Abstract
Background
Regular blood pressure (BP) measurement is crucial for the diagnosis and management of hypertensive disorders in pregnancy, such as pre‐eclampsia. BP can be measured in various settings, such as conventional clinics or self‐measurement at home, and with different techniques, such as using auscultatory or automated BP devices. It is important to understand the impact of different settings and techniques of BP measurement on important outcomes for pregnant women.
Objectives
To assess the effects of setting and technique of BP measurement for diagnosing hypertensive disorders in pregnancy on subsequent maternal and perinatal outcomes, women's quality of life, or use of health service resources.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) on 22 April 2020, and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) involving pregnant women, using validated BP devices in different settings or using different techniques.
Data collection and analysis
Two authors independently extracted data, assessed risk of bias, and used the GRADE approach to assess the certainty of the evidence.
Main results
Of the 21 identified studies, we included three, and excluded 11; seven were ongoing. Of the three included RCTs (536,607 women), one was a cluster‐RCT, with a substantially higher number of participants (536,233 deliveries) than the other two trials, but did not provide data for most of our outcomes. We generally judged the included studies at low risk of bias, however, the certainty of the evidence was low, due to indirectness and imprecision. Meta‐analysis was not possible because each study investigated a different comparison.
None of the included studies reported our primary outcome of systolic BP greater than or equal to 150 mmHg. None of the studies reported any of these important secondary outcomes: antenatal hospital admissions, neonatal unit length of stay, or neonatal endotracheal intubation and use of mechanical ventilation.
Setting of BP measurement: self‐measurement versus conventional clinic measurement (one study, 154 women)
There were no maternal deaths in either the self‐monitoring group or the usual care group. The study did not report perinatal mortality.
Self‐monitoring may lead to slightly more diagnoses of pre‐eclampsia compared with usual care (risk ratio (RR) 1.49, 95% confidence interval (CI) 0.87 to 2.54; 154 women; 1 study; low‐certainty evidence) but the wide 95% CI is consistent with possible benefit and possible harm.
Self‐monitoring may have little to no effect on the likelihood of induction of labour compared with usual care (RR 1.09, 95% CI 0.82 to 1.45; 154 women; 1 study; low‐certainty evidence).
We are uncertain if self‐monitoring BP has any effect on maternal admission to intensive care (RR 1.54, 95% CI 0.06 to 37.25; 154 women; 1 study; low‐certainty evidence), stillbirth (RR 2.57, 95% CI 0.13 to 52.63; 154 women; 1 study; low‐certainty evidence), neonatal death (RR 1.54, 95% CI 0.06 to 37.25; 154 women; 1 study; low‐certainty evidence) or preterm birth (RR 1.15, 95% CI 0.37 to 3.55; 154 women; 1 study; low‐certainty evidence), compared with usual care because the certainty of evidence is low and the 95% CI is consistent with appreciable harms and appreciable benefits.
Self‐monitoring may lead to slightly more neonatal unit admissions compared with usual care (RR 1.53, 95% CI 0.65 to 3.62; 154 women; 1 study; low‐certainty evidence) but the wide 95% CI includes the possibility of slightly fewer admissions with self‐monitoring.
Technique of BP measurement: Korotkoff phase IV (K4, muffling sound) versus Korotkoff phase V (K5, disappearance of sound) to represent diastolic BP (one study, 220 women)
There were no maternal deaths in either the K4 or K5 group.
There may be little to no difference in the diagnosis of pre‐eclampsia between using K4 or K5 for diastolic BP (RR 1.16; 95% CI 0.89 to 1.49; 1 study; 220 women; low‐certainty evidence), since the wide 95% CI includes the possibility of more diagnoses with K4.
We are uncertain if there is a difference in perinatal mortality between the groups because the quality of evidence is low and the 95% CI is consistent with appreciable harm and appreciable benefit (RR 1.14, 95% CI 0.16 to 7.92; 1 study, 220 women; low‐certainty evidence).
The trial did not report data on maternal admission to intensive care, induction of labour, stillbirth, neonatal death, preterm birth, or neonatal unit admissions.
Technique of BP measurement: CRADLE intervention (CRADLE device, a semi‐automated BP monitor with additional features, and an education package) versus usual care (one study, 536,233 deliveries)
There may be little to no difference between the use of the CRADLE device and usual care in the number of maternal deaths (adjusted RR 0.80, 95% CI 0.30 to 2.11; 536,233 women; 1 study; low‐certainty evidence), but the 95% CI is consistent with appreciable harm and appreciable benefit.
The trial did not report pre‐eclampsia, induction of labour, perinatal mortality, preterm birth, or neonatal unit admissions. Maternal admission to intensive care and perinatal outcomes (stillbirths and neonatal deaths) were only collected for a small proportion of the women, identified by an outcome not by baseline characteristics, thereby breaking the random allocation. Therefore, any differences between the groups could not be attributed to the intervention, and we did not extract data for these outcomes.
Authors' conclusions
The benefit, if any, of self‐monitoring BP in hypertensive pregnancies remains uncertain, as the evidence is limited to one feasibility study. Current practice of using K5 to measure diastolic BP is supported for women with pregnancy hypertension. The benefit, if any, of using the CRADLE device to measure BP in pregnancy remains uncertain, due to the limitations and instability of the trial study design.
Plain language summary
Do different settings and techniques for measuring blood pressure during pregnancy help to improve outcomes for women and babies?
What is the issue?
Regular blood pressure (BP) measurements are crucial during pregnancy for the diagnosis and management of high BP. BP can be measured in various settings (e.g. self‐measurement at home versus in clinic) and using different techniques (e.g. measurement based on different blood flow sounds). They may have different effects on diagnosing and monitoring high BP, and reducing the risk of serious illness or death in both woman and baby.
Why is this important?
If high BP in pregnancy is not detected and managed in a timely fashion, serious complications can develop. This review is needed to establish the benefits and risks of these settings and techniques for women and their babies.
What evidence did we find?
We searched for evidence from randomised controlled trials in April 2020, and identified three studies (involving 536,607 women). Overall, the studies were conducted in such a way that we are not certain of the findings, mainly due to the small size of two of the studies and the design of the other.
One study (154 women) compared BP settings in the UK: self‐monitoring at home versus usual measurement in clinic. The other two studies compared BP techniques: one (220 women) compared two different blood flow sounds to determine diastolic BP (the bottom number) in Australia, and the other (536,233 deliveries) investigated the introduction of a semi‐automated BP monitor and an education package (CRADLE intervention) compared with usual care across Africa, India and Haiti.
None of the studies measured high BP, the number of women admitted to hospital before birth, how long babies stayed in the neonatal unit, or what extra help babies received for their breathing.
Self‐measurement of BP at home compared with usual BP measurement in clinic
Self‐monitoring BP may lead to more women being diagnosed with pre‐eclampsia compared with usual care but the evidence is uncertain.
We are uncertain if self‐monitoring BP increases the likelihood of stillbirth, baby deaths (after birth), women giving birth early, or women admitted to the intensive care unit.
Self‐monitoring BP may have little to no effect on the likelihood of women having their labour induced compared with usual care.
Self‐monitoring BP may lead to slightly more newborns being admitted to a neonatal unit compared with usual care.
This trial had no maternal deaths, and did not report the number of baby deaths, before or shortly after birth.
Measuring BP using different blood flow sounds ‐ Korotkoff phase IV (K4, softer, muffled sound) compared with Korotkoff phase V (K5, when the sound disappears) to measure diastolic BP
There may be little to no difference between using K4 or K5 to diagnose pre‐eclampsia; the evidence is uncertain.
We are uncertain if there is an effect on baby deaths, before or shortly after birth.
This trial had no maternal deaths, and did not report the number of women admitted to intensive care, women who needed their labour induced, women giving birth early, stillbirths, baby deaths (after birth), or babies admitted to the neonatal unit.
CRADLE intervention (semi‐automated BP monitor and an education package)compared with usual care
The CRADLE BP monitor may make little or no difference to the risk of maternal death.
The trial did not report the number of women with pre‐eclampsia, women who needed their labour induced, women giving birth early, baby deaths (before and after birth), or the number of babies admitted to the neonatal unit. The number of women admitted to intensive care, stillbirths, and baby deaths (after birth) were only reported for a subgroup of women in this trial, so we did not include these results.
What does this mean?
More evidence is needed on whether self‐monitoring BP in pregnant women with high BP is beneficial, because the study exploring this was small.
Current practice of using K5 (no blood flow sound) to measure diastolic BP is supported in pregnant women with high BP.
The trial using the CRADLE device to monitor BP in pregnancy had limitations in its design, and we are uncertain about its benefit.
Summary of findings
Background
Description of the condition
Hypertensive disorders of pregnancy (HDP) are common, with pre‐eclampsia complicating 2% to 3% of pregnancies, pre‐existing hypertension complicating 1%, and isolated gestational hypertension complicating 5% to 6% of pregnancies (Hutcheon 2011). Hypertension is defined as a systolic blood pressure (BP) of 140 mmHg or higher, or a diastolic BP of 90 mmHg or higher, or both; severe hypertension is defined as a BP of 160/110 mmHg; and non‐severe (or mild to moderate) hypertension, as a systolic BP of 140 to 159 and diastolic BP of 90 to 109 mmHg (NICE 2019).
HDP are leading causes of maternal and perinatal morbidity and mortality, particularly in low‐ and middle‐income countries (Duley 2009). NICE 2019 defines pre‐eclampsia as gestational hypertension, or maternal end‐organ complication(s), or both, or fetal involvement; it is the cause of most HDP‐associated morbidity and mortality (Khan 2006). Although there are still gaps in understanding around the pathophysiology of pre‐eclampsia, it has been established as a systemic syndrome, characterised by placental and maternal vascular dysfunction, which if severe, may lead to adverse outcomes, such as severe hypertension, stroke, seizures (eclampsia), coagulopathy, renal and hepatic injury, haemorrhage, or death (Brown 2000). Two small, yet landmark cohort studies, have suggested that neurological complications of pre‐eclampsia and eclampsia, including stroke and posterior reversible encephalopathy syndrome, are more prevalent in women with systolic hypertension as opposed to diastolic hypertension (Martin 2005; Wagner 2011). These findings support the 2019 NICE guidelines that recommend treatment if systolic BP is 140 mmHg or higher (NICE 2019). Perinatal mortality and morbidity is also high as a result of pre‐eclampsia; the blood supply to the placenta is disrupted, which may result in intrauterine growth restriction, or fetal death secondary to placental abruption. Furthermore, pre‐eclampsia is an antecedent for one‐fifth of infants born prematurely (Hewitt 1988). Hypertension in pregnancy, independent of pre‐eclampsia, is also associated with increased risk of stroke and other maternal and perinatal complications, including pulmonary oedema, placental abruption, and perinatal death (Brown 2000).
Accurate and regular BP measurement is crucial to the diagnosis and management of HDP, which are often asymptomatic. Most deaths associated with HDP are considered preventable with timely and effective detection, along with management of hypertension and associated complications (MacKay 2001). The 2006–2008 UK Confidential Enquiries into Maternal Deaths report found that the most common reason for substandard care in deaths secondary to pre‐eclampsia and eclampsia was failure to recognise and treat hypertension (Cantwell 2011). The 2009–2014 report found that although the proportion of women dying secondary to hypertensive disorders had decreased, in 93% of those women, improvements in the care may have made a difference in their outcome (Knight 2016). Therefore, BP measurement and subsequent management, including treatment of hypertension and timing of birth, are critical in preventing avoidable maternal deaths.
Description of the intervention
BP measurement has traditionally taken place within an antenatal clinic environment, conventionally with one or two mercury sphygmomanometer (gold standard) measurements, taken to guide further investigation and management. In recent years, there has been a shift away from mercury sphygmomanometry, owing to environmental and safety concerns of mercury in clinical settings, and issues with user error (Nathan 2015). Likewise, some authors consider aneroid devices (which also rely on auscultation, and therefore, are also associated with user and calibration error) as unattractive options for BP measurement (Canzanello 2001). With this, there has been a move towards the use of automated devices, which rely on the detection of oscillometric waveforms produced within the cuff during BP measurement, giving a digital BP reading and minimising user error (Perry 1991). This shift has coincided with growing concern over the accuracy of automated devices, most of which have not been formally validated as accurate. This is particularly problematic in pre‐eclamptic women, for whom automated devices have been shown to significantly underestimate BP, possibly due to the pathophysiological changes of pre‐eclampsia that can alter the oscillometric waveform (Nataranjan 1999; Shennan 1993). The British & Irish Hypertension Society (BIHS), the European Society of Hypertension (ESH), and the International Organisation for Standardisation (IOS), each recommend that automated BP devices be independently validated, to ensure accuracy according to a recognised protocol (ISO 2013; O'Brien 1993; O'Brien 2010). Even if a device is validated as accurate in a non‐pregnant population, these studies recommend formally validating it in a specific group of pregnant women before using it in pregnancy (O'Brien 1993; O'Brien 2002; O'Brien 2010).
How the intervention might work
Setting of BP measurement
Relying only on clinic or home BP readings may limit the diagnostic potential of BP measurement. White coat hypertension (elevated BP in a hospital setting, yet normotension in a home setting) may occur in nearly 30% of pregnant women. Its recognition can prevent unnecessary admission to hospital or antihypertension treatment; however, it is associated with an increased risk of developing hypertension in pregnancy and pre‐eclampsia, so clinicians should not ignore it (Bellomo 1999; Brown 2005). In women with hypertension in pregnancy (including pre‐eclampsia), the diurnal variation in BP may be altered (Hermida 2000). Alternatives to conventional clinical BP measurement include self (or home) BP measurement (SBPM) and ambulatory BP measurement (ABPM). In SBPM, people are trained to use the device and interpret readings, in order to alert a health professional if they obtain abnormal readings, or readings can be automatically sent to a central facility for evaluation by a health professional (O'Brien 2001). In ABPM, a portable automated device performs repeated measurements, typically over 24 hours, during the woman's normal daily routine (Greer 1993). Both methods may increase reproducible measurements, minimise misleading results, identify impaired diurnal variation, and improve BP control and prediction of adverse clinical outcomes (Bellomo 1999; Halligan 1993; Higgins 1997).
Technique of BP measurement
It is well recognised that only BP devices that have been validated as accurate through formal validation, according to recognised protocols, should be used clinically. Even if validation has occurred, other variables outside of the test environment may influence BP measurement, including BP measurement technique. The BHIS and ESH have published several recommendations related to BP measurement technique to ensure reliability: resting for at least five minutes prior to BP measurement, sitting upright with the arm supported and the cuff placed at the level of the heart, using the appropriately sized cuff, deflating the cuff at a rate of 2 mmHg to 3 mmHg, using Korotkoff phase V, and recording BP to the nearest 2 mmHg (O'Brien 1993; O'Brien 2003). Unfortunately, correct technique may not be practised outside of the test environment, yet it may impact on BP measurement, and therefore, outcome. Universal cuffs (that can be used in women with a wide range of arm circumferences) may eliminate the potential error introduced by using an inappropriately sized cuff; however, their impact on outcome is not known.
