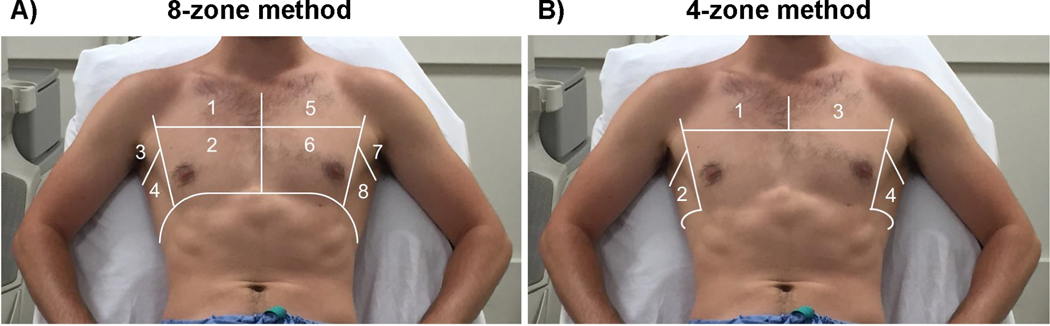
LUS examinations were performed by trained investigators employing a standardized imaging protocol using regular echocardiographic equipment with a phased-array transducer in sagittal orientation at an imaging depth of 18 cm with patients in semi-recumbent position. Patients were assessed with a simplified 4-zone imaging protocol (2 zones on each hemithorax: Figure 1B; 6 seconds per clip), in addition to examination of pleural effusions laterally at the level of the diaphragm. In Boston, the expanded and previously recommended 8-zone protocol (4 zones on each hemithorax: Figure 1A) was employed for validation purposes.10 Offline image analysis was performed on de-identified videos centrally at a core imaging laboratory in Boston by two investigators (EP, JP) with experience in LUS analysis. Readers were blinded to clinical data, timing of LUS, short- and long-term outcomes. The highest number of B-lines visualized in one intercostal space was quantified in a freeze frame after review of the entire clip for each zone and the sum of B-lines across 4 zones was used for the primary analysis. This count-based approach to B-line quantification has been employed in several prior LUS studies in HF cohorts.8, 11–13 Inter- and intra-reader agreement and B-line imputation are described in the Supplements.
Figure 1. Overview of 8 and 4-zone lung ultrasound technique.
“A) 8-zone and B) 4-zone lung ultrasound method.
Adapted from: Platz E et al. Eur J Heart Fail. 2017;19(9):1154-1163.
Clinical and demographic data
Clinical and demographic data were abstracted from medical records by trained investigators (RTC, AAM, MS, VS) at each site. Dyspnea assessment is described in the Supplements. Left ventricular EF was measured using Simpson’s biplane method by one experienced investigator at each site (EP, RTC) according to current guidelines.14
Outcomes
In-hospital outcomes:
For in-hospital events, the primary endpoint was a composite of first occurrence of all-cause death, intensive care unit (ICU) admission for worsening HF or cardiac arrest, need for intravenous inotropes (other than digoxin) or left ventricular assist device placement during baseline admission. Events were adjudicated by review of the electronic medical records by investigators at each site, blinded to LUS data.
Long-term outcomes:
Patients were followed for 6 months and time-to-first-event was used for all outcomes. The primary endpoint was a composite of HF readmission or all-cause death. All HF hospitalizations were adjudicated by two experienced physicians at each site (EFL, JDG, KFD, MMYL) using pre-specified criteria for HF events (see Supplements). HF hospitalizations were confirmed through patient follow-up phone calls, contacting primary care physicians/cardiologists and review of electronic medical records. All-cause mortality was confirmed through review of medical records, social security index and obituaries in Boston, and through electronic medical records in Glasgow.
Statistical analyses
For the in-hospital event analysis, we divided patients into three groups on the basis of the sum of baseline B-lines (LUS1) in tertiles in 4 zones: Tertile 1: 0–4 B-lines, Tertile 2: 5–9 B-lines, Tertile 3: ≥10 B-lines. Continuous variables are presented as medians (interquartile range, IQR) or means (standard deviation, SD) and categorical variables as counts and percentages. We assessed trends in baseline characteristics across B-line tertiles with modified Wilcoxon rank-sum tests and regression analysis, as appropriate. Multivariable regression analysis with stepwise forward selection was used to examine the relationship between B-lines and baseline characteristics.
Logistic regression models (unadjusted and adjusted) were used to assess the continuous association between B-line tertile and in-hospital events. Models were adjusted for potential confounding variables, including age, sex, study site, baseline systolic blood pressure, baseline log-transformed creatinine and baseline log-transformed NT-proBNP (based on risk models from ASCEND-HF and PROTECT).15, 16 These covariates were chosen based on their clinical importance in relation to the outcome, using a limited number of variables to prevent overfitting. The direction and significance of the results remained stable when adjusting for EF and body mass index (BMI) (Table S6) and when B-line number was treated as count variable (instead of tertiles).