Why it is important to do this review
As well as confirming or predicting disease, a screening and diagnostic test should also show clear benefit according to robust evidence, in terms of improved survival, function, or quality of life. The Cochrane Review 'Ambulatory versus conventional methods for measuring BP during pregnancy', published in 2002, highlighted the absence of randomised controlled trial (RCT) evidence comparing the two methods of BP measurement according to clinical outcome, use of healthcare resources, and women's views (Bergel 2002).
The BHS and ESH have made recommendations on various aspects of BP measurement technique, and a number of studies have assessed the effectiveness of SBPM, ABPM and aspects of BP measurement technique in non‐pregnant and pregnant populations. However, no systematic review has evaluated their impact on survival, health service resources or quality of life. Many of the current guidelines recommendations related to BP techniques derive from extrapolations from other populations and expert consensus; whilst many of these techniques have been adopted into clinical practice, it is important to examine the evidence base specifically for clinically relevant pregnancy outcomes.
This review aims to identify all new published and unpublished RCTs of ABPM, SBPM, and all aspects of BP measurement technique, to establish the benefits and risks of each for pregnant women. This review will not assess the accuracy of BP devices, but rather the clinical performance of BP equipment that has been previously validated according to recognised protocols, in terms of technique and interpretation.
Objectives
To assess the effects of setting and technique of blood pressure measurement for diagnosing and managing hypertensive disorders in pregnancy on subsequent maternal and perinatal outcomes, women's quality of life, or use of health service resources.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCT), including cluster‐RCTs, that use either automated blood pressure (BP) devices previously validated (as per The Association for the Advancement of Medical Instrumentation, the British & Irish Hypertension Society (BIHS), and the European Society of Hypertension (ESH)) in a pregnant population, or calibrated mercury or aneroid sphygmomanometers and appropriate cuffing. We excluded cross‐over randomised controlled designs and quasi‐randomised designs. Outcomes were not part of the criteria for including studies.
Types of participants
Studies involving pregnant women (including all gestations of pregnancy), including RCTs delivering interventions at the population level that were primarily intended for pregnant women.
Types of interventions
Eligible interventions were the use of the following listed settings and techniques for assessment, decision making, or management.
Setting
Ambulatory measurement (using automated devices that provide a large number of device‐initiated BP measurements over a period of time, usually a 24‐hour period) versus conventional clinic BP measurement
Self‐measurement (using an automated BP device at home) versus conventional clinic BP measurement
Technique
Auscultatory technique (using calibrated aneroid or mercury sphygmomanometer) versus automated technique (using oscillometric devices) to measure BP
Korotkoff phase IV (K4, muffling sound) versus Korotkoff phase V (K5, disappearance of sound) to represent diastolic BP
Resting for at least five minutes from arrival at clinic prior to BP measurement versus immediate BP measurement on arrival in clinic
Multiple BP measurements at one clinic visit versus one BP measurement at one clinic visit
Appropriately sized cuff versus universal cuff (cuff designed to be used in all women, regardless of arm circumference)
Systolic BP versus diastolic BP
Systolic or diastolic BP, or both, versus mean arterial pressure
Types of outcome measures
Primary outcomes
Systolic BP greater than or equal to 150 mmHg
Secondary outcomes
Maternal outcomes
Maternal death: death during pregnancy, or up to 42 days after end of pregnancy
-
Maternal morbidity
Pre‐eclampsia
Eclampsia
Cerebrovascular event
Renal failure
Hepatic failure
HELLP syndrome (haemolysis, elevated liver enzymes, and low platelet count)
Disseminated intravascular coagulation
Pulmonary oedema
Placental abruption
Obstetric haemorrhage
Neonatal outcomes
-
Death
Stillbirth: death in utero, at or after 20 weeks' gestation
Neonatal death: death in the first 28 days after birth
Perinatal mortality: stillbirth and early neonatal deaths (death in the first seven days after birth)
-
Neonatal morbidity
Preterm birth: birth before 37 completed weeks' gestation
Use of health service resources
-
Maternal
Number of antenatal clinic visits
Number of antenatal hospital admissions
Induction of labour
Operative delivery
Maternal admission to intensive care
Maternal length of stay in intensive care
Ventilation
Dialysis
-
Neonatal
Neonatal unit admission
Neonatal unit length of stay
Endotracheal intubation and use of mechanical ventilation
Measures of maternal quality of life
Maternal experiences and views of the interventions
Maternal anxiety
Maternal self‐confidence
Search methods for identification of studies
The methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (22 April 2020).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase, and CINAHL; the list of hand searched journals and conference proceedings; and the list of journals reviewed via the current awareness service; please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist, and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE Ovid;
weekly searches of Embase Ovid;
monthly searches of CINAHL EBSCO;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals, plus monthly BioMed Central email alerts.
Two people screen search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, they assign each trial report a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and then add it to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO ICTRP for unpublished, planned, and ongoing trial reports (22 April 2020) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We endeavoured to contact the study authors if the information we required was not included in the full article.
We did not apply any language or date restrictions.
Data collection and analysis
We used the following methods to assess the reports identified in the search. The methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. They resolved any disagreement through discussion, or if required, through consultation with a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion, or if required, through consultation with a third review author. We entered data into Review Manager 5 software (RevMan 5), and checked it for accuracy (Review Manager 2014).
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion, or by involving a third review author.
For cluster‐randomised trials, we considered recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials.
Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment, and assessed whether intervention allocation could have been foreseen in advance of or during recruitment or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results.
We assessed the methods as being at:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
We assessed methods used to blind outcome assessment as being at low, high, or unclear risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data, including attrition and exclusions from the analysis. We stated whether studies reported attrition and exclusions, as well as the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups, or were related to outcomes. We contacted authors where there were missing data, and included them, were appropriate, information was provided.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it was clear that the study reported all prespecified outcomes and all expected outcomes of interest to the review);
high risk of bias (where authors did not report all the study's prespecified outcomes; did not prespecify one or more reported primary outcomes; incompletely reported outcomes of interest, rendering them unfit for analysis; failed to include results of a key outcome that they would have been expected to report);
unclear risk of bias.
Other bias (checking for bias due to problems not covered by the domains described above)
For each included study, we described any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias, assigning it as being at:
low risk of other bias;
high risk of other bias;
unclear risk of other bias.
Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in Higgins 2011. With reference to the risk of bias domains, we assessed the likely magnitude and direction of the bias, and whether we considered it likely to impact on the findings.
If the quantity of data had allowed, we would have explored the impact of the level of bias by undertaking sensitivity analyses – see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as a summary risk ratio (RR) with 95% confidence intervals (CI).
For one of the trials, we reported adjusted data to account for the stepped‐wedge cluster design (Vousden 2019). In data and analysis tables, we reported summary RRs, as planned, instead of odds ratios (ORs), as reported by trial authors.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials. We would have used the standardised mean difference to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We identified one cluster‐randomised trial (Vousden 2019), which was not combined with any other trials in a meta‐analysis because it did not address the same comparison as any other trial. Within this trial, for our outcome of interest (maternal death), we used reported data (which had been adjusted using a 'bent stick' approach, adapted from the approach used for interrupted time series for a stepped wedge cluster‐randomised trial (Bernal 2017)). We entered these data into RevMan 5 using the generic inverse variance (Review Manager 2014). Although reported as ORs by the trial authors, we presented them as RRs, as the outcome was rare (maternal death), and thus the OR approximated the RR closely (McNutt 2003).
In future updates, if we identify both cluster‐randomised trials and individually randomised trials investigating the same comparisons, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs, and we consider the interaction between the effect of intervention and the choice of randomisation unit to be unlikely. We will adjust their standard errors using the methods described in Section 16.3.4 or 16.3.6 of Higgins 2011, using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this, and conduct sensitivity analyses to investigate the effect of variation in the ICC.
We will also acknowledge heterogeneity in the randomisation unit, and perform a subgroup analysis to investigate the effects of the randomisation unit.
Other unit of analysis issues
We did not identify any other unit of analysis issues in this version of the review. In future updates, we may include multi‐arm studies in relevant analyses, for example, ambulatory versus self (automated) versus conventional clinic BP measurement. We would include factorial studies in all relevant analyses, as two separate studies. For example, we may include ambulatory versus self BP measurement (SBPM), and appropriate cuff size versus universal cuff factorial studies in a meta‐analysis of ambulatory versus self in the appropriate cuff size group and the universal cuff group.
Dealing with missing data
We noted levels of attrition in the included studies. If combining studies in meta‐analyses had been possible, we would have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using a sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis; we attempted to include all participants randomised to each group in the analyses, and we analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome, in each trial, is the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
Since combining studies in meta‐analyses was not possible, it was not necessary to consider heterogeneity. If meta‐analysis is possible in future updates, we will assess statistical heterogeneity in each meta‐analysis using Tau², and I², and Chi² statistics. We will regard heterogeneity as substantial if I² is greater than 30%, and either Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If meta‐analysis is possible in future updates, and if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and at any suggestion of asymmetry, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using RevMan 5 (Review Manager 2014). If meta‐analysis is possible in future updates, we will use the fixed‐effect model to combine data, where it is reasonable to assume that studies are estimating the same underlying treatment effect, that is, where trials are examining the same intervention, and the trials' populations and methods are sufficiently similar. If there is sufficient clinical heterogeneity to suggest that the underlying treatment effects differ between trials, or if we detect substantial statistical heterogeneity, we will use the random‐effects model to produce an overall summary, if we determine an average treatment effect across trials is considered clinically meaningful. We will treat the random‐effects summary as the average range of possible treatment effects, and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use the random‐effects model, we will present the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If meta‐analysis is possible in future updates, and we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses.
We plan to carry out the following subgroup analyses.
Women at low or average risk of hypertension in pregnancy (unselected) versus women defined as being at high risk of hypertension in pregnancy (these will include women selected by the trial authors on the basis of an increased risk of hypertension in pregnancy, e.g. women with previous pre‐eclampsia).
Women with hypertension, without other signs of pre‐eclampsia versus women with established pre‐eclampsia or eclampsia.
Low‐ and middle‐income country setting versus high‐income country setting.
We will assess the primary outcome (systolic BP ≥ 150 mmHg) in subgroup analysis.
We will assess subgroup differences by interaction tests available in RevMan 5, reporting the results of subgroup analyses along with the Chi² statistic, P value, and the interaction test I² value (Review Manager 2014).
Sensitivity analysis
If meta‐analysis is possible in future updates, we will perform sensitivity analysis for the studies at high (or unclear) risk of selection bias (allocation concealment and sequence generation) and attrition bias. If studies are at high or unclear risk of bias for one or more of these domains, we will exclude them from the sensitivity analysis. It is likely that most studies will not have blinded participants, so this will not be one of our key domains for sensitivity analysis.
In future updates, where meta‐analysis is possible, we will also explore the robustness of our findings by excluding studies with high levels of missing data.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach, as outlined in the GRADE handbook, in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
Maternal outcomes
Systolic BP greater than or equal to 150 mmHg
Maternal death
Pre‐eclampsia
Number of antenatal hospital admissions
Maternal admission to intensive care
Induction of labour
Neonatal outcomes
Stillbirth
Neonatal death
Perinatal mortality
Preterm birth
Neonatal unit admission
Neonatal unit length of stay
Endotracheal intubation and use of mechanical ventilation
We used GRADEpro GST to import data from RevMan 5, in order to create ’Summary of findings’ tables (GRADEpro GDT; Review Manager 2014). A summary of the intervention effect and a measure of quality for each of the above outcomes were produced using the GRADE approach.
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Results
Description of studies
Results of the search
The search (22 April 2020) retrieved 37 trial reports to assess. One additional report was identified through contact with one of the trial authors. Of the 38 trial reports, there were: 16 peer‐reviewed papers, nine abstracts, and 13 study registrations. These 38 reports represented 21 individual studies, with multiple reports related to the same study. We successfully contacted eight authors when we required additional information.
Of the 21 studies identified, we included three in the review, excluded 11, and seven were ongoing. Figure 1 shows the study flow diagram.
1.
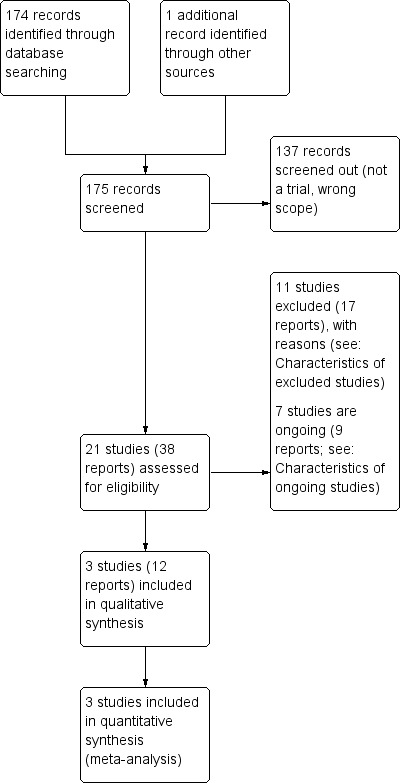
Study flow diagram
Included studies
We included three studies were included in this review (Brown 1998; Pealing 2019a; Vousden 2019). Study details are shown in Characteristics of included studies.
Design
All three included studies were prospective, multi‐centred, two‐arm randomised controlled trials (RCT), including one feasibility study (Pealing 2019a), and a pragmatic cluster‐RCT (Vousden 2019). One study was published in 1998, and the other two over two decades later in 2019 (participants recruited between 2015 and 2017).
Sample sizes
Of the three included studies: two were small (with a total of 374 women recruited across six maternity units), and one was large (a total of 536,233 deliveries across 10 clusters).
Setting
Two studies took place in high‐income countries; one in Australia (Brown 1998), and the other in the UK (Pealing 2019a). The third study was conducted in middle‐ or low‐income countries (Ethiopia, Sierra Leone, Zimbabwe, Uganda, Zambia, Malawi, India and Haiti; Vousden 2019).
Participants
Two studies recruited pregnant women with hypertension, and the third, pregnant or postpartum women presenting for maternity care (Vousden 2019).
Interventions and comparisons
Of the three included studies, one explored setting of blood pressure (BP) measurement, two explored techniques of BP measurement for diagnosing and managing hypertensive disorders in pregnancy on subsequent maternal and perinatal outcomes (Brown 1998; Vousden 2019). For all three included studies, we were able to successfully contact trial authors for additional information including outcome definitions (Pealing 2019a), details of BP device calibration and cuffing used (Brown 1998), and secondary outcome data capture (Vousden 2019).
Setting of BP measurement: self‐measurement (using an automated BP device at home) versus conventional clinic BP measurement
The OPTIMUM‐BP study assessed the feasibility of self‐monitoring BP, versus usual care, for the management of pregnancy hypertension in 154 women (Pealing 2019a).
Technique of BP measurement: Korotkoff phase IV (K4, muffling sound) versus Korotkoff phase V (K5, disappearance of sound) to represent diastolic BP
Brown 1998 explored the management of pregnancy hypertension in 220 pregnant women with diastolic hypertension, using different arterial sounds, either Korotkoff phase IV (K4, muffling sound) or Korotkoff phase V (K5, disappearance of sound), and its influence on maternal and fetal outcomes, and the likelihood of episodes of severe hypertension.
Technique of BP measurement: auscultatory technique (using calibrated aneroid or mercury sphygmomanometer) versus automated technique (using oscillometric devices) to measure BP
The CRADLE‐3 trial aimed to determine whether implementation of the CRADLE device (a hand‐held, upper‐arm, semi‐automated BP device that incorporates a traffic‐light early warning system that alerts healthcare professionals to abnormalities in BP and pulse) and an education package, into maternity care in low‐resource settings could reduce a composite of all‐cause maternal mortality or major morbidity, with information collected on 536,233 deliveries from 10 clusters across Africa, India, and Haiti (Vousden 2019).
We decided that the CRADLE intervention addressed the objectives of this review (automated oscillometric device compared with previous usual care). The intervention was an automated oscillometric device with additional features (early warning system and an eduction package), compared to usual care, in which a variety of locally available BP devices were used and clinical management followed local guidelines. We recognise that the main component of the CRADLE intervention was the BP monitoring device. Typically, all BP devices come with training, delivered either generically or specifically. As the CRADLE intervention was being introduced into settings where previous training was uncertain, training was an explicit part of the intervention.
Outcomes
None of the included studies report our primary outcome of systolic BP greater than or equal to 150 mmHg. All of the included studies reported maternal death, and only one included study reported on any one of the following outcomes: maternal admission to intensive care, induction of labour, stillbirth, neonatal death, perinatal mortality and preterm birth, and neonatal unit admission.
None of the studies reported the following secondary outcomes:
renal failure;
hepatic failure;
disseminated intravascular coagulation;
pulmonary oedema;
placental abruption;
obstetric haemorrhage;
number of antenatal clinic visits;
number of antenatal hospital admissions;
maternal length of stay in intensive care;
ventilation (maternal);
dialysis (maternal);
neonatal unit length of stay;
neonatal endotracheal intubation and use of mechanical ventilation;
women's experiences and views of the interventions;
maternal anxiety;
maternal self confidence.
Excluded studies
We excluded eleven studies. Study details are shown in Characteristics of excluded studies. Studies were excluded because:
1. BP monitors used are not validated (automated BP devices) or calibrated (sphygmomanometers) for use in the hypertensive pregnant population (7 studies). When the BP monitor used was not fully documented in the identified reports, we contracted trial authors for further information. Excluded BP monitors were as follows.
Brown 2012 and Denolle 2008 used the Omron HEM‐705CP. Although both studies reported the device was validated in pregnancy, they did not reference a validation paper, and the review authors found peer‐reviewed papers invalidating this device (Gupta 1997; Lo 2002). A systematic review of BP validation studies does not recommend the use of this BP device (Bello 2018).
Cartwright 1992 used the Dinamap 1846SX. No validation paper was referenced, and the review authors found peer‐reviewed papers invalidating this device (Franx 1994; Green 1996), alongside a review that does not recommended use of this BP device (Bello 2018).
Duggan 1999 used the QuietTrak ambulatory. No validation paper was referenced; the review authors found peer‐reviewed papers invalidating this device (Penny 1996; Nataranjan 1999). The European Society of Hypertension Working Group on Blood Pressure Monitoring did not recommend this BP devise in their clinical review (O'Brien 2001a).
Rhodes 2018 used the Spacelabs 90207. Although a validation paper was referenced in the study paper (Shennan 1993), the review authors found peer‐reviewed papers invalidating this device (Nataranjan 1999), or not recommending its use (O'Brien 1993a; Franx 1997; Shennan 1996). This BP device is also not recommended by reviews of BP devices (Bello 2018; O'Brien 2001a), or on the Dabl education website.
Ross‐McGill 2000 used the Omron Rx (author contacted to establish BP device details). No validation paper has been published in the pregnant population (adult population validation; Cuckson 2004).
Leung 1998 used unspecified home BP monitors (author contacted to establish BP device details).
We used a variety of sources to assess validation, including published literature and websites, including: STRIDE BP, the BIHS, and Dabl education website. The latter is supported by manufacturers of BP devices, however, this did not appear to have influenced the list of validated devices for use in pregnancy, as none of our excluded BP devices were listed as validated for use in pregnancy.
2. Reference type not eligible for the review (1 study): a protocol (Aslani 2014).
3. Study type not eligible for the review (3 studies): cross‐over RCTs (Holm 2019; Mangos 2012; NCT03222414).
Ongoing studies
Of the seven ongoing studies, estimated study completion dates ranged from December 2019 to April 2023. Study details are shown in Characteristics of ongoing studies.
Risk of bias in included studies
We judged the included studies to be generally at low risk of bias (Figure 2; Figure 3).
2.

Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Random sequence generation: All three studies described random components in the sequence generation, all detailing computer‐generated methods, and thus were assessed at low risk of bias.
Allocation concealment: two of the three studies described the methods used to conceal the allocation sequence and we assessed them at low risk of bias, because it was unlikely that the intervention allocation could have been foreseen. Pealing 2019a used secure web‐based software to conceal allocation; Vousden 2019 stated that "all clusters were masked to the order until two months before receiving the intervention, when the next cluster to receive the intervention was informed". It was unclear whether allocation was concealed in the remaining study, as it did not provide details of the method of concealment.
Blinding
Blinding of participants and personnel: due to the nature of the interventions, all three studies were unblinded, with study participants, healthcare professionals, and investigators aware of the intervention allocated. Blinding is not possible when exploring BP setting with a self‐monitoring BP intervention (OPTIMUM‐BP (Pealing 2019a), and is difficult when exploring BP techniques, due to the influence of BP on clinical management decisions, and the high frequency of BP measurements taken throughout pregnancy by a variety of different staff members. We assessed the risk of performance bias for all three studies at low risk of bias, because we decided that lack of blinding was unlikely to influence trial outcomes.
Blinding of outcome assessment: one of the three studies conducted a blinded endpoint analysis, and we assessed it at low risk of detection bias (Brown 1998). Outcome assessors in the two remaining trials were aware of intervention allocation, as outcomes were ‘hard clinical outcomes’. We decided that lack of blinding was unlikely to influence outcome assessment. We assessed Pealing 2019a at low risk of bias, and Vousden 2019 as unclear risk, because in balance, although the outcomes were ‘hard clinical outcomes’ and thus, unlikely to be influenced by lack of blinding, the authors also stated that a limitation was that the same team implemented and collected data, introducing possible measurement bias.
These assessments are further supported by a recent meta‐epidemiological study that explored the impact of blinding on estimated treatment effects in a wide range of meta‐analyses, which concluded that "No evidence was found for an average difference in estimated treatment effect between trials with and without blinded patients, healthcare providers, or outcome assessors" (Moustgaard 2020).
Incomplete outcome data
All three studies reported outcome data and analysis for all or almost all women randomised, and thus we assessed them at low risk of attrition bias.
Selective reporting
With no study protocol or trial registration for Brown 1998, we assessed the risk of selective reporting bias as unclear, as we did not know the expected outcomes and outcome measures. We assessed the remaining two studies at low risk of selective reporting as outcomes and outcome measures were prespecified in an ISRCTN registry (Pealing 2019a), or in a published protocol (Vousden 2019).
Other potential sources of bias
We identified other potential sources of bias for all three studies, but judged none of them at high risk of bias. In Pealing 2019a, other biases related to the evidence of cross‐over and imbalance between groups. The trial authors addressed both of these potential sources of bias; they reported that the imbalance between groups was expected because it was a feasibility study, and they minimised the effects of cross‐over by using an intention‐to‐treat analysis.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Setting of BP measurement: self‐measurement versus conventional clinic measurement.
| Setting of BP measurement: self‐measurement versus conventional clinic measurement | ||||||
| Patient or population: pregnant women with chronic or gestational hypertension Setting: 4 National Health Service maternity units in England Intervention: self‐measurement (using an automated BP device at home); daily measurements recorded in a study diary or via telemonitoring Comparison: usual care; BP monitoring by local clinical team | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with usual care | Corresponding risk with self‐monitoring | |||||
| Maternal outcomes | ||||||
| Systolic BP ≥ 150 mmHg | Not reported | |||||
| Maternal death | 0/102 maternal deaths in the self‐monitoring group, compared with 0/52 in the usual care group | ‐ | 154 (1 study) | ‐ | ||
| Pre‐eclampsia | Study population | RR 1.49 (0.87 to 2.54) | 154 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 250 per 1000 | 373 per 1000
(218 to 635) 123 more per 1000 (from 33 fewer to 385 more) |
|||||
| Number of antenatal hospital admissions | Not reported | |||||
| Maternal admission to intensive care | 1/102 maternal admission to intensive care in the self‐monitoring group, compared with 0/52 in the usual care group | RR 1.54 (0.06 to 37.25) | 154 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| Induction of labour | Study population | RR 1.09 (0.82 to 1.45) | 154 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 558 per 100 | 608 per 1000
(457 to 809) 50 more per 1000 (from 100 fewer to 251 more) |
|||||
| Neonatal outcomes | ||||||
|
Stillbirth (death after 24 weeks inutero) |
2/102 stillbirths in the self‐monitoring group, compared with 0/52 in the usual care group | RR 2.57 (0.13 to 52.63) | 154 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| Neonatal death | 1/102 neonatal death in the self‐monitoring group compared with 0/52 in the usual care group | RR 1.54 (0.06 to 37.25) | 154 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| Perinatal mortality | Not reported | |||||
|
Preterm birth (birth before 34 completed weeks' gestation) |
Study population | RR 1.15 (0.37 to 3.55) | 154 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 77 per 1000 | 88 per 1000
(28 to 273) 12 more per 1000 (from 48 fewer to 196 more) |
|||||
| Neonatal unit admission | Study population | RR 1.53 (0.65 to 3.62) | 154 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 115 per 1000 | 177 per 1000
(75 to 418) 61 more per 1000 (from 40 fewer to 302 more) |
|||||
| Neonatal unit length of stay | Not reported | |||||
| Neonatal endotracheal intubation and use of mechanical ventilation | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded two levels for very serious imprecision: few participants, wide 95% confidence interval spanning possible harm and possible benefit, based on a feasibility trial not powered to measure clinical outcomes.
Summary of findings 2. Technique of BP measurement: Korotkoff phase IV versus Korotkoff phase V to represent diastolic BP.
| Technique of BP measurement: Korotkoff phase IV versus Korotkoff phase V to represent diastolic BP | ||||||
| Patient or population: pregnant women with diastolic hypertension Setting: 2 obstetric units in Australia Intervention: Korotkoff phase IV (K4, muffling) to represent diastolic BP Comparison: Korotkoff phase V (K5, disappearance of sound) to represent diastolic BP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with K5 | Corresponding risk with K4 | |||||
| Maternal outcomes | ||||||
| Systolic BP ≥ 150 mmHg | Not reported | |||||
| Maternal death | 0/103 maternal deaths in the K4 group, compared with 0/117 in the K5 group | ‐ | 220 (1 RCT) | ‐ | ||
| Pre‐eclampsia | Study population | RR 1.16 (0.89 to 1.49) | 220 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 479 per 1000 | 555 per 1000
(426 to 713) 77 more per 1000 (from 53 fewer to 235 more) |
|||||
| Number of antenatal hospital admissions | Not reported | |||||
| Maternal admission to intensive care | Not reported | |||||
| Induction of labour | Not reported | |||||
| Neonatal outcomes | ||||||
| Stillbirth | Not reported | |||||
| Neonatal death | Not reported | |||||
| Perinatal mortality | Study population | RR 1.14 (0.16 to 7.92) | 220 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 17 per 1000 | 19 per 1000
(3 to 135) 2 more per 1000 (from 14 fewer to 118 more) |
|||||
| Preterm birth | Not reported | |||||
| Neonatal unit admission | Not reported | |||||
| Neonatal unit length of stay | Not reported | |||||
| Neonatal endotracheal intubation and use of mechanical ventilation | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded two levels for very serious imprecision: few participants and wide 95% confidence intervals spanning possible harm and possible benefit
Summary of findings 3. Technique of BP measurement: CRADLE intervention versus usual care.
| Technique of BP measurement: CRADLE intervention versus usual care | ||||||
| Patient or population: pregnant women, or those within 42 days of delivery Setting: 10 clusters (1 urban or peri‐urban secondary or tertiary health facility providing comprehensive emergency obstetric care, plus referring peripheral facilities) across 8 countries (Ethiopia, Haiti, Sierra Leone, Zimbabwe, India, Uganda, Zambia, and Malawi) Intervention: CRADLE device (a semi‐automated BP monitor, Vital Sign Alert, Microlife 3AS1‐2) and an education package Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with usual care | Corresponding risk with CRADLE intervention | |||||
| Maternal outcomes | ||||||
| Systolic BP ≥ 150 mmHg | Not reported | |||||
| Maternal death | Study population | RR 0.80 (0.30 to 2.11) | 536,233 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Effect estimate is adjusted RR, taking into account cluster design. | |
| 2 per 1000 | 1 per 1000
(1 to 4) 0 fewer per 1000 (from 1 fewer to 2 more) |
|||||
| Pre‐eclampsia | Not reported | |||||
| Number of antenatal hospital admissions | Not reported | |||||
| Maternal admission to intensive care | Not reported | |||||
| Induction of labour | Not reported | |||||
| Neonatal outcomes | ||||||
| Stillbirth | Not reported | |||||
| Neonatal death | Not reported | |||||
| Perinatal mortality | Not reported | |||||
| Preterm birth | Not reported | |||||
| Neonatal unit admission | Not reported | |||||
| Neonatal unit length of stay | Not reported | |||||
| Neonatal endotracheal intubation and use of mechanical ventilation | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for serious indirectness: intervention and comparator were not directly representative of the prespecified settings and techniques. The intervention was an oscillometric device with additional features (early warning system and an eduction package) and the comparator usual care, which varied across the clusters according to local context. bDowngraded one level for serious imprecision: wide 95% CI spanning possible benefit and possible harm.
Comparison 1: Setting. Self‐measurement (using an automated BP device at home) versus conventional clinic measurement
One study (154 women) investigated the effects of self‐measurement of BP to manage pregnancy hypertension (Pealing 2019a; Table 1). Pealing 2019a calculated results separately by hypertensive status (chronic hypertension and gestational hypertension); we combined the data in our analyses.
Primary outcomes
Systolic BP greater than or equal to 150 mmHg
Not reported.
Secondary outcomes
Maternal outcomes
Maternal death
No women in either the self‐monitoring (0/102) or the usual care group died (0/52; Analysis 1.1).
Pre‐eclampsia
Self‐monitoring may lead to slightly more diagnoses of pre‐eclampsia compared with usual care (risk ratio (RR) 1.49, 95% CI 0.87 to 2.54; 1 study, 154 women; low‐certainty evidence; Analysis 1.1) but the 95% CI is wide and includes the possibility of fewer diagnoses of pre‐eclampsia with self‐monitoring. It may be that 12.3% more women will have pre‐eclampsia diagnosed with self‐monitoring than with usual care (from 3.3% fewer to 38.5% more).