For the long-term event analysis, we divided patients into three groups on the basis of the sum of pre-discharge B-lines (LUS2) in tertiles in all 4 zones: Tertile 1: 0–3 B-lines, Tertile 2: 4–6 B-lines, Tertile 3: ≥7 B-lines. Patients with >3 days between LUS2 and hospital discharge were excluded from this analysis (n=8). The changes in congestion markers between admission and discharge were assessed with paired t-tests, sign rank test or McNemar’s test as appropriate. Cox proportional hazard models were used to assess the association between B-line number by tertile and event-free survival. Results of these analyses were considered exploratory to inform future prospective analyses. Models were adjusted for potential confounding variables, including age, sex, study site, baseline systolic blood pressure, baseline log-transformed creatinine, baseline EF and baseline log-transformed NT-proBNP. 15, 16 Harrell’s C-statistic was calculated for each model. All models were checked for interaction between B-line tertile and each covariate. The assumption of proportionality of hazards was tested by allowing a time-varying coefficient for the primary exposure variable (B-line tertile). The same models were used to compare previously published 8-zone B-line quantification methods (count and score based) with the simplified 4-zone method with respect to prediction of long-term outcomes. In addition, Spearman correlation was used to examine the association between B-line number using the 8-zone vs. the 4-zone method.
In order to examine the average time lost due to HF hospitalization or death for each of the three B-line groups we performed similar unadjusted and adjusted analyses using restricted mean time lost (RMTL), where 180 days, 90 days and 60 days were used as truncation time. In contrast to hazard ratios, this analysis method allows for an estimation of the risk in all three groups, including Tertile 1.17 The incremental value of B-lines beyond a model using crackles on auscultation was assessed using area under the receiver operating characteristic curve (AUC) and continuous NRI for 90-day HF hospitalization and all-cause death. The sample size justification is described in the Supplements. Two-sided significance levels of 0.05 were used for all analyses. Data were analyzed using STATA SE 14.2 (StataCorp, Texas) and R version 3.5.1 (continuous NRI).
Results
Baseline characteristics
Among all 370 eligible patients, 360 had a LUS1 performed (349 with interpretable images, 97%), and 138 patients had a LUS2 performed within 72 hours of hospital discharge (132 with interpretable images, 96%) (Figure S1). Baseline characteristics for this cohort, stratified by tertiles of B-line number (LUS1), are presented in Table 1.
Table 1.
Patient characteristics by baseline B-line tertiles (n=349)
| 0–4 B-lines (n=121) | 5–9 B-lines (n=131) | ≥10 B-lines (n=97) | P (trend) | |
|---|---|---|---|---|
| B-line count, 4 zones | 2 [1, 3] | 7 [6, 8] | 12 [10, 13] | - |
| Age, years | 71 [62, 79] | 75 [68, 83] | 76 [69, 82] | 0.013 |
| Men, n (%) | 70 (58) | 77 (59) | 58 (60) | 0.77 |
| Hispanic, n (%) | 5 (4) | 4 (3) | 2 (2) | 0.38 |
| Non-Hispanic White, n (%) | 100 (83) | 116 (89) | 87 (90) | 0.12 |
| Non-Hispanic Black, n (%) | 14 (12) | 12 (9) | 6 (6) | 0.17 |
| BMI, kg/m2 | 29 [24, 36] | 27 [24, 30] | 26 [23, 30] | 0.001 |
| NYHA class, n (%) | 0.012 | |||
| I and II | 40 (33) | 43 (33) | 20 (21) | |
| III | 65 (54) | 63 (48) | 52 (54) | |
| IV | 16 (13) | 25 (19) | 25 (26) | |
| Medical history, n (%) | ||||
| Prior HF | 80 (66) | 82 (63) | 56 (58) | 0.21 |
| Prior HF hospitalization | 55 (46) | 60 (46) | 37 (38) | 0.30 |
| Hypertension | 95 (79) | 104 (79) | 71 (73) | 0.38 |
| Diabetes | 40 (33) | 52 (40) | 39 (40) | 0.26 |
| Atrial fibrillation/flutter | 62 (51) | 74 (57) | 56 (58) | 0.33 |
| COPD | 25 (21) | 26 (20) | 18 (19) | 0.70 |
| PCI | 27 (22) | 26 (20) | 19 (20) | 0.61 |
| CABG | 22 (18) | 35 (27) | 23 (24) | 0.29 |
| Myocardial infarction | 42 (35) | 46 (35) | 37 (38) | 0.61 |
| CRT | 9 (7) | 10 (8) | 6 (6) | 0.74 |
| Depression | 25 (21) | 17 (13) | 10 (10) | 0.032 |