1.1. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 1: Pre‐eclampsia
Eclampsia
Two women in the usual care group had eclampsia diagnosed compared with none in the self‐monitoring group (RR 0.10, 95% CI 0.01 to 2.11; 1 study, 154 women; Analysis 1.2).
1.2. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 2: Eclampsia
Cerebrovascular event
Not reported.
Renal failure
Not reported.
Hepatic failure
Not reported.
HELLP syndrome
One woman in the self‐monitoring group developed HELLP syndrome compared with none in the usual care group (RR 1.54, 95% CI 0.06 to 37.25; 1 study, 154 women; Analysis 1.3).
1.3. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 3: HELLP syndrome
Disseminated intravascular coagulation
Not reported.
Pulmonary oedema
Not reported.
Placental abruption
Not reported.
Obstetric haemorrhage
Not reported.
Neonatal outcomes
Stillbirth
Pealing 2019a defined stillbirth as death after 24 weeks in utero.
There were 2/102 stillbirths in the self‐monitoring group compared with 0/52 in the usual care group. We are uncertain if there is any difference between groups in the incidence of stillbirth, because the certainty of evidence is low, and the 95% CI is very wide, and includes both appreciable harm and appreciable benefit (RR 2.57, 95% CI 0.13 to 52.63; 1 study, 154 babies; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 4: Stillbirth (death after 24 weeks in utero)
Neonatal death
There was 1/102 death in the self‐monitoring group compared with 0/52 in the usual care group. We are uncertain if there is any difference between groups in the incidence of neonatal death, because the certainty of evidence is low, and the 95% CI is very wide and includes both appreciable harm and appreciable benefit (RR 1.54, 95% CI 0.06 to 37.25; 1 study, 154 babies; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 5: Neonatal death
Perinatal mortality
Not reported.
Preterm birth
Pealing 2019a defined preterm birth as birth before 34 completed weeks' gestation.
There were 9/102 preterm births in the self‐monitoring group compared with 4/52 in the usual care group. We are uncertain if there is any difference between groups in the incidence of preterm birth, because the certainty of evidence is low, and the 95% CI is wide and includes both appreciable harm and appreciable benefit (RR 1.15, 95% CI 0.37 to 3.55; 1 study, 154 babies; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 6: Preterm birth (birth before 34 completed weeks' gestation)
Use of health service resources
Maternal: number of antenatal clinic visits
Not reported.
Maternal: number of antenatal hospital admissions
Not reported.
Maternal: induction of labour
There may be little to no difference between self‐monitoring and usual care for induction of labour (RR 1.09, 95% CI 0.82 to 1.45; 1 study, 154 women; low‐certainty evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 7: Induction of labour
Maternal: operative delivery
In the self‐monitoring group, 64/102 women had a caesarean section birth compared with 24/52 in the usual group (RR 1.36, 95% CI 0.98 to 1.89; 1 study, 154 women; Analysis 1.8).
1.8. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 8: Operative delivery
Maternal: admission to intensive care
In the self‐monitoring group, 1/102 woman was admitted to intensive care, compared with 0/52 in the usual care group. We are uncertain if there is any difference between groups in admission to intensive care, because the certainty of evidence is low, and the 95% CI is very wide and includes both appreciable harm and appreciable benefit (RR 1.54, 0.06 to 37.25; 1 study, 154 women; low‐certainty evidence; Analysis 1.9).
1.9. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 9: Maternal admission to intensive care
Maternal: length of stay in intensive care
Not reported.
Maternal: ventilation
Not reported.
Maternal: dialysis
Not reported.
Neonatal: neonatal unit admission
Self‐monitoring may lead to slightly more neonatal unit admissions compared with usual care (RR 1.53, 0.65 to 3.62; 1 study, 154 women; low‐certainty evidence; Analysis 1.10) but the 95% CI is wide and includes the possibility of fewer admissions with self‐monitoring. Self‐monitoring may lead to 6.1% more neonatal unit admissions with self‐monitoring compared with usual care (from 4% fewer to 30.2% more).
1.10. Analysis.

Comparison 1: Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement, Outcome 10: Neonatal unit admission
Neonatal: neonatal unit length of stay
Not reported.
Neonatal: endotracheal intubation and use of mechanical ventilation
Not reported.
Measures of maternal quality of life
Not reported.
Comparison 2: Technique. Korotkoff phase IV (K4, muffling sound) versus Korotkoff phase V (K5, disappearance of sound) to represent diastolic BP
One study (220 women) investigated management of hypertensive pregnancies according to either Korotkoff phase IV (K4, muffling sound) or Korotkoff phase V (K5,disappearance of sound) to represent diastolic BP (Brown 1998; Table 2). The primary outcome was severe hypertension, defined as either systolic BP 170 mmHg, or diastolic BP 110 mmHg, or both. As systolic and diastolic severe hypertension were not reported separately, our primary outcome could not be extracted.
Primary outcomes
Systolic BP greater than or equal to 150 mmHg
Not reported.
Secondary outcomes
Maternal outcomes
Maternal death
There were no maternal deaths in either the K4 group (N = 103 women) or the K5 group (N = 117; Analysis 2.1).
Pre‐eclampsia
There may be little to no difference between K4 and K5 in terms of pre‐eclampsia diagnosis (RR 1.16, 95% CI 0.89 to 1.49; 1 study, 220 women; low‐certainty evidence; Analysis 2.1) but the wide 95% CI includes the possibility of more pre‐eclampsia diagnoses with K4.
2.1. Analysis.

Comparison 2: Technique of BP measurement: Korotkoff phase IV (K4, muffling sound) versus Korotkoff phase V (K5, disappearance of sound) to represent diastolic BP, Outcome 1: Pre‐eclampsia
Eclampsia
Not reported.
Cerebrovascular event
Not reported.
Renal failure
Not reported.
Hepatic failure
Not reported.
HELLP syndrome
Not reported.
Disseminated intravascular coagulation
Not reported.
Pulmonary oedema
Not reported.
Placental abruption
Not reported.
Obstetric haemorrhage
Not reported.
Neonatal outcomes
Stillbirth
Not reported.
Neonatal death
Not reported.
Perinatal mortality
We are uncertain if the use of K4 reduces or increases perinatal mortality, because the certainty of evidence is low, and the 95% CI is wide and includes both appreciable harm and appreciable benefit (RR 1.14, 95% CI 0.16 to 7.92; 1 study, 220 women; low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Technique of BP measurement: Korotkoff phase IV (K4, muffling sound) versus Korotkoff phase V (K5, disappearance of sound) to represent diastolic BP, Outcome 2: Perinatal mortality
Preterm birth
Not reported.
Use of health service resources
Not reported.
Measures of maternal quality of life
Not reported.
Comparison 3: Technique. CRADLE intervention (CRADLE device, a semi‐automated BP monitor with additional features, and education package) versus usual care
One stepped‐wedge cluster‐RCT investigated the introduction of the CRADLE device (Microlife 3AS1‐2) and an education package (Vousden 2019; 536,233 deliveries; Table 3).
Here we present adjusted data (reported in the trial report) as RRs (as planned), as the outcomes were rare, in which case, odds ratio (OR) closely approximated the RR.
Primary outcomes
Systolic BP greater than or equal to 150 mmHg
Not reported.
Secondary outcomes
Maternal outcomes
Maternal death
There may be little to no difference in the number of maternal deaths following the introduction of the CRADLE device compared with usual care (adjusted RR 0.80, 95% CI 0.30 to 2.11; 1 study, 536,233 deliveries; low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Technique of BP measurement: CRADLE intervention (CRADLE device: semi‐automated BP monitor with additional features, plus an education package) versus usual care, Outcome 1: Maternal death
Pre‐eclampsia
Not reported.
Eclampsia
The number of women who were diagnosed with eclampsia following the introduction of the CRADLE device was 1378/288,995, compared with 1314/247,238 following usual care (adjusted RR 1.91, 95% CI 0.91 to 4.02; 1 study, 536,233 deliveries; Analysis 3.2).
3.2. Analysis.

Comparison 3: Technique of BP measurement: CRADLE intervention (CRADLE device: semi‐automated BP monitor with additional features, plus an education package) versus usual care, Outcome 2: Eclampsia
Cerebrovascular event
The number of women who had a stroke was only collected for women with the primary outcome (a selected subdivision of the main study population identified by an outcome, not by baseline characteristics, thereby, breaking the random allocation). Therefore, trial groups would not have comparable populations, and any differences between the groups could not be attributed to the intervention, so, we did not use data relating to secondary maternal outcomes.
Renal failure
Not reported.
Hepatic failure
Not reported.
HELLP syndrome
Not reported.
Disseminated intravascular coagulation
Not reported.
Pulmonary oedema
Not reported.
Placental abruption
Not reported.
Obstetric haemorrhage
Not reported.
Neonatal outcomes
Stillbirth
The number of stillbirths was only collected from women with the primary outcome (a selected subdivision of the main study population identified by an outcome, not by baseline characteristics, thereby, breaking the random allocation). Therefore, trial groups would not have comparable populations, and any differences between the groups could not be attributed to the intervention, so, we did not use data relating to secondary neonatal outcomes.
Neonatal death
The number of neonatal deaths was only collected from women with the primary outcome (a selected subdivision of the main study population identified by an outcome, not by baseline characteristics, thereby, breaking the random allocation). Therefore, trial groups would not have comparable populations, and any differences between the groups could not be attributed to the intervention, so, we did not use data relating to secondary neonatal outcomes.
Perinatal mortality
Not reported.
Preterm birth
Not reported.
Use of health service resources
Maternal: number of antenatal clinic visits
Not reported.
Maternal: number of antenatal hospital admissions
Not reported.
Maternal: induction of labour
Not reported.
Maternal: operative delivery
The number of women birthing by caesarean section was only collected from women with the primary outcome (a selected subdivision of the main study population identified by an outcome, not by baseline characteristics, thereby, breaking the random allocation). Therefore, trial groups would not have comparable populations, and any differences between the groups could not be attributed to the intervention, so, we did not use data relating to secondary maternal outcomes.
Maternal: intensive care admission
Maternal admissions to an intensive care unit were only collected for women with the primary outcome (a selected subdivision of the main study population identified by an outcome, not by baseline characteristics, thereby, breaking the random allocation). Therefore, trial groups would not have comparable populations, and any differences between the groups could not be attributed to the intervention, so, we did not use data relating to secondary maternal outcomes.
Maternal: length of stay in intensive care admission
Not reported.
Maternal: ventilation
Not reported.
Maternal: dialysis
Not reported.
Neonatal: neonatal unit admission
Not reported.
Neonatal: neonatal unit length of stay
Not reported.
Neonatal: endotracheal intubation and use of mechanical ventilation
Not reported.
Measures of maternal quality of life
Not reported.
Discussion
Summary of main results
We identified three randomised controlled trials (RCT), with a total of 536,607 women (one of which was a cluster‐RCT involving 536,233 deliveries), to assess the effects of setting and technique of blood pressure (BP) measurement for diagnosing and managing hypertensive disorders in pregnancy, on maternal and perinatal outcomes, and use of health service resources. We judged the studies to be generally at low risk of bias, and the certainty of evidence low.
Of the nine prespecified settings and techniques we wished to assessed, studies addressed three: one examined setting (self‐monitoring BP versus usual care), and two examined techniques (Korotkoff phase IV (K4) versus Korotkoff phase V (K4); and the CRADLE device versus usual care).
Self‐measurement (using an automated BP device at home) versus conventional clinic BP measurement
Low‐certainty evidence shows that self‐monitoring BP compared with usual care may lead to slightly more diagnoses of pre‐eclampsia, may make little to no difference to the likelihood of induction of labour, and may lead to slightly more neonatal unit admissions.
As the certainty of the evidence is low (downgraded two levels for imprecision due to the small sample size, few events and wide 95% CI) we are uncertain if self‐monitoring BP changes the risk of stillbirth, neonatal death, preterm birth, or maternal admission to intensive care compared with usual care.
Korotkoff phase IV (K4, muffling sound) versus Korotkoff phase V (K5, disappearance of sound) to represent diastolic BP
Low‐certainty evidence shows that there may be little to no difference between K5 compared with K4 in terms of pre‐eclampsia diagnosis. We are uncertain if there is a difference in perinatal mortality between the groups because of the low‐certainty of evidence.
CRADLE intervention (CRADLE device, a semi‐automated BP monitor with additional features, and an education package) versus usual care
Low‐certainty evidence shows there is probably little or no difference between the CRADLE device and usual care in the risk of maternal death compared with usual care.
Other outcomes of interest were either not measured, or were not measured in the whole population.
Overall completeness and applicability of evidence
Although we conducted a comprehensive search of the worldwide literature for RCTs on the setting and technique of BP measurement for diagnosing and managing hypertensive disorders in pregnancy, the evidence we identified was limited to three of the nine settings and techniques we set out to investigate. Moreover, we did not identify any evidence relating to our primary outcome, or to several of our important prespecified secondary outcomes.
The healthcare context of two of the three included studies suggests that the effects of the interventions in other contexts may be different, and the applicability of this evidence limited. This is evident for self‐monitoring BP and the introduction of the CRADLE device, whereby both were compared with usual care. With usual care as a comparator, the evidence from the introduction of the CRADLE device is likely only applicable to the low‐ and middle‐income country settings, whereas self‐monitoring BP is only applicable to a high‐income healthcare context. The evidence from K5 versus K4 is likely to be highly applicable, because of the universal intervention and comparator, which are independent of healthcare context.
Quality of the evidence
Overall, we judged the studies to have a low risk of bias. One of the three included studies (K4 versus K5), was judged to have an unclear risk of selection bias and reporting bias due to lack of information reported in the study paper. Another study (introducing the CRADLE device), was judged to have an unclear risk of detection bias due to the pragmatic, large study design.
We assessed the body of evidence to be of low‐certainty. For self‐monitoring versus usual care and K4 versus K5 for diastolic BP, we downgraded two levels for imprecision due to the small sample sizes, few events, and wide 95% confidence intervals (CI) that crossed the line of no effect.
We downgraded the evidence relating to the introduction of the CRADLE device one level for impression (wide 95% CI that crossed the line of no effect), and one level for serious indirectness (intervention and comparator were not directly representative of the prespecified settings and techniques). The intervention was an oscillometric device with additional features (early warning system and an eduction package) and the comparator, usual care, which varied across clusters according to local context.
Potential biases in the review process
To minimise potential biases in the review process, we conducted a comprehensive literature search and ensured that data extraction, and assessment of risk of bias and GRADE were done independently, by at least two review authors.
Authors of two of the three included studies are review authors. These authors had no part in assessment, data extraction, or data entry.
Without a core outcome set for hypertensive disorders in pregnancy, there is potential for the introduction of bias in the selection of the prespecified outcomes. Also, there were only a few of the prespecified outcomes investigated in the included studies. Furthermore, the study exploring self‐monitoring BP is a small feasibility study, which was not powered to detect differences between groups for clinical outcomes, and thus, we were unable to draw definitive conclusions from this study.