| Home medications, n (%) | ||||
| β-Blocker | 91 (75) | 95 (73) | 65 (67) | 0.19 |
| ACE-I/ARB | 58 (48) | 62 (47) | 50 (52) | 0.62 |
| ARNI | 0 | 2 (2) | 2 (2) | 0.15 |
| Spironolactone | 18 (15) | 18 (14) | 8 (8) | 0.15 |
| Diuretic(s) | 83 (69) | 88 (67) | 55 (57) | 0.08 |
| Digoxin | 6 (5) | 16 (12) | 9 (9) | 0.22 |
| Calcium channel blocker | 28 (23) | 26 (20) | 24 (25) | 0.82 |
| Amiodarone | 7 (6) | 12 (9) | 8 (8) | 0.47 |
| Insulin | 18 (15) | 25 (19) | 20 (21) | 0.26 |
| Anticoagulation | 55 (46) | 59 (45) | 30 (31) | 0.037 |
| Baseline laboratory results | ||||
| Sodium, mmol/l | 138 (4) | 137 (5) | 137 (5) | 0.08 |
| Potassium, mmol/l | 4.2 (0.6) | 4.2 (0.6) | 4.3 (0.6) | 0.75 |
| Hemoglobin, g/dl | 12.1 (2.2) | 11.6 (2.2) | 11.6 (1.9) | 0.045 |
| BUN, mg/dl | 22 [17, 45] | 26 [20, 40] | 25 [18, 41] | 0.25 |
| Creatinine, mg/dl | 1.2 [0.9, 1.9] | 1.2 [1.0, 1.8] | 1.3 [0.9, 1.9] | 0.62 |
| Albumin, g/dl | 3.6 (0.5) | 3.5 (0.4) | 3.3 (0.5) | <0.001 |
| Troponin T, ng/ml (n=167)* | 0 [0, 0.06] | 0.03 [0, 0.09] | 0.03 [0, 0.07] | 0.044 |
| Troponin I, μg/l (n=152)† | 0.04 [0.04, 0.11] | 0.05 [0.04, 0.14] | 0.04 [0.04, 0.14] | 0.73 |
| 0–4 B-lines (n=121) | 5–9 B-lines (n=131) | ≥10 B-lines (n=97) | P (trend) | |
| NT-proBNP, pg/ml | 2610 [1319, 5315] | 3965 [2143, 10163] | 5552 [2796, 10225] | <0.001 |
| Admission CXR ‡ | ||||
| Vascular congestion, n (%) | 76 (70) | 105 (83) | 80 (87) | 0.002 |
| Interstitial edema, n (%) | 56 (51) | 79 (63) | 64 (69) | 0.011 |
| Alveolar edema, n (%) | 12 (11) | 28 (22) | 35 (38) | <0.001 |
| Echocardiography | ||||
| EF, % | 40 (16) | 39 (15) | 38 (15) | 0.23 |
| HFrEF (EF<40%), n (%) | 61 (50) | 71 (54) | 55 (57) | 0.35 |
Undetectable values: 0.0
Undetectable values: 0.039
Often not performed on same day as LUS (median −1 day, range +/−4 days)
ACE-I: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor neprilysin inhibitor; BMI: body mass index; BUN: blood urea nitrogen; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; PCI: percutaneous coronary intervention; CRT: cardiac resynchronization therapy; CXR: chest x-ray; EF: ejection fraction; HF: heart failure; HFrEF: HF with reduced EF; NYHA: New York Heart Association.
The median age of all 349 patients was 75 years (range 21–102), 59% were men, 87% White, mean EF 39% (±16). The sum of B-lines in 4 zones ranged from 0 to 18 (median 6, IQR 3, 10). Overall, 123 patients (35%) had no crackles on auscultation on the day of LUS1 and 39 patients (11%) demonstrated no radiologic evidence of pulmonary congestion (vascular congestion, interstitial or alveolar edema) on admission, whereas only 21 (6%) had no B-lines on LUS1, although LUS was performed up to 4 days after admission. Patients with a higher number of B-lines were more likely to be older, have a lower body mass index (BMI) and higher New York Heart Association (NYHA) class, were more likely to have signs of pulmonary congestion on admission CXR, had lower hemoglobin and albumin levels, and higher NT-proBNP levels. There were no significant differences in history of chronic obstructive pulmonary disease (COPD), ischemic heart disease, renal function or baseline EF across B-line tertiles (Table 1). Among those with complete biomarker data, lower BMI, higher log NT-proBNP, lower albumin level, ACE-inhibitor/ARB/ARNI use, lack of diuretic use, and vascular congestion or alveolar edema on admission CXR were independently associated with B-lines in tertiles.
In-hospital events
There were 35 in-hospital events (10%). Unadjusted and adjusted risks of this short-term composite outcome by baseline B-line tertile are presented in Table 2. The risk of the in-hospital event increased with increasing B-line number, with each B-line tertile associated with an odds ratio of 1.82 (95% CI: 1.14, 2.88; P=0.011). This association persisted after adjusting for age, sex, baseline creatinine, baseline systolic blood pressure and NT-proBNP (adjusted OR 2.25; 95% CI: 1.24, 4.07; P=0.007). There was a trend towards longer length of stay with increasing baseline B-line number (P trend=0.008).
Table 2.