Agreements and disagreements with other studies or reviews
A number of the BP monitoring techniques (multiple BP measurements at one clinic visit, using an appropriately sized BP cuff, resting prior to BP measurement) are already detailed in clinical guidelines: the NICE Antenatal care for uncomplicated pregnancies (NICE 2008), the NICE Hypertension in adults: diagnosis and management (NICE 2019a), and the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy (ACOG 2013). As these are already good practice recommendations, it is unlikely that new research will be funded or generated to explore these BP techniques.
It is important to note that dynamic BP changes in pregnancy are distinct, and occur in a shorter time‐frame than outside of pregnancy, therefore, some of the BP settings and techniques recommended in adult hypertension guidance cannot be extrapolated for hypertension in pregnancy. This includes the use of ambulatory BP monitors, and self‐monitoring BP in pregnancy, for which there is ongoing research.
Authors' conclusions
Implications for practice.
Based on the evidence in this review:
Any potential benefit, if present, of self‐monitoring BP in hypertensive pregnancies remains uncertain, as the evidence is limited to a feasibility study with a small sample size. It is uncertain whether labelling women with hypertension (using self‐monitored BP measurements or clinic measurement) is associated with clinical benefit, in the absence of an effect on clinical outcomes.
Current practice of using K5 to measure diastolic BP is supported in women with hypertension in pregnancy. It is uncertain whether labelling women with hypertension is associated with clinical benefit; however, since the results for clinical outcomes were inconclusive in the K5 and K4 groups, K5 was deemed preferable to K4. Use of K5 is further supported by the smaller differences between K4 and K5 in the hypertensive pregnant population compared with normotensive women. Additionally, K5 is noted to be closer to intra‐arterial pressure and is more reliably detected.
Any potential benefit, if present, of using the CRADLE device to monitor BP in pregnancy remains uncertain, due to the limitations and instability of the trial study design.
Antenatal guidelines addressing settings and techniques for measuring BP in pregnancy (a cornerstone of antenatal care as advised by the World Health Organization) will likely need to be made based on consensus, reflecting the committee’s experience, since current evidence is lacking.
Implications for research.
Of the BP techniques and settings intended for review, there was a paucity of appropriately designed studies using BP monitors validated for use in pregnancy. Ideally, there would be robust evidence for all settings and techniques, but some may be prioritised above others, relating to perceived importance by healthcare professionals and lay people. This has been addressed in the recent James Lind Alliance Priority Setting Partnership for Hypertension in Pregnancy (www.jla.nihr.ac.uk/priority-setting-partnerships/hypertension-in-pregnancy/).
There is active research ongoing for both settings (ambulatory and self‐monitoring of BP) both relating to management of high BP (or those at higher risk). The research question of whether there is benefit to self‐monitoring BP is being examined in a definitive trial, which is ongoing, with an estimated study completion date at the end of 2020 (Dougall 2020). The use of ambulatory BP monitoring in pregnancy is hampered, because currently, there is only one validated ambulatory BP monitor for use in pregnancy (stridebp.org/bp-monitors/).
It may be that clinical practice has evolved, such that addressing a number of the techniques set out to be reviewed are no longer directly relevant. For example, the greater use of new technology and automated BP monitors in high‐income settings will likely reduce the need to decide on systolic BP versus diastolic BP, or mean arterial pressure. Despite this, there remains uncertainty around whether systolic, diastolic, or mean arterial pressure better predicts pregnancy outcomes.
History
Protocol first published: Issue 8, 2017 Review first published: Issue 8, 2020
Acknowledgements
We would like to acknowledge the contribution that Natasha L Hezelgrave and Mariana Widmer made towards the protocol for this review (Nathan 2017). We would also like to thank Cochrane Methods Suport for their help with data extraction for the Vousden 2019 study.
This project was supported by the National Institute for Health Research, via Cochrane Reviews of NICE Priority funding to Cochrane Pregnancy and Childbirth (award number 131142). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
The authors retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), members of our international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Dr Edgardo Abalos, Vice Director, Centro Rosarino de Estudios Perinatales (CREP), Rosario, Argentina; Anne Alice Chantry, Cress Universite de Paris Inserm U1153, Epope Team, Paris, France; and a referee who wishes to remain anonymous.
Appendices
Appendix 1. search terms for ICTRP and ClinicalTrials.gov
Each line was run separately and manually de‐duplicated
blood pressure AND monitoring AND pregnancy
blood pressure AND monitor AND pregnancy
blood pressure AND measurement AND pregnancy
blood pressure AND measure AND pregnancy
ambulatory AND pregnancy
Data and analyses
Comparison 1. Setting of BP measurement: self‐measurement (with automated BP device at home) versus conventional clinic measurement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pre‐eclampsia | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.87, 2.54] |
| 1.2 Eclampsia | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 2.11] |
| 1.3 HELLP syndrome | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.06, 37.25] |
| 1.4 Stillbirth (death after 24 weeks in utero) | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.13, 52.63] |
| 1.5 Neonatal death | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.06, 37.25] |
| 1.6 Preterm birth (birth before 34 completed weeks' gestation) | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.37, 3.55] |
| 1.7 Induction of labour | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.82, 1.45] |
| 1.8 Operative delivery | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.98, 1.89] |
| 1.9 Maternal admission to intensive care | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.06, 37.25] |
| 1.10 Neonatal unit admission | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.65, 3.62] |
Comparison 2. Technique of BP measurement: Korotkoff phase IV (K4, muffling sound) versus Korotkoff phase V (K5, disappearance of sound) to represent diastolic BP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pre‐eclampsia | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.89, 1.49] |
| 2.2 Perinatal mortality | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.16, 7.92] |
Comparison 3. Technique of BP measurement: CRADLE intervention (CRADLE device: semi‐automated BP monitor with additional features, plus an education package) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Maternal death | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.30, 2.11] | |
| 3.2 Eclampsia | 1 | Risk Ratio (IV, Fixed, 95% CI) | 1.91 [0.91, 4.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 1998.
| Study characteristics | ||
| Methods | Prospective, multi‐centre, 2‐arm RCT, participants recruited from 2 obstetric units in Australia. Authors state "they were randomly assigned management with K4… or K5… for the remainder of the pregnancy". Authors state "Management was randomly assigned to individual pregnant women from a computer‐generated list of random numbers, determined before the start of the study and maintained centrally in a closed file in the maternity wards… Randomisation was stratified for centre before the study". |
|
| Participants | 220 pregnant women with diastolic hypertension, K4 ≥ 90 mm Hg (following 1 night as an inpatient or the average of 4 to 6 BP measurements following 4 hours in day‐assessment unit), after 20 weeks of gestation. | |
| Interventions |
Intervention: Korotkoff phase IV (K4, muffling) in pregnancy (N = 103) Comparison: Korotkoff phase V (K5, disappearance of sound) in pregnancy (N = 117) |
|
| Outcomes |
Maternal: maternal death, maternal morbidity (eclampsia, renal failure, cerebrovascular disease), diagnosis (pre‐eclampsia, proteinuria pre‐eclampsia, gestational, essential hypertension, essential hypertension, pre‐eclampsia, renal causes), severe hypertension, need for antihypertensive treatment, maternal laboratory data before delivery (plasma creatinine, plasma urate, plasma aspartate aminotransferase, platelet count, haemoglobin) and medication score Birth: gestational age at delivery, length of pregnancy from study entry and delivery for the indication of uncontrolled maternal hypertension Perinatal: birthweight, small‐for‐gestational age and perinatal mortality |
|
| Notes |
Trial dates: not reported Funding sources: not reported Ethical approval: yes, study approval by the Southern Sydney Area Health Service Ethics Committee Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors state "Management was randomly assigned to individual pregnant women from a computer‐generated list of random numbers, determined before the start of the study and maintained centrally in a closed file in the maternity wards… Randomisation was stratified for centre before the study". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors state "…open study… Accordingly, patients, doctors, and midwives were all aware of the random allocation". The outcome is unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded endpoint analysis. Authors state "Endpoint assessment was undertaken by a research assistant experienced in documenting the outcomes of hypertensive pregnancies but not involved in the conduct of this study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 220 women randomised, completed the trial |
| Selective reporting (reporting bias) | Unclear risk | Protocol publication was not common practice at the time so we do not know if the trial reports all the pre‐specified outcomes. |
| Other bias | Low risk | Authors state "We decided against measuring both K4 and K5 in each woman in this study because we thought it might introduce bias, in either the person recording the blood pressure or the clinician making management decisions". "To facilitate accuracy of recordings, extensive in‐service meetings were held among junior medical officers and midwives, who did the vast majority of readings in this study. Diastolic blood pressure has traditionally been recorded as K4 in these units. Before the study started, each midwife in the day‐assessment unit and antenatal wards had to record blood pressure accurately in more than 85% of case examples used in the British Hypertension Society video on blood‐pressure measurement methods. These examples were modified to include K4 and K5 diastolic blood‐pressure recordings and all participants were instructed to record the blood pressure as K4, if a woman had been randomised to that group but no K4 was audible." |
Pealing 2019a.
| Study characteristics | ||
| Methods | Prospective, multi‐centre 2‐arm RCT, participants recruited from 4 NHS maternity units in England. Authors state "Women were randomised, stratified for recruiting centre and type of hypertension, in permuted blocks of random size (2 or 4) in a 2:1 ratio to BP self‐monitoring or usual care… The randomisation sequence was computer generated, and secure web‐based software was used to ensure allocation concealment, although due to the nature of the intervention, neither participants nor investigators or clinicians were masked". |
|
| Participants | 158 women aged ≥ 18 years with a singleton pregnancy and chronic hypertension (a prenatal diagnosis of hypertension or sustained BP readings ≥ 140 mmHg systolic, or ≥ 90 mmHg diastolic, or both, before 20 weeks’ gestation) or gestational hypertension (sustained BP readings ≥ 140 mmHg systolic, or ≥ 90 mmHg diastolic, or both after 20 weeks’ gestation and without proteinuria), without pre‐eclampsia. Women with chronic hypertension were recruited between booking and 23 + 6 weeks’ gestation. Women with gestational hypertension were recruited between 20 and 37 + 6 weeks’ gestation. Women were excluded if they were unwilling to self‐monitor, had insufficient understanding of the study. 158 women were randomised however four women experienced either fetal loss, were lost to follow up or withdrew from the study, therefore 154 women were included in the analysis. | |
| Interventions |
Intervention: self‐monitoring of BP involving daily home BP measurements recorded in a study diary or telemonitoring (N = 104) Comparison: usual care involving BP monitoring by their local clinical team (N = 54) |
|
| Outcomes |
Feasibility: recruitment, discontinuation, adherence to study visits and persistence with the self‐monitoring protocol Birth: time between randomisation and delivery, need for intravenous antihypertensive drugs week before delivery, method of delivery onset, induction of labour by indication of pre‐eclampsia, hypertension, or fetal growth restriction, gestation at delivery, preterm birth, mode of delivery. Maternal: diastolic BP, systolic BP, pre‐eclampsia, adverse maternal outcome (eclampsia, intracranial haemorrhage or infarct, HELLP syndrome, or death), admission to intensive care unit, admission nights, antihypertensive medication prescribing and behaviour, self‐reported medication beliefs and adherence, self‐reported quality of life scores, and BP preference Perinatal: birthweight, small‐for‐gestational age, perinatal death, admission to neonatal unit, and admission nights |
|
| Notes |
Trial dates: December 2015 to December 2017 Funding sources: study supported by Research Professorships from the National Institute for Health Research, NIHR Oxford Collaboration for Leadership in Applied Health Research and Care, NIHR Oxford Biomedical Research Centre, and 1 of the authors is part‐time employed by Sensyne Health plc. Ethical approval: yes, study approval by the UK Research Ethics Committee Declarations of interest: reported, the authors state "The authors declare the following conflicts of interest: LM receives consultancy fees from Drayson Technologies". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors state "Women were randomised, stratified for recruiting centre and type of hypertension, in permuted blocks of random size (2 or 4) in a 2:1 ratio to BP self‐monitoring or usual care… The randomisation sequence was computer‐generated, and secure web‐based software was used to ensure allocation concealment, although due to the nature of the intervention, neither participants nor investigators or clinicians were masked". |
| Allocation concealment (selection bias) | Low risk | Authors state "The randomisation sequence was computer‐generated, and secure web‐based software was used to ensure allocation concealment…" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors state "…due to the nature of the intervention, neither participants nor investigators or clinicians were masked". The outcome is unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors state "Outcome measurement was not masked". Outcomes were ‘hard clinical outcomes’ and thus outcome assessment unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors state "…outcome data were available from 154 (97%) and were included in the analysis...Three women (7%) from the intervention group provided no home readings at all (2 who formally discontinued with intervention very early in follow‐up and one who never supplied home data)." |
| Selective reporting (reporting bias) | Low risk | Trial retrospectively registered on the ISRCTN registry. All outcomes detailed were reported, including within supplementary tables. |
| Other bias | Unclear risk | Authors state "The study detected evidence of cross‐over between groups, (17% to 39% of our usual care group with gestational and chronic hypertension respectively taking up some form of home monitoring); however, the evidence from the non‐pregnant hypertensive population has shown that informal self‐monitoring alone has limited impact on BP control as opposed to self‐monitoring combined with active intervention". Bias noted and minimised by an intention‐to‐treat analysis. "Chance imbalances in outcomes between randomisation groups are likely in feasibility trials with smaller sample sizes; we saw evidence for this within 2 known important predictors of perinatal outcome (ethnicity and previous pregnancy hypertensive disorder) within the group with chronic hypertension. The small numbers used for feasibility studies also preclude them being able to successfully minimise or adjust for all important confounders in the randomisation and analyses of such studies". Bias noted but expected for a feasibility study. |
Vousden 2019.