In-hospital outcomes by admission B-line tertile (n=349)
| 0–4 B-lines (n=121) | 5–9 B-lines (n=131) | ≥10 B-lines (n=97) | P (trend) | |
|---|---|---|---|---|
| Hospital length of stay, days | 5 [3, 9] | 7 [3, 13] | 8 [4, 13] | 0.008 |
| In-hospital all-cause death, n (%) | 1 (0.8) | 2 (1.5) | 4 (4.1) | - |
| ICU admission for worsening HF or cardiac arrest, n (%) | 3 (2.5) | 4 (3.1) | 1 (1.0) | - |
| LVAD during baseline admission, n (%) | 1 (0.8) | 1 (0.8) | 1 (1.0) | - |
| Intravenous inotropes, n (%) | 4 (3.3) | 12 (9.2) | 9 (9.3) | - |
| Composite outcome, n (%) | 5 (4.1) | 16 (12.2) | 14 (14.4) | - |
| Unadjusted OR (95% CI) | Reference | 1.82 (1.14, 2.88) | 3.29 (1.31, 8.30) | 0.011 |
| Model 1: Adjusted OR (95% CI) | Reference | 2.18 (1.31, 3.64) | 4.75 (1.70, 13.25) | 0.003 |
| Model 2: Adjusted OR (95% CI) | Reference | 2.25 (1.24, 4.07) | 5.05 (1.54, 16.54) | 0.007 |
| Model 3: Adjusted OR (95% CI) | Reference | 2.10 (1.20, 3.70) | 4.43 (1.43, 13.67) | 0.010 |
Composite in-hospital outcome: All-cause death, ICU admission for worsening HF or cardiac arrest, LVAD placement during admission, need for intravenous inotropes
Model 1: Age, sex, study site, baseline log creatinine, baseline systolic blood pressure
Model 2: Age, sex, study site, baseline log creatinine, baseline systolic blood pressure, baseline log NT-proBNP
Model 3*: Age, baseline systolic blood pressure, log baseline NT-proBNP
In this model only those covariates were retained that were significant in Model 2.
HF: heart failure; ICU: intensive care unit; LVAD: left ventricular assist device; OR: odds ratio.
Dynamic changes in clinical and congestion markers
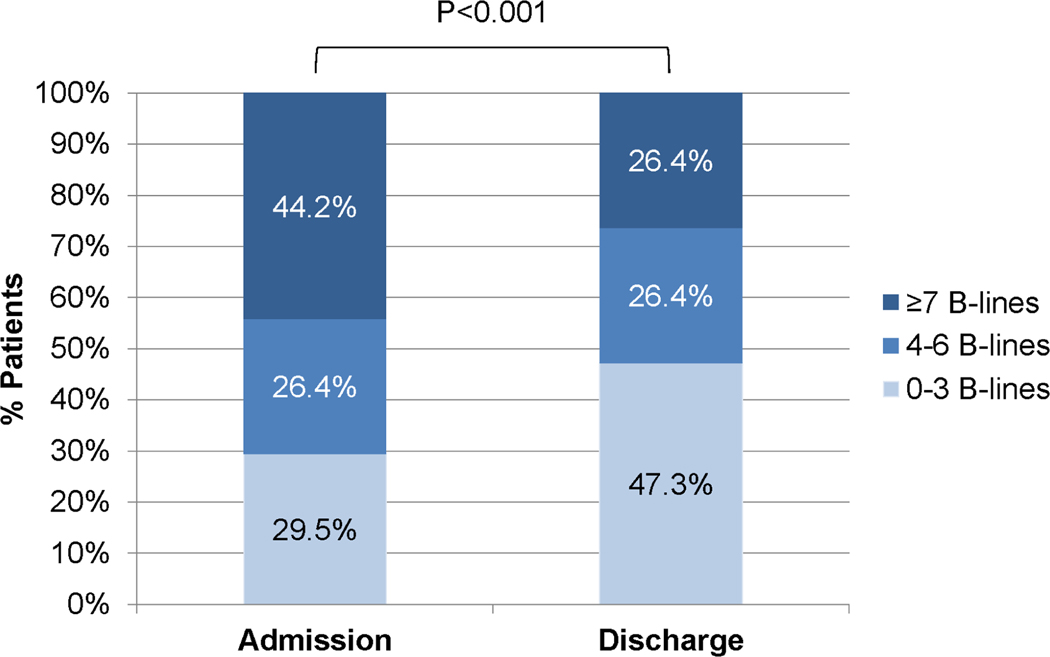
For the subset of 129 patients with both LUS on admission (LUS1) and on discharge (LUS2), dynamic changes in clinical and congestion markers are presented in Table S3. The median number of days between LUS1 and LUS2 was 6 (IQR 3, 10). Patient-reported dyspnea and most physical exam findings improved significantly from admission to discharge, with exception of S3. B-line count decreased in 4 zones from a median of 6 to 4 (P<0.001) and in 8 zones from a median of 12 to 9 (P<0.001) (Figure 2).
Figure 2. Dynamic changes in B-lines (n=129).
4-zone LUS early during hospitalization and at discharge.
Long-term outcomes
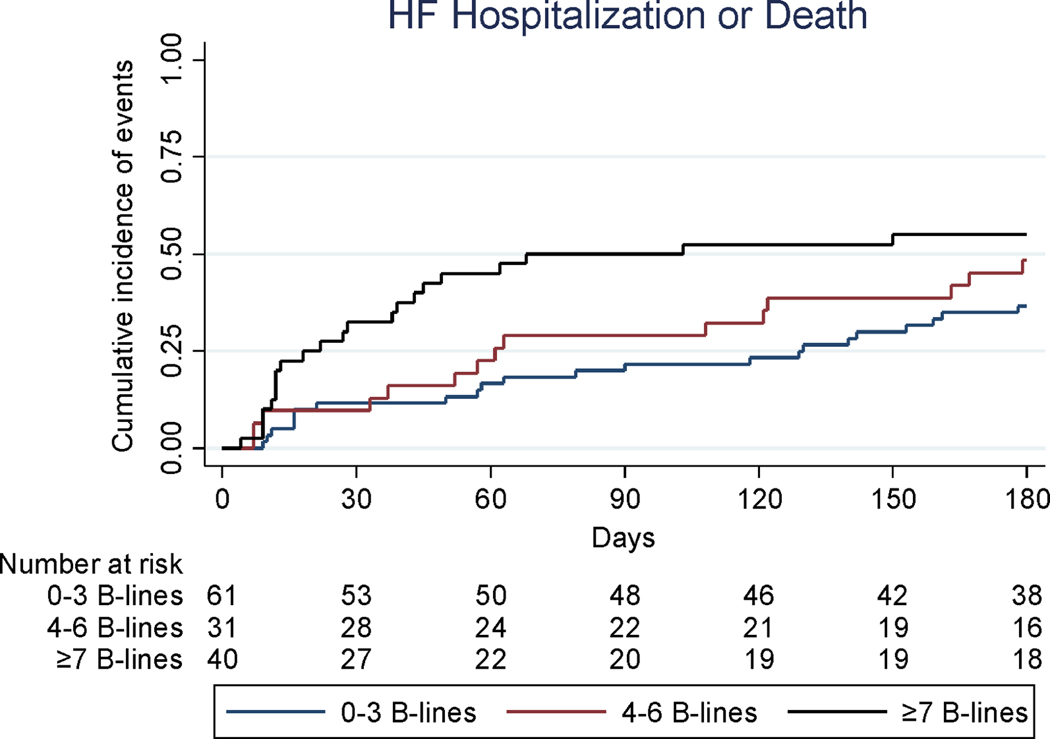
There were 59 long-term outcome events (45%) among 132 patients with available pre-discharge LUS during the 6-month follow up time period (Table S4). Baseline characteristics of these patients are presented in Table S2. Outcomes were analyzed by tertiles of B-line number on LUS2 (Central Illustration). The unadjusted and adjusted risk of the composite outcome (HF hospitalization or all-cause death) over 180 days is shown in Table 3. This outcome occurred in 36% in the first, 48% in the second and 55% in the third B-line tertile over that time period. The risk of the composite outcome diminished with time after discharge, i.e. a stronger relationship existed between B-lines and earlier events after hospital discharge and this association attenuated over time (P=0.022). Because of this time-varying association, results are also shown for 60 and 90 days after hospital discharge. All models were checked for interaction by B-line tertile and no significant interactions were found. The number of days lost due to HF hospitalization or death, as assessed by the RMTL, was significantly lower in the third B-line tertile at 60 days (6 vs. 17 days; adjusted P=0.003), 90 days (11 vs. 32; adjusted P=0.001) and 180 days (36 vs. 79 days; adjusted P=0.004), when compared to the first tertile. In a model including baseline NT-proBNP (Model 2), NT-proBNP was not a significant predictor of long-term outcomes at 60, 90 or 180 days. Data of a subset of patients from Boston with both NT-proBNP and LUS2 data are reported in Table S5.
Central Illustration: Cumulative incidence of HF hospitalization or death by pre-discharge B-line tertiles (n=132).
Cumulative incidence plot of composite long-term outcome.
Table 3.
Long-term outcomes up to 60, 90 and 180 days (n=132)
| 0–3 B-lines (n=61) | 4–6 B-lines (n=31) | ≥7 B-lines (n=40) | |
|---|---|---|---|
| Outcomes at 180 days | |||
| Unadjusted model (C=0.59) | |||
| Restricted mean time lost (RMTL) (95% CI) | 36 days (21, 51) | 49 days (27, 71) | 79 days (55, 104) |
| RMTL difference (95% CI) | (Reference) | +13 days (−14, +40) P=0.34 | +44 days (+15, +73) P= 0.003 |
| Hazard ratio (95% CI) | (Reference) | 1.43 (0.74, 2.76) P=0.28 | 2.01 (1.11, 3.64) P=0.021 |
| Model 1 (C=0.70) | |||
| RMTL difference (95% CI) | (Reference) | +16 days (−9, +41) P=0.21 | +38 days (+12, +65) P=0.004 |
| Hazard ratio (95% CI) | (Reference) | 1.42 (0.72, 2.78) P=0.31 | 1.89 (1.01, 3.51) P=0.045 |
| Outcomes at 90 days | |||
| Unadjusted model (C=0.63) | |||
| RMTL (95% CI) | 11 days (5, 18) | 16 days (7, 24) | 32 days (21, 43) |
| RMTL difference (95% CI) | (Reference) | +4 days (−7, +15) P=0.43 | +21 days (8, 34) P=0.002 |
| Hazard ratio (95% CI) | (Reference) | 1.42 (0.61, 3.32) P=0.42 | 2.94 (1.46, 5.93) P=0.003 |
| Model 1 (C=0.75) | |||
| RMTL difference (95% CI) | (Reference) | +5 days (−5, +16) P=0.32 | +20 days (8, 31) P=0.001 |
| Hazard ratio (95% CI) | (Reference) | 1.45 (0.60, 3.46) P=0.41 | 3.03 (1.45, 6.31) P=0.003 |
| Outcomes at 60 days | |||
| Unadjusted model (C=0.64) | |||
| RMTL (95% CI) | 6 days (2, 9) | 7 days (2, 12) | 17 days (10, 24) |
| RMTL difference (95% CI) | (Reference) | +2 days (−5, +8) P=0.65 | +11 days (+4, +19) P=0.005 |
| Hazard ratio (95% CI) | (Reference) | 1.42 (0.54, 3.72) P=0.48 | 3.30 (1.52, 7.17) P=0.002 |
| Model 1 (C=0.76) | |||
| RMTL difference (95% CI) | (Reference) | +2 days (−5, +8) P=0.62 | +11 days (+4, +18) P=0.003 |
| Hazard ratio (95% CI) | (Reference) | 1.46 (0.54, 3.96) P=0.46 | 3.57 (1.56, 8.20) P=0.003 |
C: Harrell’s C statistic
Model 1: Adjusted for age, log creatinine, systolic blood pressure (stratified by sex).