| Study characteristics | ||
| Methods | Prospective, multi‐centre cluster‐RCT in 10 clusters across Africa, India, and Haiti: Addis Ababa in Ethiopia, Cap Haitien in Haiti, Freetown in Sierra Leone, Harare in Zimbabwe, Gokak in India, Kampala and Mbale in Uganda, Lusaka and Ndola in Zambia, and Zomba plus the Southern Region in Malawi. A pragmatic, stepped‐wedge, cluster‐RCT with 2 arms. Authors state "A computer‐generated randomly allocated sequence run by the CRADLE statistician… determined the order in which the clusters received the intervention". |
|
| Participants | Among 536,233 deliveries, the primary outcome occurred in 4067 women. Pregnant women or those within 42 days of delivery, presenting for maternity care in a cluster facility or community healthcare providers, were exposed to the intervention. No exclusion criteria. | |
| Interventions |
Intervention: CRADLE Vital Sign Alert (Microlife 3AS1‐2) and an education package Comparison: usual care |
|
| Outcomes |
Primary composite maternal outcome: maternal death, eclampsia, or emergency hysterectomy rate Secondary maternal outcomes: eclampsia, emergency hysterectomy, and maternal death, intensive care unit admission, stroke Secondary perinatal outcomes: stillbirth, neonatal death Secondary outcomes including maternal admission to intensive care and perinatal outcomes (stillbirths and neonatal deaths) were only collected for women with the primary outcome (a selected subdivision of the main study population identified by an outcome not by baseline characteristics, thereby breaking the random allocation). Therefore, these trial groups would not be comparable populations and any differences between the groups could not be attributed to the intervention; we did not extract and include data for these outcomes in our analyses. |
|
| Notes |
Trial dates: 1 April 2016 to 30 November 2017 Funding sources: Newton Fund Global Research Programme: UK Medical Research Council; Department of Biotechnology, Ministry of Science & Technology, Government of India; and UK Department of International Development. Ethical approval: yes, ethical approval granted by King’s College London Research Ethics Subcommittee, all countries before the start of the trial, additionally all institutional‐level consent on behalf of the cluster was obtained. Declarations of interest: reported, no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors state "Clusters crossed over from existing routine care to the CRADLE intervention in 1 of 9 steps at 2‐monthly intervals, with CRADLE devices replacing existing equipment at the randomly allocated time point. A computer‐generated randomly allocated sequence determined the order in which the clusters received the intervention". |
| Allocation concealment (selection bias) | Low risk | Authors state "All clusters were masked to the order until 2 months before receiving the intervention, when the next cluster to receive the intervention was informed". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors state "Because of the nature of the intervention, this trial was not masked". The outcomes are unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes were ‘hard clinical outcomes’ and thus outcome assessment unlikely to be influenced by lack of blinding. However, authors state "A limitation was that implementation and data collection were by the same team, introducing possible measurement bias". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full data available for maternal death outcome. Missing data for other outcomes does not impact risk of attrition bias for the review. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and prospectively registered on ISRCTN registry. All outcomes detailed were reported. Authors state "It is also plausible that in some clusters, use of the intervention might have resulted in increased reporting of the primary outcome if previously occurring without documentation in the community, with a bias against the intervention. However, case finding and data collection were carefully optimised in the feasibility phase and closely monitored by the local investigator and research team". |
| Other bias | Low risk | Models were adjusted for time trends. Authors state "we adjusted for... time from start of study (continuous; with an interaction between cluster and time so that each cluster had its own underlying time trend), and total time on the randomised intervention, with time before intervention given as zero (continuous). The analysis resulted in separate linear time trends in each cluster, as prespecified. The model for intervention analysis allowed for separate linear trends in each cluster before and after the intervention (or a change in trend at the time the intervention was introduced, known as linear splines or bent stick)". |
BP: blood pressure RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aslani 2014 | Inappropriate report type: study protocol. Inappropriate intervention type: authors state "We designed a computer‐based decision aid (CDA) for use by pregnant women at home to investigate and participate in solving their pregnancy problems related to pregnancy‐induced hypertension (PIH) and gestational diabetes (GD)". Methods: 2‐arm unblinded cluster‐RCT Participants: 420 healthy pregnant women attending participating gynaecologists Interventions: gynaecologists were randomised to either the intervention arm, providing pregnant women with a computer‐based decision aid for use at home, or to the control arm, providing usual care. Outcomes: primary outcomes: self‐efficacy and knowledge. Secondary outcomes: type and frequency of visits, BP, blood sugar changes and anxiety. |
| Brown 2012 | BP device not validated in pregnant population Methods: 2‐arm unblinded RCT with blinded end points design Participants: 220 pregnant women with hypertension, detected by using mercury sphygmomanometry Interventions: women were randomised to have all subsequent BPs recorded for the remainder of their pregnancy with either mercury sphygmomanometry or an automated BP device Outcomes: primary maternal outcome: severe hypertension. Primary fetal outcome: small‐for‐gestational age |
| Cartwright 1992 | BP device not validated in pregnant population Methods: 2‐arm unblinded RCT Participants: 99 pregnant hypertensive women Interventions: women were randomised to either hospital for conventional BP management (hospital group) or were allowed to go home with the Cambridge BP telemetry system (home group) Outcomes: number of episodes of monitoring, duration of monitoring, mean BP during monitoring, gestational age at delivery, trait and state anxiety levels |
| Denolle 2008 | BP device not validated in pregnant population Methods: 2‐arm unblinded RCT Participants: 57 women with recently discovered hypertension in pregnancy without albuminuria or a history of hypertension Interventions: all women measured their BP at home and BP results were transmitted to a centralized centre. Randomisation was to either transmit the results daily to the obstetrician or store them. Outcomes: diagnosis and prognosis of white coat hypertension |
| Duggan 1999 | BP device not validated in pregnant population Methods: 2‐arm RCT. Authors state "Women were alternately allocated to one of 2 groups". Unblinded Participants: 100 pregnant women attending antenatal clinics Interventions: women were allocated to either group 1. BP measured as soon as possible on entering the clinic room and again after 5‐minutes rest, or group 2. 5‐minute rest on entry to the clinic room prior to the first BP measurement and again after a further 5‐minute rest. Outcomes: systolic BP and diastolic BP |
| Holm 2019 | Inappropriate study type: cross‐over RCT Methods: cross‐over unblinded RCT Participants: 112 pregnant women attending ultrasound at 12 weeks' gestational age Interventions: women were randomised to start with either 2 office BP measurements taken on both arms by staff, or self‐measuring using an automated BP self‐measurement station Outcomes: primary outcome: difference in mean arterial pressure. Secondary outcomes: safety and practicality issues |
| Leung 1998 | BP device unspecified, not validated in pregnant population Methods: 2‐arm unblinded RCT Participants: 90 pregnant women with diastolic BP between 90 mmHg and 100 mmHg Interventions: women were randomised to either inpatient (routine) or outpatient (home monitoring) management of diastolic BP Outcomes: diagnosis of hypertension, severe hypertension, proteinuria, gestational age at delivery, induction of labour, mode of delivery, use of antihypertensive therapy, birthweight of baby, Apgar score at 1 minute and 5 minutes, admission into the special care nursery, stillbirth or neonatal death, length of antenatal and postnatal hospital stay, and views on the management of hypertension and their preferences for outpatient or inpatient care |
| Mangos 2012 | Inappropriate study type: cross‐over RCT. Available as a poster session conference abstract only Methods: cross‐over unblinded RCT Participants: 220 pregnant and 224 non‐pregnant participants Interventions: participants had 8 BP measurements taken, in random order; they had 4 using standard and 4 using large BP cuffs Outcomes: diagnosis of hypertension, diastolic BP and systolic BP |
| NCT03222414 | Inappropriate study type: cross‐over RCT Methods: 2‐arm cross‐over unblinded RCT Participants: 25 morbidly obese (BMI > 40 kg/m²), severely hypertensive (systolic BP > 160 mmHg), pregnant women. Recruitment terminated (stopped early and will not start again) because changes in standard of care altered the feasibility of the study with 3 recruited participants. Interventions: women were randomised to either conical Ultracheck Curve BP cuff (BP recording with conical Ultracheck Curve BP cuff and direct arterial pressure monitoring) then traditional cylindrical BP cuff (BP recording with traditional cylindrical BP cuff and direct arterial pressure monitoring) or cylindrical then conical Outcomes: comparison of: systolic BP readings, mean arterial pressures, diastolic BP readings, mean of 2 systolic BP readings, mean of 2 arterial pressures and mean of 2 systolic BP readings |
| Rhodes 2018 | BP device not validated in pregnant population. Methods: 2‐arm unblinded RCT Participants: 100 pregnant women with hypertension Interventions: following 24‐hour ambulatory BP monitoring women were randomised to either have their results ‘revealed’ to, or ‘withheld’ (concealed) from, the managing obstetric team Outcomes: primary outcome: admission to hospital. Secondary outcome: antenatal attendances, use of antihypertensive drugs, duration of hospital stay and induction of labour |
| Ross‐McGill 2000 | BP device not validated in pregnant population Methods: a pilot 2‐arm unblinded RCT Participants: 80 low‐risk pregnant women Interventions: women were randomised to either standard antenatal visit schedule (9) or a reduced schedule (3) and weekly home BP self‐monitoring Outcomes: recruitment, number of clinic visits, frequency of BP measurement, schedule preference and anxiety |
BMI: body mass index BP: blood pressure RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Dougall 2020.
| Study name | Blood pressure monitoring in high‐risk pregnancy to improve the detection and monitoring of hypertension |
| Methods | 2‐arm parallel unblinded RCT |
| Participants |
Actual enrolment: 3042 pregnant women BUMP 1: 2262 women at higher risk of hypertension in pregnancy Inclusion criteria:
Exclusion criteria:
BUMP 2: 512 women with hypertension in pregnancy (including chronic hypertension) Inclusion criteria: • Women developing pregnancy hypertension previously randomised in BUMP 1 (regardless of gestation) OR
OR
AND
Exclusion criteria: Anticipated inpatient admission considered likely to lead to imminent delivery (within the next 48 hours) Women in BUMP 1 will move into BUMP 2 if they develop hypertension |
| Interventions |
Intervention arm: self‐monitoring of BP BUMP 1: home self‐monitoring of BP at least 3 times a week BUMP 2: home daily self‐monitoring of BP monitoring Women in the intervention groups will be encouraged to use a simple mobile telemonitoring system Control arm: usual care, BP monitored by the clinical team at antenatal assessments |
| Outcomes |
Primary outcome measures: 1. Time from recruitment to diagnosis of raised BP 2. Mean systolic BP Secondary outcome measures: 1. Severe hypertension 2. Serious maternal complications 3. Onset of labour 4. Quality of life 5. Stillbirth and early neonatal deaths 6. Gestation at delivery 7. Mode of delivery 8. Birthweight including centile and small‐for‐gestational age 9. Neonatal unit admissions 10. Health behaviours 11. Fidelity to monitoring schedule 12. Anxiety questionnaire 13. Health service costs 14. Cost per quality‐adjusted life year gained over trial period 15. Qualitative data gathered from participating women and healthcare professionals 16. Mean diastolic BP 17. Mean area under the BP over time curve 18. Mean proportion of BP readings above 140 mmHg 19. Adherence to medication |
| Starting date | 22 November 2017 |
| Contact information | Richard J McManus (Principal Investigator), University of Oxford |
| Notes | clinicaltrials.gov/show/NCT03334149 Estimated study completion date: 30 December 2020 |
NCT03509272.
| Study name | LimPrOn: Limburg pre‐eclampsia investigation |
| Methods | 2‐arm parallel unblinded RCT |
| Participants |
Estimated enrolment: 1000 pregnant women from the prenatal consultations of 8 different hospitals Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention arm: remote monitoring ‐ NICCOMO device (which records haemodynamic parameters uninterrupted and reliable in an objective manner by using Impedance Cardiography), maternal venous doppler echography and Maltron measurements, taken up to 3 times in pregnancy. Wireless BP monitoring, pulse oxygen activity, weight analyser Control: no intervention |
| Outcomes |
Primary outcome measures:
Secondaryoutcome measures:
|
| Starting date | May 2016 |
| Contact information | Dorien Lanssens dorien.lanssens@uhasselt.be |
| Notes | clinicaltrials.gov/ct2/show/NCT03509272 Estimated study completion date: December 2019 |
NCT03648645.
| Study name | Hypertensive pregnant women monitored by teletransmitted self‐measurements of blood pressure |
| Methods | 2‐arm parallel unblinded RCT |
| Participants |
Estimated enrolment: 270 pregnant women with confirmed mild to moderate hypertension without pre‐eclampsia Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention arm: home daily self‐monitoring of BP with results transmitted to the healthcare team Women perform the measurement of BP at their home, on a daily basis, 3 consecutive measurements in the morning and in the evening, after a period of rest; measurements are performed using a BP monitor Bluetooth‐connected to a telecommunicating box, which automatically and securely transmits the data to the healthcare team via a secure web platform. A trained nurse or midwife participating in the project will daily consult the BP values and alert history through a secure web interface. If a threshold value is exceeded (and depending on the alert), a consultation will be scheduled (unexpected consultation) and the woman notified. Control arm: standard follow‐up consultation in the hospital Standard care includes several follow‐up visits on the maternity wards, where the woman is cared for, and at a frequency set by the coordinating physician on a case‐by‐case basis, depending on the severity of the arterial hypertension. During the follow‐up visits, the following examinations will be carried out on the maternity services in charge of the woman. Examinations will be conducted routinely as part of a pregnancy follow‐up. |
| Outcomes |
Primary outcome measures: 1. Hypertension specific intervention, proportion of women requiring at least 1 extended consultation, or unplanned hospitalisation due to pregnancy hypertension Secondary outcome measures: 1. Number of significant alerts (that result in additional consultation, initiation or modification of antihypertensives) 2. Number of alerts 3. Likert scale to assess woman's satisfaction |
| Starting date | 9 April 2020 |
| Contact information | Thierry Denolle, (+33)299 16 88 88, denolle.thierry@wanadoo.fr Cathy Drexler, cdrexler@softelem.com |
| Notes | clinicaltrials.gov/ct2/show/NCT03648645 Estimated study completion date: 9 September 2022 |
NCT03858595 2019.
| Study name | Optimizing gestational weight gain, birthweight, and other perinatal outcomes among pregnant women at risk of hypertension in pregnancy, by regular monitoring of weight gain and blood pressure: a pilot randomised controlled trial |
| Methods | 2‐arm parallel unblinded RCT |
| Participants |
Esimated enrolment: 70 high‐risk pregnant women Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention arm: self‐monitoring of BP using Health Gauge Device and monthly monitoring of weight gain Control arm: conventional antenatal and postnatal care only |
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 1 March 2019 |
| Contact information | Syed Imran Ahmed, (+88 02) 9827001‐10 ext 2216, syed.ahmed@icddrb.org S. M. Tafsir Hasan, (+88 02) 9827001‐10 ext 2216, afsir.hasan@icddrb.org |
| Notes | clinicaltrials.gov/ct2/show/NCT03858595 Estimated study completion date: 31 December 2020 |
NCT03978429.