Creatinine, systolic blood pressure and NT-proBNP were measured at baseline. Composite long term outcome: Heart failure hospitalization or all-cause death.
Note: Additional adjusted models accounting for left ventricular ejection fraction and body mass index are presented in the Supplements (Table S6).
The number of B-lines using the 4- and 8-zone method was highly correlated both for LUS1 (rho=0.94, P<0.001) and LUS2 (rho=0.88, P<0.001). In the subset of 123 patients with both 4- and 8-zone LUS2 data similar results were found for count-based B-line quantification methods in 4- and 8-zones and the score based 8-zone method with respect to long-term events (Table S7).
The incremental prognostic value of LUS (by B-line tertiles) in 4 zones beyond crackles on auscultation pre-discharge, significantly improved the AUC: AUC auscultation 0.528 (95% CI: 0.435, 0.621); AUC auscultation plus LUS 0.653 (95% CI: 0.549, 0.756); AUC delta P=0.033 for the 90-day composite outcome. The addition of B-line tertiles to auscultation demonstrated significant improvement in risk reclassification at 90 days: continuous NRI = +0.16 (95% CI: +0.02, +0.37), P=0.027.
Discussion
In this two-site study of patients hospitalized for AHF, the vast majority of patients had a measurable number of B-lines on a simplified 4-zone lung ultrasound (LUS) after admission to the hospital, despite the absence of clinical or radiologic signs of congestion in many. The risk of an in-hospital event increased with higher B-line number, with each B-line tertile associated with a nearly two-fold higher risk, independent of other important clinical variables, including NT-proBNP. B-line number decreased from hospital admission to discharge, but more than half of patients still had >3 B-lines on 4-zone LUS at discharge. Patients with the highest B-line number had a higher risk of HF readmission or all-cause death independent of age, sex, creatinine, systolic blood pressure, EF and NT-proBNP. There was a time-varying association between B-line number and outcomes, such that the association was strongest during the initial part of follow-up and seemed to attenuate beyond 90 days. Over a 90-day period following discharge, an additional 21 days were lost due to HF readmission or death in the highest compared to the lowest B-line group.
Prior literature suggests that B-lines can be detected in patients with AHF using either an 8- or 28-zone scanning method and that those with ≥3 B-lines in at least one zone on each hemithorax (8-zone method) or with >15 B-lines (28-zone method) at the time of discharge have a more than 5-fold risk of HF readmission or death over the course of 90 to ~160 days.2, 7, 8 The prognostic value of LUS performed early during the admission on in-hospital events, has not been assessed in a well-defined AHF cohort, to our knowledge. The implementation of a simplified LUS imaging protocol in the in-hospital setting is attractive due to its shorter performance time and lower likelihood of missing data for count-based quantification approaches in which the sum of all B-lines in all zones is used. With increasing prevalence of obesity among patients with HF the time to acquire adequate quality images could potentially be higher in obese patients than previously reported and simplified imaging protocols may provide an attractive strategy.18 Furthermore, if the simplified protocol provides similar prognostic information in patients with AHF it is difficult to argue that more zones should be evaluated with LUS. A lower number of zones could facilitate a more cost-efficient approach and the integration of this method as a congestion measure in clinical trials in AHF.
Prevalence and dynamic changes of pulmonary congestion at baseline and pre-discharge
Prior AHF classification systems have defined categories of patients with and without pulmonary edema based on the presence of findings on clinical examination and CXR. Our data suggest that pulmonary congestion identified on LUS presents a spectrum rather than a binary category. In this study only 6% of patients had no B-lines on LUS early during hospital admission (compared to 35% of patients without crackles on lung auscultation) suggesting that most patients with AHF have some degree of pulmonary congestion early during their hospital admission which can be quantified by LUS. Importantly, we did not find any significant differences in patients’ common co-morbidities, such as COPD or differences in baseline EF suggesting that LUS may be equally useful in all HF subgroups. The precision of the B-line quantification method was high in this study with a mean B-line difference for the sum of B-lines in 4 zones of 0.4 (intra-reader agreement) and 1.1 B-lines (inter-reader agreement). Compared with crackles on auscultation, B-lines can be objectively quantified with high reproducibility.11, 13, 19
In-hospital outcomes
In our study a higher number of B-lines early during admission marked patients at increased risk for adverse events during the hospitalization. LUS might, therefore, be useful as a tool for triaging patients early during the admission, with either closer monitoring of these patients or even admission to a higher level of care unit (e.g. inpatient ward vs. ED Observation Unit, or Cardiac ICU vs. general ward). Of course, this possibility remains to be tested prospectively. Similarly, reduction in B-line number might seem an obvious goal of therapy, although recent experience using NT-proBNP as a means of tailoring therapy in this setting shows that what seems obvious may not be. Specifically, in the GUIDE-IT trial, patients randomly assigned to a NT-proBNP-guided strategy did not do better than those receiving usual care. Even if therapy targeted at reducing B-lines is a worthwhile strategy, whether that strategy should use percent reduction from baseline (admission) or an absolute number of B-lines is uncertain. Future randomized trials are needed to address this question.
Long-term outcomes
Prior studies using imaging protocols involving a larger number of chest zones demonstrated the prognostic importance of a higher number of B-lines at the time of discharge from a hospital after admission for AHF.6–8, 13 Our study expands on these earlier findings and demonstrates that a simplified 4-zone protocol may provide similar information. We estimated the pooled hazard ratio for recent studies, including the current one, in patients with AHF who underwent 8-zone pre-discharge LUS.7, 8 When considered together, these studies collectively suggest that the presence of ≥3 B-lines in at least one zone on each hemithorax (of 8 zones) is associated with a three-fold higher risk of readmission for HF or death over 90–100 days. We also showed that the prognostic importance of pre-discharge B-lines diminishes with time after discharge which stands in contrast to prior studies in ambulatory HF patients.11, 12 Both findings have important implications for clinical practice and for the design of future trials involving LUS. Our simplified imaging protocol should allow for rapid initial and repeated assessments of patients with AHF in the clinical setting. Studies aimed at assessing the effect of interventions to reduce B-lines pre-discharge on post-hospital outcomes may need to focus on an earlier post-discharge time-frame, i.e. up to 90 days.
Study strengths and limitations
To the best of the authors’ knowledge, the current study is the first to comprehensively investigate the prognostic importance of LUS findings with a simplified 4-zone method on both in-hospital and post-discharge outcomes in a larger cohort of patients with AHF (Table 4). This study also employed a rigorous methodological approach including off-line analysis of all LUS videos, temporal blinding during the image analysis and standardized endpoint adjudication.2 Although this was a preplanned two-site LUS study in patients with AHF, operational differences at the sites led to the need for slight modification of eligibility criteria. For instance, point-of-care BNP testing was available in Glasgow but not in Boston and the clinical laboratory in Boston was using NT-proBNP. While we cannot exclude that eligibility criteria may have led to slight differences between the study cohorts, we believe that both cohorts are representative of AHF patients at academic centers in the US and the UK. Although pre-discharge LUS was not feasible in Glasgow due to study logistics in the majority of patients, the baseline LUS from this site allowed for investigation of the relationship with in-hospital events. The ineligibility of patients who were admitted to an ICU at the time of screening may have contributed to a low in-hospital event rate. CXRs were performed on the day of admission in the majority of patients and not always on the same day as the LUS1. This limits the direct comparison of these two imaging modalities due to the variable time intervals and de-congestive treatment that occurred between the two tests and likely affected LUS findings. As pre-discharge NT-proBNP was not routinely performed at either of the two sites, NT-proBNP measurements were only available on admission.
Table 4.
Novel methodological aspects and findings
| Novel methodological aspects | Novel findings | |
|---|---|---|
| Lung ultrasound image acquisition | Use of a simplified 4-zone imaging protocol. | Among patients with acute heart failure (AHF) B-lines on lung ultrasound can be detected and quantified with a simplified 4-zone protocol in the majority of patients. |
| Lung ultrasound image analysis | Use of offline image analysis at a core laboratory Use of temporal blinding | |
| Outcome analysis | Assessment of inpatient events | B-lines detected and quantified by the simplified protocol in patients hospitalized for AHF provided prognostic information regarding short-term in-hospital and longer term adverse events. |
| Data analysis | There was a time-varying association between B-line number and long term outcomes, such that the association was strongest during the initial part of follow-up and seemed to attenuate beyond 90 days post-discharge. |
Conclusions
Pulmonary congestion using a simplified 4-zone LUS method was found to be common in patients hospitalized for AHF and decreased with therapy. A higher number of B-lines early during admission and at discharge identified patients at increased risk of both in-hospital and longer-term events. This relationship between B-lines and outcomes was stronger closer to hospital discharge and diminished over time.