| Study name | Cluster‐randomized, parallel‐group, superiority study to compare the effectiveness of a community‐based mHealth strategy versus enhanced usual care in the detection and management of pre‐eclampsia or eclampsia in Tanzania |
| Methods | 2‐arm parallel blinded (triple blinded: participants, investigators, and outcome assessors) cluster‐RCT |
| Participants |
Estimated enrolment: 3000 pregnant women Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention arm: Community‐based pre‐eclampsia/eclampsia detection and management 3 Community Health Workers (CHWs) per health facility (cluster) will be equipped with android smartphones and BP monitors and visit participant's homes once per month until 6 weeks post‐partum, and complete a case report form for signs and symptoms of pre‐eclampsia and BP. CHWs, antenatal care nurses (ANC) at the woman's health facility and study co‐ordinator will receive SMS message if the algorithm on our platform (informed by the Tanzanian Standard Treatment Guidelines (TSTG) deems she is at risk for pre‐eclampsia. This message will indicate that the woman needs to be referred to her health facility if: participant has high BP, a significant intra‐patient rise in BP, or a combination of factors according to an algorithm. Woman's condition will be assessed and ANC will decide on the management, including rest at home with CHW monitoring. Women found to be at risk for pre‐eclampsia will be visited twice monthly. Strengthened referral network from community to referral hospital levels ANC nurses and CHWs will play complementary roles in performing activities to meet key indicators and facilitate referrals. They will deliver a plan using the pre‐eclampsia application tool on their smartphone, and then they can refer the pregnant woman for enrolment for facility care and track them as per key indicators. The key innovation is that the mHealth platform can detect increases in BP within each person, and this ability to detect is brought down to the community level through CHWs. This will allow for earlier detection of pre‐eclampsia as singular BPs. Nurses will receive protocolised instructions and education regarding when to refer a woman to a higher‐level facility for further management. The program consists of SMS component delivered to provide information about participant's condition to relevant members of the referral pathway to enhance referrals and facilitate community level follow up. Control arm: enhanced usual care
|
| Outcomes |
Primary outcome measures: A composite indicator reflecting activities (1) associated with recognition of pre‐eclampsia by recognizing 1 of these 4 items; systolic BP reading higher than 140 mmHg, diastolic BP higher than 90 mmHg, intra‐patient BP rise of 30 mmHg systolic or 15 mmHg diastolic, proteinuria (+) and headache, visual disturbance, or both, or epigastric pain and vomiting, or both, or edema and (2) activities associated with the management and treatment of pre‐eclampsia Measured at 1 month post delivery |
| Starting date | 31 August 2019 |
| Contact information | Karen E Yeates, 6135403735, yeatesk@queensu.ca |
| Notes | clinicaltrials.gov/ct2/show/NCT03978429 Estimated study completion date: 1 July 2020 |
NCT04031430.
| Study name | PREMOM II: Pregnancy remote monitoring of women with gestational hypertensive disorders |
| Methods | 3‐arm parallel unblinded RCT |
| Participants |
Estimated enrolment: 6107 women at risk of development of gestational hypertensive disorders (GHD) Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention arm 1: telemonitoring — home, twice daily self‐monitoring of BP and activity tracker. These data, alongside weight, inputted weekly into the app, are transmitted to the hospital Intervention arm 2: participant self‐monitoring — home, twice daily self‐monitoring of BP and activity tracker. These data, alongside weight, inputted weekly into the app, are transmitted to the hospital Control arm: no intervention |
| Outcomes |
Primary outcome measures: 1. Gestational age 2. Number of hospitalisations during pregnancy Secondary outcome measures: 1. Number of prenatal consults, ultrasounds, CTGs 2. Number of hospitalisations of the mother at the maternal intensive care (MIC) department 3. Number of days admitted to the maternal intensive care (MIC) 4. Number of medication adaptations during pregnancy 5. Development of gestational hypertensive disorders 6. Onset and mode of delivery 7. Birthweight 8. Apgar at 1 and 5 minutes 9. Admission to and length of stay in the neonatal intensive care 10. Cost for the healthcare services 11. Number of phone calls from the women to the midwife for technical or medical issues 12. Number of phone calls from the midwife to the women for technical or medical issues 13. Number of starts or adjustments to the antihypertensive medication |
| Starting date | 18 June 2019 |
| Contact information | Wilfried Gyselaers (Principal Investigator), + 32 89 32 15 57, wilfried.gyselaers@zol.be Dorien Lanssens (Study Chair), + 32 89 32 15 57, dorien.lanssens@uhasselt.be |
| Notes | clinicaltrials.gov/ct2/show/NCT04031430 Estimated study completion date: 14 April 2023 |
NTR6076.
| Study name | HOspital care versus TELemonitoring in high risk pregnancy: the HOTEL trial |
| Methods | 2‐arm parallel unblinded RCT |
| Participants |
Target: 200 pregnant women Inclusion criteria: Necessity for hospital admittance for maternal or fetal surveillance because of 1 of the following indications: intrauterine growth retardation, pre‐eclampsia, premature rupture of membranes, recurrent reduced fetal movements, fetal anomalies requiring daily monitoring, or fetal demise in obstetric history Exclusion criteria:
• Pregnancy complications requiring intravenous therapeutics or obstetric intervention within 48 hours • BP > 160/110 mmHg • Antepartum haemorrhage or signs of placental abruption • CTG registration with abnormalities indicating fetal distress or hypoxia • Multiple pregnancies • Place of residence 30 minutes drive away from a hospital • Insufficient knowledge of Dutch or English language, or inability to understand the training or instructions of the devices |
| Interventions |
Intervention arm: telemonitoring for daily monitoring of maternal and fetal parameters. The telemonitoring group will use a wireless CTG registration device and a home BP monitor, and will have daily telephone calls with an obstetric healthcare professional in the hospital. Weekly outpatient visits for real‐time contact and ultrasound assessment, blood sampling, or urinary analysis if necessary. Control arm: traditional hospital admittance |
| Outcomes |
Primary outcomes: 1. Participant safety 2. Composite measure of perinatal outcome: perinatal mortality, a 5‐minute Apgar score below 7, or an arterial pH below 7.05, or both 3. Maternal morbidity (such as eclampsia, HELLP syndrome, thromboembolic events) 4. Neonatal intensive care admissions 5. Emergency caesarean section Secondary outcome: Participant satisfaction, quality of life, cost effectiveness, preferences for the new strategy |
| Starting date | 1 November 2016 |
| Contact information | JFM (Hans) van den Heuvel, 0887577249, hotelstudie@umcutrecht.nl |
| Notes |
www.trialregister.nl/trial/5888 Stop date: 31 January 2020 |
BP: blood pressure CTG: cardiotocograph RCT: randomised controlled trial
Differences between protocol and review
There are some differences between the published protocol for our review (Nathan 2017), and the full review; these are listed below.
Natasha L Hezelgrave and Mariana Widmer have left the review team. Danielle C Ashworth, Sophie P Maule, and Fiona Stewart joined the review team.
We amended the objective slightly to include 'managing' as well as diagnosing hypertensive disorders. We clarified eligible interventions and participants, addressing the inclusion of cluster‐RCTs delivered at a population level, where the intervention is primarily intended for pregnant women.
We clarified outcome measures and slightly altered GRADE outcomes to reflect clinically important outcomes.
We removed the following outcomes.
Neonatal outcomes:
Death within the first 28 days after birth
Admission to a special care nursery for more than seven days
We clarified the following outcomes were clarified.
Cerebrovascular accident edited to cerebrovascular event
Maternal measures of anxiety levels and self‐confidence split into maternal anxiety and maternal self‐confidence
Special care nursery edited to neonatal unit
Admissions split into admission and length of stay for both maternal admission to intensive care and neonatal unit admissions
We removed the following outcomes from the 'Summary of findings' tables.
Maternal
Eclampsia
Cerebrovascular event
Renal failure
HELLP syndrome
Pulmonary oedema
Neonatal
Admission to a special care nursery for more than seven days
We added the following outcomes to the 'Summary of findings' tables.
Maternal
Pre‐eclampsia
Number of antenatal hospital admissions
Maternal admission to intensive care
Induction of labour
Neonatal
Perinatal mortality
Neonatal unit admission
Neonatal unit length of stay
Neonatal endotracheal intubation and use of mechanical ventilation
Contributions of authors
Danielle C Ashworth, Sophie P Maule, Fiona Stewart, and Lucy C Chappell contributed to the co‐ordination and writing of the review. Danielle C Ashworth, Sophie P Maule, and Fiona Stewart undertook assessment, data extraction, and data entry (including data extraction, assessment of risk of bias and assessment of the certainty of the evidence).
Hannah Nathan contributed to the design of the review, contributed to the writing of the protocol, and made substantial contributions when reviewing and revising the review. Lucy C Chappell and Andrew Shennan contributed to conceiving and designing the review: providing a methodological, clinical, policy, and consumer perspective; writing the protocol; made substantial contributions when reviewing and revising the review, and provided general advice on the review. All authors reviewed and approved the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Reviews of NICE Priority funding to Cochrane Pregnancy and Childbirth (award number 131142)
Declarations of interest
This project was supported by the National Institute for Health Research, via Cochrane Reviews of NICE Priority funding to Cochrane Pregnancy and Childbirth (award number 131142).
Danielle C Ashworth ‐ none known
Sophie P Maule ‐ none known
Fiona Stewart ‐ none known
Hannah Nathan ‐ Dr Nathan was involved in the development of the CRADLE VSA device with Professor Shennan and is co‐author of the CRADLE‐3 study paper (Vousden 2019).
Andrew Shennan – Professor Shennan is the inventor of the CRADLE VSA device and co‐author of the CRADLE‐3 study paper (Vousden 2019).
Lucy C Chappell ‐ Professor Chappell is a co‐author of the OPTIMUM‐BP study paper (Pealing 2019a), and the CRADLE‐3 study paper (Vousden 2019).
Hannah Nathan, Andrew Shennan, and Lucy C Chappell had no part in the assessment, data extraction, or data entry for either the OPTIMUM‐BP study paper (Pealing 2019a), or the CRADLE‐3 study paper (Vousden 2019).
New
References
References to studies included in this review
Brown 1998 {published data only}
- Brown MA, Buddle ML, Farrell T, Davis G, Jones M. Randomised trial of management of hypertensive pregnancies by Korotkoff phase IV or phase V. Lancet 1998;352(9130):777-81. [DOI] [PubMed] [Google Scholar]
- Brown MA. Does it matter which Korotkoff sound is used to determine diastolic blood pressure in hypertensive pregnancies? In: 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 Aug 4-8; Seattle, Washington, USA. 1996:Abstract no: O86.
Pealing 2019a {published data only}ISRCTN16018898
- ISRCTN16018898. OPTIMUM: Optimising titration and monitoring of maternal blood pressure. isrctn.com/ISRCTN16018898 (first received 4 July 2016).
- Pealing L, Crawford C, Wilson H, Tucker K, MacKillop L, Churchill D, et al. Self-monitoring blood pressure in hypertensive pregnancies: the optimum-BP pilot randomised controlled trial. BJOG 2019;126(6):e139. [Google Scholar]
- Pealing LM, Tucker KL, Mackillop LH, Crawford C, Wilson H, Nickless A, et al. A randomised controlled trial of blood pressure self-monitoring in the management of hypertensive pregnancy. OPTIMUM-BP: A feasibility trial. Pregnancy Hypertension 2019;18:141-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vousden 2019 {published data only}ISRCTN41244132
- ISRCTN41244132. Evaluation of a novel device in the management of high blood pressure and shock in pregnancy in low-resource settings. isrctn.com/ISRCTN41244132 (first received 24 November 2015).
- Nathan HL, Duhig K, Vousden N, Lawley E, Seed PT, Sandall J, et al. Evaluation of a novel device for the management of high blood pressure and shock in pregnancy in low-resource settings: study protocol for a stepped-wedge cluster-randomised controlled trial (CRADLE-3 trial). Trials 2018;19(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden N, Lawley E, Nathan HL, Seed PT, Gidiri MF, Goudar S, et al, CRADLE Trial Collaborative Group. Effect of a novel vital sign device on maternal mortality and morbidity in low-resource settings: a pragmatic, stepped-wedge, cluster-randomised controlled trial. Lancet Global Health 2019;7(3):e347-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden N, Lawley E, Seed PT, Gidiri MF, Charantimath U, Makonyola G, et al. Exploring the effect of implementation and context on a stepped wedge randomized-controlled trial of a vital sign triage device in routine maternity care in low-resource settings. BJOG: An International Journal of Obstetrics and Gynaecology 2019;126:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden N, Lawley E, Seed PT, Gidiri MF, Charantimath U, Makonyola G, et al. Exploring the effect of implementation and context on a stepped-wedge randomised controlled trial of a vital sign triage device in routine maternity care in low-resource settings. Implementation Science 2019;14(1):38. [DOI] [PMC free article] [PubMed]
- Vousden N, Lawley E, Seed PT, Gidiri MF, Goudar S, Sandall J, et al. Incidence of eclampsia and related complications across 10 low- and middle-resource geographical regions: secondary analysis of a cluster randomised controlled trial. PLoS Medicine 2019;16(3):e1002775. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden N, Lawley E, Seed PT, Sandall J, Chappell L, Shennan AH, et al. CRADLE-3: a trial of a novel device to reduce maternal mortality/ morbidity in low resource settings. International Journal of Gynaecology and Obstetrics 2018;143:233.
References to studies excluded from this review
Aslani 2014 {published data only}
- Aslani A, Tara F, Ghalighi L, Pournik O, Ensing S, Abu-Hanna A, et al. Impact of computer-based pregnancy-induced hypertension and diabetes decision aids on empowering pregnant women. Healthcare Informatics Research 2014;20(4):266-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2012 {published data only}
- Brown M, Roberts L, Mackenzie C, Mangos G, Davis G. A prospective randomised study of automated versus mercury blood pressure recordings in hypertensive pregnancy ('Pram' Study). Hypertension in Pregnancy 2008;27(4):521. [DOI] [PubMed] [Google Scholar]
- Brown MA, Roberts LM, Mackenzie C, Mangos G, Davis GK. A prospective randomized study of automated versus mercury blood pressure recordings in hypertensive pregnancy (PRAM Study). Hypertension in Pregnancy 2012;31(1):107-19. [DOI] [PubMed] [Google Scholar]
- Davis GK, McHugh L, Brown MA, Mangos GJ, Bowyer L, Buddel ML. A prospective study of automated versus mercury ('PRAM') recording in pregnancy. Hypertension in Pregnancy 2002;21(Suppl 1):37. [DOI] [PubMed] [Google Scholar]
- NCT00809666. A prospective randomised study of automated versus mercury blood pressure recordings in pregnancy. clinicaltrials.gov/ct2/show/NCT00809666 (first received 17 December 2008).
Cartwright 1992 {published data only}
- Cartwright W, Dalton KJ, Swindells H, Rushant S, Mooney P. Objective measurement of anxiety in hypertensive pregnant women managed in hospital and in the community. British Journal of Obstetrics and Gynaecology 1992;99:182-5. [DOI] [PubMed] [Google Scholar]
Denolle 2008 {published data only}
- Denolle T, Weber JL, Calvez C, Getin Y, Daniel JC, Lurton O, et al. Diagnosis of white coat hypertension in pregnant women with teletransmitted home blood pressure. Hypertension in Pregnancy 2008;27(3):305-13. [DOI] [PubMed] [Google Scholar]
Duggan 1999 {published data only}
- Duggan PM. Quiet resting is not necessary prior to routine antenatal blood pressure measurement. Australian & New Zealand Journal of Obstetrics & Gynaecology 1999;39(1):19-20. [DOI] [PubMed] [Google Scholar]
Holm 2019 {published data only}
- Holm L, Stucke-Brander T, Wagner S, Sandager P, Schlutter J, Lindahl C, et al. Automated blood pressure self-measurement station compared to office blood pressure measurement for first trimester screening of pre-eclampsia. Health Informatics Journal 2019;25(4):1815-24. [DOI] [PubMed] [Google Scholar]
Leung 1998 {published data only}
- Leung KY, Sum TK, Tse CY, Law KW, Chan MY. Is in-patient management of diastolic blood pressure between 90 and 100 mmHg during pregnancy necessary? Hong Kong Medical Journal 1998;4:211-7. [PubMed] [Google Scholar]
Mangos 2012 {published data only}
- Mangos GJ, Ram J, Brown MA. The effect of cuff size on automated blood pressure measurement. Journal of Hypertension 2012;30:e287. [Google Scholar]
NCT03222414 {published data only}
- NCT03222414. Non-invasive versus invasive blood pressure measurement in the morbidly obese parturient with severe preeclampsia. clinicaltrials.gov/ct2/show/record/NCT03222414 (first received 19 July 2017).