Supplementary Material
Clinical perspectives
Among patients hospitalized for AHF adjustment of inpatient diuretic therapy and discharge timing are currently based on symptom improvement, physical examination findings, urine output and weight loss. Reliance on these imprecise congestion markers can result in under- or over-diuresis at the time of discharge, with potentially detrimental consequences after discharge. Lung ultrasound might offer a more reliable method of assessing pulmonary congestion in patients with AHF. Findings from this current study suggest that a simplified 4-zone lung ultrasound method allows for detection and monitoring of so called ‘B-lines’ (which are markers of pulmonary congestion) in AHF. An increased number of B-lines was associated with adverse in-hospital and longer term outcomes in this cohort.
Translational outlook
As a simplified 4-zone lung ultrasound method seems sufficient to detect residual pulmonary congestion in hospitalized patients treated for AHF, the efficacy of this strategy should be examined in prospective clinical trials with the goal to optimize decongestive therapy and reduce readmissions for heart failure.
Acknowledgements:
The authors would like to thank Julie Peck, MD for her assistance in the B-line analysis, and Hajime Uno, PhD for his assistance with the RMTL analysis.
Funding:
This project was supported by a grant from the National Heart, Lung and Blood Institute (grant number K23HL123533) (EP) and a project grant from the British Heart Foundation (grant number PG/13/17/30050) (RTC/JJM).
Dr. Platz reports a grant from NIH/NHLBI, during the conduct of the study; Dr. Groarke reports grants from Amgen Pharmaceuticals, outside the submitted work; Dr. Solomon reports grants and personal fees from Alnylam, grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants from Bellerophon, grants and personal fees from BMS, grants from Celladon, grants and personal fees from Gilead, grants and personal fees from GSK, grants from Ionis, grants from Lone Star Heart, grants from Mesoblast, grants from MyoKardia, grants from NIH/NHLBI, grants and personal fees from Novartis, grants from Sanofi Pasteur, grants from Theracos, personal fees from Akros, personal fees from Bayer, personal fees from Corvia, personal fees from Ironwood, personal fees from Merck, personal fees from Pfizer, personal fees from Roche, personal fees from Takeda, personal fees from Theracos, personal fees from Quantum Genetics, personal fees from AoBiome, personal fees from Janssen, personal fees from Cardiac Dimensions, outside the submitted work;
Abbreviations:
- AHF
Acute heart failure
- COPD
Chronic obstructive pulmonary disease
- CXR
Chest x-ray
- ED
Emergency department
- EF
Ejection fraction
- HF
Heart failure
- ICU
Intensive care unit
- LUS
Lung ultrasound
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- NYHA
New York Heart Association
- RMTL
Restricted mean time lost
Footnotes
Conflict of Interest:
all other authors report no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944–56. [DOI] [PubMed] [Google Scholar]
- 2.Platz E, Merz AA, Jhund PS, Vazir A, et al. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail. 2017;19:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43. [DOI] [PubMed] [Google Scholar]
- 4.Fudim M, Parikh KS, Dunning A, et al. Relation of Volume Overload to Clinical Outcomes in Acute Heart Failure (From ASCEND-HF). Am J Cardiol. 2018;122:1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler J, Gheorghiade M, Metra M. Moving away from symptoms-based heart failure treatment: misperceptions and real risks for patients with heart failure. Eur J Heart Fail. 2016;18:350–2. [DOI] [PubMed] [Google Scholar]
- 6.Gargani L, Pang PS, Frassi F, et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound. 2015;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coiro S, Rossignol P, Ambrosio G, et al. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail. 2015;17:1172–81. [DOI] [PubMed] [Google Scholar]
- 8.Cogliati C, Casazza G, Ceriani E, et al. Lung ultrasound and short-term prognosis in heart failure patients. Int J Cardiol. 2016;218:104–8. [DOI] [PubMed] [Google Scholar]
- 9.Campbell RT, Jackson CE, Wright A, et al. Palliative care needs in patients hospitalized with heart failure (PCHF) study: rationale and design. ESC Heart Fail. 2015;2:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–91. [DOI] [PubMed] [Google Scholar]
- 11.Platz E, Lewis EF, Uno H, et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J. 2016;37:1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwyer KH, Merz AA, Lewis EF, et al. Pulmonary Congestion by Lung Ultrasound in Ambulatory Patients With Heart Failure With Reduced or Preserved Ejection Fraction and Hypertension. J Card Fail. 2018;24:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palazzuoli A, Ruocco G, Beltrami M, et al. Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol. 2018;107:586–596. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 15.Felker GM, Hasselblad V, Tang WH, et al. Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail. 2012;14:1257–64. [DOI] [PubMed] [Google Scholar]
- 16.Cleland JG, Chiswell K, Teerlink JR, et al. Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: a report from the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Study. Circ Heart Fail. 2014;7:76–87. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Claggett B, Tian L, et al. On the restricted mean survival time curve in survival analysis. Biometrics. 2016;72:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 19.Platz E, Lattanzi A, Agbo C, et al. Utility of lung ultrasound in predicting pulmonary and cardiac pressures. Eur J Heart Fail. 2012;14:1276–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.