Rhodes 2018 {published data only}
- Rhodes C, Churchill D. A randomised comparison of ambulatory blood pressure measurement (ABPM), versus conventional blood pressure measurement, for the management of pregnant hypertensive women. Hypertension in Pregnancy 2006;25(Suppl 1):166. [Google Scholar]
- Rhodes C, Churchill D. Randomized comparison of ambulatory blood pressure measurement (ABPM), versus conventional blood pressure measurement, for the management of pregnant hypertensive women. Journal of Obstetrics and Gynaecology 2005;25 Suppl 1:S16. [Google Scholar]
- Rhodes CA, Beevers DG, Churchill D. A randomized trial of ambulatory blood pressure monitoring versus clinical blood pressure measurement in the management of hypertension in pregnancy. A feasibility study. Pregnancy Hypertension 2018;11:142-4. [DOI] [PubMed] [Google Scholar]
Ross‐McGill 2000 {published data only}ISRCTN20643323
- ISRCTN20643323. Home blood pressure recording in pregnancy - a pilot study for a randomised trial. isrctn.com/ISRCTN20643323 (first received 23 January 2004).
- Ross-McGill H, Hewison J, Hirst J, Dowswell T, Holt A, Brunskill P, et al. Antenatal home blood pressure monitoring: a pilot randomised controlled trial. British Journal of Obstetrics and Gynaecology 2000;107(2):217-21. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Dougall 2020 {published data only}
- Dougall G, Franssen M, Tucker KL, Yu L-M, Hinton L, Abel L, et al. Blood pressure monitoring in high-risk pregnancy to improve the detection and monitoring of hypertension (the BUMP 1 and 2 trials): protocol for two linked randomised controlled trials. BMJ Open 2020;10(1):e034593. [CENTRAL: CN-02072405] [EMBASE: 630719140] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03334149. Blood pressure monitoring in high risk pregnancy to improve the detection and monitoring of hypertension. clinicaltrials.gov/show/NCT03334149 (first received 7 November 2017).
- Tucker K, Dougall G, Hinton L, Arias OR, Wilson H, Crawford C, et al. Self-monitoring of blood pressure during pregnancy: the BUMP trials. BJOG 2019;126(S2):159.
NCT03509272 {published data only}
- NCT03509272. LimPrOn: Limburg Pre-eclampsia Investigation. clinicaltrials.gov/show/NCT03509272 (first received 2018 April 26). [CENTRAL: CN-01586280]
NCT03648645 {published data only}
- NCT03648645. Hypertensive pregnant women monitored by teletransmitted self-measurements of blood pressure. clinicaltrials.gov/ct2/show/NCT03648645 (first received 27 August 2018).
NCT03858595 2019 {published data only}
- NCT03858595. Optimizing gestational weight gain, birth weight and other perinatal outcomes among pregnant women at risk of hypertension in pregnancy by regular monitoring of weight gain and blood pressure: a pilot randomized controlled trial. clinicaltrials.gov/show/NCT03858595 (first received 2019 February 28). [CENTRAL: CN-02089793]
NCT03978429 {published data only}
- NCT03978429. An mHealth strategy to reduce pre-eclampsia and infant death in Tanzania. clinicaltrials.gov/show/nct03978429 (first received 2019 Jun 7). [CENTRAL: CN-01945374]
NCT04031430 {published data only}
- NCT04031430. PREMOM II: Pregnancy remote monitoring of women with gestational hypertensive disorders. clinicaltrials.gov/ct2/show/NCT04031430 (first received 24 July 2019).
NTR6076 {published data only}
- NTR6076. HOspital care versus TELemonitoring in high risk pregnancy: the HOTEL trial. trialregister.nl/trialreg/admin/rctview.asp?TC=6076 (first received 19 September 2016).
Additional references
ACOG 2013
Bello 2018
- Bello NA, Woolley JJ, Cleary KL, Falzon L, Alpert BS, Oparil S, et al. Accuracy of blood pressure measurement devices in pregnancy: a systematic review of validation studies. Hypertension 2018;71(2):326-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellomo 1999
- Bellomo G, Narducci PL, Rondoni F, Pastorelli G, Stangoni G, Angeli G, et al. Prognostic value of 24-hour blood pressure in pregnancy. JAMA 1999;282(15):1447-52. [DOI] [PubMed] [Google Scholar]
Bergel 2002
- Bergel E, Carroli G, Althabe F. Ambulatory versus conventional methods for monitoring blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD001231. [DOI: 10.1002/14651858.CD001231] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernal 2017
- Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. International Journal of Epidemiology 2017;46(1):348-55. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2000
- Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J, et al. The detection, investigation and management of hypertension in pregnancy: executive summary. Australian & New Zealand Journal of Obstetrics & Gynaecology 2000;40:133–8. [DOI] [PubMed] [Google Scholar]
Brown 2005
- Brown MA, Mangos G, Davis G, Homer C. The natural history of white coat hypertension during pregnancy. BJOG 2005;112(5):601–6. [DOI] [PubMed] [Google Scholar]
Cantwell 2011
- Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011;118(Suppl 1):1–203. [DOI] [PubMed] [Google Scholar]
Canzanello 2001
- Canzanello VJ, Jensen PL, Schwartz GL. Are aneroid sphygmomanometers accurate in hospital and clinic settings? Archives of Internal Medicine 2001;161(5):729-31. [DOI] [PubMed] [Google Scholar]
Cuckson 2004
- Cuckson AC, Moran P, Seed P, Reinders A, Shennan AH. Clinical evaluation of an automated oscillometric blood pressure wrist device. Blood Pressure Monitoring 2004;9(1):31-7. [DOI] [PubMed] [Google Scholar]
Duley 2009
- Duley L. The global impact of pre-eclampsia and eclampsia. Seminars in Perinatology 2009;33(3):103-7. [DOI] [PubMed] [Google Scholar]
Franx 1994
- Franx A, Post JA, Elfering IM, Veerman DP, Merkus HM, Boer K, et al. Validation of automated blood pressure recording in pregnancy. British Journal of Obstetrics and Gynaecology 1994;101(1):66-9. [DOI] [PubMed] [Google Scholar]
Franx 1997
- Franx A, Post JA, Montfrans GA, Bruinse HW. Comparison of an auscultatory versus an oscillometric ambulatory blood pressure monitor in normotensive, hypertensive, and preeclamptic pregnancy. Hypertension in Pregnancy 1997;16(2):187-202. [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version Version accessed March 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Green 1996
- Green LA, Froman RD. Blood pressure measurement during pregnancy: auscultatory versus oscillatory methods. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1996;25(2):155-9. [DOI] [PubMed] [Google Scholar]
Greer 1993
- Greer IA. Ambulatory blood pressure in pregnancy: measurement and machines. British Journal of Obstetrics and Gynaecology 1993;100(10):887-9. [DOI] [PubMed] [Google Scholar]
Gupta 1997
- Gupta M, Shennan AH, Halligan A, Taylor DJ, Swiet M. Accuracy of oscillometric blood pressure monitoring in pregnancy and pre-eclampsia. British Journal of Obstetrics and Gynaecology 1997;104(3):350-5. [DOI] [PubMed] [Google Scholar]
Halligan 1993
- Halligan A, O'Brien E, O'Malley K, Mee F, Atkins N, Conroy R, et al. Twenty-four-hour ambulatory blood pressure measurement in a primigravid population. Journal of Hypertension 1993;11(8):869-73. [DOI] [PubMed] [Google Scholar]
Hermida 2000
- Hermida RC, Ayala DE, Mojon A, Fernandez JR, Alonso I, Silva I, et al. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension 2000;36(2):149–58. [DOI] [PubMed] [Google Scholar]
Hewitt 1988
- Hewitt B, Newnham J. A review of the obstetric and medical complications leading to the delivery of infants of very low birthweight. Medical Journal of Australia 1988;149(5):234. [DOI] [PubMed] [Google Scholar]
Higgins 1997
- Higgins JR, Walshe JJ, Halligan A, O'Brien E, Conroy R, Darling MR. Can 24-hour ambulatory blood pressure measurement predict the development of hypertension in primigravidae? British Journal of Obstetrics and Gynaecology 1997;104(3):356-62. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hutcheon 2011
- Hutcheon JA, Lisonkova S, Joseph K. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Practice & Research. Clinical Obstetrics & Gynaecology 2011;25(4):391-403. [DOI] [PubMed] [Google Scholar]
ISO 2013
- ISO– International Standard. Non-invasive Sphygmomanometers. Part 2: Clinical Investigation of Automated Measurement Type. Geneva: International Organization of Standardisation, 2013. [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066-74. [DOI] [PubMed] [Google Scholar]
Knight 2016
- Knight M NM, Tuffnell D, Kenyon S, Shakespeare J, Brocklehurst P, Kurinczuk JJ, editors, on behalf of MBRRACE-UK. Saving Lives, Improving Mothers’ Care - Surveillance of Maternal Deaths in the UK 2012-14 and Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009-14. Oxford: National Perinatal Epidemiology Unit, University of Oxford, 2016. [Google Scholar]
Lo 2002
- Lo C, Taylor RS, Gamble G, McCowan L, North RA. Use of automated home blood pressure monitoring in pregnancy: is it safe? American Journal of Obstetrics and Gynecology 2002;187(5):1321-8. [DOI] [PubMed] [Google Scholar]
MacKay 2001
- MacKay AP, Berg CJ, Atrash HK. Pregnancy‐related mortality from preeclampsia and eclampsia. Obstetrics and Gynecology 2001;97(4):533-8. [DOI] [PubMed] [Google Scholar]
Martin 2005
- Martin JN Jr, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstetrics and Gynecology 2005;105(2):246–54. [DOI] [PubMed] [Google Scholar]
McNutt 2003
- McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American Journal of Epidemiology 2003;157(10):940-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moustgaard 2020
- Moustgaard H, Clayton GL, Jones HE, Boutron I, Jørgensen L, Laursen DRT, et al. Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study. BMJ 2020;368:l6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nataranjan 1999
- Natarajan P, Shennan A, Penny J, Halligan A, De Swiet M, Anthony J. Comparison of auscultatory and oscillometric automated blood pressure monitors in the setting of preeclampsia. American Journal of Obstetrics and Gynecology 1999;181(5 Pt 1):1203–10. [DOI] [PubMed] [Google Scholar]
Nathan 2015
- Nathan HL, Duhig K, Hezelgrave NL, Chappell LC, Shennan AH. Blood pressure measurement in pregnancy. The Obstetrician & Gynaecologist 2015;17(2):91-8. [Google Scholar]
NICE 2008
- National Institute for Health and Care Excellence. Antenatal Care for Uncomplicated Pregnancies (CG62). National Institute for Health and Care Excellence, 2008. [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. Hypertension in Pregnancy: Diagnosis and Management (NG133). National Institute for Health and Care Excellence, 2019. [PubMed] [Google Scholar]
NICE 2019a
- National Institute for Health and Care Excellence. Hypertension in Adults: Diagnosis and Management (NG136). National Institute for Health and Care Excellence, 2019. [Google Scholar]
O'Brien 1993
- O’Brien E, Petrie J, Littler W, Swiet M, Padfield PL, Altman D, et al. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. Journal of Hypertension 1993;11(2):S43-62. [DOI] [PubMed] [Google Scholar]
O'Brien 1993a
- O'Brien E, Mee F, Atkins N, Halligan A, O'Malley K. Accuracy of the SpaceLabs 90207 ambulatory blood pressure measuring system in normotensive pregnant women determined by the British Hypertension Society protocol. Journal of Hypertension 1993;11 Suppl(5):S282-3. [PubMed] [Google Scholar]
O'Brien 2001
- O'Brien E, Beevers G, Lip GY. ABC of hypertension: blood pressure measurement. Part IV - Automated sphygmomanometry: self blood pressure measurement. BMJ 2001;322(6):1167-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2001a
- O'Brien E, Waeber B, Parati G, Staessen J, Myers M G. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ (Clinical Research Ed.) 2001;322(7285):531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2002
- O'Brien E, Pickering T, Asmar R, Myers M, Parati G, Staessen J, et al, Working Group on Blood Pressure Monitoring of the European Society of Hypertension. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Pressure Monitoring 2002;7(1):3-17. [DOI] [PubMed] [Google Scholar]
O'Brien 2003
- O'Brien E, Asmar R, Beilin L, Imai Y, Mallion J-M, Mancia G, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. Journal of Hypertension 2003;21(5):821-48. [DOI] [PubMed] [Google Scholar]
O'Brien 2010
- O'Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Pressure Monitoring 2010;15:23–38. [DOI] [PubMed] [Google Scholar]
Penny 1996
- Penny JA, Shennan AH, Rushbrook J, Halligan Aidan W, Taylor DJ, Swiet M. Validation of the Welch Allyn Quiettrak ambulatory blood pressure monitor in pregnancy. Hypertension in Pregnancy 1996;15(3):313-21. [Google Scholar]
Perry 1991
- Perry IJ, Wilkinson LS, Shinton RA, Beevers DG. Conflicting views on the measurement of blood pressure in pregnancy. British Journal of Obstetrics and Gynaecology 1991;98(3):241-3. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shennan 1993
- Shennan AH, Kissane J, Swiet M. Validation of the SpaceLabs 90207 ambulatory blood pressure monitor for use in pregnancy. British Journal of Obstetrics and Gynaecology 1993;100(10):904-8. [DOI] [PubMed] [Google Scholar]
Shennan 1996
- Shennan A, Halligan A, Gupta M, Taylor D, Swiet M. Oscillometric blood pressure measurements in severe pre-eclampsia: validation of the SpaceLabs 90207. British Journal of Obstetrics and Gynaecology 1996;103(2):171-3. [DOI] [PubMed] [Google Scholar]
Wagner 2011
- Wagner SJ, Acquah LA, Lindell EP, Craici IM, Wingo MT, Rose CH, et al. Posterior reversible encephalopathy syndrome and eclampsia: pressing the case for more aggressive blood pressure control. Mayo Clinic Proceedings 2011;86(9):851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Nathan 2017
- Nathan HL, Hezelgrave NL, Widmer M, Chappell LC, Shennan AH. Setting and techniques for monitoring blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD012739. [DOI: 10.1002/14651858.CD012739] [DOI] [PMC free article] [PubMed] [Google Scholar]


